Abstract
Doenjang has been reported to exhibit antioxidant, fibrinolytic, antimutagenic, anticancer, and antiobesity effects. In our preliminary study, doenjang decreased fecal lipopolysaccharide (LPS) levels in mice. Therefore, we investigated the effect of doenjang on the composition of gut microbiota in mice. Treatment with doenjang significantly increased the number of bifidobacteria cultured in BL media, compared with mice not treated with doenjang. However, doenjang decreased the number of Enterobacteriaceae cultured in DHL media. Doenjang significantly suppressed the β-glucuronidase activity, but did not influence α-/β-glucosaminidase and α-/β-glucosidase activities. When gut microbiota in mice treated with or without doenjang was analyzed by pyrosequencing, doenjang induced a significant modulation of the populations of the dominant gut microbiota. At the phylum level, doenjang treatment resulted in a significant decrease of Firmicutes and an increase of Bacteroidetes, which led to a decrease in the Firmicutes to Bacteroidetes ratio in gut microbiota. At the family level, the number of Ruminococcaceae and Lachnospiraceae were significantly decreased, while the number of Odoribacter_f was increased in doenjang-treated mice. Of colonic tight junction proteins, occludin, ZO-1, and claudin-1 in mice, occludin alone was significantly increased by treatment with doenjang. Although treatment with doenjang seemed to suppress NF-κB activation, it was not significant. Doenjang significantly suppressed tumor necrosis factor-α expression, whereas it did not influence interleukin (IL)-1β and IL-6 expression. However, doenjang increased IL-10 expression. Based on these findings, doenjang may promote gut health by regulating gut microbiota and its LPS concentrations and suppressing harmful enzyme production.
Key Words: : doenjang, gut microbiota, inflammation, lipopolysaccharide
Introduction
Human gut microbiota consists of 10 to 100 trillion microorganisms, which is 10-fold more than the number of cells that compose the human body.1 Although infants grow and develop in the sterile environment of the uterus of their mothers before birth, the external surfaces of infants such as gut and skin are colonized by maternal, environmental, and dietary microbes during delivery and immediately afterward. Human gut microbiota has been usually regarded as relatively stable throughout adulthood. However, gut microbiota is disturbed by exogenous and endogenous factors, such as diet, antibiotics, and stress. Gut microbiota is pivotal for the development of gastrointestinal mucosa and immune system.2,3 Therefore, gut microbiota plays an important role in energy balance,4,5 glucose metabolism,6,7 low-grade inflammation8,9 and drug metabolism.10,11 Recently, we found that gut microbiota-derived lipopolysaccharide (LPS) involves in the onset and progression of inflammation and metabolic diseases.6 For example, high-fat diets increase gram-negative bacteria, which induce the production of LPS in the intestine and cause inflammation, obesity, and cancer.6,9,12
Concentrations of LPS, a component of gram-negative bacterial cells, are increased by a high-fat diet (HFD).6,12 We and others have confirmed that HFD could contribute to higher plasma LPS levels and low-grade inflammation.13,14 Thus, HFD induces LPS production in the intestinal contents of humans and animals, supporting that gut microbiota initiates and perpetuates colonic inflammation. More specifically, gut bacterial LPS penetrates the epithelial barrier and stimulates mucosal immune reactions.15,16 Subsequently, this toxin induces the production of proinflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, leading to inflammatory activation using distinct signaling pathways through toll-like receptor 4.17,18 Regulating the expression of these inflammatory cytokines is therefore beneficial for treating inflammatory and metabolic diseases.
Doenjang is a traditional Korean soybean paste that has been used for centuries as a protein source and flavoring ingredient in Korea, like that of miso in Japan and tempeh in Indonesia.19 Doenjang is prepared by fermenting moldy cooked soybeans (meju) in brine, resulting in the degradation of soy proteins and production of organic acids, amino acids, and minerals.20 Recently, doenjang research has focused on its excellent nutritional value as well as its health-promoting properties, such as its antioxidant,21 fibrinolytic,22 antimutagenic,23 anticancer,24 and antiobesity effects.25 However, its effect on gut microbiota, particularly endotoxins, has not been studied.
Therefore, in the present study, we investigated the effect of doenjang on the composition of gut microbiota in mice.
Materials and Methods
Materials
p-Nitrophenyl-β-D-glucopyranoside, 4-nitrophenyl-N-acetyl-α-D-glucosaminide, 4-nitrophenyl-N-acetyl-β-D-glucosaminide, 4-nitrophenyl-β-D-glucuronide, 4-nitrophenyl-α-D-glucopyranoside, and 4-nitrophenyl-β-D-glucopyranoside were purchased from Sigma-Aldrich (St Louis, MO, USA). The diazo-coupled limulus amoebocyte lysate (LAL) assay kit was purchased from Associates of Cape Cod, Inc. (East Falmouth, MA, USA). Antibodies for p-p65, p65, ZO-1, occludin, claudin, and β-actin were purchased from Cell Signaling Technology (Beverly, MA, USA). All antibodies were used at dilutions of 1:1000. Enzyme-linked immunosorbent assay (ELISA) kits for cytokines were purchased from R&D Systems (Minneapolis, MN, USA).
Animals
Male C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). All animals were fed standard laboratory chow (Samyang Co., Seoul, South Korea), housed in wire cages at 20–22°C and 50%±10% humidity, and allowed water ad libitum. All experiments were performed in accordance with the NIH and Kyung Hee University guides for Laboratory Animals Care and Use and approved by the Committee for the Care and Use of Laboratory Animals in the College of Pharmacy, Kyung Hee University (KHP-2013-07-2).
After 7 days of acclimation, mice were separated into two groups, the AIN-93G diet (Harlan Teklad Test Diets, Madison, WI, USA) with or without freeze-dried 5% doenjang (prepared according to the method of Hwang et al.26 and freeze-dried) and treated for 7 days. The food intake and body weight were then measured daily. At the end of 7 days, mice were anesthetized and blood collected by cardiac puncture and the colonic contents removed.
Preparation of fecalase
The mouse colon contents (about 0.3 g) were prepared according to a previous method of Lee et al.27 by collecting after sacrifice, carefully mixing with a spatula, and suspending in cold 2.7 mL saline. Fecal suspensions were centrifuged at 500 g for 5 min. The resulting supernatants were sonicated for 10 min and then centrifuged at 10,000 g for 20 min. The supernatants were used as fecalase suspensions.
Fecalase activity assay
Fecalase activities were assayed according to the previous reported method of Yeo et al.28 Briefly, the reaction mixture (total volume of 0.5 mL) containing 0.2 mL of 1 mM p-nitrophenyl-β-D-glucopyranoside for β-glucosidase (or 1 mM p-nitrophenyl- β -D-glucuronide for β -glucuronidase, 1 mM 4-nitrophenyl-N-acetyl-α-D-glucosaminide for α-glucosaminidase, 1 mM 4-nitrophenyl-N-acetyl-β-D-glucosaminide for β-glucosaminidase, and 1 mM 4-nitrophenyl-α-D-glucopyranoside for α-glucosidase), 0.2 mL of 0.1 M phosphate buffer, pH 7.4, and 0.1 mL of the fecal suspension (wet weight, 4 mg) was incubated at 37°C for 15 min. Then, the reaction mixture was stopped by the addition of 0.5 mL of 0.5 N NaOH, centrifuged at 2000 g for 10 min, and the absorbance measured at 405 nm (a BioTek spectrophotometer, London, England).
Determination of LPS
Plasma endotoxin contents were determined by a LAL assay kit (Cape Cod) according to the manufacturer's protocol. Briefly, plasma was diluted 1:10 in pyrogen-free water, inactivated for 10 min at 70°C, and then incubated with LAL for 30 min at 37°C. Addition of reagents led to the formation of a magenta derivative that absorbs light at 545 nm.
Myeloperoxidase activity assay
An aliquot (50 μL) of the colon supernatant was added to a reaction mixture of 1.6 mM tetramethyl benzidine and 0.1 mM H2O2 and then incubated at 37°C. The absorbance was obtained at 650 nm over time. The myeloperoxidase activity was defined as the quantity of enzyme degrading 1 μmol/mL of peroxide at 37°C and expressed in unit/mg protein.29
ELISA and immunoblotting
For the ELISAs of IL-1β, IL-6, IL-10, and TNF-α, plasma and colon tissue homogenates were transferred to 96-well ELISA plates. IL-1β, IL-6, IL-10, and TNF-α concentrations were determined using commercial ELISA kits (Pierce Biotechnology, Inc., Rockford, IL, USA).
For immunoblot analyses of p65, p-p65, ZO-1, occludin, claudin-1, and β-actin, colon tissue homogenates were resuspended in 1 mL of RIPA lysis buffer containing 1% protease inhibitor cocktail and 1% phosphatase inhibitor cocktail. After centrifugation, the supernatant was used for the immunoblot assay. The proteins were subjected to electrophoresis on an 8–10% sodium dodecyl sulfate–polyacrylamide gel, and then transferred to a nitrocellulose membrane and immunoblotted using standard procedures.29
Bacterial culture of mouse stools
Fresh mouse stools (0.1 g) from each group were collected separately in sterilized plastic cups, carefully suspended in 10 volumes of peptone water, diluted 10-fold in a stepwise manner, and inoculated in agar plates of blood liver medium (BL; Nissui Pharm. Co., Ltd., Tokyo, Japan) and hydrogen sulfate lactose medium (DHL; Eiken Chem. Co., Ltd., Tokyo, Japan). DHL agar plates were cultured aerobically for 1 day at 37°C and BL agar plates were cultured anaerobically for 3 days at 37°C.
DNA extraction, pyrosequencing, and data analysis
Genomic DNA was extracted from fecal samples using a commercial DNA isolation kit (QIAamp DNA stool mini kit; Qiagen, Hilden, Germany) by following the manufacturer's protocol. For pyrosequencing, amplification of genomic DNA was performed using barcoded primers, which targeted the V1 to V3 region of the bacterial 16S rRNA gene. The amplification and sequencing were performed according to the methods described by Chun et al.30 and completed by Chunlab, Inc. (Seoul, Korea) using a 454 GS FLX Titanium Sequencing System (Roche, Branford, CT, USA). Sequence reads were identified using EzTaxon-e database (http://eztaxon-e.ezbiocloud.net)31 on the basis of 16S rRNA sequence data. Number of sequences analyzed, observed diversity richness (operational taxonomic units [OTUs]), estimated OTU richness (ACE and Chao1), and coverage in the present pyrosequencing are indicated in Table 1.
Table 1.
Number of Sequences Analyzed, Observed Diversity Richness, Estimated Operational Taxononic Unit Richness for ACE and Chao1, and Coverage
| Phylotype | ||||||
|---|---|---|---|---|---|---|
| Group | Number | Total reads | OTUs | ACE | Chao1 | Goods coverage |
| NC | 1 | 4920 | 376 | 704.93 | 604 | 0.97 |
| 2 | 4981 | 347 | 640.32 | 519.12 | 0.97 | |
| 3 | 2976 | 220 | 282.27 | 274.03 | 0.98 | |
| 4 | 4669 | 292 | 401.87 | 387.12 | 0.98 | |
| 5 | 731 | 106 | 132.48 | 123.4 | 0.96 | |
| Mean±SD | 3655.4±1830 | 268.2±108.4 | 432.4±240.3 | 381.5±191.5 | 0.97±0.01 | |
| DJ | 1 | 4997 | 309 | 619.57 | 512.95 | 0.97 |
| 2 | 2854 | 200 | 275.96 | 263.55 | 0.98 | |
| 3 | 1197 | 128 | 289.53 | 196.14 | 0.95 | |
| 4 | 1246 | 114 | 154.36 | 142.96 | 0.97 | |
| 5 | 4844 | 370 | 645.84 | 530.62 | 0.97 | |
| Mean±SD | 3027.6±1853 | 224.2±112.3 | 397.1±221.7 | 329.2±181.0 | 0.97±0.01 | |
The cutoff value of phylotype is equal to or greater than 97% similarity.
OTUs, operational taxonomic units; NC, normal control group; DJ, doenjang-treated group.
Statistics
The data are expressed as the mean±standard deviation. Statistical analysis of the data was performed with the Student's t-test. Differences with a P<.05 were considered to be statistically significant.
Results
Effect of doenjang on fecal and plasma LPS levels in mice
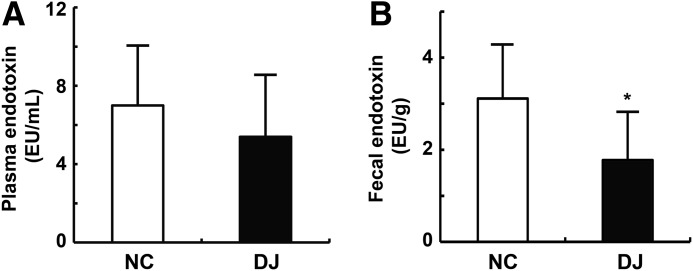
To understand the possible role of gut microbiota in the antiobesity properties of doenjang, we orally administered doenjang with diet in mice for 7 days and measured fecal and plasma LPS levels (Fig. 1). Treatment with doenjang reduced body weight and fecal and plasma LPS levels, compared with those of untreated normal control mice. The fecal LPS level alone was significantly decreased by treatment with doenjang.
FIG. 1.
Effect of doenjang on fecal and plasmatic lipopolysaccharide levels in mice. (A) Limulus amoebocyte lysate assay was used to measure the plasma (A) and fecal endotoxin concentrations (B). NC, normal control group; DJ, doenjang-treated group. All values are indicated as the mean±S.D. (n=8). *P<.05 compared with NC.
Effect of doenjang on gut microbiota composition in mice
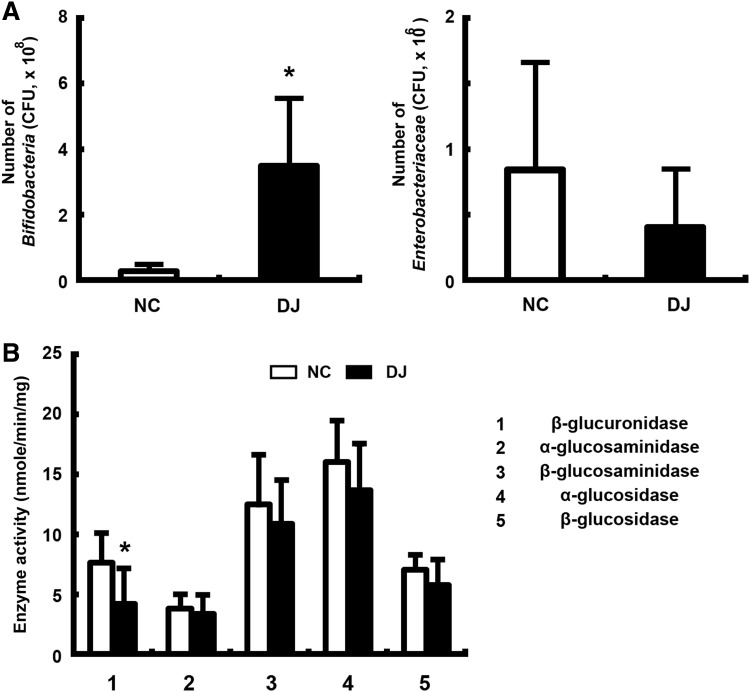
To understand the fecal LPS production inhibitory mechanism of doenjang, we analyzed the gut microbiota in mice treated with or without doenjang by the culture method (Fig. 2A). Treatment with doenjang significantly increased the number of Bifidobacteria cultured in BL media, compared with that of mice untreated with doenjang, however, fewer Enterobacteriaceae were cultured in DHL media. Then, we analyzed the activities of harmful enzymes β-glucuronidase and α-/β-glucosaminidases, which caused inflammation or cancer,32,33 in the gut content of mice treated with or without doenjang (Fig. 2B). Treatment with doenjang significantly decreased the β-glucuronidase activity, but did not influence α-/β-glucosaminidase and α-/β-glucosidase activities.
FIG. 2.
Effect of doenjang on the number of Bifidobacteria and Enterobacteriaceae (A) and harmful enzyme activities (B) in fecal samples from mice treated with or without doenjang. The fresh feces were suspended in 9 volumes of dilution media, inoculated in BL agar plates and DHL agar plates. DHL agar plates were aerobically cultured for 1 day at 37°C, and BL agar plates were anaerobically cultured for 3 days at 37°C. The fecal enzyme activities were measured as described in Materials and Methods. All values were indicated as the mean±S.D. (n=5). *P<.05 compared with NC.
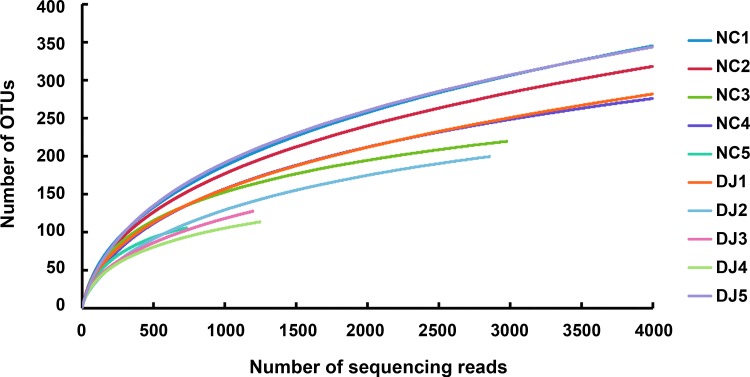
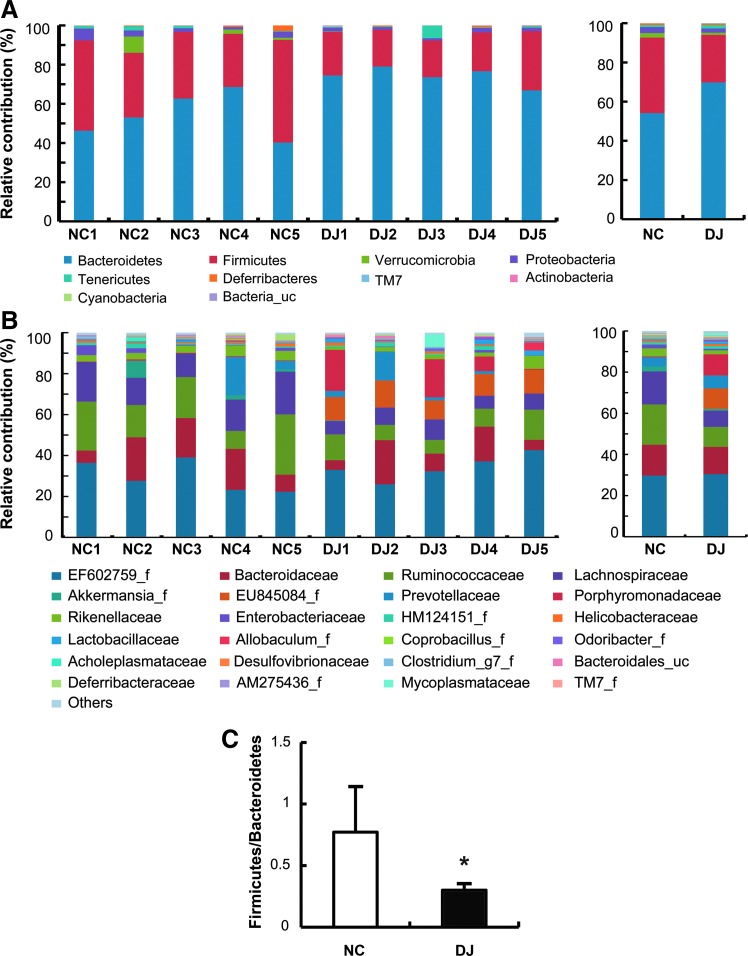
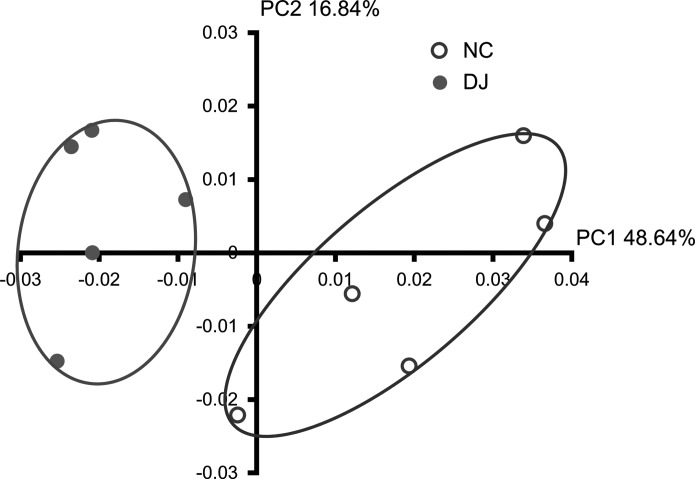
Next, we analyzed the gut microbiota in mice treated with or without doenjang by a 454 pyrosequencing method. As demonstrated by the rarefaction curves (Fig. 3) and the number of sequences analyzed, estimated OUT richness, coverage (Table 1), bacterial richness, and diversity were not different between normal control and doenjang-treated mice. Taxomomy-based analysis showed that treatment with doenjang induced a significant modulation in the populations of dominant gut microbiota as compared with vehicle treatment. At the phylum level, doenjang treatment resulted in a significant decrease of Firmicutes and an increase of Bacteroidetes, which led to a decrease in the Firmicutes to Bacteroidetes ratio in the gut microbiota (Fig. 4A and 4C). At the family level, the number of Ruminococcaceae and Lachnospiraceae was significantly decreased, while the number of Odoribacter_f was increased in doenjang-treated mice (Fig. 4B). Interestingly, EU845084_f (Bacteroidetes phylum) was only observed in doenjang-treated mice. In addition, treatment with doenjang lowered the number of Enterobacteriaceae, Clostridium_g7_f, and Rikenellaceae, whereas it expanded the populations of Porphyromonadaceae and Lactobacillaceae. At the genus level, doenjang significantly increased the number of EF603735_g, DQ815871_g (Bacteroidales, order), and Odoribacter, whereas decreased the number of Pseudoflavonifractor, DQ789121_g, and Clostridium_g9 (Clostridiales, order) (Table 2). At the species level, doenjang increased the number of EF406456_s, 4P003085_s, and EF406459_s (Bacteroidales, order), but decreased the number of EF406686_s, EF603419_s (Bacteroidales, order), and EF604627_g (Clostridiales, order) significantly (Table 3). We also processed all these sequences at the same length and position to match the length and position of the gut microbiota 16S rRNA gene sequences, computed all pairwise distances among normal control and doenjang-treated groups, and performed principal coordinate analysis to cluster these communities along axes of maximal variance (Fig. 5). Gut microbial communities of each group member were clustered and the maximum variations were 48.64% (PC1) and 16.84% (PC2).
FIG. 3.
Rarefaction curves. Rarefaction analysis of V1–V3 pyrosequencing tags of the 16S rRNA gene in fecal microbiota from normal control group (NC1–5) and doenjang-treated group (DJ1–5).
FIG. 4.
The composition of gut microbiota in phylum and family levels. Taxonomy compositions: (A) phylum and (B) family levels are shown (individual samples are on the left panels and pooled samples are on the right panels). Genomic DNA was extracted from the cecal samples taken from mice maintained for 7 days on diets with or without doenjang. Samples were analyzed for the bacterial composition by pyrosequencing of the bacterial 16S rRNA fragments. (C) The Firmicutes to Bacteroidetes ratio (n=5). *P<.05 compared with NC.
Table 2.
The Difference Between Normal Control and Doenjang-Treated Groups in the Composition of Fecal Bacterial Genera
| Compositiona(%) | |||
|---|---|---|---|
| Genus | NC | DJ | P value |
| Bacteroides | 16.24±6.93 | 11.53±6.88 | .321 |
| Akkemansia | 2.60±3.92 | 0.84±1.73 | .183 |
| E603735_g (Bacteroidales) | nd | 8.95±5.20 | <.001 |
| Pseudoflavonifractor | 6.04±3.14 | 2.11±1.04 | .047 |
| Oscillibacter | 4.57±1.21 | 2.23±0.87 | .480 |
| Prevotellaceae_uc | 4.69±9.37 | 5.84±5.67 | .891 |
| Parabacteroides | 0.41±0.21 | 9.21±9.69 | .114 |
| Alistipes | 3.72±0.98 | 1.70±0.51 | .424 |
| DQ815871_g (Bacteroidales) | 0.48±0.17 | 5.4±3.5 | .006 |
| DQ789121_g (Clostridiales) | 3.70±1.34 | 1.59±0.95 | .031 |
| Clostridium_g9 | 2.02±0.41 | 0.81±0.29 | .035 |
| Escherichia | 2.00±2.13 | 0.71±0.96 | .136 |
| Lactobacillus | 0.43±0.29 | 1.02±1.07 | .129 |
| Clostridium_g6 | 0.26±0.33 | 0.07±0.15 | .125 |
| Odoribacter | 0.33±0.21 | 0.70±0.42 | .049 |
| Clostridium_g7 | 0.50±0.20 | 0.17±0.12 | .084 |
Mean±SD (n=5).
nd, not detected.
Table 3.
The Difference Between Normal Control and Doenjang-Treated Groups in the Composition of Fecal Bacterial Species
| Compositiona(%) | |||
|---|---|---|---|
| Species | NC | DJ | P value |
| Bacteroides acidifaciens | 14.51±6.93 | 10.97±7.16 | .178 |
| EF406456_s (Bacteroidales) | 5.24±3.87 | 9.17±2.65 | .047 |
| 4P003085_s (Bacteroidales) | nd | 11.11±1.36 | .005 |
| EF406686_s (Bacteroidales) | 3.35±1.63 | 1.75±1.25 | .042 |
| EU504499_s (Bacteroidales) | 3.56±4.63 | 0.09±0.20 | .068 |
| EF603419_s (Bacteroidales) | 2.79±1.33 | 1.60±0.68 | .009 |
| EF406459_s (Bacteroidales) | 0.12±0.07 | 3.84±2.36 | .005 |
| Escherichia fergusonii | 1.39±1.64 | 0.05±0.11 | .157 |
| Clostridium cocleatum | 0.24±0.29 | 0.01±0.03 | .568 |
| Lactobacillus reuteri | 0.04±0.06 | 0.42±0.47 | .104 |
| Blautia_uc | 0.31±0.39 | 0.06±0.08 | .073 |
| Lactobacillus johnsonii | 0.08±0.13 | 0.57±0.82 | .444 |
| Pseudoflavonifractor_uc | 0.27±0.22 | 0.09±0.11 | .42 |
| EF604627_g (Clostridiales) | 0.32±0.17 | 0.02±0.05 | .026 |
Mean±SD (n=5).
FIG. 5.
Principal coordinate analysis (PCoA) plot. The plot showed the clustering pattern between normal control and doenjang-treated groups based on weighted pairwise Fast UniFrac analysis. (n=5).
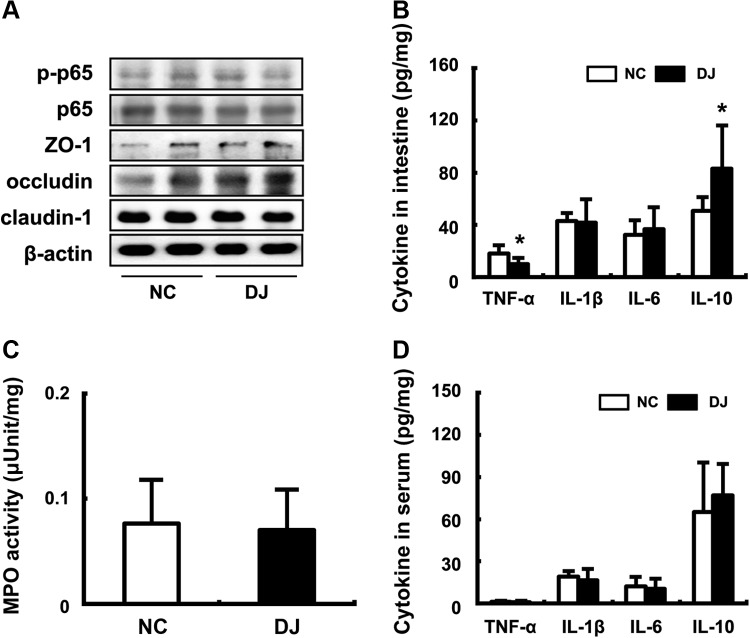
Effect of doenjang on the expression of colonic tight junction proteins and the activation of NF-κB in mice
Next, we measured the effect of doenjang on expression of colonic tight junction proteins—occludin, ZO-1, and claudin-1—in mice (Fig. 6A). Generally, treatment with doenjang increased expressions of tight junction proteins. Especially, occludin alone was increased most potently by treatment with doenjang. We also measured its effect on NF-κB activation. Although treatment with doenjang suppressed NF-κB activation, it was not significant. Next, we measured TNF-α, IL-1β, IL-6, and IL-10 levels and myeloperoxidase activity in the colon of mice treated with or without doenjang (Fig. 6B). Although IL-1β and IL-6 expression was not influenced by treatment with doenjang, a proinflammatory cytokine TNF-α expression was significantly suppressed, but an anti-inflammatory cytokine IL-10 expression was significantly increased. However, the colonic inflammation marker myeloperoxidase activity was not influenced by treatment with doenjang. Furthermore, treatment with doenjang did not influence plasma TNF-α, IL-1β, IL-6, and IL-10 levels.
FIG. 6.
Effect of doenjang on colonic p65, p-p65, ZO-1, occludin, claudin-1, β-actin (A), cytokine expression levels (B), myeloperoxidase activities (C), and plasma cytokine levels (D) in mice. p65, p-p65, ZO-1, occludin, claudin-1, and β-actin were measured by Western blot analysis. Proinflammatory cytokine levels were analyzed by ELISA. All values were indicated as the mean±S.D. (n=5). *P<.05 compared with NC. ELISA, enzyme-linked immunosorbent assay.
Discussion
Doenjang has been documented as having antimutagenic,23 anticancer,24 antioxidative,21 and anti-inflammatory properties.34 Recently, antiobesity effects of doenjang have been observed in animals,35,36 which may have been due to constituents such as genistein activating the transcription of carnitine palmitoyltransferase-I, a rate regulating enzyme for fatty acid oxidation, and peroxisome proliferator-activated receptor-alpha target genes involved in fatty acid beta oxidation.25,34,35 However, doenjang is a unique soybean paste fermented by diverse microorganisms, which uses Bacillus subtilis and B. licheniformis and molds such as Rhizopus, Mucor, and Aspergillus oryzae from rice straw and local environments instead of inoculation.37,38 Recently, 16S rRNA cloning and sequencing, PCR-DGGE, and 454 pyrosequencing were used to analyze the bacterial community of finally fermented doenjang and revealed that its dominant microbes are Staphylococcus equorum, Enterococcus faecium, Tetragenococcus halophilus, Leuconostoc mesenteroides, and Staphylococcus gallinarum.39,40 Most of these bacteria belong to phylum Firmicutes. Thus, the composition of microbiota and consituents of doenjang was not significantly different according to rice straw and local environments. Therefore, of doenjang constituents, microbes may play an important role in expressing their biological activities.
Endotoxins such as LPS of gut microbiota constantly promote the aging process and metabolic diseases such as obesity, diabetes, and colitis.6,10 Therefore, to maintain a healthy condition, LPS production of gut microbiota should be decreased.
In the present study, we found that doenjang decreased plasma and gut bacterial LPS levels as well as a harmful gut bacterial enzyme β-glucuronidase activity, which is increased in colitis,31,32,41 in normal mice. Furthermore, doenjang stimulated the growth of LAB, particularly Bifidobacteria. However, doenjang inhibited the number of Enterobacteriaceae, which potently produces the endotoxin, LPS.42 These results suggest that the stimulation of significant bifidobacterial growth by doenjang may suppress the growth of Enterobacteriaceae, such as Escherichia coli and LPS production.
We also found that treatment with doenjang inhibited proinflammatory cytokine TNF-α expression, which significantly activates inflammatory reactions, such as NF-κB activation, and increased anti-inflammatory cytokine IL-10 expression, which inhibits inflammatory responses. Therefore, these effects may also be due to the decrease in Enterobacteriaceae in the intestine by treatment with doenjang.
In addition, Ley et al. reported that the decrease in the Bacteroidetes phylum and the increase in Firmicutes were observed in gut microbiota of ob/ob mice as compared with those in lean littermates, as well as in those of obese human as compared with those in healthy humans.5,43 We and others also observed that HFD resulted in an increase in Firmicutes and a decrease in Bacteroidetes, which resulted in an increase in the Firmicutes/Bacteroidetes ratio in the gut microbiota according to the pyrosequencing method.6,12 Although doenjang exhibits antiobesity and body weight reduction effects as Cha et al.35 reported, it did not significantly reduce body weight in the present study. The difference may be due to the duration of doenjang administration. Doenjang induced growth of Porphyromonadaceae and Lactobacillaceae, which is related to obesity. Nevertheless, doenjang decreased fecal LPS levels, which are absorbed into the blood and cause obesity and body weight increase.6,10 Furthermore, doenjang may be a potent prebiotic and ameliorates disturbed gut microbiota.
Based on these findings, doenjang may promote gut health by regulating gut microbiota and its LPS and harmful enzyme productions.
Acknowledgment
This study was supported by a grant from the BK21 Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012).
Author Disclosure Statement
The authors state no conflict of interests.
References
- 1.Savage DC: Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 1977;31:107–133 [DOI] [PubMed] [Google Scholar]
- 2.Power SE, O'Toole PW, Stanton C, Ross RP, Fitzgerald GF: Intestinal microbiota, diet and health. Br J Nutr 2013;110:1–16 [DOI] [PubMed] [Google Scholar]
- 3.Purchiaroni F, Tortora A, Gabrielli M, Bertucci F, Gigante G, Ianiro G, Ojetti V, Scarpellini E, Gasbarrini A: The role of intestinal microbiota and the immune system. Eur Rev Med Pharmacol Sci 2013;17:323–333 [PubMed] [Google Scholar]
- 4.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI: The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 2004;101:15718–15723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ley RE, Turnbaugh PJ, Klein S, Gordon JI: Microbial ecology: human gut microbes associated with obesity. Nature 2006;444:1022–1023 [DOI] [PubMed] [Google Scholar]
- 6.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R: Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–1772 [DOI] [PubMed] [Google Scholar]
- 7.Cani PD, Dewever C, Delzenne NM: Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr 2004;92:521–526 [DOI] [PubMed] [Google Scholar]
- 8.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R: Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008;57:1470–1481 [DOI] [PubMed] [Google Scholar]
- 9.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM: Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009;58:1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KA, Jung IH, Park SH, Ahn YT, Huh CS, Kim DH: Comparative analysis of the gut microbiota in people with different levels of ginsenoside Rb1 degradation to compound K. PLoS One 2013;8:e62409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DH: The possible role of intestitnal microflora in pharmacological activities of ginseng. Int J Biomed Pharmceut Sci 2012;6:90–96 [Google Scholar]
- 12.Kim KA, Gu W, Lee IA, Joh EH, Kim DH: High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One 2012;7:e47713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferriéres J: Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 2008;87:1219–1223 [DOI] [PubMed] [Google Scholar]
- 14.Erridge C, Attina T, Spickett CM, Webb DJ: A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr 2007;86:1286–1292 [DOI] [PubMed] [Google Scholar]
- 15.Radema SA, van Deventer SJ, Cerami A: Interleukin 1 beta is expressed predominantly by enterocytes in experimental colitis. Gastroenterology 1991;100:1180–1186 [PubMed] [Google Scholar]
- 16.Rafii F, van Embdin R, van Lieshout LMC: Changes in bacterial enzymes and PCR profiles of fecal bacteria from a patient with ulcerative colitis before and after antimicrobial treatments. Dig Dis Sci 1999;44:637–642 [DOI] [PubMed] [Google Scholar]
- 17.Cario E, Podolsky DK: Differential alteration in intestinal epithelial cell expression of Toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun 2000;68:7010–7017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F: Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem 1999;274:10689–10692 [DOI] [PubMed] [Google Scholar]
- 19.Golbitz P: Traditional soyfoods: processing and products. J Nutr 1995;125:570S–572S [DOI] [PubMed] [Google Scholar]
- 20.Namgung HJ, Park HJ, Cho IH, Choi HK, Kwon DY, Shim SM, Kim YS: Metabolite profiling of doenjang, fermented soybean paste, during fermentation. J Sci Food Agric 2010;90:1926–1935 [DOI] [PubMed] [Google Scholar]
- 21.Park JS, Park HY, Kim DH, Kim DH, Kim HK: Ortho-dihydroxyisoflavone derivatives from aged doenjang (Korean fermented soypaste) and its radical scavenging activity. Bioorg Med Chem Lett 2008;18:5006–5009 [DOI] [PubMed] [Google Scholar]
- 22.Choi NS, Chung DM, Han YJ, Kim SH, Song JJ: Purification and characterization of a subtilisin D5, a fibrinolytic enzyme of Bacillus amyloliquefaciens DJ-5 isolated from doenjang. Food Sci Biotechnol 2009;18:500–505 [Google Scholar]
- 23.Kim JG: Antigenotoxic effects of water extract from Korean fermented soybean paste (doen-jang). J Food Prot 2004;67:156–161 [DOI] [PubMed] [Google Scholar]
- 24.Jung KO, Park SY, Park KY: Longer aging time increases the anticancer and antimetastatic properties of doenjang. Nutrition 2006;22:539–545 [DOI] [PubMed] [Google Scholar]
- 25.Kwak CS, Park SC, Song KY: Doenjang, a fermented soybean paste, decreased visceral fat accumulation and adipocyte size in rats fed with high fat diet more effectively than nonfermented soybeans. J Med Food 2012;15:1–9 [DOI] [PubMed] [Google Scholar]
- 26.Hwang KM, Jung KO, Song CH, Park KY: Increased antimutagenic and anticlastogenic effects of doenjang (Korean fermented soybean paste) prepared with bamboo salt. J Med Food 2008;11:717–722 [DOI] [PubMed] [Google Scholar]
- 27.Lee DS, Kim YS, Ko CN, Cho KH, Bae HS, Lee KS, Kim JJ, Park EK, Kim DH: Fecal metabolic activities of herbal components to bioactive compounds. Arch Pharm Res 2002;25:165–169 [DOI] [PubMed] [Google Scholar]
- 28.Yeo HK, Hyun YJ, Jang SE, Han MJ, Lee YS, Kim DH: Development of fecal microbial enzyme mix for mutagenicity assay of natural products. J Microbiol Biotechnol 2012;22:838–848 [DOI] [PubMed] [Google Scholar]
- 29.Joh EH, Kim DH: Kalopanaxsaponin A ameliorates experimental colitis in mice by inhibiting IRAK-1 activation in the NF-κB and MAPK pathways. Br J Pharmacol 2011;162:1731–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chun J, Kim KY, Lee JH, Choi Y: The analysis of oral microbial communities of wild-type and toll-like receptor 2-deficient mice using a 454 GS FLX Titanium pyrosequencer. BMC Microbiol 2010;10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J: Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 2012;62:716–721 [DOI] [PubMed] [Google Scholar]
- 32.Kim DH, Jin YH: Intestinal bacterial beta-glucuronidase activity of patients with colon cancer. Arch Pharm Res 2001;24:564–567 [DOI] [PubMed] [Google Scholar]
- 33.Lee JH, Lee B, Lee HS, Bae EA, Lee H, Ahn YT, Lim KS, Huh CS, Kim DH: Lactobacillus suntoryeus inhibits pro-inflammatory cytokine expression and TLR-4-linked NF-kappaB activation in experimental colitis. Int J Colorectal Dis 2009;24:231–237 [DOI] [PubMed] [Google Scholar]
- 34.Choi J, Kwon SH, Park KY, Yu BP, Kim ND, Jung JH, Chung HY: The anti-inflammatory action of fermented soybean products in kidney of high-fat-fed rats. J Med Food 2011;14:232–239 [DOI] [PubMed] [Google Scholar]
- 35.Cha YS, Yang JA, Back HI, Kim SR, Kim MG, Jung SJ, Song WO, Chae SW: Visceral fat and body weight are reduced in overweight adults by the supplementation of doenjang, a fermented soybean paste. Nutr Res Pract 2012;6:520–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park NY, Rico CW, Lee SC, Kang MY: Comparative effects of doenjang prepared from soybean and brown rice on the body weight and lipid metabolism in high fat-fed mice. J Clin Biochem Nutr 2012;51:235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoo SK, Cho WH, Kang SM, Lee SH: Isolation and identification of microorganisms in Korean traditional soybean paste and soybean sauce. Korean J Appl Microbiol Biotechnol 1999;27:113–117 [Google Scholar]
- 38.Cho KM, Seo WT: Bacterial diversity in a Korean traditional soybean fermented foods (doenjang and ganjang) by 16S rRNA gene sequence analysis. Food Sci Biotechnol 2007;16:320–324 [Google Scholar]
- 39.Kim TW, Lee JH, Kim SE, Park MH, Chang HC, Kim HY: Analysis of microbial communities in doenjang, a Korean fermented soybean paste, using nested PCR-denaturing gradient gel electrophoresis. Int J Food Microbiol 2009;131:265–271 [DOI] [PubMed] [Google Scholar]
- 40.Nam YD, Lee SY, Lim SI: Microbial community analysis of Korean soybean pastes by next-generation sequencing. Int J Food Microbiol 2012;155:36–42 [DOI] [PubMed] [Google Scholar]
- 41.Kinoshita N, Gelboin HV: beta-Glucuronidase catalyzed hydrolysis of benzo(a)pyrene-3-glucuronide and binding to DNA. Science 1978;199:307–309 [DOI] [PubMed] [Google Scholar]
- 42.Wells CL, Barton RG, Wavatne CS, Dunn DL, Cerra FB: Intestinal bacterial flora, intestinal pathology, and lipopolysaccharide-induced translocation of intestinal bacteria. Circ Shock 1992;37:117–123 [PubMed] [Google Scholar]
- 43.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI: Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 2005;102:11070–11075 [DOI] [PMC free article] [PubMed] [Google Scholar]