Abstract
This study was designed to evaluate the effect of Korean red ginseng (KRG) supplementation on glucose control in subjects with impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or newly diagnosed type 2 diabetes mellitus (T2DM). The study was a 12-week randomized, double-blinded, placebo-controlled (5 g of KRG [n=21] or placebo [n=20] in tablet form) trial. Glucose-related biomarkers, including serum and whole blood levels of glucose, insulin, and C-peptide, were measured by 2-h oral glucose tolerance tests (OGTTs) at baseline and after the 12-week intervention. After the intervention, the test group showed a significant decrease in serum levels of glucose at 30 min (−22.24±10.77 mg/dL) and whole blood levels of glucose at 30 min (−17.52±5.22 mg/dL). In addition, the test group tended to have lower whole blood levels of glucose at 0 min and glucose area under curve (AUC). However, the placebo group did not show any changes in blood glucose-related indices. The changes (difference from baseline) in serum glucose levels at 30 min, whole blood glucose levels at 60 min, and glucose AUC during OGTTs in the test group exhibited a tendency toward a decrease from those in the placebo group. There were significant decreases or trends toward a decrease in both serum insulin and C-peptide concentrations at most time intervals in the test group. In conclusion, KRG supplementation (5 g/day) may be beneficial for controlling serum and whole blood glucose levels compared with placebo among patients with IFG, IGT, or T2DM.
Key Words: : diabetes mellitus, glycemia, impaired fasting glucose, impaired glucose tolerance, insulin, Korean red ginseng
Introduction
Global diabetes diagnosis rates continue to rise due to a myriad of social factors, such as obesity, stress, overeating, and lack of exercise.1 According to the Korean National Health and Nutrition Examination Survey (KNHANES) conducted in 2010, the estimated prevalence of diabetes mellitus for adults over age 30 was about 10%, and the number of people with impaired fasting glucose (IFG) and impaired glucose tolerance (IGT), which are prediabetic conditions, is increasing.2
Korean red ginseng (KRG, Panax ginseng C.A. Meyer), a herb that populations have used for more than 2000 years as a general tonic in traditional oriental medicine, has been reported to have various pharmacological and physiological effects, such as lowering serum blood glucose, aiding hematogenesis and the recovery of liver function, and promoting immune function. Claims for the fatigue-diminishing, immune system-enhancing, and blood circulation-promoting (by preventing blood platelet aggregation) functions of KRG have been approved by the Korean Food and Drug Administration (KFDA).3
Until now, there have been few clinical studies on the efficacy of red ginseng extract or powder for blood glucose control, and past studies mostly included people with type 2 diabetes mellitus (T2DM) or normal blood glucose levels.4–9 There has not been a clinical trial on the effect of Korean red ginseng on regulation of blood glucose in subjects with IFG, IGT, or newly diagnosed T2DM.
This study was a randomized, double-blind, placebo-controlled clinical trial designed to evaluate the effect of Korean red ginseng supplementation on glucose control in subjects with IFG, IGT, or newly diagnosed T2DM and to establish clinical evidence of the glucose control effect of KRG.
Materials And Methods
Subjects
Study participants between 20 and 70 years of age were recruited from the health checkup center at Ilsan Hospital (Goyang-si, Gyeonggi-do, Korea) and by advertisements in a local newspaper. After the glucose screening test, subjects with IFG (100 mg/dL ≤fasting blood glucose ≤125 mg/dL), IGT (2-h OGTT ≥140 mg/dL), or newly diagnosed T2DM (fasting glucose ≥126 mg/dL) were enrolled in this study. The exclusion criteria included the following: glucose-lowering medications or insulin injections; chronic alcoholism or evidence of alcoholism; pregnancy or breast feeding; chronic gastrointestinal disorders; signs of nutrient deficiency of malnutrition; serious kidney problems; serious liver problems; an occupation that could be dangerous if hypoglycemia should occur; complications, such as headache, insomnia, heart palpitations, or elevated blood pressure, after previously consuming red ginseng; or other factors that the researchers considered unsuitable for this study.
Sixty patients with IFG, IGT, and T2DM were enrolled in this study and provided their written informed consent for the study, which was approved by the Institutional Review Board of Yonsei University (IRB-765) and registered in the international clinical trial registry (NCT01911663).
Study design
This study was designed as a 12-week randomized, double-blinded, placebo-controlled trial. Sixty subjects were randomly assigned to receive placebo (corn starch) or 500 mg KRG. Both groups consumed a total of 10 capsules per day (totaling 5.0 g) at three times during the day: after breakfast (three capsules), lunch (three capsules), and dinner (four capsules). Red ginseng and placebo capsules were provided by the Korea Ginseng Corporation (KGC, Daejeon, Korea). The red ginseng capsules contained 16.58 mg/g total ginsenosides, and the ratio of protopanaxadiol ginsenosides (Rb1, Rb2, Rc, Rd, and Rg3) to protopanaxatriol ginsenosides (Rg1, Re, and Rf ) was 1.65:1. Analyses of common ginsenosides were performed in quadruplicate using standard HPLC-UV techniques10 at the Korean Ginseng Research Institute in Daejeon, Korea. Subjects met with the investigational team at four different time points: screening (Week-1), randomization and treatment baseline (Week 0), the treatment midpoint (Week 6), and the treatment endpoint (Week 12). Daily intake by a 24-h recall method and physical activity were measured at baseline, midpoint, and endpoint of the treatment period. Compliance with study restrictions and capsule consumption were monitored by means of daily documentation by subjects on individualized study calendars and end-study count of returned capsules.
Anthropometric parameters, blood pressure measurements, and blood collection
Body weight and height were measured in the morning with the subjects unclothed and without shoes. The body mass index (BMI) was calculated as body weight in kilograms divided by the square of height in meters (kg/m2). Blood pressure was measured using the left arm of the seated patient with an automatic blood pressure monitor (TM-2654; A&D, Tokyo, Japan) after a 10-min rest. The mean of two measurements was recorded for each subject. Venous blood specimens were collected in EDTA-treated and plain tubes after a 12-h fast. The tubes were placed on ice until they arrived at the laboratory (within 1–3 h) and were stored at −70°C until analysis.
Glucose-related biomarkers
The oral glucose tolerance test (OGTT, 75 g glucose) was performed. Subjects drank a 75-g glucose solution after an overnight fast. Venous blood samples were then collected at 30-min intervals for 2 h (0, 30, 60, 90, and 120 min) and blood sugar was measured at the same time points. Tubes were centrifuged to isolate plasma or serum, and stored at −70°C until analysis.
Fasting glucose and blood glucose concentrations during OGTTs were measured using the glucose oxidase method with a Beckman Glucose Analyzer (Beckman Instruments, Irvine, CA, USA). Insulin was measured by radioimmunoassay using commercial kits from Immuno Nucleo Corporation (Stillwater, MN, USA). Insulin resistance (IR) was calculated by the homeostasis model assessment (HOMA) using the following equation: HOMA-IR=(fasting insulin [μIU/mL]×fasting glucose [mM])/22.5. The C-peptide concentration was determined by two-site sandwich immunoassay using an ADVIA Centaur XP immunoassay system (Siemens, Princeton, NJ, USA). HbA1c was measured by an immonoturbidimetic analyzer using a turbidimeter.
Serum lipid profiles
Fasting serum concentrations of total cholesterol and triglyceride (TG) were measured using commercially available kits with the Hitachi 7150 Autoanalyzer (Hitachi Ltd., Tokyo, Japan). High-density lipoprotein (HDL) cholesterol was measured from the supernatant by enzymatic methods. Low-density lipoprotein (LDL) cholesterol was estimated indirectly when serum TG levels were below 400 mg/dL using the Friedewald formula. In subjects with serum TG concentrations ≥400 mg/dL, LDL cholesterol levels were determined directly by an enzymatic method on a Hitachi 7150 Autoanalyzer.
Assessment of food intake and physical activity
Food intake was assessed by a 24-h recall method; a semiquantitative food frequency questionnaire was used to confirm that the data collected by the 24-h recall method were representative of the usual dietary pattern.11 Nutrient intake data were calculated as mean values from the database referenced above. Total energy expenditure (TEE; kcal/d) was calculated from the basal metabolic rate, 24-h physical activity,12 and food-specific dynamic action. The basal metabolic rate for each subject was calculated using the Harris–Benedict equation.13
Statistical analysis
All analyses were performed using SPSS software package version 18.0 for Windows (SPSS, Inc., Chicago, IL, USA). Descriptive statistics are used to describe the basic features of the demographic data in this study. Continuous variables are presented as means with standard errors of the mean, and frequencies are shown for categorical variables. We compared baseline characteristics between the intervention and control groups by using the Mann–Whitney test (nonparametric independent t-test) for continuous variables and the chi-square test or the Fisher's exact test for categorical variables.
We evaluated the changes from baseline to follow-up in glucose-related parameters for both the test and control groups using the nonparametric Wilcoxon signed-rank test. Then, we compared the changes from baseline to follow-up in glucose-related parameters in the intervention and control groups by using the nonparametric Mann–Whitney test. Results are expressed as mean±SE. P-values<.05 were considered statistically significant.
Results
Baseline characteristics and dietary intake of the study participants
Among the enrolled subjects (n=60), 19 subjects dropped out and 41 subjects completed the study. Among the 19 dropouts, 6 were excluded because they took glucose-related medications during the intervention and 13 dropped out for personal reasons. The baseline characteristics for the 41 subjects were evaluated and are presented in Table 1. There were no significant differences between the groups at baseline in age, BMI, or systolic or diastolic blood pressure. When the pre- and post-treatment parameters of the subjects were compared, there were no significant baseline to end-of-study changes in BMI or blood pressure within either group, and these changes were not significantly different when compared between groups. In the placebo group after 12 weeks, serum TG concentrations significantly decreased compared with the baseline. However, this change was not significantly different when compared with the corresponding change in the test group. Other serum lipid profiles, including the concentrations of total cholesterol and HDL cholesterol, did not show any significant changes in either group.
Table 1.
General Characteristics of Study Subjects Before and After the 12-Week Intervention
| Placebo (n=20) | Test (n=21) | |||
|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | |
| Age (years) | 56.10±2.18 | 58.81±1.72 | ||
| Sex (male/female) | 12/8 | 16/5 | ||
| BMI (kg/m2) | 23.80±0.61 | 23.78±0.68 | 23.52±0.48 | 23.57±0.47 |
| SBP (mmHg) | 128.78±2.99 | 127.73±2.69 | 125.76±3.55 | 124.00±2.91 |
| DBP (mmHg) | 82.05±2.01 | 83.18±1.89 | 79.90±2.04 | 78.95±1.67 |
| TG (mg/dL) | 120.00±16.01 | 110.33±22.90* | 120.06±16.75 | 129.11±21.31 |
| TC (mg/dL) | 181.35±6.47 | 186.05±7.27 | 188.76±7.40 | 185.00±6.09 |
| HDL-c (mg/dL) | 54.05±2.31 | 55.65±2.10 | 54.38±2.38 | 53.38±2.71 |
| LDL-c (mg/dL) | 100.32±5.68 | 107.44±6.53 | 108.66±7.40 | 103.12±7.91 |
Data values are reported as mean±SE or frequency. Tested by the Wilcoxon signed-rank test (intragroup comparison) and by the Mann–Whitney test (intergroup comparison for initial value).
P<.05 compared with baseline in each group.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; TC, total cholesterol; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol.
TEE also did not significantly change in either group (Table 2). Dietary intake such as TCI, percentage total carbohydrates, percentage total proteins, and percentage total lipids did not show significant differences in either group.
Table 2.
Daily Dietary Intake at Baseline and After the 12-Week Intervention
| Placebo (n=20) | Test (n=21) | |||
|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | |
| TEE (kcal/d) | 2157.1±48.52 | 2170.1±47.16 | 2163.3±30.89 | 2172.6±29.39 |
| Estimated daily nutrient intake | ||||
| TEI (kcal/d) | 2146.7±49.04 | 2132.8±46.54 | 2161.7±30.30 | 2169.0±34.86 |
| CHO (%) | 61.91±0.25 | 62.13±0.27 | 61.91±0.22 | 61.85±0.19 |
| Protein (%) | 16.60±0.16 | 16.43±0.16 | 16.40±0.13 | 16.63±0.19 |
| Fat (%) | 21.98±0.20 | 21.88±0.21 | 21.70±0.16 | 21.49±0.16 |
Data values are reported as mean±SE. Tested by the Wilcoxon signed-rank test (intragroup comparison) and by the Mann–Whitney test (intergroup comparison for initial value).
TEE, total energy expenditure; TEI, total energy intake; CHO, carbohydrate.
Serum and whole blood glucose levels
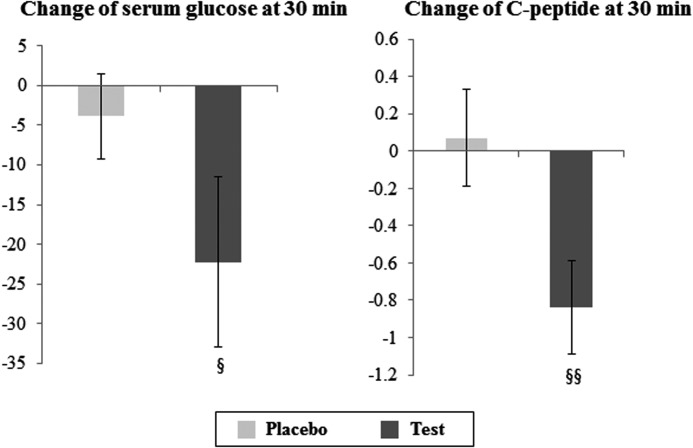
Test group serum glucose levels at 30 min significantly decreased from a baseline of 208.90±11.53 mg/dL to an endpoint value of 186.67±8.95 mg/dL (P=.016), while the placebo group did not exhibit such a statistically significant decrease (Table 3). When these changes between two groups were compared, the test group (−22.24±10.77 mg/dL) trended toward a more substantial decrease than the placebo group (−3.90±5.32 mg/dL; P=.078; Fig. 1).
Table 3.
Serum and Whole Blood Glucose Levels at Baseline and After the 12-Week Intervention
| Placebo (n=20) | Test (n=21) | |||
|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | |
| Serum glucose (mg/dL) | ||||
| Glu_0 min | 109.75±3.57 | 107.95±4.39 | 110.57±3.50 | 107.43±4.66 |
| Glu_30 min | 184.75±8.09 | 180.85±8.68 | 208.90±11.53 | 186.67±8.95* |
| Glu_60 min | 195.10±14.51 | 195.85±14.38 | 216.14±15.45 | 210.52±14.97 |
| Glu_120 min | 158.15±16.95 | 165.45±17.38 | 163.24±11.53 | 166.52±20.62 |
| Glu_AUC (mg/dL×h) | 345.30±22.55 | 347.10±23.38 | 375.86±19.92 | 361.43±26.32 |
| Whole blood glucose (mg/dL) | ||||
| Glu_0 min | 128.40±3.44 | 124.80±4.51 | 127.90±3.50 | 122.33±4.85† |
| Glu_30 min | 203.05±7.83 | 195.25±8.13 | 217.29±6.48 | 199.76±8.38** |
| Glu_60 min | 204.65±13.46 | 212.80±12.18 | 230.90±13.87 | 219.95±13.23 |
| Glu_120 min | 177.00±16.11 | 174.45±14.88 | 179.62±12.31 | 179.14±18.70 |
| Glu_AUC (mg/dL×h) | 375.75±20.90 | 375.70±20.79 | 403.86±17.94 | 385.19±23.60† |
Data values are reported as mean±SE. Tested by the Wilcoxon signed-rank test (intragroup comparison) and by the Mann–Whitney test (intergroup comparison for initial value).
P<.1, *P<.05, **P<.01 compared with baseline in each group.
Glu, glucose; Glu_AUC, glucose area under curve.
FIG. 1.
Changes in glucose and C-peptide levels at 30 min during the oral glucose tolerance test before and after the Korean red ginseng supplementation intervention. §P<.1, §§P<.05, tested by a nonparametric test (Mann–Whitney test).
With respect to whole blood glucose concentrations, the test group showed a tendency toward a decrease in fasting glucose (P=.070) and response areas of glucose (P=.059) and a significant reduction in whole blood glucose levels at 30 min (P=.002). The changes (difference from baseline) in levels of whole blood glucose at 60 min (P=.070) and glucose AUC (P=.080) during OGTTs in the test group trended toward a more substantial decrease from those in the placebo group (data not shown).
We also analyzed the results between subjects with IFG/IGT and subjects newly diagnosed with T2DM. The test group subjects with IFG/IGT (n=11) experienced significant decreases in the levels of serum (P=.041) and whole blood glucose (P=.016) at 30 min. The test group subjects with newly diagnosed T2DM (n=10) showed a tendency toward a decrease in whole blood glucose levels at 30 min (P=.059). However, the placebo group (IFG/IGT: n=9, T2DM: n=11) did not exhibit such statically significant decreases in the levels of serum and whole blood glucose.
Whole blood glucose levels at 60 min in the test group with IFG/IGT trended toward a decrease compared with those in the placebo group with IFG/IGT (P=.056; data not shown).
Levels of glucose-related biomarkers
Table 4 shows serum concentrations of insulin and C-peptide between the test and placebo groups before and after the intervention. After the intervention, serum concentrations of insulin at 0 min (P=.024) and 30 min (P=.007) and insulin AUC (P=.006) significantly decreased and there was a trend toward a decrease in serum concentrations of insulin at 60 min (P=.070) and 120 min (P=.070) in the test group. In addition, serum concentrations of C-peptide at 0 min (P=.042) and 30 min (P=.008) and C-peptide AUC (P=.039) significantly decreased and there was a trend toward a decrease in serum concentrations of C-peptide at 60 min (P=.085) in the test group. The net change of values of C-peptide at 30 min (P=.013) before and after the intervention was significantly different between the test group and the placebo group (Fig. 1). HbA1C levels did not change significantly in either group. HOMA-IR significantly decreased from baseline to endpoint in both test and placebo groups, and the baseline to end-of-study changes were not significant in either group or between groups (Table 4).
Table 4.
Glucose-Related Biomarkers at Baseline and After the 12-Week Intervention
| Placebo (n=20) | Test (n=21) | |||
|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | |
| HOMA-IR | 1.89±0.12 | 1.66±0.10* | 1.81±0.12 | 1.53±0.12* |
| HbA1c (%) | 6.19±0.18 | 6.18±0.18 | 6.21±0.16 | 6.30±0.18 |
| Ins_0 min (μIU/dL) | 6.98±0.45 | 6.25±0.32† | 6.71±0.45 | 5.81±0.37* |
| Ins_30 min (μIU/dL) | 23.68±4.09 | 22.37±4.26 | 26.43±4.33 | 18.76±3.22** |
| Ins_60 min (μIU/dL) | 32.26±5.91 | 28.68±4.31 | 40.70±6.60 | 33.20±5.54† |
| Ins_120 min (μIU/dL) | 35.68±5.47 | 35.32±5.48 | 39.24±6.72 | 31.0±5.29† |
| Ins_AUC (μIU/dL×h) | 55.68±8.15 | 51.89±7.56 | 66.10±9.88 | 51.65±7.87** |
| C-pep_0 min (μEq/L) | 1.77±0.15 | 1.81±0.15 | 2.10±0.18 | 1.79±0.19* |
| C-pep_30 min (μEq/L) | 5.09±0.62 | 5.10±0.55 | 5.45±0.47 | 4.66±0.45** |
| C-pep_60 min (μEq/L) | 7.66±0.73 | 7.93±0.63 | 8.67±0.69 | 7.99±0.67† |
| C-pep_120 min (μEq/L) | 8.11±0.62 | 8.19±0.59 | 9.45±0.68 | 8.72±0.78 |
| C-pep_AUC (μEq/L×h) | 12.85±1.09 | 13.05±1.00 | 14.57±1.03 | 13.19±1.03* |
Data values are reported as mean±SE. Tested by the Wilcoxon signed-rank test (intragroup comparison) and by the Mann–Whitney test (intergroup comparison for initial value).
P<.1, *P<.05, **P<.01 compared with baseline in each group.
HOMA-IR, homeostasis model assessment-insulin resistance; HbA1c, hemoglobin A1c; Ins, insulin; C-pep, C-peptide; Ins_AUC, insulin area under curve; C-pep_AUC, C-peptide area under curve.
When we subdivided the results between IFG/IGT and newly diagnosed T2DM, the levels of glucose-related biomarkers, serum concentrations of insulin at 30 min (P=.041) and 60 min (P=.028), insulin AUC (P=.033), and C-peptide at 30 min (P=.050) significantly decreased in the test group subjects with IFG/IGT. In addition, serum concentrations of insulin (P=.012) and C-peptide (P=.022) at 120 min and C-peptide AUC (P=.010) significantly decreased and serum concentrations of insulin at 0 (P=.066) and 30 min (P=.079), AUC (P=.092), and C-peptide at 30 (P=.059) and 60 min (P=.074) trended toward a decrease in the test group in subjects with newly diagnosed T2DM. The net changes of values of C-peptide at 0 min (P=.024), 30 min (P=.020), and 120 min (P=.024) and AUC (P=.006) before and after the intervention were significantly different between the test and placebo groups in subjects with newly diagnosed T2DM (data not shown).
Discussion
This study demonstrates that supplementation with KRG over 12 weeks can improve glucose control among individuals with IFG, IGT, or newly diagnosed T2DM. Subjects consuming 5 g of KRG capsules per day attained significant decreases in serum glucose at 30 min during a 75-g OGTT over the intervention period relative to the placebo group. In addition, the test group experienced significant improvements or trends toward improvements in fasting glucose, glucose at 60 min, and glucose AUC in whole blood samples relative to the placebo group. There were also significant decreases or tendencies to decrease in both serum insulin and C-peptide concentrations at most time intervals in the test group. However, there were no significant decreases in the placebo group except fasting serum insulin levels. There were no significant differences between the test and control groups in the insulin index. However, the net change in values of C-peptide at 30 min before and after the intervention was significantly different between the test group and the placebo group. HOMA-IR, an IR biomarker, and HbA1C were unchanged over the course of the study.
The absence of improvement in HbA1C, the marker of long-term glycemic control, was likely due to the great glycemic control subjects achieved. The pre- and post-treatment HbA1C values of below 6.3% in both groups were at the upper limit of the normal range for the assay and within a<6.5% treatment goal.14 Moreover, changes in postprandial glycemia tend to be found more easily in poorly controlled T2DM, whereas our subjects were prediabetic or had been recently diagnosed with T2DM.
It has been suggested that KRG supplements improve glucose control; however, there are few studies that have evaluated the hypoglycemic effect of KRG in human subjects. Sievenpiper et al. showed that 2 g of KRG rootlets was enough to attain reproducible reductions in postprandial glycemia.5 Vuksan et al. reported glucose and insulin regulation effects of KRG in well-controlled T2DM. In this clinical trial, 12 weeks of supplementation with the selected KRG treatment improved plasma glucose levels (decreased 75-g OGTT PG indices by 8–11%) and plasma insulin (decreased fasting and 75-g OGTT PI indices by 33–38% and increased insulin sensitivity index by 33%) in subjects with well-controlled T2DM. However, these improvements in fasting and postprandial glucose (PPG) metabolism were reflected in neither the intra- nor the intertreatment differences in HbA1C.6 On the other hand, Reed et al. were unable to find evidence that oral ginseng or ginsenoside Re therapy improves B-cell function or insulin sensitivity in subjects with IGT or newly diagnosed T2DM.7 De Souza et al. reported differential effects of KRG root fractions on postprandial glycemia in healthy individuals.8 Their study found that KRG rootlets contained more than sixfold total ginsenosides than the KRG body fraction, but did not show significant differences in PPG levels. Although the KRG body fraction had a lower ginsenoside profile, it lowered PPG levels during OGTTs and reduced glucose AUC by 27%.
The results from studies that investigated the effects of KRG on glucose control have varied considerably. Therefore, future studies are required to determine the contribution of KRG and different ginsenosides to glycemic control in humans.
It is not clear through which mechanisms different ginseng sources and their various components have an effect on glucose regulation. Four theories have been supported by a growing body of evidence from in vitro and in vivo studies: modulation of glucose absorption, insulin secretion and binding, glucose transport, and glucose disposal.9 Modulation of glucose absorption is the way different sources of ginseng might affect the rate of digestion by inhibiting gastric secretion15 and neuronal discharge frequency from the gastric compartment of the brain stem.16 Those actions may slow the digestion of food, decreasing the rate of absorption of carbohydrates, such as glucose, into portal hepatic circulation. There is evidence of insulin secretion and binding effects of ginseng from several animal studies. Insulin release from rat islets was increased by total ginsenosides from Asian ginseng.17 Insulin binding in rat liver, brain, and bone marrow was also increased by ginsenoside Rg1 or Asian ginseng extract.18–19 The third possible mechanism, modulation of glucose transport, suggests that ginseng extracts and compounds may increase glucose transport in various cell lines. In the livers of mice, glucose transporter (GLUT)-2 protein expression was increased by Asian ginseng extract.20 Glucose uptake in isolated sheep erythrocytes by GLUT-1 was increased by diverse ginsenosides.21 A glucose disposal mechanism has also been suggested. Several enzymes related to glucose disposal, such as isomerase and lactate dehydrogenase, were increased in human diploid fibroblasts by the saponin fraction,22 and Asian ginseng decreased the activity of the rate-limiting gluconeogenic enzyme, glucose-6-phosphatase, in the livers of diabetic mice.23
In the present study, the mechanism can be derived from the measured indices. A glucose transport mechanism coupled with insulin sensitization is suggested by the decreases in serum and whole blood glucose levels and decrease in serum insulin concentrations.
The limitations of this study include the relatively small number of participants and the lack of testing for a dose–response relationship between the KRG supplement and glucose control. Thus, further large-scale research in which several KRG doses are examined is required to obtain detailed and accurate results. In addition, subjects taking glucose-lowering medications or insulin injections were excluded from this study to accurately determine the glucose-lowering efficacy of KRG alone. Thus, the potential for greater blood glucose reductions in subjects taking both hypoglycemic medications and KRG supplements could be explored.
Despite these limitations, the present study indicates that KRG supplementation improves serum glucose levels compared with a placebo supplement among patients with IFG, IGT, and T2DM. Additionally, KRG supplementation led to improvements in glucose-related biomarkers, including serum insulin and C-peptide levels.
Further studies are still required to identify how KRG affects glucose control, and the results of those studies may be used to develop dietary management strategies to prevent and treat DM.
Acknowledgments
The authors sincerely thank the research subjects who participated in the studies described in this report. This work was supported by a 2011 grant from the Korean Society of Ginseng funded by the Korea Ginseng Corporation and the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2006-2005306, 2010-0015017, and 2012M3A9C4048762).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Whiting DR, Guariguata L, Weil C, et al. : IDF Diabetes Atlas: global estimates of the prevalence of diabetes for 2011 and 2030. J Diabetes Res 2011;94:311–321 [DOI] [PubMed] [Google Scholar]
- 2.Ministry of Health and Welfare: The Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV). Ministry of Health and Welfare, Seoul, Korea, 2010, pp. 56–57 [Google Scholar]
- 3.Kwak YS, Park JD, Yang JW: Present and its prospect of red ginseng efficacy rearch. Food Ind Nutr 2003;8:30–37 [Google Scholar]
- 4.Vuksan V, Sievenpiper JL: Herbal remedies in the management of diabetes: lessons learned from the study of ginseng. J Numecd 2005;15:149–160 [DOI] [PubMed] [Google Scholar]
- 5.Sievenpeper JL, Sung MK, Buono MD, et al. : Korean red ginseng rootlets decrease acute postgrandial glycemia: results from equential preparation-and dose-finding studies. J Am Coll Nutr 2006;25:100–107 [DOI] [PubMed] [Google Scholar]
- 6.Vuksan V, Sung MK, Sievenpiper JL, et al. : Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. J Numecd 2006;18:46–56 [DOI] [PubMed] [Google Scholar]
- 7.Reed DN, Holloszy JO, Patterson BW: Ginseng and ginsenoside Re do not improve B-cell function or insulin sensitivity in overweight and obese subjects with impaired glucose tolerance or diabetes. Diabetes Care 2011;34:1071–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Souza L, Jenkins AL, Sievenpiper JL: Korean red ginseng (Panax ginseng C.A. Meyer) root fraction: differential effects on postprandial glycemia in healthy individuals. J Ethnopharmacol 2011;137:245–250 [DOI] [PubMed] [Google Scholar]
- 9.Pasupuleti VK, Anderson JW: Ginseng in type 2 diabetes mellitus: a review of the evidence in humans. In: Nutraceuticals, Glycemic Health and Type 2 Diabetes. (Pasupuleti VK, Anderson JW, eds.) Ames, Iowa: Wiley-Blackwell/IFT Press, New York, 2008, pp. 245–283 [Google Scholar]
- 10.Fitzloff JF, Yat P, Lu ZZ, et al. : Perspectives on the quality control assurance of ginseng products in North America. In: Proceedings of the 7th International Ginseng Symposium, Seoul, Korea, September22–25, 1998, pp. 138–145 [Google Scholar]
- 11.Shim JS, Oh KW, Suh I, et al. : A study on validity of a 299 semiquantitative food frequency questionnaire of Korean adults. Korean J Commun Nutr 2002;7:484–494 [Google Scholar]
- 12.Christian JL, Greger JH: Nutrition for Living. The Benjamin/Cummings Publishing Company, Inc., San Francisco: 1991, p 111 [Google Scholar]
- 13.American Dietetic Association: Handbook of Clinical Dietetics, 2nd ed. Yale University Press, New Haven, 1992, pp. 5–39 [Google Scholar]
- 14.The International Expert Committee: International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki Y, Ito Y, Konno C, et al. : Effects of tissue cultured ginseng on gastric secretion and pepsin activity. Yakugaku Zasshi 1991;111:770–774 [DOI] [PubMed] [Google Scholar]
- 16.Yuan CS, Wu JA, Lowell T, et al. : Gut and brain effects of American ginseng root on brainstem neuronal activities in rats. Am J Chin Med 1998;26:47–55 [DOI] [PubMed] [Google Scholar]
- 17.Li G, Lu Z: Effect of ginseng saponins on insulin release from isolated pancreatic islets of rat. Zhongguo Zhong Zi Yi Jie He Za Zhi 1987;7:357–359 [PubMed] [Google Scholar]
- 18.Tchilian EZ, Zhelezarov IE, Hadjiivanova CL: Effect of ginsenoside Rg1 on insulin binding in mice liver and brain membranes. Phytother Res 1991;5:46–48 [Google Scholar]
- 19.Yushu CS, Yuzhen H: The effect of Panax ginseng extract (GS) on insulin and corticosteroid receptors. J Trad Chin Med 1988;8:292–295 [PubMed] [Google Scholar]
- 20.Ohnishi Y, Takagi S, Miura T, et al. : Effect of ginseng radix on GLUT2 protein content in mouse liver in normal and epinephrine-induced hyperglycemic mice. Biol Pharm Bull 1996;19:1238–1240 [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa H, Matsumiya S, Murakami C, et al. : Interactions of ginseng extract, ginseng separated fractions, and some triterpenoid saponins with glucose transporters in sheep erythrocytes. Planta Med 1994;60:153–157 [DOI] [PubMed] [Google Scholar]
- 22.Shia GT, Ali S, Bittles AH: The effects of ginseng saponins on the growth and metabolism of human diploid fibroblasts. Gerontology 1982;28:121–124 [DOI] [PubMed] [Google Scholar]
- 23.Chung SH, Choi CG, Park SH: Comparisons between white ginseng radix and rootlet for antidiabetic activity and mechanism in KKAy mice. Arch Pharm Res 2001;24:214–218 [DOI] [PubMed] [Google Scholar]