Abstract
Purpose
The purpose of this study is to describe the county-level geographic distribution of human papillomavirus (HPV) vaccine coverage among young women aged 18–26 in Texas using multilevel, small area estimation.
Methods
Multilevel (individual, county, public health region) random-intercept logit models were fit to HPV vaccination data (receipt of ≥ 1 dose Gardasil®) from the 2008 Behavioral Risk Factor Surveillance System and a number of secondary sources. Using the parameters from the final model, we simulated HPV vaccine coverage in each county.
Results
Indirect county-level estimates ranged from 1.9–23.8%, with a weighted state average of 11.4%. The counties with the highest and lowest coverage estimates were Orange County, TX and Webb County, TX respectively. Significant correlations were observed between HPV vaccination and age, Hispanic ethnicity, and the percentage of uninsured at the county and public health region levels.
Conclusions
Small area analyses have been used in a variety of settings to assess a variety of health outcomes, and as shown in this study, can be used to highlight geographic disparities and opportunities for intervention in HPV vaccine coverage.
INTRODUCTION
The U.S. Food and Drug Administration (FDA) approved the use of Gardasil® (HPV4), a quadrivalent vaccine against four prevalent strains of human papillomavirus (HPV), for females 9–26 years old in June 2006 1. The Advisory Committee on Immunization Practices soon thereafter recommended routine vaccination of females aged 11–12 and catch-up vaccination of females aged 13–262. Since 2008, over 30 peer-reviewed articles have examined HPV vaccine uptake in the U.S. and other countries. Most have focused on adolescent females. Surveillance of young adult women has not kept pace, leaving gaps in our knowledge of uptake and its predictors in this population. Although national uptake is known (by 2007, 10% of women aged 18–26 had received ≥1 dose HPV4; 3, 4), no studies to our knowledge have explored geographic variation in uptake among young adult women. This information is critical for 1) identifying areas with low vaccine uptake, 2) developing geographic-specific interventions to increase vaccine uptake, and 3) strategic resource allocation, program planning, and policy-making.
The purpose of this study was to describe the geographic distribution of HPV vaccine coverage in Texas. We applied a multilevel, small area model of HPV vaccine initiation using the 2008 Texas Behavioral Surveillance System (TX BRFSS5, 6) to provide county-level estimates of HPV vaccine coverage among females aged 18–26 years. Given the results of previous studies, we expected to find a negative association between age and HPV vaccination and a greater likelihood of vaccination among whites than among racial/ethnic minorities4,7–9.
METHODS
Small area estimation (SAE) operates on the assumption that areas with the same characteristics will have similar outcomes. Thus, statistically we could borrow information from the population-based model to derive local estimates. Place-based random-effects are increasingly included in these population-based models to allow for location-specific patterns. In this study, we utilized multilevel (individual, county, and health service region (HSR)) SAE to derive county-level estimates of HPV vaccine coverage among Texas females aged 18–26. In Texas, there are 254 counties nested within 11 HSRs, which represent state-designated boundaries for the administration of public health services and resources. Hierarchical level data and a random intercept term were included to improve model fit and ensure the correct selection of group-level covariates and parameter estimates.
We used multiple data sources to facilitate our methodological approach. The individual-level dataset used to obtain the outcome (i.e., ever received ≥ 1 dose HPV4) and level 1 covariates (i.e., age, race/ ethnicity, and educational attainment) was the 2008 TX BRFSS. The group-level covariates (i.e., county and HSR, where available) to be tested were obtained from the U.S. Census Bureau (percentage of uninsured adults aged 18–64 and percentage of total population in poverty), the Texas Cancer Registry (cervical cancer incidence rate), the Association of Religion Data Archives (evangelical religious adherence rate), and U.S. Office of Management & Budget (rurality index). The auxiliary dataset, which enumerates the population in each county by age, sex, and race/ethnicity, was obtained from the National Vital Statistic System10. This dataset serves as the foundation for predicting vaccine coverage for both sampled and unsampled counties.
We constructed a series of increasingly complex, multilevel random-intercept logit models of HPV vaccination (ever received ≥1 dose HPV4, yes or no) in MLwiN Version 2.2011. Adding covariates sequentially by level allows one to examine the contribution of covariate sets and how their addition changes the parameter estimates of the previous model. A total of 277 sampled women aged 18–26 from 61 Texas counties were included. Weights were incorporated at each level and scaled as recommended by Carle (Method A;12) and Goldstein13. A variance component term was included at the county and HSR level to ensure the correct selection of covariates and the accuracy of their parameter values.
After fitting the empty model, we added all level 1 covariates to the model. Retaining them regardless of statistical significance, we then evaluated level 2 covariates one by one. County-level covariates with p-values ≤0.10 were retained for further examination. Next, we evaluated level 3 (HSR level) covariates one by one, retaining only those with p-values ≤0.10. First order marginal quasi-likelihood was used to estimate all models in MLwiN. The preliminary model included all level 1 covariates and statistically significant (p-value ≤0.05) group-level covariates. Because the individual-level educational attainment was not available in the auxiliary dataset, it was removed in the final model and later adjusted for at the county level, as suggested by Congdon14. The regression coefficients for educational attainment from the preliminary model were used to calculate the weighted total of the regression coefficients in county j (i.e., variable L). This weighted total is used to adjust the county-level estimates by their respective educational characteristics in the simulation process described below.
Using the estimated regression coefficients and standard errors from the final model, we simulated 10,000 datasets for each regression coefficient from the normal distribution and applied them to the logit model to estimate the vaccination rate for each Race- and Age-specific group in county j (RAj), adjusted for county j’s educational characteristics (multiplied by L). Subsequently, we calculated the mean and standard deviation of the 10,000 RAj adjusted probabilities. To obtain summary estimates for each county, we took the population weighted mean of the 10,000 RAj adjusted probabilities (technical documentation available upon request). Thus, we weighted the probability for each demographic subgroup by their respective population distribution in county j. HSR random effects were not estimated due to insignificant variability in the final model and were ignored in the simulations. County random effects were only estimated for the 61 sampled counties in the 2008 TX BRFSS (non-sampled counties were assigned the simulated mean value). Simulations were conducted using SAS Version 9.2 15.
RESULTS
In the TX BRFSS study population of 277 young women aged 18–26 years, the mean age was 22.7 years. Other demographic characteristics were as follows: 42.5% were non-Hispanic white, 8.3% were non-Hispanic black, 38.3% were Hispanic, 19.8% had less than a high school education, and 38.9% reported a lack of insurance. Compared to the state level estimates obtained from U.S. Census Bureau, BRFSS demographic estimates were similar.
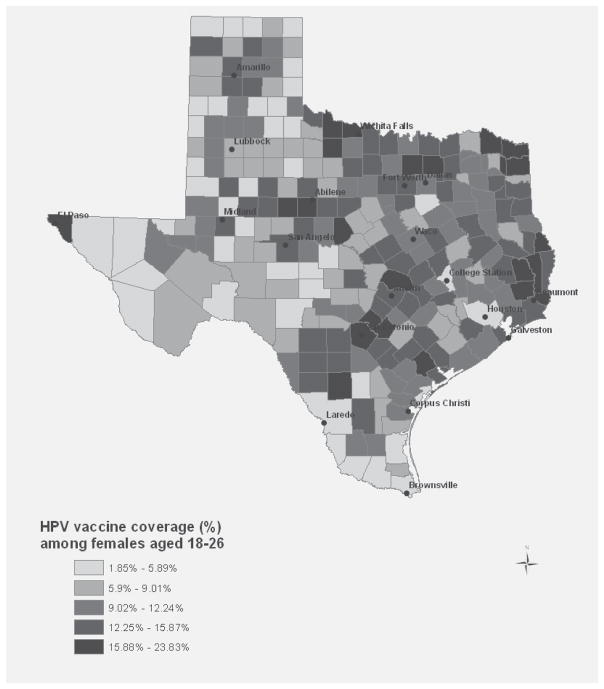
In 2008, 12.0% (95% CI: 6.2, 17.7) of Texas women aged 18–26 reported having ever received at least one dose of HPV4 (direct estimation from the 2008 TX BRFSS). At the county-level, indirect estimates ranged from 1.9–23.8% (Figure 1; county-specific estimates available upon request). Aggregated to the state level, we obtained a population weighted average of 11.4% (95% CI: 10.3, 12.5), a value which, as expected, lies within the confidence interval of the direct estimate of HPV vaccine coverage in Texas. The county with the lowest vaccine coverage (Webb) was about 10 percent lower than the direct state estimate, while the county with the highest vaccine coverage (Orange) was about 12 percent higher than the direct state estimate.
Figure 1.
County-level estimates of HPV vaccine coverage among young women, aged 18–26: Texas: 2008
Although the purpose of model-based SAE is to provide small area estimates, model results are also important because they contribute to the formulation of the indirect estimates. Table 1 provides the results of the series of increasingly-complex multilevel models previously described. Compared to non-Hispanic whites, Hispanics were significantly more likely to be vaccinated against HPV (OR = 1.83, 95% CI: 1.35, 2.46). For age, we found an inverse association (OR = 0.80, 95% CI: 0.66, 0.95), indicating that the odds of receiving ≥1 dose HPV4 decreased with increasing age. This association was consistent across all models. Among tested group-level covariates, we found significant inverse associations between county- and HSR-level lack of insurance and HPV vaccination. The statistical significance and relative effect size of these covariates (i.e., age, Hispanic ethnicity, and county- and HSR-level lack of insurance) did not change with the removal of education from the final model.
Table 1.
Random-intercept logit model of HPV vaccination (≥ 1 dose HPV4) among young women, aged 18–26: Texas, 2008
| Model Parameters | Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
Model 4 OR (95% CI) |
Model 5 OR (95% CI) |
|---|---|---|---|---|---|
| Fixed Effects | |||||
| Intercept | 0.13 (0.09,0.20) | 0.04 (0.01, 0.14) | 2.63 (0.11, 60.76) | 23.59 (1.49, 374.13) | 46.85 (4.18, 525.15) |
| Mean Centered Age | _ | 0.78 (0.64, 0.95) | 0.78 (0.66, 0.94) | 0.78 (0.65, 0.94) | 0.80 (0.66, 0.95) |
| Non-Hispanic white | _ | Ref | Ref | Ref | Ref |
| Non-Hispanic black | _ | 1.59 (0.57, 4.48) | 1.89 (0.44, 8.11) | 1.86 (0.51, 6.74) | 1.54 (0.40, 5.95) |
| Hispanic | _ | 1.53 (1.00, 2.35) | 2.08 (0.84, 5.17) | 1.95 (1.39, 2.72) | 1.83 (1.35, 2.46) |
| Other races | _ | 0.58 (0.18, 1.85) | 0.95 (0.13, 7.00) | 0.90 (0.37, 2.14) | 1.15 (0.44, 2.98) |
| < HS1 education | _ | Ref | Ref | Ref | |
| HS graduate | _ | 1.87 (0.71, 4.94) | 1.81 (0.46, 7.15) | 1.88 (0.68, 5.21) | |
| Some college or more | _ | 2.96 (1.22, 7.16) | 2.58 (0.68, 9.82) | 2.65 (1.09, 6.47) | |
| Lack of insurance (County) | _ | _ | 0.86 (0.77, 0.95) | 0.87 (0.83, 0.92) | 0.87 (0.83, 0.91) |
| Lack of insurance (HSR) | _ | _ | _ | 0.91 (0.85, 0.97) | 0.92 (0.86, 0.98) |
HS = High School
DISCUSSION
County-level estimates of HPV vaccine coverage among women aged 18–26 varied from 1.9–23.8% (Figure 1), with a population weighted state average of 11.4%. In this study, Hispanic women were more likely to be vaccinated against HPV than their White counterparts. This relationship was not found in other studies conducted nationally or in other regions4, 7–9, which suggests either fundamental differences in the characteristics of Hispanics living in Texas versus other regions or conversely, better education and outreach initiatives targeting these individuals. In a previous study done by JME, Hispanic ethnicity was also a significant correlate among adolescent Texas females16. The group-level covariates of importance differed, however. For example, county-level poverty was strongly and positively associated with vaccination in adolescent females16, but not in young adult women. We suspect this is because high poverty counties have a large proportion of low income families, and income is inversely associated with eligibility for publicly-financed health insurance and services for children and adolescents. Through these publicly-funded programs (e.g., Children’s Health Insurance Program and Vaccines for Children), children and adolescents whose families meet certain income limits can received free or low cost vaccines including HPV4. Given that women aged 18 years and older are generally not covered by these programs, it is not surprising that county-level poverty does not equate to greater likelihood of HPV vaccination in this age group. Insurance coverage, on the other hand, may be very important for young women aged 18 years and older given the high cost of the vaccine series (currently $360 for 3 doses). Our research supports this association, although we were only able to include insurance coverage at the county level. Additional factors such as awareness of and access to Merck’s Vaccine Patient Assistance Program and other financing options (e.g., under/ uninsured young women can receive HPV4 from Federally Qualified Health Centers or Family Planning Clinics in Texas) may also be important, although these variables were not available for examination in our sample. Due to the self-reported nature of the survey used in this study, it is possible that the data are subject to recall bias. We should also note that our primary outcome, initiation of the HPV vaccine, is only a preliminary measure of vaccine coverage; future studies should use more current data to examine estimates of vaccine completion at the county-level. Finally, the seemingly contradictory finding that Hispanic ethnicity is positively associated with vaccination, while many border counties with large Hispanic populations have low vaccination rates is likely due to the strong influence of insurance coverage in the model.
Small sample size and privacy concerns often plague local health studies. Through the application of model-based SAE to survey data on HPV vaccination, we have shown how local health statistics can be derived and discussed their potential uses for both research and public health surveillance and control activities. In this study, we utilized a multilevel SAE framework to highlight geographic disparities and opportunities for intervention in HPV vaccine coverage among young women aged 18–26. This outcome is particularly well-suited for small area analysis because 1) receipt of the HPV vaccine and documentation of vaccination status are not required by most states (including Texas) making it difficult to accurately estimate HPV vaccine coverage in local areas, 2) geographic variation in adolescent vaccine coverage has been documented in recent studies17,18, and 3) interest in increasing HPV vaccine uptake through local advocacy efforts, health education programs, and policies is on the rise. Although our study showed county-level differences in HPV vaccine coverage, even the county with the highest estimate had <25% of women aged 18–26 initiating HPV4, indicating that state-level policies might be more effective at bringing up coverage across the board. We propose that future small area studies on HPV vaccination examine the sensitivity of other modeling strategies in estimating county HPV vaccine coverage, estimate county HPV vaccine coverage among males, who can now receive the vaccine19, and assess whether coverage is increasing over time.
Acknowledgments
Dr. Jan M. Eberth was the recipient of two National Cancer Institute fellowships during the course of this study: the Cancer Education and Career Development Pre-doctoral Fellowship (R25-CA057712, Patricia Dolan-Mullen, PhD, Principal Investigator) and the Cancer Prevention Training Program (R25T-CA57730, Shine Chang, PhD, Principal Investigator). Additionally, we acknowledge support from the National Institutes of Health MD Anderson Cancer Center Support Grant (CA016672, Ronald DePinho, MD, Principal Investigator). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Cancer Institute, the National Institutes of Health, or the Centers for Disease Control and Prevention. Gardasil® is a registered trademark of Merck & Co., Inc.
References
- 1.Approval Letter - Human Papillomavirus Quadrivalent (Types 6, 11, 16, 18) Vaccine, Recombinant [Internet] Rockville (MD): Food and Drug Administration; Jun 8, 2006. [updated 2009 May 1; cited 2012 Jan 31]. Available from: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm111283.htm. [Google Scholar]
- 2.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 3.Caskey R, Lindau ST, Alexander GC. Knowledge and early adoption of the HPV vaccine among girls and young women: results of a national survey. J Adolesc Health. 2009;45(5):453–62. doi: 10.1016/j.jadohealth.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Jain N, Euler GL, Shefer A, Lu P, Yankey D, Markowitz L. Human papillomavirus (HPV) awareness and vaccination initiation among women in the United States, National Immunization Survey-Adult 2007. Prev Med. 2009;48(5):426–431. doi: 10.1016/j.ypmed.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 5.BRFSS Annual Survey Data: Survey Data and Documentation. Atlanta (GA): Centers for Disease Control and Prevention, Office of Surveillance, Epidemiology, and Laboratory Services; 2008. [updated 2011 May 16; cited 2012 Jan 31]. Available from: http://www.cdc.gov/brfss/technical_infodata/surveydata.htm. [Google Scholar]
- 6.Texas Behavioral Risk Factor Surveillance System. Austin (TX): Texas Department of State Health Services; [updated 2011 Mar 1; cited 2012 Jan 31]. Available from: http://www.dshs.state.tx.us/chs/brfss/ [Google Scholar]
- 7.Chao C, Velicer C, Slezak JM, Jacobsen SJ. Correlates for completion of 3-dose regimen of HPV vaccine in female members of a managed care organization. Mayo Clin Proc. 2009;84(10):864–70. doi: 10.4065/84.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao C, Velicer C, Slezak JM, Jacobsen SJ. Correlates for human papillomavirus vaccination of adolescent girls and young women in a managed care organization. Am J Epidemiol. 2010;171(3):357–67. doi: 10.1093/aje/kwp365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Licht AS, Murphy JM, Hyland AJ, Fix BV, Hawk LW, Mahoney MC. Is use of the human papillomavirus vaccine among female college students related to human papillomavirus knowledge and risk perception? Sex Transm Infect. 2010;86(1):74–78. doi: 10.1136/sti.2009.037705. [DOI] [PubMed] [Google Scholar]
- 10.Rabe-Hesketh S, Skrondal A. Multilevel modelling of complex survey data. J Roy Stat Soc A Sta. 2006;169(4):805–827. [Google Scholar]
- 11.Rasbash J, Charlton C, Browne WJ, Healy M, Cameron B. MLwiN Version 2.20. Centre for Multilevel Modeling, University of Bristol; 2005. [Google Scholar]
- 12.Carle AC. Fitting multilevel models in complex survey data with design weights: Recommendations. BMC Med Res Methodol. 2009;9:49. doi: 10.1186/1471-2288-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein H. Multilevel statistical models. John Wiley & Sons; New York: 2010. pp. 91–92. [Google Scholar]
- 14.Congdon P. A multilevel model for cardiovascular disease prevalence in the US and its application to micro area prevalence estimates. Int J Health Geogr. 2009;8:6. doi: 10.1186/1476-072X-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SAS Version 9.2. Cary, NC: SAS Institute Inc; 2008. [Google Scholar]
- 16.Eberth JM, Hossain MM, Tiro JA, Zhang X, Holt JB, Vernon SW. Applying multilevel, small area estimation to the examination of human papillomavirus (HPV) vaccine coverage among females aged 11–17 in Texas counties. Women Health Iss. 2012 doi: 10.1016/j.whi.2012.12.005. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorell C, Stokley S, Yankey D, Liang JL, Markowitz L. National and state vaccination coverage among adolescents aged 13 through 17 years --- United States, 2010. MMWR. 2011;60(33):1117–1123. [PubMed] [Google Scholar]
- 18.pruitt SL, Schootman M. Geographic Disparity, Area Poverty, and Human Papillomavirus Vaccination. Am J Prev Med. 2010;38(5):525–533. doi: 10.1016/j.amepre.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Approval Letter – Gardasil. Rockville (MD): Food and Drug Administration; Oct 16, 2009. [updated 2009 Oct 16; cited 2012 Jan 31]. Available from: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186991.htm. [Google Scholar]