Abstract
Background
Dynamic visual acuity (DVA) testing may be a useful, indirect indicator of vestibulo-ocular reflex function. Previous evidence shows that acuity for 2 m targets differs little between patients and normals using a 75 ms display duration and that healthy subjects do not differ in acuity when standing and walking while viewing a far target but they do differ when viewing a near target.
Objective
Improve the protocol of a screening tool by testing the hypothesis that healthy control subjects and patients and with unilateral peripheral vestibular weakness differ on DVA when viewing far targets while seated.
Methods
Controls and patients were tested while they were seated in a chair that oscillated vertically at 2 Hz. They viewed a computer screen 4 m away, while stationary and while moving, with viewing times of either 75 ms or 500 ms.
Results
The amount of change between static and dynamic conditions did not differ significantly between patients and controls for the 75 ms condition but controls had lower difference scores than patients when using the 500 ms duration. The ROC value was low, 0.68. Compared to historical data using the 75 ms duration at a distance of 2 m, subjects in both diagnostic groups had better visual acuity at the 75 ms/ 4 m distance.
Conclusions
These results suggest that using the longer duration is better for differentiating patients from healthy controls and they support previous evidence showing that near target viewing is more challenging.
Keywords: gaze stabilization, vestibulo-ocular reflex, epidemiologic screening, vestibular disorders, vestibular hypofunction
1.0 Introduction
Dynamic visual acuity (DVA) is an indirect indicator of vestibulo-ocular reflex function (VOR) and thus is an indirect indicator of the health of the vestibular system. The first report of DVA testing in patients with vestibular disorders was by Longridge and Mallinson [11]. They held an eye chart with an “E” at 1.8 m from normals and patients with vestibular disorders, moved their heads in yaw at 1 Hz, and noted any decreased acuity in terms of decrease ability to read a line on the chart. They reported particular difficulty in patients with aminoglycoside toxicity.
To perform dynamic visual acuity tests with the head moving the performer must have a functioning VOR to enable stable gaze. In patients who have unilateral vestibular impairments DVA is decreased during unpredictable head movements [6, 19, 20]. It is also decreased in patients with bilateral vestibular hypofunction [7, 9, 17].
Active head movement has been used in some tests of DVA [3, 21]. In computerized DVA tests the head movement triggers the visual stimulus [8, 16]. The use of active head movements may facilitate the use of predictive saccades. Therefore a DVA test that uses active head movements may measure aspects of motor control other than the VOR. Use of passive head movement is a better paradigm, such as passively shaking the patient’s head while he or she views a Snellen chart [4]. Having the examiner move the patient’s head makes the test inexpensive and easy to perform, which is desirable, but the examiner’s hands provide an uncontrolled stimulus. Also, the Snellen chart presents some perceptual disadvantages.
Peters and Bloomberg tested a DVA paradigm using a Landolt C in several sizes and orientations on a computer screen that subjects viewed during treadmill walking [13] at 4 m and 50 cm viewing distances. They found greater change in DVA scores at the shorter distance and surmised that VOR compensation was better during the far target walking condition, when the angular head movements are the primary threat to clear vision. In the near target condition, compensating for the vertical translation is the primary challenge. More recently Peters and his colleagues tested subjects while seated in a movable chair and viewing the computer screen at 2 m. They found that during passive, vertical chair movements subjects had decreased acuity compared to a static condition and patients with vestibular disorders were worse than healthy controls [14]. The present study tried to sharpen the test, altering the test distance and the duration of the stimulus viewing time, to improve its usefulness for screening.
2.0 Methods
2.1 Subjects
The study population included 50 healthy control subjects and 25 subjects with chronic vestibular impairments as indicated by unilateral weakness scores on bi-thermal caloric testing of ≥ 20% (UW) in our laboratory and diagnosed by board-certified physicians at Baylor College of Medicine. See Table 1. Younger and older UW subjects did not differ significantly by level of vestibular impairment. Healthy control subjects had no history of hearing loss, vestibular impairment, vertigo, gait abnormality, artificial joints, or neurologic problems. Head shaking in yaw rotations did not elicit vertigo; head thrusts and Dix-Hallpike maneuvers were all negative, bilaterally; and gait showed no ataxia. Patients and normals did not differ significantly by age, p=0.36.
Table 1.
Demographic details. Mean age (standard deviation, ranges) and length of illness are reported in years. Mean unilateral weakness (standard deviation, ranges) is reported in percent. Both groups are subdivided at age 50, 25 subjects per subgroup in the controls, 9 younger UW and 16 older UW.
| Group | Age | Unilateral weakness |
Length of illness | Females/ Males |
|---|---|---|---|---|
| Controls | 49.1 (15.9, 21.0 to 89.2) | 29F/ 21 M | ||
| Younger Older |
35.4 (7.4, 21.0 to 47.5) 62.9 (8.2), 50.9 to 89.2) |
12F/13M 17F/8M |
||
| UW | 52.5 (13.9, 24.2 to 77.5) | 48.0 (25.0, 20 to 100) | 2.3 (3.1, 0.01 to 13.0) | 11F/ 14M |
| Younger Older |
37.5 (8.4, 24.2 to 48.3) 61.0 (7.7, 51.2 to 77.5) |
42 (20, 25 to 80) 51 (29, 20 to 100) |
0.95 (1.0, 0.16 to 3.5) 3.0 (3.5, 0.01 to 13.0) |
2F/7M 9F/7M |
This study was approved by the Institutional Review Board for Human Subjects Research for Baylor College of Medicine and Affiliated Hospitals. Subjects gave informed consent prior to participation.
2.2 Apparatus
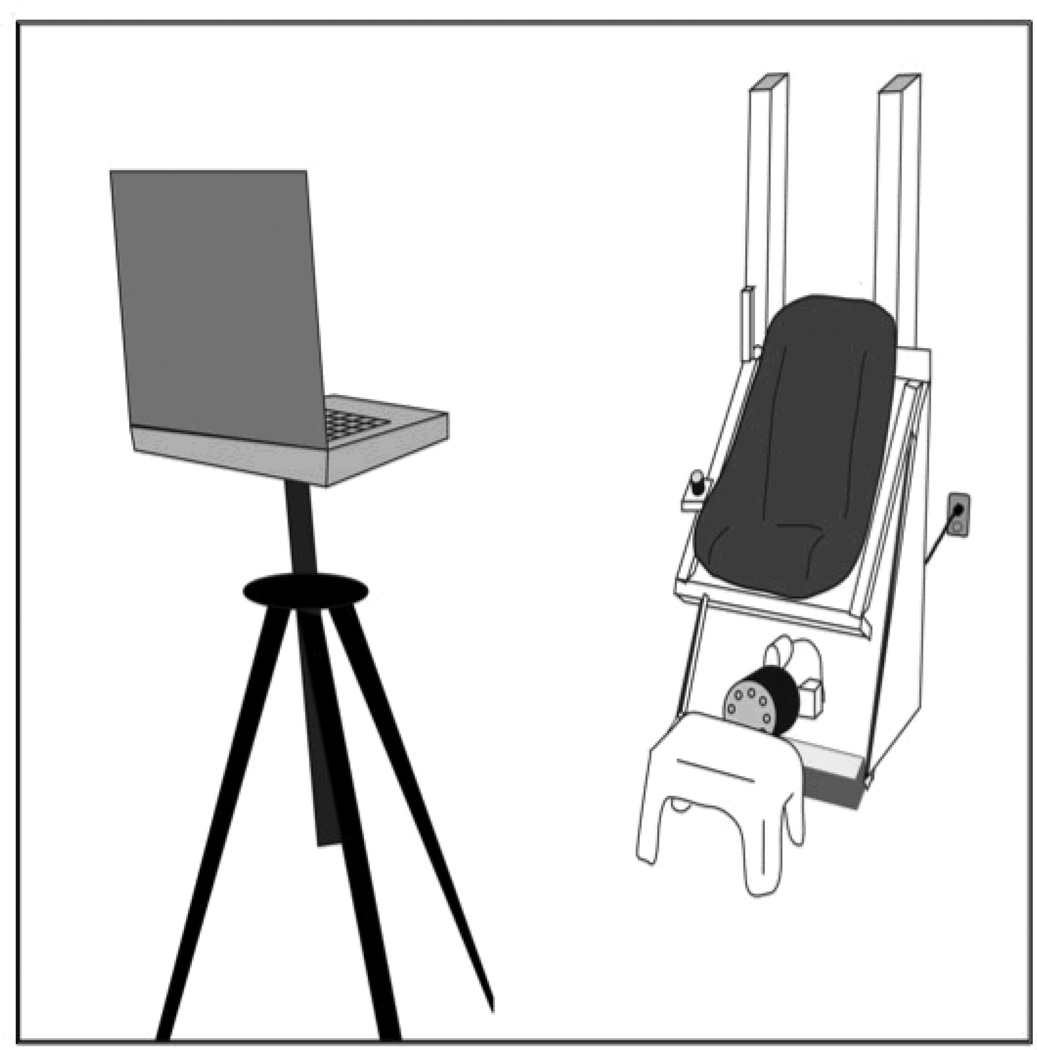
Subjects sat in an automobile-type bucket seat and were strapped in using a standard automobile lap belt. The head was not restrained. The chair was attached to vertically-oriented linear rails and ran through a cam mechanism to an electric motor positioned beneath the chair. When the motor was turned on the chair oscillated vertically at a frequency of 2 Hz with maximal displacements of ± 2.5 cm, which simulated the vertical displacement of the head during locomotion [5]. A personal computer set upon a tripod 4 m away from the chair was used to display visual acuity optotypes. For each subject the center of the computer screen was set at eye level when the chair was stationary, in the resting position, at the lowest point of it’s range. See Figure 1.
Figure 1.
The testing apparatus, including the chair on the right, and the computer on the left. The chair includes a standard automobile bucket seat. At rest the edge of the seat is 58.5 cm high so the small footstool in front of the chair is available for subjects who are too short to keep their feet on the floor. The subject wears a standard lap-style seatbelt during testing. The motor is the small round object at the base of the chair. The seat rides vertically on the rails located behind it.
2.3 Procedure
Visual acuity was assessed using custom-written software that sequentially presented Landolt C optotypes on a computer screen. The presentation of the optotypes in the dynamic condition was triggered based on the motion of the chair. In both the static and dynamic testing phases the Landolt C flashed on the computer screen for one of two durations, either 75 ms or 500 ms. During each of the four trials 32 optotype presentations were used to determine the acuity threshold. The trial order was always static trial followed by dynamic trial using the same durations. The order of durations was varied randomly, using a coin-flipping procedure that was determined a priori. When the chair was in motion, a mechanical switch on the chair triggered the optotype presentation. For the 75 ms display duration conditions the presentation was triggered to occur before the peak velocity and it remained on for an equivalent time after the peak velocity occurred. The presentation for the 500 ms display duration conditions was triggered by the same mechanism, but the longer duration permitted the optotype to be visible during one complete oscillation of the chair. The number of presentations was equal for both upward travel and downward travel and the presentations were randomized. Due to the randomization and slight inconsistency by staff from trial to trial in typing the subject’s responses on the keypad, the time between successive presentations varied unpredictably.
Optotypes in one of 8 orientations -- up, down, right, left, up-right, up-left, down-right and down-left -- ranged in size from −0.4 to 1.0 logMAR (log of the Minimum Angle Resolvable) or 20/8 to 20/200 Snellen ratios. Subjects were instructed to state the orientation of each C. After the first, the optotype size used for each successive presentation was based on the accuracy of the subject’s responses to all previous presentations. A PEST (parameter estimation by sequential testing) algorithm was used to estimate the subject’s acuity after each presentation and the next presentation size corresponded with that calculated threshold. A slightly modified version of the same algorithm was used in data post processing to determine the subject’s acuity threshold for each condition. The modification allowed the final measure to be calculated with a resolution of 0.02 logMAR. Therefore the dependent measures were the logMAR scores. We also calculated the differences scores between static and dynamic conditions as dependent measures.
2.4 Statistical analyses
Correlation coefficients were used to examine the level of association between age and DVA score at each condition (static, dynamic) within patients and normals. Differences in acuity scores between groups (controls vs patients) and across conditions (static vs. dynamic) were tested via multilevel and mixed effect models using maximum likelihood estimation techniques. In each model, interactions were included and tested for significance. Adjustments were made for multiple comparisons. p <0.05 was considered statistically significant. Receiver Operator Characteristic analysis (ROC analysis) and sensitivity/ specificity were calculated on the difference between static and dynamic scores at the 500 ms duration in order to find an optimal level for classifying patients vs. normals. All analyses were performed in SAS Statistical software (SAS, Cary, NC).
3.0 Results
3.1 Differences between conditions and diagnostic groups
For the raw logMAR scores, paired comparisons within healthy controls showed no significant differences between static and dynamic conditions at the 500 ms duration, p=0.52, but did show a significant difference between static and dynamic conditions at the 75 ms duration, p=0.001. Significant differences were also found within healthy controls, between the 500 ms and 75 ms durations on the static and the dynamic tests, p<0.001 for each comparison. Similarly, paired comparisons showed a significant difference within patients, on static tests at 75 ms and 500 ms. No significant differences were found within the patients, or between the control and patient groups for any of the 4 measures. See Table 2.
Table 2.
Raw LogMAR scores and difference scores between static and dynamic conditions; means (SD, ranges) by group and trial duration.
| 75 ms duration | 500 ms duration | |||||
|---|---|---|---|---|---|---|
| Static | Dynamic | Difference | Static | Dynamic | Difference | |
| Controls | 0.07 (0.17, −0.28 to 0.46) | 0.12 (0.16, −0.16 to 0.56) | 0.05 (0.10, −0.16 to 0.24 | −0.07 (0.16, −0.40 to 0.30) | −0.07 (0.17, −0.32 to 0.38) | *0.00 (0.09, −0.18 to 0.20) |
| UW | 0.06 (0.16, −0.22 to 0.44) | 0.11 (0.15, −0.20 to 0.36) | 0.04 (0.14, −0.18 to 0.34) | −0.10 (0.14, −0.32 to 0.24) | −0.06 (0.12, −0.28 to 0.22) | *0.05 (0.07, −0.14 to 0.24) |
= significant differences.
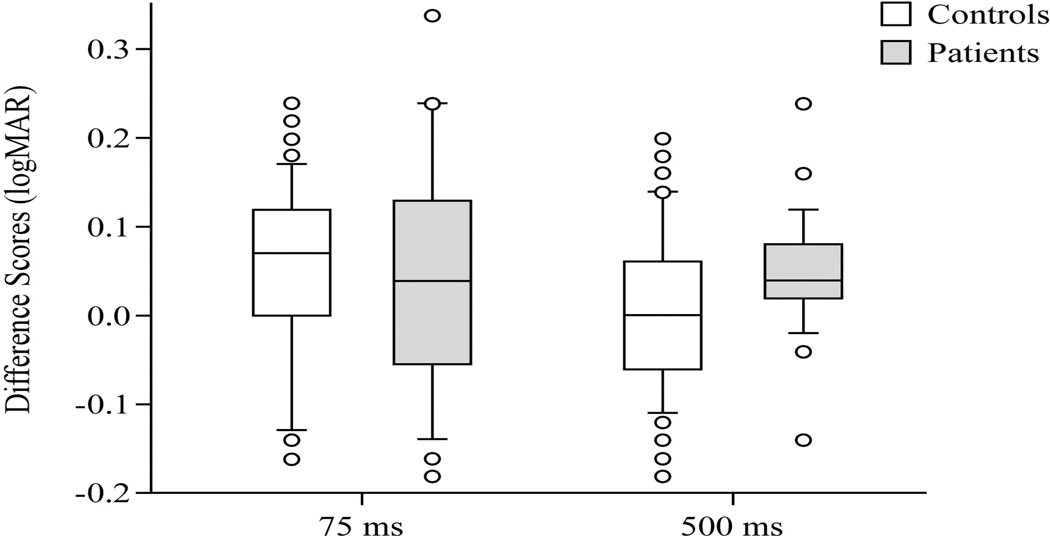
We also examined the difference scores between static and dynamic conditions comparing the diagnostic groups. At the 75 ms duration no difference was found between healthy controls and patients, p=0.87. At the 500 ms duration, however, a significant difference was found between the groups, p=0.02. See Figure 2.
Figure 2.
Difference scores in logMar units, 75 ms duration and 500 ms duration, at 4 m distance, between healthy controls and patients. Center horizontal bars are medians, rectangle ends are interquartile ranges, error bars are 10th and 90th deciles, and circles are outliers.
Based on these results ROC analysis was performed using the values of the difference scores for the 500 ms duration, and ROC=0.685. When the age range was cut at 50 years, as in the previous study [14], the ROC values were unchanged: age < 50 years, ROC=0.689; age > 50 years, ROC= 0.688. At that level the best sensitivity/ specificity values were slightly better, 0.68/0.66 with a cut-point of 0.04.
3.2 Age-related differences
The ages of the control and patient groups did not differ significantly, p=0.37. For the raw scores in normals age was weakly but significantly correlated with all tests: 75 ms static, r=0.37, p<0.008; dynamic, r= 0.44, p < 0.001; 500 ms static, r = 0.47, p = 0.0006; dynamic, r = 0.45, p < 0.001. For patients correlations between age and raw scores were lower overall (range, r=0.12 – 0.40) and with the exception of raw scores at the 75 ms duration dynamic condition (r=−.40, p=0.04) no correlations reached statistical significance. Age was not correlated with difference scores in normals or patients for either the 75 ms or 500 ms durations.
When the two groups were divided at age 50 some differences were found between age groups. At the 75 ms duration for the static condition, healthy controls aged < 50 and > 50 approached significant differences, p= 0.06, but older and younger patients did not differ significantly, p=0.38. At the 75 ms duration for the dynamic condition, older and younger controls differed significantly, p = 0.009, and patients approached significant differences, p = 0.08. For 500 ms duration in the static condition older and younger controls differed significantly, p = 0.02, and older and younger patients approached significant differences, p = 0.08. For the 500 ms dynamic condition older and younger controls differed significantly, p = 0.005, but patients did not differ significantly, p = 0.13. See Table 3.
Table 3.
Raw LogMAR scores by age groups. Means (standard deviations, ranges).
| 75 ms | 500 ms | |||||
|---|---|---|---|---|---|---|
| Static | Dynamic | Difference | Static | Dynamic | Difference | |
| Controls < 50 (n=25) | 0.03 (0.19, −0.28 to 0.46) | *0.06 (0.16, −0.16 to 0.44) | 0.03 (0.11, −0.16 to 0.22) | ^−0.13 (0.16, −0.40 to 0.30) | #−0.13 (0.18, −0.32 to 0.38) | 0.01 (0.09, −0.18 to 0.18) |
| Controls > 50 (n=25) | 0.12 (0.13, −0.14 to 0.32) | *0.18 (0.14, −0.06 to 0.56) | 0.06 (0.10, −0.14 to 0.24) | ^−0.01 (0.12, −0.20 to 0.24) | #−0.02 (0.15, −0.26 0.32) | 0.00 (0.09, −0.16 to 0.20) |
| UW < 50 (n=9) | 0.03 (0.17, −0.22 to 0.30) | 0.04 (0.17, −0.20 to 0.36) | 0.01 (0.12, −0.18 to 0.22) | −0.16 (0.14, −0.32 to 0.04) | −0.12 (0.15, −0.28 to 0.16) | 0.05 (0.05, 0.00 to 0.16) |
| UW > 50 (n=16) | 0.08 (0.15, −0.12 to −.44) | 0.15 (0.12, −0.10 to 0.30) | 0.06 (0.15, −0.16 to −.34) | −0.07 (0.14, −0.22 to −.24) | −0.02 (−0.11, −0.18 to −0.22) | 0.05 (0.08, −0.14 to −.24) |
= significant differences between age groups within the same condition and stimulus duration.
3.3 Comparison to historical data at 2 m
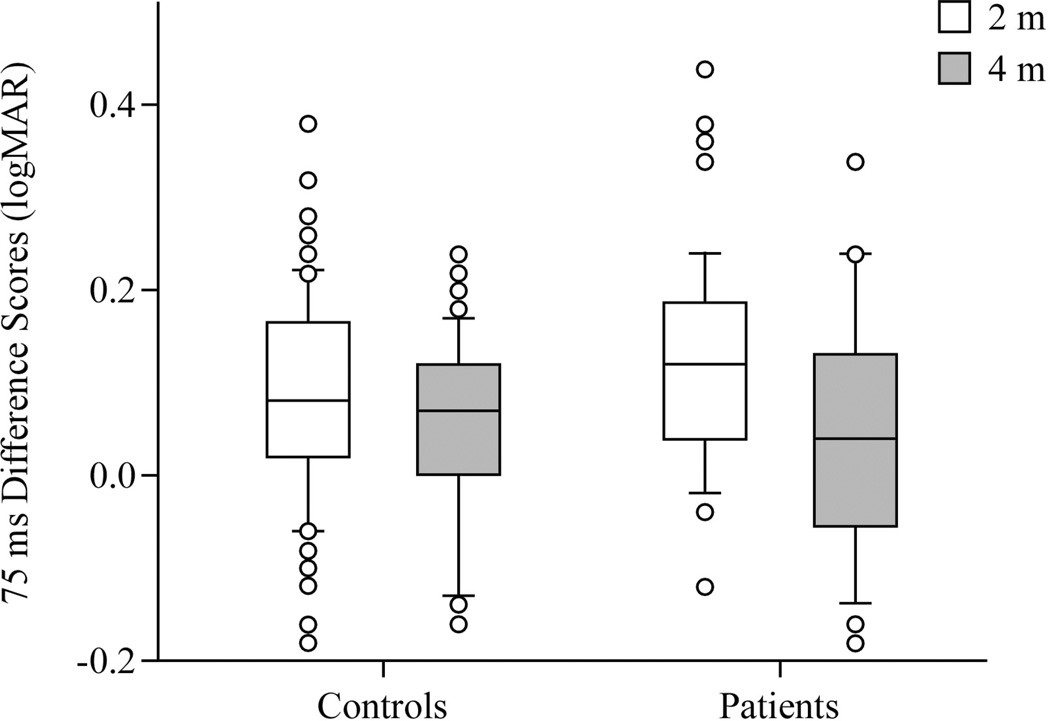
A previous study tested healthy controls and patients with vestibular disorders, using the 75 ms duration but at 2 m [14]. Within controls and patients age-adjusted tests showed a significant difference between scores of the previous study and that of the current study for difference scores (controls: p=0.02; patients: p=0.01). These data indicate that scores improved at the 4 m distance. Controls in the first and second studies did not differ significantly by age; patients in the two studies did not differ by age in each study, either. See Figure 3.
Figure 3.
Difference scores in logMar units, 75 ms duration, at 2 m and 4 distances, comparing historical [12] and current data in healthy controls and patients. Center horizontal bars are medians, rectangle ends are interquartile ranges, error bars are 10th and 90th deciles, and circles are outliers.
4.0 Discussion
Using this passive movement stimulus we compared static and dynamic visual acuity scores and the differences between them. The raw scores showed some differences by age, mostly in healthy controls. This finding supports the previous finding of an age difference in raw scores at a distance of 2 m for the 75 ms duration. Previous studies, using a stimulus linked to the velocity of active head movement also showed differences between age groups in healthy controls and differences between patients and controls [8, 17]. No differences were found by age in the difference scores. Our result is unlike previous DVA studies that have found changes with age. One of several differences between this paradigm and previous studies is the display duration. Studies that triggered the optotype display based on head movement velocity, but which used unlimited durations for viewing optotypes during static measurements, may not have accounted of the effects of display duration on the acuity measurement. In the present study we had an age effect when comparing the static scores between the 75 and 500 ms. The display duration was held constant for the static and dynamic portions of the test.
4.1 Difference scores
The use of difference scores effectively eliminated any age bias. It also eliminated any bias from differences in subjects’ static viewing ability, leaving control of the VOR as the only factor. Therefore, the difference score is a better measure than raw scores.
The significant differences between groups for the difference scores at the 500 ms duration trials suggests that a fully functioning VOR is essential for maintaining visual acuity during movement. The ROC value was relatively low but considerably better than the ROC value of 0.51 obtained in the previous study at the 75 ms duration [14]. The current ROC value and sensitivity/ specificity scores suggests that the test should be sharpened further to make it useful for screening people with vestibular disorders. Unlike some previous work [17] we did not test patients with bilateral vestibular loss, who might have shown more significant differences in DVA scores from static to dynamic conditions. We were more interested in the more common patients with somewhat more subtle problems.
4.2 Stimulus durations
We expected to find differences between the groups at both stimulus display durations. The 75 ms duration, triggered only during the high velocity portion of the movement, was intended to sharpen the test. Patients did not show differences between the 75 ms duration and the 500 ms duration but the performance of normals was degraded at the 75 ms duration. As a result, no difference was found between the patient and normal groups for the 75 ms condition. The degraded performance of healthy controls suggests that the compensatory VOR in healthy people is inadequate for stabilizing vision during primarily vertical oscillations of the head. This idea is supported by evidence showing that the vertical translational VOR has a gain of approximately 0.5 when a target is viewed at 2 m [10].
4.3 Distances
In the previous study subjects were tested with the stimulus 2 m away. At that distance the 75 ms duration stimulus showed no differences in difference scores. We still found no difference in difference scores at the 4 m distance but we did see differences at the 500 ms duration at 4 m. Because the optotype was visible throughout the movement at the 500 ms display duration the stimulus was present during both the high and low velocity portions of the oscillation. Our results suggest that healthy controls could take advantage of the low velocity portion of the movement to see clearly, whereas the patients could not do so.
During translation, larger eye movement amplitudes are required to maintain visual fixation on targets at shorter viewing distances [2, 15, 18]. Although the stimulus in the current protocol was constrained to be a sinusoidal translation, the head was unconstrained, probably inducing pitch movements of the head. Kinematic analyses done prior to this investigation showed that the frequency, magnitudes and phase relationship between the vertical oscillation and induced pitch head movement were similar to those experienced during walking. The resulting stimulation of both linear and angular VOR mechanisms was intentional. While this methodology made isolating the effects on acuity of specific mechanisms impossible it did allow for passively-induced conditions similar to those experienced during gait. At the longer 4 m distance the influence of the linear VOR was probably minimized. This decreased influence of the linear VOR may be the reason for the improved performance.
The longer viewing distance in the present study and the 500 ms duration are akin to many distance vision tasks experienced in daily life, such as reading a street sign while walking down the sidewalk. It may also be somewhat more similar to the challenge of seeing a moving object from a further distance and predicting whether or not it will be an obstacle to forward progress, such as a vehicle moving forward at an intersection. Out of concern for being confronted with such safety problems many patients with vestibular disorders limit their driving to local areas and reduce driving to distant communities in order to avoid driving on highways, where faster reactions may be needed [1].
The results comparing the 2 m and 4 m distance support previous evidence showing greater change in DVA when viewing far targets [13]. These findings support the idea that when viewing near targets the VOR is partly suppressed. When viewing far targets, however, the VOR is needed for a stable gaze. Thus, a deficit of vestibular function may not be particularly detrimental to dynamic visual acuity for near target viewing. The deficit in the VOR becomes more apparent for far target viewing. This idea is supported by evidence from Wist et al, who showed a relationship between viewing distance and oscillopsia for yaw head movements [22].
4.4 Practical implications
The functional implications of this idea are indicated by the reports from patients who feel safe to drive their cars in the neighborhoods, slowly, but do not feel safe to drive on highways because they do not see clearly [1]. When driving slowly in a familiar neighborhood the patient has time to notice landmarks and on-coming cars and may have time to read street signs. When driving on the highway, however, the faster speed of the vehicle probably increases the vibrations felt by the driver and may preclude reading signs and noticing hazards, and familiar landmarks may not be available. Also, on a street with an uneven surface high frequency head oscillations elicited in response to vehicle movement will elicit the vertical VOR [12]. If the vertical VOR is impaired then the individual will prefer to drive slowly to minimize oscillopsia.
Unlike several other studies we used a passive, whole-body, vertical movement stimulus. Patients with severe bilateral vestibular loss often complain about oscillopsia while walking. Gait elicits a combination of translations and pitch, roll and yaw head movements, creating a complex oculomotor compensation problem that the individual must solve. By restricting the perturbation to a vertical translation we reduced that complexity to only the vertical translation and the resulting pitch head movement, both of which are normal components of gait. The movement amplitudes for our test are similar the amplitudes of fast walking. The comparison between the current data and the previous data show an effect of target distance that is similar to results from treadmill walking [13]. Thus, use of this passive vertical stimulus during DVA testing was a reasonable approximation of the head movement elicited during gait and has the potential to simulate the decreased visual acuity elicited during gait.
4.5 Limitations of the study
This test was studied in the effort to develop it as a using screening tool for people who have vestibular disorders, not as a diagnostic test. Therefore we did not account for length of illness. The relatively small sample size precluded testing smaller age groups. We did not attempt to measure the VOR and determine the gain in response to the translation of the chair.
References
- 1.Cohen HS, Wells J, Kimball KT, Owsley C. Driving disability in dizziness. J Safety Res. 2003;34:361–369. doi: 10.1016/j.jsr.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Crane B, Demer JL. Human horizontal vestibulo-ocular reflex initiation: effects of accelearation, target distance, and unilateral deafferentation. Journal of Neurophysiology. 1998;80:1151–1166. doi: 10.1152/jn.1998.80.3.1151. [DOI] [PubMed] [Google Scholar]
- 3.Dannenbaum E, Paquet N, Hakim-Zadeh R, Feldman AG. Optimal parameters for the clinical test of dynamic visual acuity in patients with a unilateral vestibular deficit. Journal of Otolaryngology. 2005;34:13–19. doi: 10.2310/7070.2005.03105. [DOI] [PubMed] [Google Scholar]
- 4.Goebel JA. The ten-minute examination of the dizzy patient. Semin Neurol. 2001;21:391–398. doi: 10.1055/s-2001-19410. [DOI] [PubMed] [Google Scholar]
- 5.Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE. Frequency and velocity of rotational head perturbations during locomotion. Exp Brain Res. 1988;70:470–476. doi: 10.1007/BF00247595. [DOI] [PubMed] [Google Scholar]
- 6.Herdman S, Schubert MC, Das VE, Tusa RJ. Recovery of dynamic visual acuity in unilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg. 2003;129:819–824. doi: 10.1001/archotol.129.8.819. [DOI] [PubMed] [Google Scholar]
- 7.Herdman SJ, Schubert MC, Tusa RJ. Role of central preprogramming in dynamic visual acuity with vestibular loss. Arch Otolaryngol Head Neck Surg. 2001;127:1205–1210. doi: 10.1001/archotol.127.10.1205. [DOI] [PubMed] [Google Scholar]
- 8.Herdman SJ, Tusa RJ, Blatt P, Suzuki A, Venuto PJ, Roberts D. Computerized dynamic visual acuity test in the assessment of vestibular deficits. Amer J Otol. 1998;19:790–796. [PubMed] [Google Scholar]
- 9.Hillman EJ, Bloomberg JJ, McDonald VP, Cohen HS. Dynamic visual acuity while walking in normals and labyrinthine deficient patients. J Vestib Res. 1999;9:49–57. [PubMed] [Google Scholar]
- 10.Liao K, Walker MF, Joshi A, Reschke M, Leigh RJ. Vestibulo-ocular responses to vertical translation in normal human subjects. Exp Brain Res. 2008;185:553–562. doi: 10.1007/s00221-007-1181-z. [DOI] [PubMed] [Google Scholar]
- 11.Longridge NS, Mallinson AI. A discussion of the dynamic illegible "E" test: a new method of screening for aminoglycoside vestibulotoxicity. Otolaryngol Head Neck Surg. 1984;92:671–677. doi: 10.1177/019459988409200614. [DOI] [PubMed] [Google Scholar]
- 12.MacDougall HG, Moore ST. Functional assessment of head-eye coordination during vehicle operation. Optom Vis Sci. 2005;82:7076–7715. doi: 10.1097/01.opx.0000175623.86611.03. [DOI] [PubMed] [Google Scholar]
- 13.Peters BT, Bloomberg JJ. Dynamic visual acuity using "far" and "near" targets. Acta Otolaryngol. 2005;125:353–357. doi: 10.1080/00016480410024631. [DOI] [PubMed] [Google Scholar]
- 14.Peters BT, Mulavara AP, Cohen HS, Sangi-Haghpeykar H, Bloomberg JJ. Dynamic visual acuity testing for screening patients with vestibular impairments. J Vestib Res. 2012;22:145–151. doi: 10.3233/VES-2012-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranjbaran M, Galiana HL. The horizontal angular vestibulo-ocular reflex: a non-linear mechanism for context-dependent responses. Conf Proc IEEE Med Biol Soc. 2012;2012:3866–3869. doi: 10.1109/EMBC.2012.6346811. [DOI] [PubMed] [Google Scholar]
- 16.Rine RM, Roberts DC, Corbin BA, McKean-Cowdin R, Varma R, Beaumont J, Slotkin J, Schubert MC. New portable tool to screen vestibular and visual function - National Instiutes of Health Toolbox. J Rehabil Res Dev. 2012;49:209–220. doi: 10.1682/jrrd.2010.12.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schubert MC, Herdman SJ, Tusa RJ. Vertical dynamic visual acuity in normal subjects and patients with vestibular hypofunction. Otol Neurotol. 2002;23:372–377. doi: 10.1097/00129492-200205000-00025. [DOI] [PubMed] [Google Scholar]
- 18.Telford L, Seidman SH, Paige GD. Dynamics of squirrel monkey linear vestibuloocular reflex and interactions with fixation distance. J Neurophys. 1997;78:1775–1790. doi: 10.1152/jn.1997.78.4.1775. [DOI] [PubMed] [Google Scholar]
- 19.Tian J-R, Shubayev I, Demer JL. Dynamic visual acuity during yaw rotation in normal and unilaterally vestibulopathic humans. Ann NY Acad Sci. 2001;942:501–504. doi: 10.1111/j.1749-6632.2001.tb03781.x. [DOI] [PubMed] [Google Scholar]
- 20.Tian J-R, Shubayev I, Demer JL. Dynamic visual acuity during passive and self-generated transient head rotation in normal and unilaterally vestibulopathic humans. Exp Brain Res. 2002;142:486–495. doi: 10.1007/s00221-001-0959-7. [DOI] [PubMed] [Google Scholar]
- 21.Vital D, Hegemann SCA, Straumann D, Bergamin O, Bockisch CJ, Angehrn D, Schmitt KU, Probst R. A new dynamic visual acuity test to assess peripheral vestibular function. Arch Otolaryngol Head Neck Surg. 2010;136:686–691. doi: 10.1001/archoto.2010.99. [DOI] [PubMed] [Google Scholar]
- 22.Wist ER, Brandt T, Krafczyk S. Oscillopsia and retinal slip: evidence supporting a clinical test. Brain. 1983;106:153–168. doi: 10.1093/brain/106.1.153. [DOI] [PubMed] [Google Scholar]