Abstract
Purpose
Preclinical data suggested that bryostatin-1 (bryo) could potentiate the cytotoxicity of cisplatin when given prior to this drug. We designed a phase I study to achieve tolerable doses and schedules of bryo and cisplatin in combination and in this sequence.
Methods
Patients with non-hematologic malignancies received bryo followed by cisplatin in several schedules. Bryo was given as an 1 hour and a 24 hour continuous infusion, while cisplatin was always given over 1 hour at 50mg/m2 and 75mg/m2; the combined regimen was repeated on an every 3-week and later on an every 2-week schedule. Bryo doses were escalated until recommended phase II doses were defined for each schedule. Patients were evaluated with computerized tomography every 2 cycles.
Results
53 patients were entered. In an every 2-week schedule, the 1 hour infusion of bryo became limited by myalgia that was clearly cumulative. With cisplatin 50 mg/m2 its recommended phase II dose was 30 mcg/m2. In the 3-week schedule, dose-limiting toxicities were mostly related to cisplatin effects while myalgias were tolerable. Pharmacokinetics unfortunately proved to be unreliable due to bryo’s erratic extraction. Consistent inhibition of PKC isoform eta (η) in peripheral blood mononuclear cells was observed following bryo.
Conclusions
Bryo can be safely administered with cisplatin with minimal toxicity;, however, only 4 patients achieved an objective response. Modulation of cisplatin cytotoxicity by bryo awaits further insight into the molecular pathways involved.
Introduction
Bryostatin 1 (bryo) is a macrocyclic lactone derived from the marine animal Bugula neritina (27). Some of its anti-tumor activity has been ascribed to modulation of protein kinase C (PKC) activity (10, 28) that may antagonize functions such as tumor invasion, angiogenesis and cell adhesion. Bryo also has additional anti-tumor mechanisms such as inhibition of topoisomerase II phosphorylation (3), prevention or reversal of multidrug resistance (6), modulation of ionizing radiation damage (13), stimulation of cytokine production and activation of cytotoxic T lymphocytes (16, 29, 34, 36). Preclinical studies have shown in vitro and in vivo activity of bryo against a variety of tumors including melanoma, leukemia, lymphoma and lung cancer (4, 17, 27, 28).
Bryo underwent its initial clinical testing in the United Kingdom (29, 31). These trials established myalgia as the dose-limiting toxicity (DLT), and this problem was both schedule and dose-dependent (20, 37). The mechanism underlying this painful muscle syndrome has remained obscure. One study suggested that bryo-induced myalgias are related to muscle vasoconstriction and bryo’s direct toxic effect on mitochondria (14); these were not improved by vasodilators (35). Nonetheless, interest in bryo increased based on its unique molecular effects and preclinical experiments showing enhancement of the efficacy of cytotoxic drugs. In vitro studies demonstrated enhancement of cisplatin cytotoxicity by bryo in HeLa cells (2), and this stimulated exploration of cisplatin in combination with this agent. PKC activation by phorbol esters increases the sensitivity of human ovarian carcinoma cells to cisplatin (18), while others noted that inhibitors of PKC were synergistic with cisplatin both in vitro and in large-cell lung tumor xenografts in nude mice (8).
Bryo prevents the down-regulation of PKC-delta, a tumor suppressor important in cell cycle progression (11, 19), which is ubiquinated by phorbol esters, and in fact, it prevents the first step of multistage carcinogenesis exerted by phorbol esters (11). PKC eta (η), a PKC isoform that promotes cell proliferation through the mammalian target of rapamycin (mTOR) pathway (1), is preferentially expressed in epidermis, lung and brain (12, 25, 40), and its expression in invasive breast cancer is associated with lymph node positive status (22). Bryo downregulates the activity of PKCη (12). Possibly related to its PKC modulation, bryo has been shown also to potentiate the cytotoxic effects of cytosine arabinoside, mitomycin C, BCNU and vincristine in cell line studies (2, 8, 9, 18, 23, 26, 30).
A clinical study of bryo in combination with cytotoxic drugs is particularly appealing because of its near lack of myelosuppression, gastrointestinal toxicity, neuropathy or alopecia. In pursuing a combination of bryo with cisplatin, we had a particular interest in developing a regimen for possible activity against metastatic melanoma. Cisplatin has only a 10% response rate in this disease (7), bryo has activity in melanoma cell lines (20), and the hypothesis underlying this trial was that successful modulation would be relatively easy to detect.
Patients and Methods
The study was activated in November 1997 utilizing a 24 hour bryo infusion followed by a 1 hour infusion of cisplatin administered every 3 weeks. After 21 patients were entered, the study was amended in May 1999 to evaluate 1 hour bryo infusions, as there had been bryo 1 hour infusion data in a phase I study involving patients with heterogeneous advanced cancers, and this short infusion schedule had a tolerable side effect profile (38). An additional 18 patients were enrolled on this schedule, and once a Maximal Tolerated Dose (MTD) had been identified, the protocol was further amended to evaluate an every 2-week schedule, completing accrual in November 2001. This schedule change was based on a study that bryo can be given every 2 weeks (37). A 3 + 3 dose-escalation design was used so that the MTD was considered the dose where no more than 2 out of 6 patients in an expanded level had dose-limiting toxicities (DLTs). Routine peripheral blood tests and symptom and sign survey took place at the time of every dosing. DLTs were defined by utilizing the NCI Common Toxicity Criteria version 2.0 and taking into account any grade 3 or 4 non-hematologic toxicities, including nausea, vomiting, alopecia or any grade 4 hematologic toxicities such as anemia to expand a dose level. Tumor assessments were carried out every 2 cycles with computerized tomography (CT) scans, where recist criteria was utilized (39).
Bryo was supplied by the National Cancer Institute Division of Cancer Treatment and Diagnosis. The protocol was approved by Cancer Therapy Evaluation Program (CTEP), the Protocol Review and Monitoring Committee, Institutional Review Board (IRB) at New York University School of Medicine, and the IRB’s of all participating institutions. Written informed consent was obtained from each patient.
Eligibility Criteria
Enrollment criteria included a histological diagnosis of a disseminated malignancy not amenable to standard treatments, imaging studies including the relevant body CT scans (and brain CTs in patients with melanoma) documenting at least one bidimensionally measurable lesion of 2 cm or greater. Each patient had adequate organ functions defined as: absolute neutrophil count ≥ 1500/mm3, platelets ≥ 100,000/mm3, serum creatinine ≤ 1.5mg/dl, total bilirubin ≤ 1.5 upper limit of normal and AST / ALT / alkaline phosphatase ≤ 2.5 × upper limit of normal. These patients had Eastern Cooperative Oncology Group (ECOG) performance status of ≤ 2, age ≥18 and peripheral neuropathy < Grade 1. No more than 3 prior chemotherapeutic regimens were allowed, and radiation therapy should not have encompassed more than 25% of bone marrow-bearing areas (i.e., no whole pelvic radiation). Patients had to complete prior chemotherapy at least 3 weeks prior and radiation at least 2 weeks prior to entry. Previous cisplatin treatment was not an exclusion. Patients with metastatic malignant melanoma were preferentially entered into this trial based on the paucity of available effective therapies, and the ability to be able to detect early effective modulation should any responses become apparent (Tables 1 and 2). The possibility of overcoming cisplatin resistance with the addition of bryo in metastatic melanoma makes this drug combination a suitable option for patients with metastatic melanoma.
Table 1.
Patient Characteristics (n=53)
| Age (years) | Median | 54 |
| Range | 25–83 | |
| Gender | Male | 29 |
| Female | 24 | |
| Performance Status | 0 | 14 |
| 1 | 36 | |
| 2 | 3 | |
| Prior chemotherapy | 0 | 15 |
| 1 | 18 | |
| 2 | 20 | |
| Prior radiation | Y/N | 19/34 |
| Prior biologic/immunotherapy | Y/N | 20/33 |
Table 2.
Tumor Types (n=53)
| Melanoma | 26 |
| Sarcoma | 6 |
| Head and Neck | 6 |
| Squamous Cell (Unknown Primary) | 2 |
| Ovarian | 2 |
| Cervix | 2 |
| Esophageal | 2 |
| Pancreatic | 2 |
| Appendiceal | 1 |
| Renal | 2 |
| Lung | 2 |
Treatment Regimens (Table 2)
The study design was to have 2 therapeutic doses of cisplatin (50 and 75 mg/m2), preceded by bryo. Initially, a 24 hour infusion of bryo beginning on day 1 followed by cisplatin on day 2 was used (Dose levels 1–7). Subsequently, based on clinical experience with shorter infusions and better patient acceptance of the schedule, we shifted to 1 hour infusions just prior to cisplatin infusion (dose levels 8–11). Each cycle was 3 weeks apart for dose levels 1–11. After concluding this portion of the study, we evaluated the combination given every 2 weeks (Dose levels 1A–2A). All patients had hydration and antiemetic measures including HT3 antagonists prior to cisplatin. Treatment schedules are shown in Table 3. A patient was considered evaluable if this patient completed at least 2 cycles of treatments and had clinical and radiographic assessments every 2 cycles. Patients were taken off protocol either due to progression of disease (PD), adverse events from the treatments (AE) or patient’s or physician’s choice.
Table 3.
Treatment Schedules
| 24 hour infusion of Bryo every 3 weeks | ||||
|---|---|---|---|---|
| Dose level | Bryo (mcg/m2) |
Cisplatin (mg/m2) |
# of patients (Total/Eval) |
Cycles (Total) |
| 1 | 10 | 50 | 3/3 | 11 |
| 2 | 15 | 50 | 3/3 | 6 |
| 3 | 20 | 50 | 3/3 | 10 |
| 4 | 25 | 50 | 3/3 | 9 |
| 5 | 30 | 50 | 3/3 | 5 |
| 6 | 30 | 75 | 3/3 | 7 |
| 7 | 40 | 75 | 3/3 | 8 |
| 1 hour infusion of Bryo every 3 weeks | ||||
|---|---|---|---|---|
| Dose level | Bryo (mcg/m2) |
Cisplatin (mg/m2) |
# of patients (Total/Eval) |
Cycles (Total) |
| 8 | 40 | 50 | 3/3 | 9 |
| 9 | 50 | 50 | 3/3 | 6 |
| 10 | 65 | 50 | 6/3 | 19 |
| 11 | 80 | 50 | 7/6 | 13 |
| 1 hour infusion of Bryo every 2 weeks | ||||
|---|---|---|---|---|
| Dose level | Bryo (mcg/m2) |
Cisplatin (mg/m2) |
# of patients (Total/Eval) |
Cycles (Total) |
| 1A | 40 | 50 | 7/6 | 28 |
| 2A | 30 | 50 | 6/6 | 28 |
Pharmacokinetics and Correlative Studies
In addition to the usual clinical end-points of tolerance and response, we sought to characterize the time course of effect from bryo on a specific PKC isoform in peripheral blood mononuclear cells (PBMCs). Also, we attempted to develop and validate assays for the detection of bryo by mass spectroscopy. PBMCs were collected for study of effects on protein kinase C isoform η, as there is a commercially reliable antibody to detect the signal of this isoform. Samples were collected prior to commencement of the bryo infusion (time 0), at the completion of the bryo infusion (time 1 hour), and 3 hours after the completion of the bryo infusion (time 4 hours). PBMCs were prepared by centrifugation following collection, then samples for DNA content were processed using a modification of the diphenylamine colorimetric determination for use in a microtiter plate reader (15). Protein mixtures obtained from the cell lysates were separated by 6% polyacrylamide gel electrophoresis (PAGE) with 50 µL of each sample loaded per well using Western Blot methodology (15, 21). Plasma samples were collected for bryo pharmacokinetics assay. Samples were collected prior to commencement of the bryostatin-1 infusion (time 0) and at the completion of the bryostatin-1 infusion (time 1 hour).Bryo levels were difficult to detect with 24 hour infusions, but its detection became possible during 1 hour infusions. A solid phase plasma extraction procedure was developed employing a 1:1 addition of Dimethyl Sulfoxide (DMSO) and used to simultaneously extract bryo while minimizing any protein interactions from plasma. Detection of bryo was achieved by High Performance Liquid Chromatography (HPLC) using Ultraviolet (UV) detection at 266 nm along with parallel detection by mass spectroscopy (MS). The UV HPLC assay has a sensitivity of 20 ng/mL but additional sensitivity down to 1 ng/ml was achievable through the use of electrospray MS detection, but its detection was erratic. Subsequent to this study, a validated method employing liquid-liquid extraction followed by reversed phase HPLC and tandem MS (HPLC/MS/MS) detection by Zhao et al reported a lower limit of detection of bryo down to 50 pg/ml (41). However, our study clinical plasma samples were expended in our less sensitive methodology.
PKCη isoform was detected in PBMCs after their isolation from baseline and from 1 hour and 24 hour samples after exposure to bryo by Western blot. To interpret detection of these changes in tumor, three patient cohorts were treated with cisplatin alone, followed three weeks later by the usual bryo-cisplatin sequence. This was abandoned, however, because only a paucity of patients had accessible lesions.
Results
Patient Characteristics
There were 53 patients enrolled in this trial, 29 were men and 24 were women. The median age was 54 years, and most patients had an ECOG performance status (PS) of 0 or 1. Thirty eight patients had at least 1 prior chemotherapy, 22 patients were treated with prior biologic or immunotherapy and 19 patients had prior radiation therapy. Nearly half the patients had melanoma; the next common tumor types were sarcoma and head and neck carcinoma.
Dose escalation and dose limiting toxicities
The first 7 dose levels administered a 24 hour infusion of bryo followed by a 1 hour infusion of cisplatin. Bryo was escalated from 10 to 40 mcg/m2 and the cisplatin from 50 to 75 mg/m2 IV every 3 weeks. No dose limiting toxicities were observed. The protocol was then modified to administer bryo as a 1 hour infusion at 40–80 mcg/m2 with a 1 hour infusion of 50 mg/m2 of cisplatin. Dose limiting myalgias occurred at the 80 mcg/m2 dose level of bryo, while bryo at 65 mcg/m2 was well tolerated (Table 4).
Table 4.
Toxicity
| Treatment Related Toxicity (every 3 weeks) | ||||||
|---|---|---|---|---|---|---|
| Dose Level |
# Patients |
Myalgia | Nausea/vomiting | Fatigue | Headache | Neuropathy |
| G1– 2/G3 |
G1–2/G3 | G1– 2/G3 |
G1–2/G3 | G1–2/3 | ||
| 1 | 3 | 0/0 | 2/0 | 0/0 | 0/0 | 0/0 |
| 2 | 3 | 0/0 | 1/0 | 1/0 | 1/0 | 0/0 |
| 3 | 3 | 0/0 | 2/0 | 2/0 | 0/0 | 0/0 |
| 4 | 3 | 0/0 | 1/0 | 1/0 | 0/0 | 1/0 |
| 5 | 3 | 0/0 | 1/0 | 1/0 | 0/0 | 0/0 |
| 6 | 3 | 0/0 | 2/1 | 1/0 | 0/0 | 1/0 |
| 7 | 3 | 0/0 | 3/0 | 0/0 | 2/0 | 1/0 |
| 8 | 3 | 0/0 | 1/0 | 2/0 | 1/0 | 0/0 |
| 9 | 3 | 0/0 | 1/1 | 3/0 | 0/0 | 0/0 |
| 10 | 6 | 1/1 | 3/0 | 3/0 | 0/0 | 0/0 |
| 11 | 6 | 2/2 | 2/0 | 2/0 | 0/0 | 0/0 |
| Treatment Related Toxicity (every 2 weeks) | ||||||
|---|---|---|---|---|---|---|
| Dose Level |
# Patients |
Myalgia | Nausea/vomiting | Fatigue | Headache | Neuropathy |
| G1– 2/G3 |
G1–2/G3 | G1– 2/G3 |
G1–2/G3 | G1–2/3 | ||
| 1A | 6 | 1/3 | 3/3 | 4/2 | 0/1 | 0/0 |
| 2A | 6 | 2/0 | 3/1 | 2/1 | 1/0 | 4/0 |
We then investigated an every 2-week schedule based on safety data of every 2-week bryo infusion in hematological malignancies (37). Noteworthy were dose limiting myalgias, observed at the first dose level when bryo at 40 mcg/m2 over 1 hour was given with cisplatin 50 mg/m2 (Dose level 1A): By comparison to the prior experience the 2-week interval was associated with cumulative myalgias. On the amended dose level (2A) of bryo at 30 mcg/m2 over 1 hour with 50 mg/m2 of cisplatin every 2 weeks, 6 patients were treated without any dose limiting toxicity. Therefore, the tolerable Phase II dose for an every 2-week schedule is bryo 30 mcg/m2 over 1 hour with cisplatin 50 mg/m2 (See table 4).
Other toxicities such as nausea and vomiting were attributable to the administration of cisplatin, with patients who received a cycle of cisplatin alone (cycle 0) experiencing the same toxicities as when they received bryo followed by cisplatin. Symptoms resolved upon discontinuation of the study drugs. Interestingly, complaints of peripheral neuropathy and headaches were only noted in patients on the every 2-week schedule. Four of the 12 patients developed Grade 2 peripheral neuropathy and 5 patients (4-Grade 1–2 and 1-Grade 3) developed headaches, which occurred in patients who required additional doses of antiemetics.
Observations at both the 24 hour and the 1 hour infusions of bryo attested to the relatively safety and tolerance of this agent, except at the MTD (Table 4: Toxicities). Other toxicities (hematological or gastrointestinal) were limited and managed with supportive care measures and could also be attributed to cisplatin. There were 6 patients who required hospitalization during the treatment, in 3 patients, this was clearly attributable to cisplatin toxicities such as vomiting, dehydration and infection. The remaining 3 patients were hospitalized for disease-related reasons.
Response (Tables 5–6)
Table 5.
Anti-tumor Activity
| Patient ID | Dose Level | Tumor Type | Response | TTP (wks) |
|---|---|---|---|---|
| 3 | 1 | Melanoma* | PR | 15 |
| 24 | 8 | Head/Neck | PR | 22 |
| 28 | 10 | Melanoma | PR | 11 |
| 43 | 1A | Head/Neck | PR | 10 |
Prior cisplatin treatment
Table 6.
Patient characteristics with stable disease
| Time to progression (weeks) |
Total # of patients |
Most common tumor types |
Most common dose levels |
Reasons to be off protocol other than PD (AE/Noncompliance) |
|---|---|---|---|---|
| 9 | 5 | Melanoma/liposarcoma | 5 and 11 | 2/2 |
| 12 | 9 | Melanoma/ appendiceal cancer/ head and neck cancer/ NSCLC/ RCC | 2A and 1 | 3/3 |
| 15 | 3 | Melanoma/ head and neckcancer/ appendiceal cancer | 4,7,1A | 2/0 |
| 18 | 3 | Melanoma/ small cell lung cancer | 3,10, 2A | 0/1 |
| 22 | 1 | Melanoma | 2A | 0/0 |
A total of 4 patients achieved partial responses (PR), duration of response ranged from 10 to 22 weeks. One PR in melanoma lasting 15 weeks was observed following 24 hour bryo at 10 mcg/m2 and cisplatin 50 mg/m2, this was the only PR in patients who had been resistant to cisplatin (Table 5). The other 3 PRs occurred in 1 melanoma patient and 2 head and neck patients who had previously been treated with either radiation therapy or chemotherapy, but not exposed to cisplatin.
There were 20 patients who had stable disease (SD) at reassessment, although 15 patients received only 1 cycle of therapy on the every 3-week schedule. In fact, the median time to progression (TTP) was 12 weeks for patients who were classified as SD at this first assessment (Table 6). One melanoma patient remained stable for 22 weeks and 1 small cell lung cancer patient and another melanoma patient had SD for 18 weeks.
Conversely, at the time of first reassessment, 19 patients had disease progression. Ten patients were not evaluable for response: 7 due to rapid progression prior to reassessment, 2 due to toxicity with (rash and nausea vomiting, respectively); and 1 due to refusal of re-evaluation after 2 cycles). There were 9 patients taken off study due to side effects, 4 patients was off study due to noncompliance.
Correlative Science
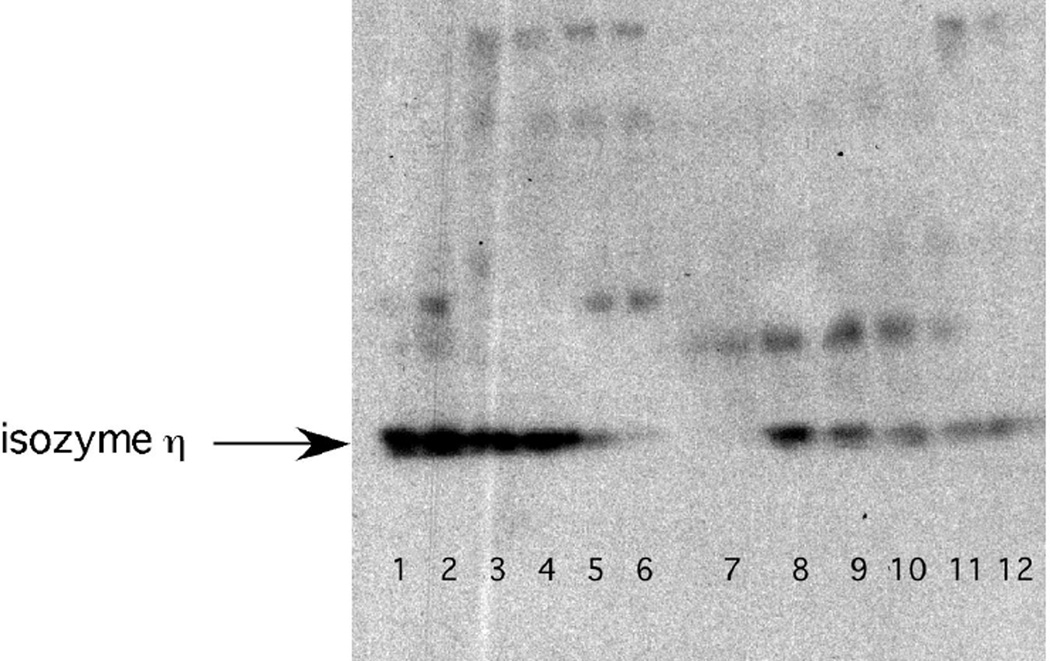
The pharmacokinetic studies among 7 patients revealed detectable bryo during 1 hour infusions; however, the levels varied widely when run at different times. In PMBC sample from 7 patients where PKC isoform signal were performed, 4 showed augmentation of the signal while 3 showed depletion. It was concluded that the inconsistencies were due to capricious behavior of the drug during extraction procedures. On the other hand, near virtual disappearance of the PKCη isoform by 3 hours followed by recovery on the subsequent day was noted consistently in the 3 PBMCs from patients examined at dose level 9 (bryo at 50 mcg/m2 over 1 hours and cisplatin at 50 mg/m2) (Figure 1), no simultaneous tumor data was obtainable.
Figure 1.
Companion Western blot analysis for the protein kinase c isoform η from PBMCs isolated from two patients (lanes 1–6; & 7–12) receiving (combination cisplatin (50 mg/m2) and Byro (50 mcg/m2 × 1hr). For both patients, a substantial depletion of the baseline signal (lanes 1, 2 and 7, 8) can be observed 28.3 and 48% respectively for the pkc isoform eta after the end of the Byro infusion (lanes 3,4 compared to 1,2 and lanes 9,10 compared to 7,8). This effect increased to respectively to 95.4 and 90% at 3 hours post Byro infusion (lanes 5,6 and 11,12).
Discussion
Prior to this study, the combination of bryo and cisplatin has not been examined in humans. Interest was stimulated by the broad spectrum of antitumor activity for cisplatin and the absence of overlapping toxicities. The study design explored 2 dose levels of cisplatin (50 and 75 mg/m2), preceded by bryo infusions. Initially, a 24-hour infusion schedule of bryo beginning on day 1 followed by cisplatin on day 2 was used. Subsequently, we administered 1-hour infusions of bryo just prior to cisplatin injection, in part with the hope of determining bryo peak levels. Several dose schedules of bryo were explored not only to simplify the regimen but also to detect whether modulatory effects could correlate with a given peak plasma level. Disappointingly, our efforts to measure plasma levels (in collaboration with the GC/MS unit at Mt Sinai of Dr. J. Roboz) did not produce any consistent results. Subsequently, the availability of new instrumentation and the development of an optimized liquid/liquid extraction has led to a validated assay by Zhao and coworkers employing a triple-quadrupoic mass spectrometric detection with an electrospray interface. This advancement will provide adequate sensitivity for the detection of bryo (41) in future studies.
The majority of patients (26 out of total of 53) had melanoma. A patient that was clearly platinum refractory responded to the combination, thus providing a hint that bryo exerted a modulatory effect on the efficacy of cisplatin. However, overall, only this patient and 3 others (one other melanoma and 2 head and neck cancers) among 53 had a PR. Although 20 patients had SD, only 5 remained stable beyond 3 months, indicating that bryo did not offer a sustained inhibition on tumor growth. On the other hand, the trial provided information on drug tolerance: myalgias appeared to be cumulative and markedly more apparent on the 2-week interval. The toxic effects of the drug at the MTD were reversible, and we defined the tolerable Phase II dose as bryostatin 30 mcg/m2 over 1 hour with cisplatin 50 mg/m2, repeated every 2 weeks.
The results of several Phase I and II trials using bryo with chemotherapy have been recently published, also following preclinical leads. Roberts et al recently showed that the combination of bryo 24 hour infusion with fludarabine produced anti-tumor effects suggestive of potentiation of drug action in patients with chronic lymphocytic leukemia or indolent lymphomas (32). Those patients were refractory to fludarabine but had response to the combination regardless if bryo was infused first or not. In pancreatic cell lines, treatment with gemcitabine followed by bryo resulted in significant more growth inhibition than treatment with bryo followed by gemcitabine; phase I study using this schedule combination showed 8 stable disease in 36 patients with pancreatic cancer (5), raising the question whether a preferred sequence for antitumor activity is actually the reverse of the one employed here.
We subsequently tested our level 1A bryo and cisplatin combination within New York Gynecologic Oncology Group in a Phase II study of patients with metastatic carcinoma of the cervix who had received prior platinum. The results were disappointing with no response observed among 14 patients (24). The dose limiting toxicity in this study was also myalgia (24). The finding of severe headaches and peripheral neuropathy for patients dosed every 2 weeks may possibly reflect bryo’s aggravation of cisplatin neurotoxicity and/or enhanced symptoms from the use of antiemetics. This may be important in future combination studies with other potentially neurotoxic drugs and with this recommended schedule. Careful neurosensory monitoring may be warranted, and guidelines to manage myalgias may be required on short bryo infusions and repetitive cycles on an every 2-week interval.
In summary, the combination of bryo and cisplatin resulted in myalgias as the dose limiting toxicity when given on an every 2-week schedule. Unfortunately, in any of the bryo schedules preceding cisplatin among 53 patients, only 4 patients experienced partial responses. Modulation of cytotoxicity by bryo awaits further insight into the molecular pathways involved, and may be facilitated by the recent introduction of a sensitive determination for byro in human plasma.
Acknowledgments
Supported by U01 CA76642, P30 CA 16087 and GCRC MO1 RR00096
The authors thank John Roboz for the GC/MS.
References
- 1.Aeder SE, Martin PM, Soh JW, Hussaini IM. PKC-η mediates glioblastoma cell proliferation through the Akt and mTOR signaling pathways. Oncogene. 2004;23:9062–9069. doi: 10.1038/sj.onc.1208093. [DOI] [PubMed] [Google Scholar]
- 2.Basu A, Lazo JS. Sensitization of human cervical carcinoma cells to cis-diammine-dichloroplatinum (II) by bryostatin 1. Cancer Res. 1992;52:3119–3124. [PubMed] [Google Scholar]
- 3.Corbett AH, Fernald AW, Osheroff N. Protein kinase C modulates the catalytic activity of topoisomerase II by enhancing the rate of ATP hydrolysis: evidence for a common mechanism of regulation by phosphorylation. Biochemistry. 1993;32:2090–2097. doi: 10.1021/bi00059a029. [DOI] [PubMed] [Google Scholar]
- 4.Dale IL, Bradshaw TD, Gescher A, Pettit GR. Comparison of effects of bryostatins 1 and 2 and 12-0-tetradecanoylphorbol-13-acetate on protein kinase C activity in A549 human lung carcinoma cells. Cancer Res. 1989;49:3242–3245. [PubMed] [Google Scholar]
- 5.El-Rayes BF, Gadgeel S, Shields AF, Manza S, Lorusso P, Philip PA. Phase I study of bryostatin 1 and gemcitabine. Clin Cancer Res. 2006;12:7059–7062. doi: 10.1158/1078-0432.CCR-06-1419. [DOI] [PubMed] [Google Scholar]
- 6.Ford JM, Hait WN. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacology Rev. 1990;42:155–199. [PubMed] [Google Scholar]
- 7.Goodnight JE, Jr, Moseley HS, Eilber FR, Sarna G, Morton DL. Cis-dichlorodiammineplatinum(II) alone and combined with DTIC for treatment of disseminated malignant melanoma. Cancer Treat Rep. 1979;63:2005–2007. [PubMed] [Google Scholar]
- 8.Grunicke H, Hofmann J, Maly K, Goddard PM, Kelland LR, Morgan SE. Enhancement of the antiproliferative effect of cis-diammine-dichloroplatinum (II) and other antitumor agents by inhibition of enzymes involved in growth factor signal transduction. In: Howell SB, editor. Platinum and other metal coordination compounds in cancer chemotherapy. New York: Plenum Press; 1991. pp. 161–172. [Google Scholar]
- 9.Grunicke H, Hofmann J, Utz I, Uberall F. Role of protein kinases in antitumor drug resistance. Ann Hematol. 1994;69:S1–S6. doi: 10.1007/BF01757347. [DOI] [PubMed] [Google Scholar]
- 10.Grunicke HH, Uberall F. Protein kinase C modulation. Semin Cancer Biol. 1992;3:351–360. [PubMed] [Google Scholar]
- 11.Gschwendt M, Furstenberger G, Rose-John S, Rogers M, Kittstein W, Pettit GR, Herald CL, Marks F. Bryostatin 1, an activator of protein kinase C mimics as well as inhibits biological effects of the phorbol ester TPA in vivo and in vitro. Carcinogenesis. 1988;9:555–562. doi: 10.1093/carcin/9.4.555. [DOI] [PubMed] [Google Scholar]
- 12.Gschwendt M, Leibersperger H, Kittstein W, Marks F. Protein kinase C zeta and eta in murine epidermis. TPA induces down-regulation of PKC eta but not PKC zeta. FEBS-Lett. 1992;307:151–155. doi: 10.1016/0014-5793(92)80756-7. [DOI] [PubMed] [Google Scholar]
- 13.Hallahan DE, Virudachalam S, Sherman ML, Huberman E, Kufe DW, Weichselbaum RR. Tumor necrosis factor gene expression is mediated by protein kinase C following activation by ionizing radiation. Cancer Res. 1991;51:4565–4569. [PubMed] [Google Scholar]
- 14.Hickman PF, Kemp GJ, Thompson CH, Salisbury AJ, Wade K, Harris AL, Radda GK. Bryostatin 1, a novel antineoplastic agent and protein kinase C activator, induces human myalgia and muscle metabolic defects: a 31P magnetic spectroscopic study. Br J Cancer. 1995;72:998–1003. doi: 10.1038/bjc.1995.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochster H, Liebes L, Speyer J, Sorich J, Taubes B, Oratz R, Wernz J, Chachoua A, Raphael B, Vinci RZ. Phase I trial of low-dose continuous topotecan infusion in patients with cancer: an active and well-tolerated regimen. J Clin Oncol. 1994;12:553–559. doi: 10.1200/JCO.1994.12.3.553. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann J, Doppler W, Jakob A, Maly K, Posch L, Uberall F, Grunicke HH. Enhancement of the antiproliferative effect of cis-diamminedichloroplatinum(II) and nitrogen mustard by inhibitors of protein kinase C. Int J Cancer. 1988;42:382–388. doi: 10.1002/ijc.2910420313. [DOI] [PubMed] [Google Scholar]
- 17.Hornung RL, Pearson JW, Beckwith M, Longo DL. Preclinical evaluation of bryostatin as an anticancer agent against several murine tumor cell lines: in vitro versus in vivo activity. Cancer Res. 1992;52:101–107. [PubMed] [Google Scholar]
- 18.Isonishi S, Andrews PA, Howell SB. Increased sensitivity to cis-diamminedichloroplatinum (II) in human ovarian carcinoma cells in response to treatment with 12-O-tetradecanoylphorbol 13-acetate. J Biol Chem. 1990;265:3623–3627. [PubMed] [Google Scholar]
- 19.Jackson DN, Foster DA. The enigmatic protein kinase C delta: complex roles in cell proliferation and survival. FASEB J. 2004;18:627–636. doi: 10.1096/fj.03-0979rev. [DOI] [PubMed] [Google Scholar]
- 20.Jayson GC, Crowther D, Prendiville J, McGown AT, Schneid C, Stern P, Young R, Brenchley P, Chang J, Owens S, et al. A Phase I trial of bryostatin 1 in patients with advanced malignancy using a 24 hour intravenous infusion. Br J Cancer. 1995;72:461–468. doi: 10.1038/bjc.1995.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liebes L, Potmesil M, Kim T, Pease D, Buckley M, Fry D, Cho J, Adler H, Dar K, Zeleniuch-Jacquotte A, Hochster H. Pharmacodynamics of topoisomerase I inhibition: Western blot determination of topoisomerase I and cleavable complex in patients with upper gastrointestinal malignancies treated with topotecan. Clin Can Res. 1998;4:545–557. [PubMed] [Google Scholar]
- 22.Masso-Welch PA, Winston JS, Edge S, Darcy KM, Asch H, Vaughan MM, Ip MM. Altered expression and localization of PKC eta in human breast tumors. Breast Cancer Res and Treat. 2001;68:211–223. doi: 10.1023/a:1012265703669. [DOI] [PubMed] [Google Scholar]
- 23.Mohammad RM, Diwakaran H, Maki A, Emara MA, Pettit GR, Redman B, AL-Katib A. Bryostatin 1 induces apoptosis and augments inhibitory effects of vincristine in human diffuse large cell lymphoma. Leuk Res. 1995;19:667–673. doi: 10.1016/0145-2126(95)00037-o. [DOI] [PubMed] [Google Scholar]
- 24.Nezhat F, Wadler S, Muggia F, Mandeli J, Goldberg G, Rahaman J, Runowicz C, Murgo AJ, Gardner GJ. Phase II trial of the combination of bryostatin-1 and cisplatin in advanced or recurrent carcinoma of the cervix: a New York Gynecologic Oncology Group study. Gynecol Oncol. 2004;93:144–148. doi: 10.1016/j.ygyno.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Osada SI, Hashimoto Y, Nomura S, Kohno Y, Chida K, Tajima O, Kubo K, Akimoto K, Koizumi H, Kitamura Y, Suzuki K, Ohno S, Kuroki T. Predominant expression of nPKCη, a Ca2+- independent isoform of protein kinase C in epithelial tissues, in association with epithelial differentiation. Cell Growth & Diff. 1993;4:167–175. [PubMed] [Google Scholar]
- 26.Perego P, Casati G, Gambetta RA, Soranzo C, Zunino F. Effect of modulation of protein kinase C activity on cisplatin cytotoxicity in cisplatin-resistant and cisplatin-sensitive human osteosarcoma cells. Cancer Lett. 1993;72:53–58. doi: 10.1016/0304-3835(93)90010-7. [DOI] [PubMed] [Google Scholar]
- 27.Pettit GR, Herald CL, Doubek DL, Herald DL, Arnold E, Clardy J. Isolation and structure of bryostatin 1. J Am Chem Soc. 1982;104:6846–6848. [Google Scholar]
- 28.Philip PA, Harris AL. Potential for protein kinase C inhibitors in cancer therapy. In: Muggia FM, editor. Concepts, mechanisms and new targets for chemotherapy. 1st ed. Boston: Kluwer Academic Publishers; 1995. pp. 3–28. [Google Scholar]
- 29.Philip PA, Rea D, Thavasu P, Carmichael J, Stuart NSA, Rockett H, Talbot DC, Ganesan T, Pettit GR, Balkwill F, Harris AL. Phase I study of Bryostatin 1: assessment of interleukin 6 and tumor necrosis factor α induction in vivo. J Natl Cancer Inst. 1993;85:1812–1818. doi: 10.1093/jnci/85.22.1812. [DOI] [PubMed] [Google Scholar]
- 30.Pollack IF, Kawecki S, Lazo JS. Blocking of glioma proliferation in vitro and in vivo and potentiating the effects of BCNU and cisplatin: UCN-01, a selective protein kinase C inhibitor. J Neurosurg. 1996;84:1024–1032. doi: 10.3171/jns.1996.84.6.1024. [DOI] [PubMed] [Google Scholar]
- 31.Prendiville J, Crowther D, Thatcher N, Woll PJ, Fox BW, McGown A, Testa N, Stern P, McDermott R, Potter M, Pettit GR. A phase I study of intravenous bryostatin 1 in patients with advanced cancer. Br J Cancer. 1993;68:418–424. doi: 10.1038/bjc.1993.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts JD, Smith MR, Feldman EJ, Cragg L, Millenson MM, Roboz GJ, Honeycutt C, Thune R, Padavic-Shaller K, Carter WH, Ramakrishnan V, Murgo AJ, Grant S. Phase I study of bryostatin 1 and fludarabine in patients with chronic lymphocytic leukemia and indolent (non-Hodgkin’s) lymphoma. Clin Cancer Res. 2006;12:5809–5816. doi: 10.1158/1078-0432.CCR-05-2730. [DOI] [PubMed] [Google Scholar]
- 33.Schuchter LM, Esa AH, May S, Laulis MK, Pettit GR, Hess AD. Successful treatment of murine melanoma with bryostatin 1. Cancer Res. 1991;51:682–687. [PubMed] [Google Scholar]
- 34.Szallasi Z, Du L, Levine R, Lewin NE, Nguyen PN, Williams MD, Pettit GR, Blumberg PM. The bryostatins inhibit growth of B16/F10 melanoma cells in vitro through a protein kinase C-independent mechanism: dissociation of activities using 26-epi-bryostatin 1. Cancer Res. 1996;56:2105–2111. [PubMed] [Google Scholar]
- 35.Thompson CH, Macaulay VM, O'Byrne KJ, Kemp GJ, Wilner SM, Talbot DC, Harris AL, Radda GK. Modulation of bryostatin 1 muscle toxicity by nifedipine: effects on muscle metabolism and oxygen supply. Br J Cancer. 1996;73:1161–1165. doi: 10.1038/bjc.1996.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trenn G, Pettit GR, Takayama H, Hu-Li J, Sitkovsky MV. Immunomodulating properties of a novel series of protein kinase C activators. The bryostatins. J Immunol. 1988;140:433–439. [PubMed] [Google Scholar]
- 37.Varterasian M, Eilender D, Mohammad R, Chen B, Hulburd K, Rodriguez D, Pluda J, Valdivieso M, Al-Katib A. Phase I trial of bryostatin 1 in relapsed lymphoma and CLL. Proc Am Soc Clin Oncol. 1996;15:481. Abstr # 1526. [Google Scholar]
- 38.Weitman S, Langevin AM, Berkow RL, Thomas PJ, Hurwitz CA, Kraft AS, Dubowy AL, Smith DL, Bernstein M. A phase I trial of bryostatin-1 in children with refractory solid tumors: a Pediatric Oncology Group study. Clin Cancer Res. 1999;5:2344–2348. [PubMed] [Google Scholar]
- 39.WHO handbook for reporting results of cancer treatment. Geneva (Switzerland): World Health Organization; 1979. Offset Publication No. 48. [Google Scholar]
- 40.Zhang R, Muller HJ, Kielassa K, Marks F, Gschwendt M. Partial purification of a type eta protein kinase C from murine brain: separation from other protein kinase C isoenzymes and characterization. Biochem. J. 1994;304:641–647. doi: 10.1042/bj3040641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao M, Rudek MA, He P, Smith BD, Baker SD. Validation and implementation of a method for determination of bryostatin 1 in human plasma by using liquid chromatography/tandem mass spectrometry. Anal Biochem. 2005;33:143–148. doi: 10.1016/j.ab.2004.10.030. [DOI] [PubMed] [Google Scholar]