Abstract
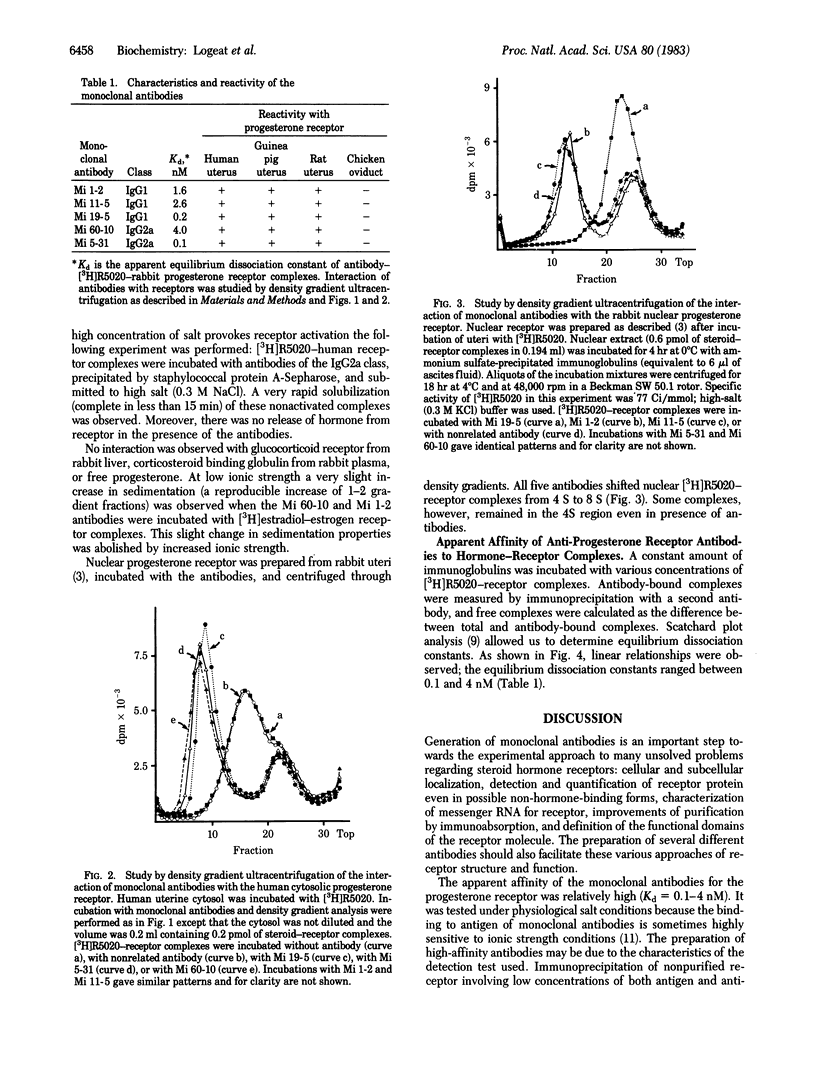
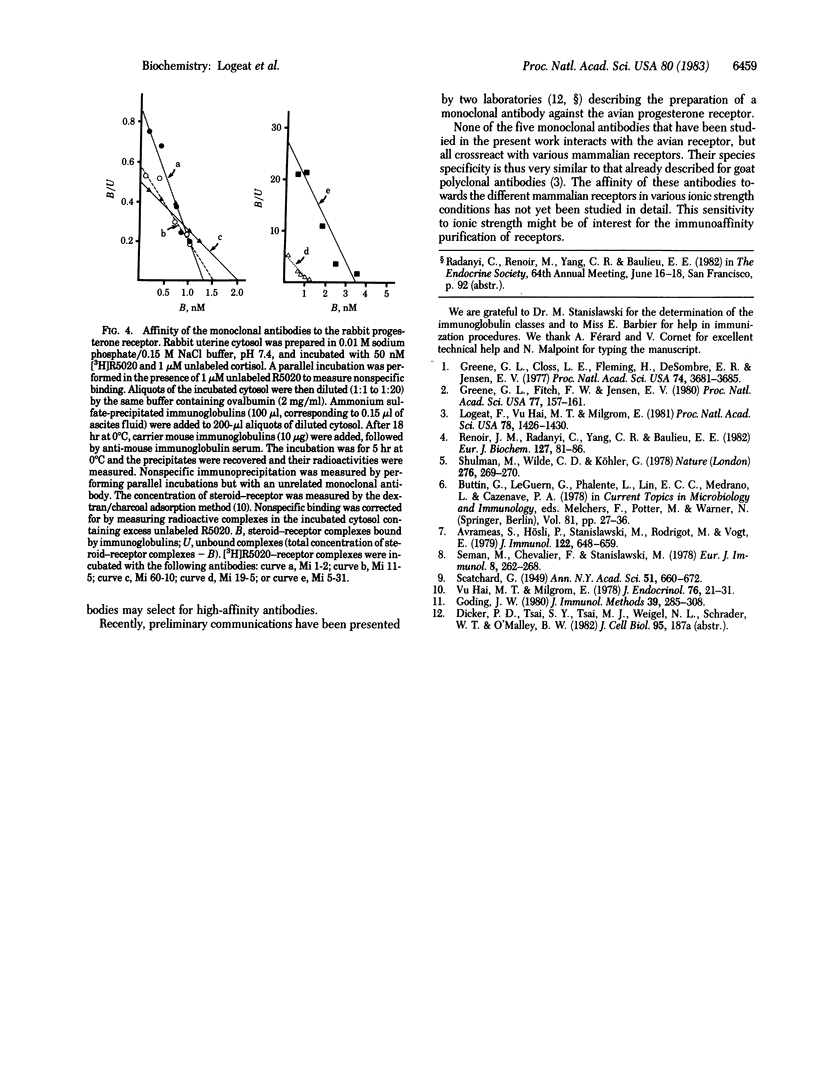
A mouse was immunized with purified rabbit uterine cytosolic progesterone receptor (specific activity: 3 nmol of steroid bound per mg of protein). After fusion of its spleen cells with Sp2-OAg myeloma cells, supernatants of 11 hybrid cultures were found to react in both an immunoenzymatic test and a double-immunoprecipitation test with the progesterone receptor. Clones were obtained from the five hybrid cells that gave the strongest response in both tests. Antibodies from cell culture supernatants and ascitic fluids were characterized. Three are of the IgG1 and two of the IgG2a isotype. Their apparent affinity for the progesterone receptor was measured by immunoprecipitation in physiological salt conditions. The equilibrium dissociation constants were between 0.1 and 4 nM. All five monoclonal antibodies crossreacted with the rabbit nuclear receptor, the human cytosolic receptor, and other mammalian (rat, guinea pig) but not avian (chicken) cytosolic progesterone receptors. There was no interaction with the glucocorticoid receptor and corticosteroid binding globulin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Hösli P., Stanislawski M., Rodrigot M., Vogt E. A quantitative study at the single cell level of immunoglobulin antigenic determinants present on the surface of murine B and T lymphocytes. J Immunol. 1979 Feb;122(2):648–659. [PubMed] [Google Scholar]
- Buttin G., LeGuern G., Phalente L., Lin E. C., Medrano L., Cazenave P. A. Production of hybrid lines secreting monoclonal anti-idiotypic antibodies by cell fusion on membrane filters. Curr Top Microbiol Immunol. 1978;81:27–36. doi: 10.1007/978-3-642-67448-8_4. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Antibody production by hybridomas. J Immunol Methods. 1980;39(4):285–308. doi: 10.1016/0022-1759(80)90230-6. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Closs L. E., Fleming H., DeSombre E. R., Jensen E. V. Antibodies to estrogen receptor: immunochemical similarity of estrophilin from various mammalian species. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3681–3685. doi: 10.1073/pnas.74.9.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene G. L., Fitch F. W., Jensen E. V. Monoclonal antibodies to estrophilin: probes for the study of estrogen receptors. Proc Natl Acad Sci U S A. 1980 Jan;77(1):157–161. doi: 10.1073/pnas.77.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logeat F., Hai M. T., Milgrom E. Antibodies to rabbit progesterone receptor: crossreaction with human receptor. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1426–1430. doi: 10.1073/pnas.78.3.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoir J. M., Radanyi C., Yang C. R., Baulieu E. E. Antibodies against progesterone receptor from chick oviduct. Cross-reactivity with mammalian progesterone receptors. Eur J Biochem. 1982 Sep;127(1):81–86. doi: 10.1111/j.1432-1033.1982.tb06840.x. [DOI] [PubMed] [Google Scholar]
- Seman M., Chevalier F., Stanislawski M. Evidence for independent genetic regulation of the expression of different antibody classes in anti-sheep red blood cell responses. Eur J Immunol. 1978 Apr;8(4):262–268. doi: 10.1002/eji.1830080409. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Vu Hai M. T., Milgrom E. Characterization and assay of the progesterone receptor in rat uterine cytosol. J Endocrinol. 1978 Jan;76(1):21–31. doi: 10.1677/joe.0.0760021. [DOI] [PubMed] [Google Scholar]



