Abstract
Background
Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has improved the survival of patients with peritoneal surface malignancy. Upon recurrence, a repeat CRS/HIPEC is a treatment option.
Study Design
A retrospective analysis of 868 CRS/HIPEC procedures was performed. Type of primary, functional status, completion of resection, hospitalization, morbidity, mortality and survival were reviewed.
Results
Sixty-two patients (7.7%) underwent a second CRS/HIPEC including thirty-three patients with appendiceal primaries, 8 ovarian, 7 mesotheliomas, 4 colon cancers and 10 various malignancies. Median follow up was 60.8 months. The median overall survival in months was 85.3 for appendiceal cancer, 52.9 for mesothelioma, 60.1 for ovarian, and 137.4 for colon cancer. R1 resection was achieved in 43.5% after both procedures. Median survival after the second cytoreduction was 52.1 months for appendiceal cancer, 21.8 for mesothelioma, 53.9 for ovarian and 55.7 for colon cancer. The median survival was 55.7 months for R1 resection, 20.3 months for R2a resection, and 15.5 months for R2b-R2c. Median ICU and hospital stay was 1 and 7.5 days, respectively. The 30 day morbidity after the second CRS/HIPEC was 48.4% and mortality was 3.2%. In multivariate analysis the R status of the second CRS/HIPEC (p=0.013) and the interval between the two procedures (p=0.009) were significant in predicting improved survival.
Conclusions
In experienced tertiary centers and for selected patients, a repeat CRS/HIPEC procedure has similar morbidity and mortality with the initial cytoreduction. Survival depends primarily on the completion of the repeat cytoreduction and favorable biology of the tumor.
Introduction
Cytoreductive surgery (CRS) followed by hyperthermic intraperitoneal chemotherapy (HIPEC), is a treatment option for peritoneal carcinomatosis (PC). This treatment modality has resulted in long-term survival for selected patients with peritoneal surface malignancies(1-3). The principle underlying this therapeutic modality is the initial surgical resection of all macroscopic peritoneal disease, and subsequently treating any residual, microscopic peritoneal disease with hyperthermic chemotherapy.
Intraperitoneal chemotherapy provides a higher local concentration of the chemotherapeutic agents than can be achieved with even the most aggressive dosing, and the addition of hyperthermia results in a potentiation of cytotoxicity. Reported 5-year survival rates for pseudomyxoma, peritoneal carcinomatosis of a colorectal origin, and peritoneal mesothelioma ranges from 60-80%, 25-51%, and 29-63%, respectively (3-5).
Unfortunately, peritoneal recurrence is noted in a substantial number of these patients within 3 years following CRS/HIPEC. Approximately 80% for patients with PC of a colorectal origin, 40% for patients with mesothelioma, and 25-44% for patients with pseudomyxoma (6-8).
The primary goal of this paper is to evaluate the utility of a repeat CRS/HIPEC by recording the procedure's specific morbidity and mortality. The secondary goal is to describe the impact of the procedure to overall survival.
Materials and Methods
This is a retrospective analysis of a prospectively maintained database of 868 CRS/HIPEC procedures.
Patient data relevant to our analysis included age, race, gender, ECOG graded functional status, date of initial and repeat CRS/HIPEC, chemoperfusion agent, R status of resection, type of malignancy, hospital and ICU stay, morbidity, mortality and median survival. Eligibility criteria for initial CRS/HIPEC were ECOG ≤3, histologic or cytologic diagnosis of peritoneal carcinomatosis and complete recovery from prior systemic chemotherapy or radiation treatments, resectable or resected primary lesion, debulkable peritoneal disease, no extra-abdominal disease and limited medical co-morbidities. Patients were considered for second CRS HIPEC if they had an initial R0, R1 or R2a resection, had a complete recovery from prior systemic chemotherapy or radiation treatments, were ECOG 0, 1 or 2, had disease considered to be resectable based upon imaging, and had no extra-abdominal disease. Selected patients with R2b and R2c were considered for second procedures if all disease near bowel and bile ducts was considered resectable. All patients had a complete history and physical, tumor markers and CT of the chest, abdomen and pelvis before all HIPEC procedures. The CRS/HIPEC procedure was as conducted as previously described by our group (9). An IRB approval was obtained for this study.
Statistical Analyses
Descriptive statistics, including frequencies and proportions for categorical data, means and standard deviations for continuous outcomes, were calculated for all study measures. To assess for differences between study groups, Fisher's Exact Tests were used as it is more efficient than a chi-square test when cell counts are small. These tests give the exact p-value, rather than an approximation, of the observed cell frequencies. P-values less than 0.05 were considered to be statistically significant. Survival estimates were calculated using the Kaplan-Meier method; to compare groups, the log-rank test of the chi-square approximation was used. To assess which factors were significant in a multivariate model, proportional hazards regression was used. Statistical analyses were performed using SPSS 19 and SAS 9.2.
Results
From 1993 to 2010, 66 repeat CRS/HIPEC procedures for isolated peritoneal tumor recurrence were performed in 62 patients in our institution. The mean age of patients was 46.4 ± 11 years. These 62 patients represent a highly selected subset (7.7%) from 868 patients who underwent a CRS/HIPEC procedure.
The origin of the primary tumors was: appendiceal 33 (53.2%), colorectal cancer 4 (6.5%), malignant mesothelioma 7 (11.3%), gallbladder cancer 1 (1.6%), gastric cancer 2 (3.2%), GIST 2 (3.2%), ovarian 8 (12.9%), sarcoma 2 (3.2%), small bowel 2 (3.2%), urachal 1 (1.6%). (Table 1)
Table 1. Clinicopathologic Characteristics of 62 Patients who Underwent 66 Repeat Cytoreductive Surgery/Hyperthermic Intraperitoneal Chemotherapy for Recurrent Peritoneal Carcinomatosis.
| Characteristics | n=62 |
|---|---|
| Age, y | 46.4 ± 11 |
| Sex | |
| Male | 27(43.5%) |
| Female | 35(56.5% |
| ECOG status | |
| 0 | 23(37.7%) |
| 1 | 30(49.2%) |
| 2 | 5(8.2%) |
| 3 | 3(4.9%) |
| BMI (kg/m2) | 27.8 ± 7.0 |
| Primary tumor (n = 62), n (%) | |
| Appendiceal | 33 (53.2) |
| Colorectal | 4 (6.5) |
| Mesothelioma | 7 (11.3) |
| Ovarian | 8 (12.9) |
| Gastric/GIST | 4 (6.5) |
| Gallbladder | 1 (1.6) |
| Sarcoma | 2 (3.2) |
| Small bowel | 2 (3.2) |
| Urachal | 1 (1.6) |
| No. of CRS+ HIPEC | |
| Two | 62 |
| Three | 4 |
| Resection status after first HIPEC, n (%) | |
| R0-R1 | 27 (43.5) |
| R2a | 25 (40.3) |
| R2b-R2c | 10 (16.1) |
| Resection status after second HIPEC, n (%) | |
| R0-R1 | 27 (43.5) |
| R2a | 16 (25.8) |
| R2b-R2c | 19 (30.6) |
| Peritoneal Carcinomatosis indexa | 9.2 ± 5.1 |
| Adjuvant therapy after previous CRS+HIPEC, n (%) | 11 (17.7) |
| Interval between previous CRS + HIPEC and diagnosis of recurrence, mo* | 27.0 +/- 29.0 |
Mean ± SD.
CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy.
The mean interval between the initial CRS/HIPEC and the diagnosis of peritoneal recurrences was 27.0 ± 29.0 months; the median estimate was 17.0 months, with a range of (4.1 - 143.0) months.
Resection status following repeat CRS/HIPEC
Of the 27 patients who had an R0-R1 resection during their first CRS/HIPEC, 16 (59.3%) had an R0-R1 resection during the repeat CRS/HIPEC, 6 (22.2%) had an R2a resection, while 5 (18.7%) had an R2b-R2c resection status. Of the 25 patients who had an R2a resection during their first CRS/HIPEC, 9 (36%) had an R0-R1 resection during the repeat CRS/HIPEC, 9 (36%) had an R2a resection, while 7 (18.7%) had an R2b-R2c resection status. Of the 10 patients who had an R2b-R2c resection during their first CRS/HIPEC, 2 (20%) had an R0-R1 resection during the repeat CRS/HIPEC, 1 (10%) had an R2a resection, while 7 (70%) had an R2b-R2c resection status. (Table 1)
Postoperative Morbidity and Mortality and Duration of Hospitalization
The thirty day post-operative mortality rate was 3.2% (2 patients). The postoperative morbidity rate was 43.5% (27 patients). Of these 27 patients, 18 (66.7%) suffered a Clavien-Dindo grade I/II complication, while 9 (33.3%) suffered a Clavien-Dindo grade III/IV complication (10) (Table 2). Median hospital stay was 7.5 days (range 4–49 days). The mean duration of postoperative hospitalization on the surgical floor was similar after the first and second cytoreduction (7 vs 7.5 days) with the repeat CRS/HIPEC patients requiring less ICU time (1.5 vs 1 day).
Table 2. Recorded Complication Rate after First and Second Cytoreductive Surgery/Hyperthermic Intraperitoneal Chemotherapy Procedure.
| No complications | Major + Minor complications | Death within 30 d | |
|---|---|---|---|
| 1ST HIPEC, n=62 | 40 | 22 | 0 |
| 2ND HIPEC, n=62 | 32 | 30 | 2 |
HIPEC, hyperthermic intraperitoneal chemotherapy.
Survival and Prognostic Factors
Median follow-up was 60.8 months after the second CRS/HIPEC (range 1–111 months). The median overall survival for the 62 patients was 32.2 months (25th and 75th percentile of 14.6, 71.2) following repeat CRS/HIPEC. Patients who had an R0-R1 resection status had a significantly better median survival: 55.7 months compared to 20.3 and 15.5 months, respectively for patients who had an R2a or R2b-R2c, respectively (p=0.027), as shown in Figure 1. Overall, one, three and five year overall survival rates were 78.7(+/- 5.5)%, 48.6 (+/-7.5)% and 31.6 (+/- 7.4)%, respectively. The median overall survival after both procedures for this group of patients was 85.3 months for appendiceal cancer, 52.9 for mesothelioma, 60.1 for ovarian and 137.4 months for colon cancer. The median survival after the second CRS/HIPEC based on type of malignancy was 52.1 months for appendiceal cancer, 21.8 months for mesothelioma, 53.9 for ovarian and 55.7 months for colon cancer. (Table 3)
Figure 1.
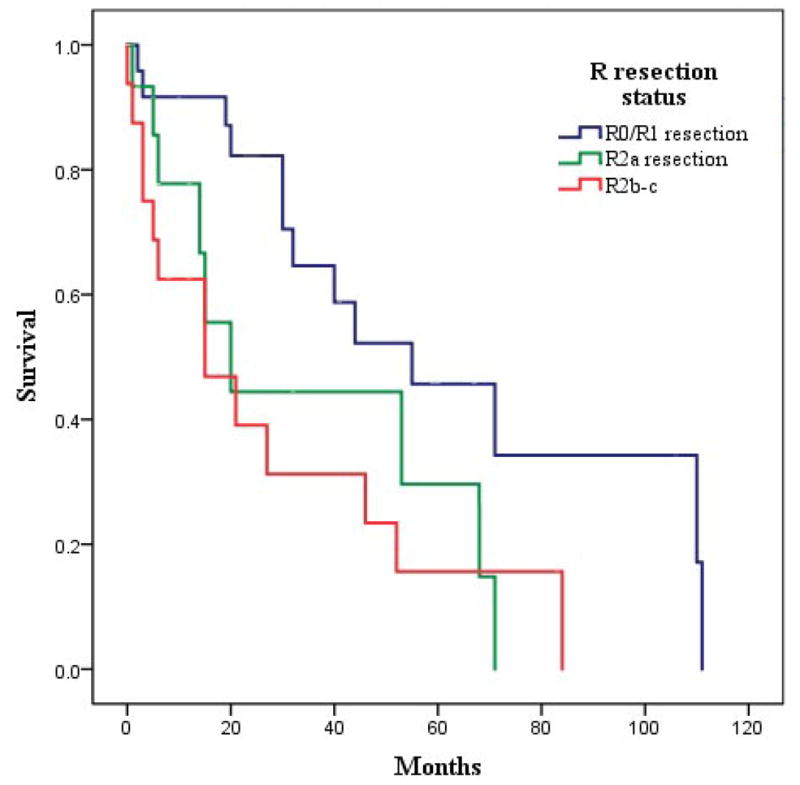
Overall survival as a function of R resection status.
Table 3. Median Survival Based on Primary and Resection Status after First and Second Cytoreductive Surgery/Hyperthermic Intraperitoneal Chemotherapy.
| First HIPEC | Range | Second HIPEC | Range | |
|---|---|---|---|---|
| Appendix (all) (n=33) | 85.3 | 11.5-148.5 | 52.1 | 0.1-111.7 |
| Low grade (appendix) (n=28) | 108.5 | 11.5-148.5 | 71.3 | 0.1-111.7 |
| Mesothelioma (n=7) | 52.9 | 10.7-67.7 | 21.8 | 3.1-44.1 |
| Ovarian (n=8) | 60.1 | 15.8-111.4 | 53.9 | 0.6+-106.1 |
| Colon (n=4) | 137.4 | 13.6-164.6 | 55.7 | 0.3-110.2 |
| R0/1 | 77.6 | 7.8-148.5 | 55.7 | 0.3+-111.7 |
| R2a | 63.5 | 9.5+-164.6 | 20.3 | 0.6+-71.3 |
| R2b | 60.3 | 11.5-71.0 | 46.8 | 1.3-84.1 |
| R2c | 12.3 | 10.7-21.0 | 4.7 | 0.1-27.7 |
Based on type of malignancy and resection status, units in months.
HIPEC, hyperthermic intraperitoneal chemotherapy.
On multivariate analysis, the R resection status after the second CRS/HIPEC (p=0,013) along with the time interval between the two cytoreductions (p=0.009), were statistically significant in predicting improved survival. On the contrary, the R resection status after the first cytoreduction and the type of primary malignancy were not significant in predicting overall survival. (Table 4)
Table 4. Multivariate Analysis of Survival Predictors.
| p Value | |
|---|---|
| Type of primary | 0.20 |
| Resection status after first HIPEC | 0.28 |
| Resection status after second HIPEC | 0.013 |
| Interval between HIPECs | 0.009 |
HIPEC, hyperthermic intraperitoneal chemotherapy.
Discussion
Peritoneal carcinomatosis can no longer be considered as the absolute synonym to unresectable, terminal metastatic disease. Clearly, a subset of PC patients will present with disease confined to the peritoneal cavity. For this specific group of patients CRS/HIPEC has emerged as a procedure that in specialized high volume centers, can provide a substantial increase in the overall survival, with reasonably good quality of life (11,12). This procedure includes resection of involved organs and stripping of infiltrated peritoneum, followed by heated intraperitoneal chemotherapy. It is a major procedure with considerable morbidity and mortality (13). A prospective randomized trial performed on patients with PC from principally colorectal primary reported an astonishing 45%, 5 year survival, when a complete cytoreduction was achieved (14). Therefore, some of these patients live long enough to present with recurrence or progression of disease that is refractory to chemotherapy. At this point they can be evaluated for a possible second CRS/HIPEC. Until now, there has been a reasonable skepticism regarding the tolerability of a repeat cytoreduction due to the extent of the operation, and the physiologic impact on a patient already compromised by an advanced stage of disease (15).
Our prior accumulated experience with initial CRS/HIPEC has demonstrated that long-term survival was adversely influenced by increased volume of disease, R2 status of resection, high ECOG, poor nutrition and high grade of the primary tumor (16). The patients we considered to be the best candidates for a successful repeat cytoreduction are those who maintain an ECOG 0-1 functional status, had a prior R0 or R1 resection, were maintaining their nutritional reserves (Albumin > 3g/dL), had a low grade tumor without nodal metastatic disease and had an interval between the two procedures that was at least a year long. The above selection criteria are mirrored by the median interval of 27 months between the two procedures. For those patients who had a prior R2 resection, the option of a second cytoreduction was considered only in cases with a prolonged progression free interval (notably more than a year), while the patient was maintaining his functional and nutritional status. These selection criteria were guidelines, and a handful of cases not meeting criteria underwent a second procedure. Interesting enough almost one out of five or18% (11/62) of the second cytoreduction patients had an ECOG status at least 2 and 20% (2/10) of the patients who initially were left with macroscopic disease after the first cytoreduction (R2 resection) had a complete second cytoreduction (R0/1 resection). This is important in peritoneal surface disease from a low grade appendiceal primary where the completeness of the second cytoreduction and not the completeness of the first cytoreduction is the one that determines long term survival (17).
The site of origin clearly impacts the outcomes after initial CRS/HIPEC. It is not surprising that favorable biological behavior such as that encountered with low grade appendiceal cancer (N=28) was associated with a prolongation of median survival of an additional 71.3 months (0.1-111.7). This resulted in a median survival of 108.5 months from the first cytoreduction. Colon cancer PC patients (N=4) had an average 55.7 months median survival after the second CRS/HIPEC that resulted in a median survival of 137.4 months after the first cytoreduction. Although highly selected, this outcome is vastly superior to any systemic chemotherapy, including oxaliplatin and irinotecan regimens (18). Similarly ovarian cancer peritoneal carcinomatosis patients (N=8) had a median of 53.9 and 60.1 months survival after second and first cytoreduction respectively. Several of the above patients continued to remain free of recurrent disease at time this paper was written.
The morbidity of the procedure for the entire group of repeat CRS/HIPEC was 43.5% and this number includes both major and minor complications. The mortality was 3.2%, which is significantly less than the 6.5% mortality rate quoted to these patients when they present for their first cytoreduction (13). The mean duration of the postoperative hospitalization both in the intensive care unit and on the surgical floor was similar after the first and second cytoreduction, with the repeat CRS/HIPEC patients requiring 33% less ICU time. This morbidity and mortality is comparable to that experienced by our patients undergoing an initial CRS/HIPEC.
On multivariate analysis, the R status of the second CRS/HIPEC (p=0.013), and the interval between the two procedures (p=0.009), were the only two significant factors in predicting improved survival indicating the ultimate importance of the biological behavior of the tumor. Patients who had an R0/1 complete cytoreduction had a median survival of 55.7 months, which was significantly better than the 20.3 months of an R2 resection. This indicates that patients who will have prolonged survival regardless of the type of primary are those with anatomic distribution of disease that is amenable to complete cytoreduction as those with favorable tumor biology. The importance of complete cytoreduction after a repeat HIPEC has also been demonstrated by Esquivel and Sugarbaker in peritoneal surface disease from appendiceal primary (17). In that manuscript, the interval between the two procedures was not prognostic of improved survival, possibly because the patient population included asymptomatic patients with normal imaging studies, and patients who went to the operating room for elective procedures such as colostomy reversal or incisional hernia repair (17).
We recognize that the current paper is limited in that it represents a retrospective review from a single institution where patients are selected based on clinical experience obtained over a 20 year period of time. Therefore, patient selection bias is inevitably part of the reported outcomes. Patients were selected based primarily on performance status and favorable biological behavior of the tumor. Despite this, however, our experience measures the surgical outcomes of the same procedure in a variety of primaries with different biological behavior and prognosis.
We do not claim that the above results apply to every patient with peritoneal surface disease. On the contrary, selecting not only the correct patient but also the correct timing to perform the procedure, is of paramount importance in achieving prolonged survival with meaningful quality of life. This is not an easy clinical decision, and the operation itself is definitely demanding for both the patient and surgeon. Considering the significant learning curve for this operation these patients should be referred to high volume tertiary care centers with expertise in the treatment of peritoneal surface disease, where the procedure can be performed with at least the same morbidity and mortality as the first cytoreduction (19). An incomplete (R2a,b) first cytoreduction is not an absolute contraindication to an attempted second cytoreduction. The type of primary is not significant as long as the clinical behavior of the disease is favorable. A repeat CRS/HIPEC should not be undertaken unless it can provide with improved survival or can control symptomatic disease with quality of life. However, in selected patients repeat CRS/HIPEC procedures can be associated with prolonged survival with the potential of disease free survival.
Abbreviations and Acronyms
- CRS
cytoreductive surgery
- HIPEC
hyperthermic intraperitoneal chemotherapy
- PC
peritoneal carcinomatosis
Footnotes
Disclosure Innformation: Nothing to disclose.
Presented at the 64th Annual Meeting of the Society of Surgical Oncology, San Antonion, TX, March 2011.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elias DM, Ouellet JF. Intraperitoneal chemohyperthermia: rationale, technique, indications, and results. Surg Oncol Clin N Am. 2001;10:915–933. xi. [PubMed] [Google Scholar]
- 2.Sugarbaker PH, Cunliffe WJ, Belliveau J, et al. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol. 1989;16:83–97. [PubMed] [Google Scholar]
- 3.Elias D, Blot F, El OA, et al. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92:71–76. doi: 10.1002/1097-0142(20010701)92:1<71::aid-cncr1293>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Feldman AL, Libutti SK, Pingpank JF, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol. 2003;21:4560–4567. doi: 10.1200/JCO.2003.04.150. [DOI] [PubMed] [Google Scholar]
- 5.Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Elias D, Honore C, Ciuchendea R, et al. Peritoneal pseudomyxoma: results of a systematic policy of complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Br J Surg. 2008;95(9):1164–1171. doi: 10.1002/bjs.6235. [DOI] [PubMed] [Google Scholar]
- 7.Smeenk RM, Verwaal VJ, Antonini N, Zoetmulder FA. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2007;245:104–109. doi: 10.1097/01.sla.0000231705.40081.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verwaal VJ, Boot H, Aleman BM, et al. Recurrences after peritoneal carcinomatosis of colorectal origin treated by cytoreduction and hyperthermic intraperitoneal chemotherapy: location, treatment, and outcome. Ann Surg Oncol. 2004;11:375–379. doi: 10.1245/ASO.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Shen P, Stewart JH, Levine EA. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancy: overview and rationale. Curr Probl Cancer. 2009;33:125–141. doi: 10.1016/j.currproblcancer.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McQuellon RP, Loggie BW, Lehman AB, et al. Long-term survivorship and quality of life after cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2003;10:155–162. doi: 10.1245/aso.2003.03.067. [DOI] [PubMed] [Google Scholar]
- 12.McQuellon RP, Russell GB, Shen P, et al. Survival and health outcomes after cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for disseminated peritoneal cancer of appendiceal origin. Ann Surg Oncol. 2008;15:125–133. doi: 10.1245/s10434-007-9678-z. [DOI] [PubMed] [Google Scholar]
- 13.Shen P, Cahagan J, Stewart JH, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis: analysis of postoperative morbidity and mortality from a high volume center. Society of Surgical Oncology 65th Annual Meeting; Orlando, Florida. March 2012. Abstract. [Google Scholar]
- 14.Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 15.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 16.Stewart JH, Shen P, Russell GB, et al. Appendiceal neoplasms with peritoneal dissemination: outcomes after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy. Ann Surg Oncol. 2006;13:624–34. doi: 10.1007/s10434-006-9708-2. [DOI] [PubMed] [Google Scholar]
- 17.Esquivel J, Sugarbaker PH. Second-look surgery in patients with peritoneal dissemination from appendiceal malignancy: analysis of prognostic factors in 98 patients. Ann Surg. 2001;234:198–205. doi: 10.1097/00000658-200108000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of North Central Cancer Treatment Group Phase III Trials N9741 and N9841. J Clin Oncol. Jan 20;30:263–7. doi: 10.1200/JCO.2011.37.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusamura S, Baratti D, Deraco M. Multidimensional analysis of learning curve for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal surface malignancies. Ann Surg. 2011 Dec 26; doi: 10.1097/SLA.0b013e3182436c28. [DOI] [PubMed] [Google Scholar]


