Abstract
Evidence suggests that the cytokine interferon (IFN)-γ released by natural killer and CD4+ T cells contributes to the conjunctival goblet cell (GC) loss in dry eye. The purpose of this study was to investigate if topical neutralization of IFN-γ prevents or alleviates GC loss in an experimental desiccating stress (DS) model of dry eye. In this study, we found that topical IFN-γ neutralization significantly decreased DS-induced conjunctival GC loss. This was accompanied by decreased epithelial apoptosis, and increased IL-13 and decreased FoxA2 expression in the forniceal conjunctiva. To establish that IFN-γ produced by pathogenic CD4+ T cells contributes to DS-induced GC loss, adoptive transfer of CD4+ T cells isolated from DS exposed donors to naïve RAG-1−/− recipient mice was performed. Similar to the donor mice, topical IFN-γ neutralization decreased conjunctival GC loss, suppressed apoptosis and increased IL-13 expression in adoptive transfer recipients. In summary, this study demonstrated that topical neutralization of IFN-γ prevents GC loss via modulating apoptosis and maintaining IL-13 signaling.
Keywords: Dry eye, IFN-γ, apoptosis, IL-13, FOXA2
1. Introduction
Dry eye is one of the most prevalent eye diseases, affecting tens of millions of people worldwide. The pathogenesis of keratoconjunctivitis (KCS), the ocular surface disease of dry eye is a multifactorial process that includes activation of stress pathways in the ocular surface epithelia by desiccation, the hyperosmolar tear film and inflammatory cytokines, such as interleukin (IL)-17 and interferon gamma (IFN-γ) that are produced by resident intraepithelial lymphocytes and infiltrating CD4+ T cells (De Paiva et al., 2007, 2009, 2010; Lam et al., 2009; Niederkorn et al., 2006). Conjunctival goblet cells (GCs) are simple columnar epithelial cells that secrete the gel-forming mucin MUC5AC that stabilizes the tear film and protects the cornea. GC loss in dry eyes is often associated with a poorly protected and irregular cornea and may lead to sight-threatening corneal ulceration and perforation (Murube and Rivas., 2003; Pflugfelder et al., 1997; Stern et al., 1998a, 1998b). The mechanisms responsible for GC loss in KCS are not completely understood; however, there is increasing evidence indicating that an altered balance of T helper cell (Th) cytokines has a prominent role this process. We previously demonstrated that CD4+ T cells activated by exposure to desiccating stress (DS), when adoptively transferred to naïve T-cell–deficient nude mice, were sufficient to elicit autoimmune KCS with pronounced conjunctival GC loss (Niederkorn et al., 2006; Zhang et al., 2011b).
Th1 and Th2 cytokines have been found to have opposing effects on conjunctival goblet cell development. The Th2 cytokine IL-13 has been found to induce1 GC hyperplasia in nonocular mucosa, such as the gut and respiratory tracts (Atherton et al., 2003; Kanoh et al., 2011; Marillier et al., 2008). We have observed that IL-13 produced by resident NKT cells has a homeostatic function in promoting conjunctival epithelial goblet cell differentiation and mucus production (De Paiva et al., 2011). IL-13 signals through STAT-6 and has been found to stimulate production of GC mucin MUC5AC directly or indirectly by suppressing production of the forkhead transcription factor FoxA2, a MUC5AC repressor (Kim et al., 2008; Oh et al., 2010; Rogers, 2003). In contrast, experimental desiccating stress increased the number of cells staining positively for the Th1 cytokine IFN-γ + in the goblet cell zones of the conjunctiva and increased the concentration of IFN-γ in tears (De Paiva et al., 2007). This was accompanied by a decrease in IL-13/IFN-γ ratio and progressive GC loss. No change in conjunctival GC density was noted in IFN-γ-knockout mice subjected to desiccating stress; however, loss of GCs similar to wild type was observed following subconjunctival injection of IFN-γ in these mice (De Paiva et al., 2007). These findings suggest that strategies to neutralize IFN-γ may prevent dry eye induced GC loss.
One mechanism for IFN-γ induced GC loss in KCS is induction of conjunctival epithelial apoptosis. IFN-γ-knockout mice were found to be resistant to DS induced conjunctival apoptosis; however, exogenous administration of IFN-γ to this strain significantly increased apoptosis after DS (Zhang et al., 2011a). Meaningfully, apoptosis was greatest in the goblet cell area, and MUC5AC expression was inversely associated with level of apoptosis in experimental murine dry eye, suggesting that IFN-γ may cause GC loss in DS by promoting apoptosis under DS (Zhang et al., 2011a).
The purpose of this study was to investigate if topical neutralization of IFN-γ would alleviate or prevent GC loss by maintaining IL-13 expression and modulating apoptosis using a murine DS model with features similar to human KCS.
2. Methods
2.1. Mouse Model of Dry Eye
This research protocol was approved by the Baylor College of Medicine Center for Comparative Medicine, and it conformed to the standards in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Desiccating stress (DS) was used to induce experimental dry eye in C57/BL6(B6) mice, 6 to 8 weeks of age of both genders, by subcutaneous injection of 0.5 mg/0.2 mL scopolamine hydrobromide (Sigma-Aldrich, St. Louis, MO) into alternating hindquarters administered four times a day (8:30 AM, 11 AM, 1 PM and 4:30 PM) with exposure to an air draft and <40% ambient humidity. Mice were euthanized after 5 days of desiccating stress treatment (DS). A group of age- and gender-matched mice that did not receive any treatment to induce dry eye served as nonstressed (NS) controls.
2.2 In Vivo Neutralization of IFN-γ in B6 Mice Subjected to DS
In vivo neutralization of IFN-γ was performed using topical application of rat anti-mouse IFN-γ IgG1 (1 mg/mL, hybridoma R4-6A2; catalog no. HB-170; American Type Culture Collection, Rockville, MD). This antibody has been previously shown to Antibody neutralization of IFN-γ on the ocular surface in our model was confirmed by comparing expression of MHC Class II Ia gene in the conjunctiva of adoptive transfer recipients of CD4+ T cells from donors exposed to DS for 5 days.
To evaluate the role of IFN-γ in GC loss in dry eye induced by DS, in vivo neutralization of IFN-γ was performed in B6 mice subjected to DS by topical application of 10 µL of anti-IFN-γ (DS+αIFNγ) or isotype control (rat IgG, 1 mg/mL; Vector Laboratories) (DS+IC) to the ocular surface four times daily from –3 to 5 days of DS.
2.3. CD4+ T Cell Isolation and Adoptive Transfer
To evaluate the role of pathogenic CD4+ T cells in conjunctival GC loss induced by DS, adoptive transfer experiments were performed after CD4+ T cell isolation.
Spleens and cervical lymph nodes were collected from NS and DS B6 donor mice, and their cell suspensions were enriched for CD4+ T cells by negative selection using a cocktail of antibodies conjugated to magnetic microbeads (MACS system; Miltenyi Biotec, Bergisch Gladbach, Germany), as previously described (Zhang et al., 2012). Untouched CD4+ T cells (5 ×106 ) were washed with PBS and adoptively transferred (i.p.) to RAG-1–deficient mice (RAG-1−/−; B6.129S7/J; Jackson Laboratories) that lack mature B and T lymphocytes. RAG-1−/− recipient mice were divided into two treatment groups: DS that received CD4+ T cells from B6 DS exposed donors (n=80) and NS (n=80) that received CD4+ T cells from NS B6 donors. All RAG-1−/− mice were euthanized 72 hours after adoptive transfer.
2.4 In Vivo Neutralization of IFN-γ in the Recipients
To evaluate the role of IFN-γ produced by pathogenic CD4+ T cells in GC loss in RAG-1−/− mice after adoptive transfer of CD4+ T cells, in vivo neutralization of IFN-γ was performed in DS RAG-1−/− mice by topical application of 10 µL of anti-IFN-γ (1 mg/mL) or vehicle isotype control (rat IgG, 1 mg/mL) four times daily from –3 to 3 days of CD4+ T cell adoptive transfer.
2.5 Histology
The eyes and adnexa of mice were excised and embedded in OCT compound (VWR, Suwanee, GA) or paraffin. OCT–embedded samples were cut into sagittal sections (8µm thick) and placed onto glass slides that were stored at –80°C. Periodic acid Schiff (PAS) staining was performed on paraffin sections and immunostaining was performed on frozen sections (2 sections per slide, 3 slides per animal, 5 animals per experimental group).
2.6 Measurement of Goblet Cell Density
The goblet cell density was measured in PAS-stained sections from the fornix to the mid-palpebral conjunctiva overlying the distal meibomian glands superiorly and inferiorly using NIS Elements software and expressed as the number of positive cells per millimeter.
2.7 Immunohistochemistry
Cryosections were immunostained for mouse CD4 (rat anti-mouse CD4 antigen; 10 µg/mL; rat IgG2a, k; clone H129.19; BD Pharmingen). Sections in three slides (at least 100 µm apart) from each experimental group were evaluated. Positively stained cells in digital images were counted in the area where the GCs are found (fornix to mid palpebral conjunctiva) over a length of at least 500 µm in the epithelium and in the stroma and to a depth of 75 µm below the epithelial basement membrane using NIS Elements (version 4.1, Nikon, Melville, NY) and were expressed as the number of positive cells per millimeter.
2.8 Immunofluorescent Staining
Cryosections were incubated with polyclonal rabbit anti–AC caspase-3 (1:100; BD Pharmingen, San Diego, CA), rabbit anti–AC caspase-8 (1:100; Novus Biologicals, Littleton, CO) or rabbit anti-FOXA2 (1:100, Abcam, Cambridge, MA) primary antibodies at 4°C overnight. The next day, samples were incubated with goat anti–rabbit FITC-conjugated antibody for 45 minutes in the dark at room temperature, washed and nuclei were counterstained using propidium iodide (PI) (0.7 µg/mL). Negative controls were performed at the same time and consisted of sections incubated with secondary antibody alone. Digital images (512 × 512 pixels) of representative areas of the conjunctiva were captured with a laser-scanning confocal microscope with krypton-argon and He-Ne laser (LSM 510; Carl Zeiss Meditec, Ltd., Thornwood, NY). They were acquired with a 40/1.3× oil immersion objective. The intensity of the staining was graded in sections on three slides that were at least 100 µm apart. Briefly, in each digital image, seven elliptical regions (of 1076 pixels2) were drawn with image analysis software (NIS Elements, version 4.1, Nikon, Melville, NY), and the integrated intensity calculated by the software was recorded on a spreadsheet (Excel; Microsoft, Redmond, WA). The results within each image were summed and are presented as the mean of all images within a group, in fluorescence unit ×105.
2.9 RNA Extraction and Real-Time PCR
Total RNA was isolated from the conjunctiva by a PicoPure RNA isolation kit (Arcturus, KIT0204). Five samples per group were used, one sample consisted of pooled conjunctiva of right and left eyes of the same animal and the experiment was repeated. Reverse transcription (RT) and relative quantitative real-time PCR were performed as previously described (Zhang et al., 2011a, 2011b;) using primers and reporter probe for GAPDH (Mm99999915), IFN-γ (Mm00801778), IL-13 (Mm00434204) and MHC II (Mm00482914) (TaqMan Gene Expression Assays; Applied Biosystems). A non-template control was included in all the experiments, to evaluate for DNA contamination. The GAPDH gene was used as an endogenous reference for each reaction. The results of real time PCR were analyzed by the comparative CT method where target change = 2−ΔΔCT. The results were normalized by the CT value of GAPDH. The mean CT of relative mRNA level in the non-stressed control group (NS) was used as the calibrator.
2.10 Statistical Analysis
Data from two independent experiments was averaged and graphs generated. Two-way analysis of variance with Tukey’s post hoc test was used for statistical comparison between groups. P ≤ 0.05 was considered statistically significant. These tests were performed using GraphPad Prism 5.0 software (GraphPad Software Inc, San Diego, CA).
3. RESULTS
3.1 Topical IFN-γ Neutralization Inhibits Conjunctival GC Loss in a DS Model of Dry Eye
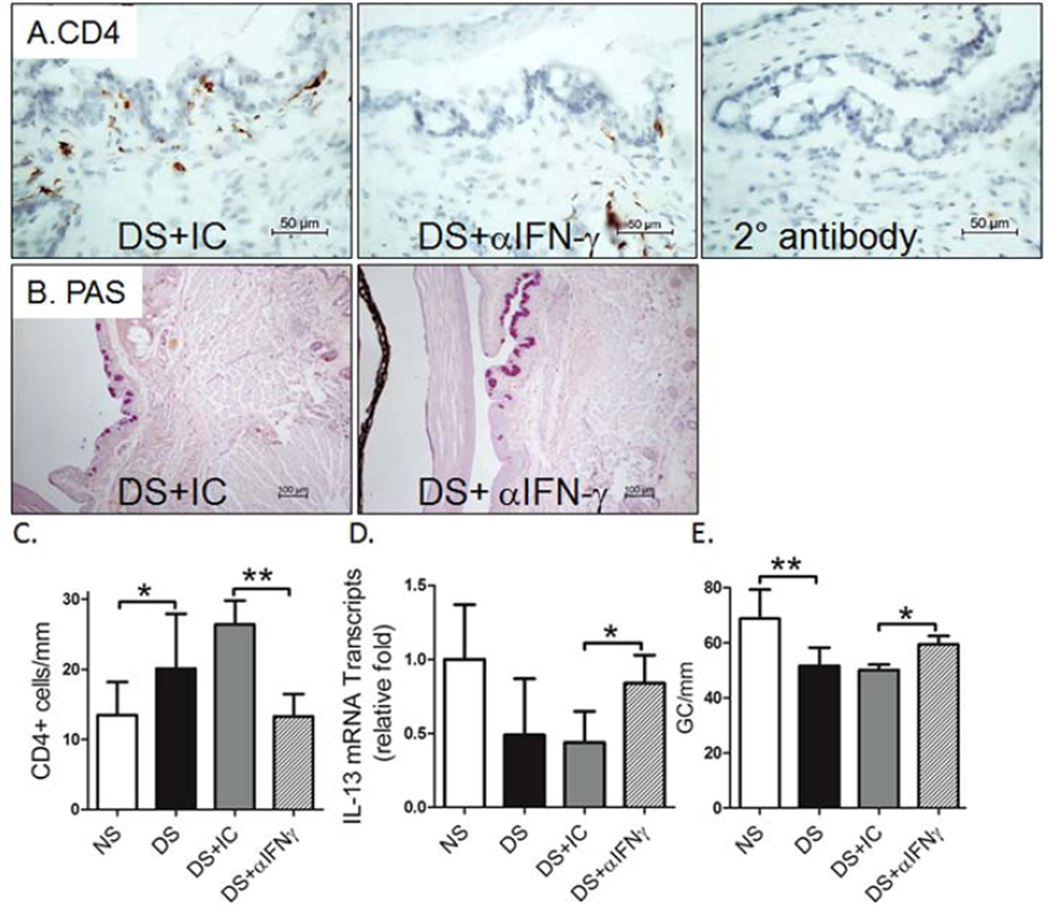
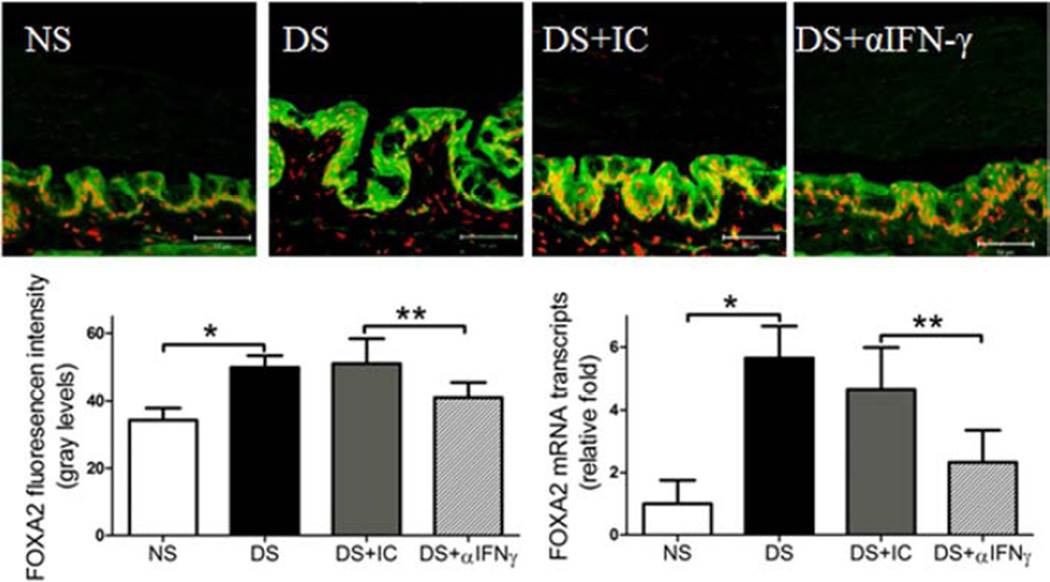
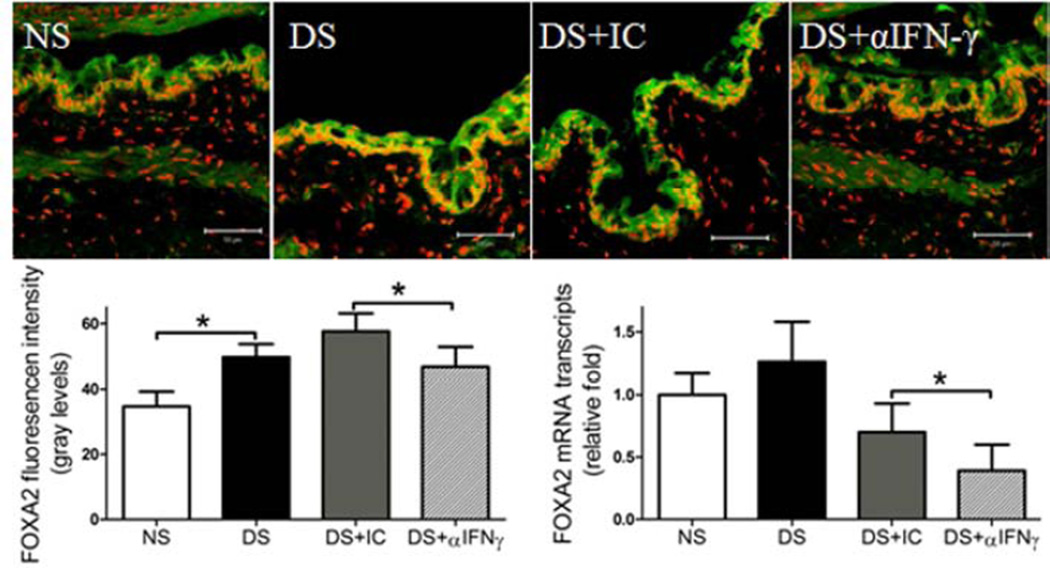
To investigate the role of IFN-γ neutralization in preventing conjunctival GC loss in dry eye, we used B6 mice to evaluate if topical IFN-γ neutralization minimizes or prevents DS-induced conjunctival GC loss. We found that topical IFN-γ neutralization significantly decreased DS-induced conjunctival GC loss. We previously found a significant inverse correlation between the density of infiltrating CD4+ T cells and GCs and this was associated with a decreased IL-13/IFN-γ ratio (De Paiva et al., 2011). IL-13 has been found to have an important homeostatic function in promoting differentiation of mucus-filled GCs in the conjunctival mucosa (De Paiva et al., 2007, 2011). In this study, we found a decreased number of CD4+ T cells infiltrating the conjunctiva (P ≤ 0.05, Fig 1A and 1C) that was accompanied by increased IL-13 mRNA expression and increased number of mucin-filled conjunctival GCs following IFN-γ neutralization (P<0.01, Fig. 1B–D). IL-13 has been found to suppress production of FoxA2, a transcriptional repressor of GC development in the airway mucosa. In this study, DS significantly increased FOXA2 immunoreactivity and level of mRNA transcripts; however, topical IFN-γ neutralization inhibited the DS-induced increase in FoxA2 (Fig. 2). This evidence suggests that topical IFN-γ neutralization mitigates DS-induced GC loss by suppressing CD4+ T cell infiltration and maintaining a homeostatic level of IL-13 in the conjunctiva.
Figure 1.
Effects of topical anti-IFN-γ neutralization on the conjunctival response to experimental desiccating stress dry eye model. (A) Immunohistochemical staining for CD4+ T cells (left and center) and control section stained with secondary antibody alone (right), (B) PAS staining of goblet cells in conjunctiva, (C) number of CD4+ T cells per mm infiltrating the conjunctiva counted over a length of at least 500 µm in the epithelium and in the stroma and to a depth of 75 µm below the epithelial basement membrane, (D) relative levels of IL-13 mRNA transcripts in the conjunctiva, and (E) density of PAS-positive goblet cells (GC) in non-stressed (NS) control mice, desiccating stress treated mice (DS) and DS treated with topical IFN-γ neutralizing antibody (DS+ αIFN-γ) or isotype antisera (DS+IC) in C57BL/6 mice. Topical IFN-γ neutralization decreased CD4+ T cells infiltration, increased levels of IL-13 mRNA transcripts levels and inhibited DS-induced conjunctiva GC loss. Data shown as mean±SEM. * P ≤ 0.05 and ** P ≤ 0.01
Figure 2.
Effects of topical anti-IFN-γ neutralization on expression of FOXA2 transcription factor in the conjunctiva. Laser scanning confocal microscopy of tissue sections immunostained for FOXA2 with propidium iodide (red) nuclear counterstaining. Immunofluorescent intensity, as well as relative mRNA levels in C57BL/6 mice is shown in graphs. Topical IFN-γ neutralization decreased FOXA2 expression in conjunctiva under DS. NS, non-stressed control mice; DS, desiccating stress treated mice; DS+ αIFNγ, DS treated with topical IFN-γ neutralizing antibody; DS+ IC, DS treated with topical isotype control antisera. Data shown as mean±SEM. * P ≤ 0.05 and ** P ≤ 0.01
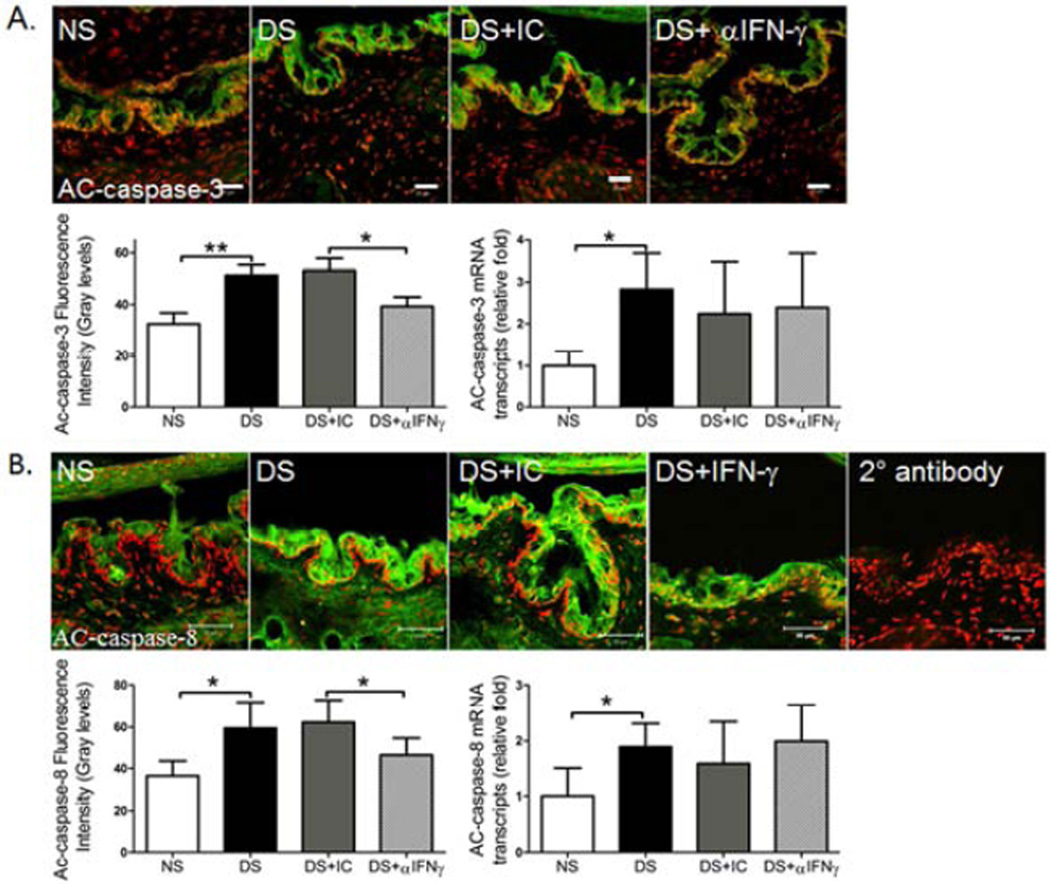
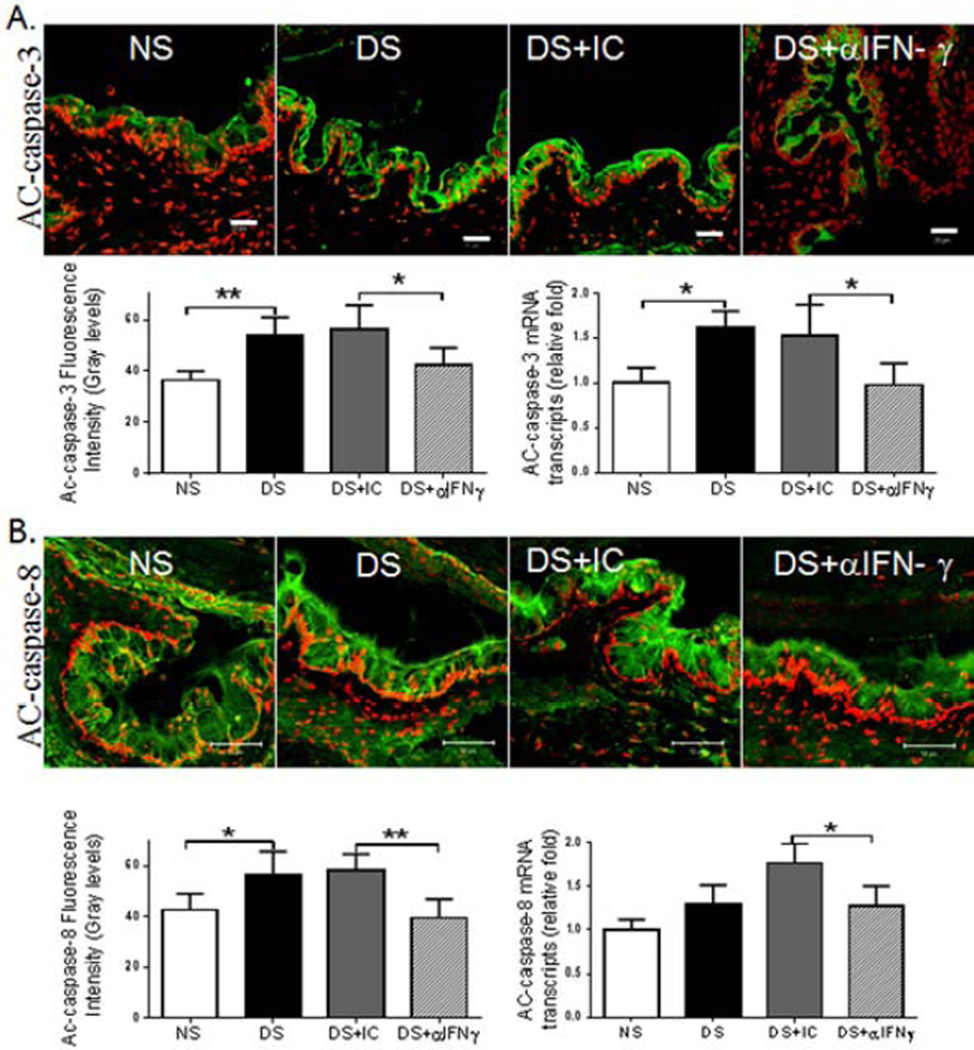
3.2 Topical IFN-γ Neutralization Suppresses DS-induced apoptosis in the Conjunctiva
Previously, we have shown that IFN-γ stimulates apoptosis in the conjunctival epithelium during DS (Zhang et al., 2011a). In this study, we found that topical IFN-γ neutralization had no effect on levels of caspase-3 and -8 mRNA transcripts, but it significantly decreased AC-caspase-3 and -8 immunoreactivity in the conjunctival epithelium (Fig. 3), suggesting topical neutralization of IFN-γ may also prevent GC loss by suppressing apoptosis in the conjunctival epithelium that includes goblet cells and their precursor cells.
Figure 3.
Effects of topical anti-IFN-γ neutralization on markers of apoptosis in the conjunctiva. Laser scanning confocal microscopy of tissue sections immunostained for active (AC)-caspase-3 and -8 (green) with propidium iodide (red) nuclear counterstaining and control section stained with secondary antibody alone. Graphs show AC-caspase-3 and -8 immunofluorescent intensity, as well as relative mRNA levels in C57BL/6 mice. NS, non-stressed control mice; DS, desiccating stress treated mice; DS+aIFNγ, DS treated with topical IFN-γ neutralization; DS+ IC, DS treated with topical isotype antisera. Although in vivo topical IFN-γ neutralization had no effect on the mRNA levels of caspase-3 and -8, it significantly decreased AC-caspase-3 and -8 immunoreactivity in the conjunctival epithelium. Data shown as mean±SEM. * P ≤ 0.05 and ** P ≤ 0.01
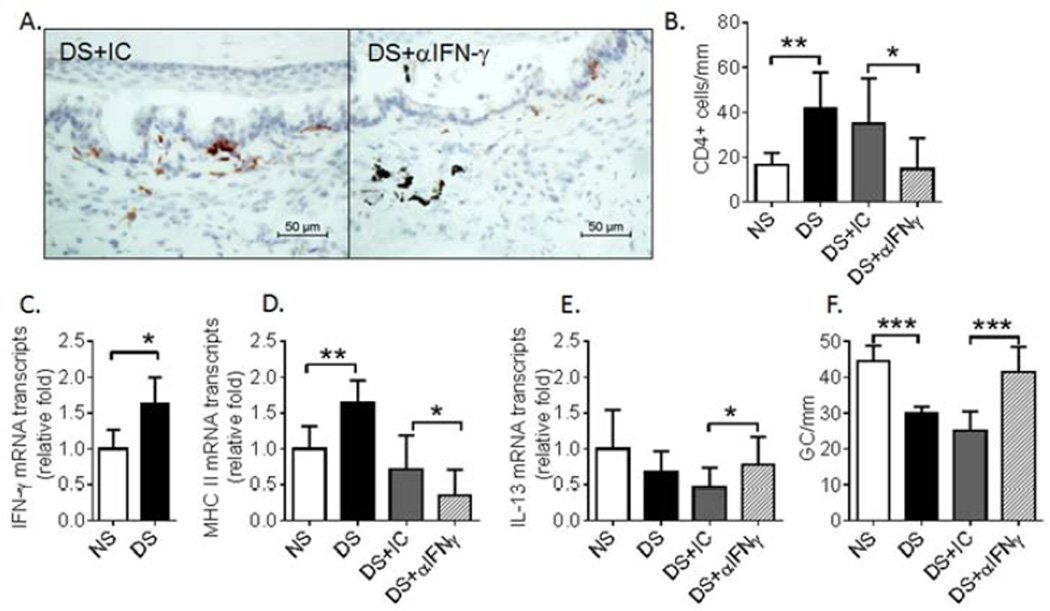
3.3 In Vivo IFN-γ Neutralization Prevents CD4+ T-Cell-Mediated Conjunctival GC Loss
We have previously demonstrated that adoptively transferred CD4+ T cells from donor mice subjected to DS to naïve immune deficient RAG-1−/− mice homed to the recipient’s conjunctiva and caused GC loss (Zhang et al., 2011b). In this study, topical neutralization of IFN-γ was performed to determine whether donor CD4+ T cells caused GC loss via IFN-γ. We found that topical neutralization of IFN-γ increased GC number in the recipient mice indicating that IFN-γ by produced pathogenic CD4+ T cells elicited by DS contributes to GC loss. Similar to the donor mice, IFN-γ neutralization decreased the number of IFN-γ producing CD4+ T cells infiltrating the recipient conjunctival epithelium (P≤0.05, Fig. 4A – C). Topical anti-IFN-γ significantly decreased expression of MHC Class II (Ia) gene transcripts in the conjunctiva (Fig 4D). MHC class II has been reported to be one of the most IFN-γ inducible genes (Young and Hardy, 1995). Topical anti-IFN-γ also increased levels of IL-13 mRNA transcripts (P≤0.05, Fig. 4E) and increased the number of mucin-filled GCs (P≤0.001, Fig. 4F). FOXA2 expression increased in the conjunctiva in the CD4+ T-cell adoptive transfer recipients and this was significantly inhibited by topical IFN-γ neutralization (Fig. 5).
Figure 4.
Effects of topical anti-IFN-γ neutralization on CD4+ T cell infiltration, MHC class II expression and GC density in the conjunctiva of adoptive transfer recipients treated with IC or αIFNγ. (A) CD4+ T cell immunostaining, (B) number of CD4+ T cells per mm infiltrating the conjunctiva counted over a length of at least 500 µm in the epithelium and in the stroma and to a depth of 75 µm below the epithelial basement membrane, (C) relative levels of IFN-γ mRNA transcripts, (D) relative levels of MHC II mRNA transcripts, (E) relative levels of IL-13 mRNA transcripts, and (F) conjunctival goblet cell density in the recipient conjunctiva. Topical IFN-γ neutralization decreased CD4+ T cell infiltration, MHC class II expression and CD4+ T-cell-mediated GC loss, while it increased mRNA levels of IL-13 in the conjunctiva. NS, RAG-1−/− mice receiving CD4+ T cells from non-stressed donor mice; DS, RAG-1−/− mice receiving CD4+T cells from desiccating stress treated donor mice; DS+ αIFNγ, DS5 RAG-1−/− adoptive transfer recipients treated with topical application of anti-IFN-γ antibody; DS+IC, DS RAG-1−/− adoptive transfer recipients treated with topical application of isotype antisera. Data shown as mean±SEM. * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001
Figure 5.
Laser scanning confocal microscopy of tissue sections immunostained for FOXA2 (green) with propidium iodide (red) nuclear counterstaining. FOXA2 immunofluorescent intensity, as well as relative mRNA transcript levels were evaluated in the recipient conjunctiva. Adoptive transfer of CD4+ T cells increased FOXA2 immunoreactivity and mRNA level, and topical IFN-γ neutralization decreased FOXA2 immunoreactivity and mRNA level. NS, RAG-1−/− mice receiving CD4+ T cells from non-stressed donor mice; DS, RAG-1−/− mice received CD4+T cells from desiccating stress treated donor mice; DS+ αIFNγ, DS RAG-1−/− adoptive transfer recipients treated with topical application of anti-IFN-γ antibody; DS+IC, DS RAG-1−/− adoptive transfer recipients treated with topical application of isotype antisera. Data shown as mean±SEM. * P ≤ 0.05
3.4 In Vivo IFN-γ Neutralization Suppresses CD4+ T-Cell-Mediated apoptosis in the Conjunctiva
We found evidence of increased apoptosis in the epithelium in the region where the goblet cells are located in the recipient conjunctiva after adoptive transfer of DS-elicited CD4+ T cells (Fig. 6). Topical neutralization of IFN-γ was performed to determine whether IFN-γ produced by the adoptively transferred CD4+ T cells contributed to the conjunctival epithelial apoptosis. Topical neutralization of IFN-γ was found to significantly decrease AC-caspase-3 and -8 immunoreactivity in the epithelium, as well as levels of caspase-3 and -8 mRNA transcripts compared to isotype-treated mice (Fig. 6).
Figure 6.
Laser scanning confocal microscopy of tissue sections immunostained for active (AC)-caspase-3 and -8 (green) with propidium iodide (red) nuclear counterstaining. AC-caspase-3 and -8 immunofluorescent intensity are shown in graphs, as well as relative levels of their mRNA transcripts in the recipient conjunctiva. Topical IFN-γ neutralization decreased conjunctival AC-caspase-3 and -8 immunoreactivity in the conjunctival epithelium, as well as levels of their mRNA transcripts. NS, RAG-1−/− mice receiving CD4+ T cells from non-stressed donor mice; DS, RAG-1−/− mice receiving CD4+T cells from desiccating stress treated donor mice; DS+ αIFNγ, DS RAG-1−/− adoptive transfer recipients treated with topical application of anti-IFN-γ antibody; DS+IC, DS RAG-1−/− adoptive transfer recipients treated with topical application of isotype antisera. Data shown as mean±SEM. * P ≤ 0.05 and ** P ≤ 0.01
4. DISCUSSION
This study demonstrated that topical neutralization of IFN-γ could prevent conjunctival GC loss in a desiccating stress model of dry eye and following adoptive transfer of DS-elicited CD4+ T cells. These cells synthesize, store, and secrete the large gel-forming mucin MUC5AC that lubricates and protects the ocular surface. Decrease or loss of mucin-filled GCs is a well-recognized feature of aqueous deficient dry eye (Dursun et al., 2002; Hori et al., 2006; Stern et al., 1998a, 1998b). Goblet cell density has been found to correlate with severity of ocular surface disease in patients with aqueous tear deficiency (Pflugfelder et al., 1997). Although the pathogenesis of dry eye induced GC loss is not completely understood, evidence in our DS-induced dry eye model indicates that IFN-γ plays a pivotal role. Previously, we have found that IFN-γ-knockout mice were resistant to DS-induced GC loss (De Paiva et al., 2007). This study evaluated if topical neutralization of IFN-γ would prevent or alleviate GC loss in KCS. We found that topical IFN-γ neutralization significantly decreased DS-induced conjunctival GC loss and conjunctival epithelial apoptosis, which offers a potentially new topical therapeutic approach.
This study identified several potential mechanisms for the goblet cell protective activity of IFN-γ neutralizing antibody. Suppression of apoptosis is one mechanism. Previously, we have shown that increased IFN-γ production in dry eye promotes conjunctival epithelial apoptosis, and IFN-γ-induced apoptosis was greatest in goblet cell area and was accompanied by decreased MUC5AC expression (Zhang et al., 2011a). In this study, in vivo topical IFN-γ neutralization not only increased GC density, but also decreased conjunctival epithelial apoptosis in areas where the GCs and their precursors are located. This evidence suggests IFN-γ-induced pathological apoptosis of the conjunctival epithelium may contribute to the GC loss in dry eye.
Another mechanism by which topical neutralization of IFN-γ may prevent GC loss is by maintaining IL-13 signaling. Increased IL-13 signaling through the IL-13/IL-4 receptor-α complex is recognized to cause GC hyperplasia in monocular mucosa, such as the gut and respiratory tracts. The principal signaling molecule activated by IL-13 is signal transducer and activator of transcription 6 (STAT6) (Kim et al., 2008; Oh et al., 2010; Rogers, 2003). IL-13 has been observed to promote GC hyperplasia in airway epithelial cells through STAT6-dependent up regulation of the SAM pointed domain-containing ETS transcription factor (SPDEF) (Park et al., 2007) or down regulation of FOXA2 transcription factor, a critical negative regulator of GC hyperplasia. Genetic deletion of FOXA2 in mice leads to constitutive hyperplasia resembling mucous metaplasia (Wan et al., 2004). In this study, topical IFN-γ neutralization significantly increased IL-13 and decreased FOXA2 expression in the conjunctiva in response to DS.
Mainly Th1 and natural killer (NK) cells secrete IFN-γ. We previously reported increased IFN-γ expression by intraepithelial lymphocytes in the conjunctiva after 5 days of DS (De Paiva et al., 2007). More recently, we observed that conjunctival intraepithelial NK+ cells are a source of IFN-γ in eyes subjected to DS, and that IFN-γ mRNA level increased in NK+ cells isolated from the conjunctiva of mice after 1 day of DS compared to those obtained from the NS conjunctiva (De Paiva et al., 2011;). These results indicate that activated NK+ cells may contribute to the IFN-γ-induced GC loss in mice subjected to DS. To establish a definite role for pathogenic CD4+ T cells in DS-induced GC loss, adoptive transfer of isolated CD4+ T cells to naïve recipients was performed. We have previously shown the purity of CD4+ T cells by our isolation method was almost 90% and that other potential IFN-γ-secreting cells, including DX5+ NK cells and CD8+ T cells, were depleted (Zhang et al., 2011b). The recipients of adoptively transferred CD4+ T cells from DS-exposed donors developed conjunctival inflammation with CD4+ T cell infiltration, increased IFN-γ expression and decreased GC density (Zhang et al., 2011b). Consistent with findings in the donor mice, in vivo topical neutralization of IFN-γ decreased conjunctival GC loss, suppressed conjunctival epithelial apoptosis and increased IL-13 levels in the adoptive transfer recipients. This provides further evidence that topical neutralization of IFN-γ produced by CD4+ T cells can rescue GC loss by suppressing apoptosis and increasing IL-13 signaling.
In conclusion, this study provides evidence that topical neutralization of IFN-γ produced by CD4+ T cells can prevent GC loss by modulating apoptosis and maintaining IL-13 levels in a mouse model dry eye model. This study provides new insight into the potential of local IFN-γ neutralizing therapy to treat mucosal autoimmune diseases.
Highlights.
Interferon gamma (IFN-γ) inhibits goblet cell differentiation
IFN-γ stimulates apoptosis and inhibits IL-13 signaling in the conjunctival epithelium
Topically applied anti-IFN-γ inhibits apoptosis and maintains IL-13 signaling
Acknowledgments
Financial Support: NIH Grant EY11915 (SCP), NEI/NIH core Grant for Vision Research EY-002520-37, an unrestricted grant from Research to Prevent Blindness, New York, NY (SCP), the Oshman Foundation, Houston, TX (SCP), the William Stamps Farish Fund, Houston, TX (SCP), Hamill Foundation, Houston, TX (SCP)
Abbreviations
- KCS
keratoconjunctivitis sicca
- GC
goblet cell
- MUC5AC
gel forming mucin secreted by goblet cells
- IFN-γ
interferon gamma, a cytokine produced by natural killer (NK) and CD4+ T cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors have no proprietary interest in any materials or methods described within this article.
REFERENCES
- 1.Atherton HC, Jones G, Danahay H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am.J.Physiol Lung Cell Mol.Physiol. 2003;285:L730–L739. doi: 10.1152/ajplung.00089.2003. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zhang X, Zhang J, et al. A murine model of dry eye induced by an intelligently controlled environmental system. Invest Ophthalmol.Vis.Sci. 2008;49:1386–1391. doi: 10.1167/iovs.07-0744. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zhang X, Liu M, et al. Trehalose protects against ocular surface disorders in experimental murine dry eye through suppression of apoptosis. Exp.Eye Res. 2009;89:311–318. doi: 10.1016/j.exer.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 4.De Paiva CS, Villarreal AL, Corrales RM, S.C., et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol.Vis.Sci. 2007;48:2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 5.De Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal.Immunol. 2009;2:243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Paiva CS, Hwang CS, Pitcher JD, III, et al. Age-related T-cell cytokine profile parallels corneal disease severity in Sjogren's syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology.(Oxford) 2010;49:246–258. doi: 10.1093/rheumatology/kep357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Paiva CS, Raince JK, McClellan AJ, et al. Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13. Mucosal.Immunol. 2011;4:397–408. doi: 10.1038/mi.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dursun D, Wang M, Monroy D, et al. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol.Vis.Sci. 2002;43:632–638. [PubMed] [Google Scholar]
- 9.Grau GE, Heremans H, Piguet P-F, et al. Monoclonal antibody against inferferon-γ can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci. 1989;86:5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hori Y, Argueso P, Spurr-Michaud S, Gipson IK. Mucins and contact lens wear. Cornea. 2006;25:176–181. doi: 10.1097/01.ico.0000177838.38873.2f. [DOI] [PubMed] [Google Scholar]
- 11.Kanoh S, Tanabe T, Rubin BK. IL-13-induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells. Clin.Exp.Allergy. 2011;41:1747–1756. doi: 10.1111/j.1365-2222.2011.03852.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin.Immunol. 2008;126:332–337. doi: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam H, Bleiden L, de Paiva CS. Tear cytokine profiles in dysfunctional tear syndrome. Am.J.Ophthalmol. 2009;147:198–205. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marillier RG, Michels C, Smith EM, et al. IL-4/IL-13 independent goblet cell hyperplasia in experimental helminth infections. BMC.Immunol. 2008;9:11. doi: 10.1186/1471-2172-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murube J, Rivas L. Impression cytology on conjunctiva and cornea of dry eye patients establishes a correlation between squamous metaplasia and dry eye clinical severity. Eur J Ophthalmol. 2003;13:115–127. doi: 10.1177/112067210301300201. [DOI] [PubMed] [Google Scholar]
- 16.Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjogren's Syndrome-like lacrimal keratoconjunctivitis. J.Immunol. 2006;176:3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 17.Oh CK, Geba GP, Molfino N. Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma. Eur.Respir.Rev. 2010;19:46–54. doi: 10.1183/09059180.00007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park KS, Korfhagen TR, Bruno MD, et al. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest. 2007;117:978–88. doi: 10.1172/JCI29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pflugfelder SC, Tseng SCG, Yoshino K, et al. Correlation of goblet cell density and mucosal epithelial mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology; 1997. 1997;104:223–235. doi: 10.1016/s0161-6420(97)30330-3. [DOI] [PubMed] [Google Scholar]
- 20.Rogers DF. The airway goblet cell. Int.J.Biochem.Cell Biol. 2003;35:1–6. doi: 10.1016/s1357-2725(02)00083-3. [DOI] [PubMed] [Google Scholar]
- 21.Stern ME, Beuerman RW, Fox RI, et al. A unified theory of the role of the ocular surface in dry eye. Adv.Exp.Med.Biol. 1998a;438:643–651. doi: 10.1007/978-1-4615-5359-5_91. [DOI] [PubMed] [Google Scholar]
- 22.Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998b;17:584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Vogel SN, Havell EA, Spitalny GL. Monoclonal antibody-mediated inhibition of interferon-γ induced macrophage antiviral resistance and surface antigen expression. J Immunol. 1986;136:2917–2923. [PubMed] [Google Scholar]
- 24.Wan H, Kaestner KH, Ang SL, et al. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development. 2004;131:953–964. doi: 10.1242/dev.00966. [DOI] [PubMed] [Google Scholar]
- 25.Young HA, Hardy KJ. Role of interferon-g in immune cell regulation. J Leukocyte Biol. 1995;58:373–381. [PubMed] [Google Scholar]
- 26.Zhang X, Chen W, De Paiva CS, et al. Interferon-gamma exacerbates dry eye-induced apoptosis in conjunctiva through dual apoptotic pathways. Invest Ophthalmol.Vis.Sci. 2011a;52:6279–6285. doi: 10.1167/iovs.10-7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Chen W, De Paiva CS, et al. Desiccating stress induces CD4+ T-cell-mediated Sjogren's syndrome-like corneal epithelial apoptosis via activation of the extrinsic apoptotic pathway by interferon-gamma. Am.J.Pathol. 2011b;179:1807–1814. doi: 10.1016/j.ajpath.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Volpe EA, Gandi NB, et al. NK cells promote Th17 mediated corneal barrier disruption in dry eye. PLoS One. 2012;5:e36822. doi: 10.1371/journal.pone.0036822. [DOI] [PMC free article] [PubMed] [Google Scholar]