Abstract
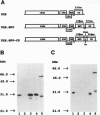
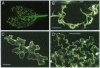
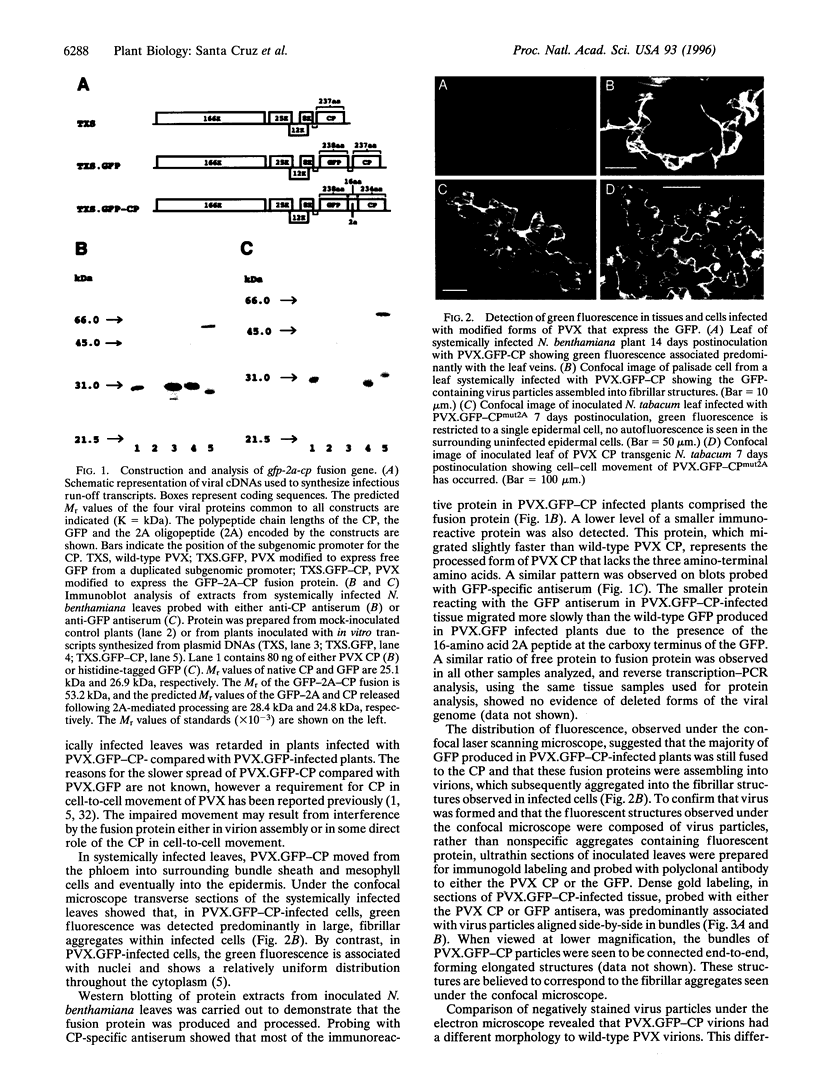
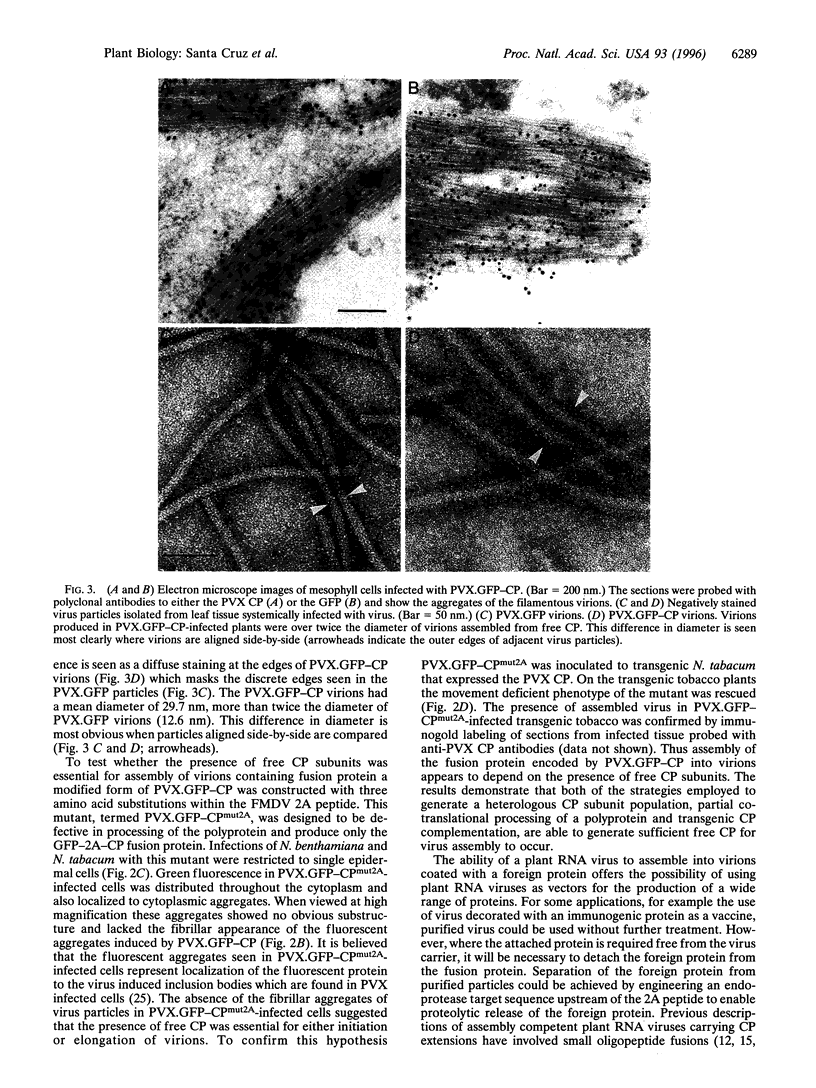
Potato virus X (PVX) is a filamentous plant virus infecting many members of the family Solanaceae. A modified form of PVX, PVX.GFP-CP which expressed a chimeric gene encoding a fusion between the 27-kDa Aequorea victoria green fluorescent protein and the amino terminus of the 25-kDa PVX coat protein, assembled into virions and moved both locally and systemically. The PVX.GFP-CP virions were over twice the diameter of wild-type PVX virions. Assembly of PVX.GFP-CP virions required the presence of free coat protein subunits in addition to the fusion protein subunits. PVX.GFP-CP virions accumulated as paracrystalline arrays in infected cells similar to those seen in cells infected with wild-type PVX The formation of virions carrying large superficial fusions illustrates a novel approach for production of high levels of foreign proteins in plants. Aggregates of PVX.GFP-CP particles were fluorescent, emitting green light when excited with ultraviolet light and could be imaged using confocal laser scanning microscopy. The detection of virus particles in infected tissue demonstrates the potential of fusions between the green fluorescent protein and virus coat protein for the non-invasive study of virus multiplication and spread.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Baratova L. A., Grebenshchikov N. I., Dobrov E. N., Gedrovich A. V., Kashirin I. A., Shishkov A. V., Efimov A. V., Järvekülg L., Radavsky Y. L., Saarma M. The organization of potato virus X coat proteins in virus particles studied by tritium planigraphy and model building. Virology. 1992 May;188(1):175–180. doi: 10.1016/0042-6822(92)90747-d. [DOI] [PubMed] [Google Scholar]
- Baulcombe D. C., Chapman S., Santa Cruz S. Jellyfish green fluorescent protein as a reporter for virus infections. Plant J. 1995 Jun;7(6):1045–1053. doi: 10.1046/j.1365-313x.1995.07061045.x. [DOI] [PubMed] [Google Scholar]
- Chapman S., Hills G., Watts J., Baulcombe D. Mutational analysis of the coat protein gene of potato virus X: effects on virion morphology and viral pathogenicity. Virology. 1992 Nov;191(1):223–230. doi: 10.1016/0042-6822(92)90183-p. [DOI] [PubMed] [Google Scholar]
- Chapman S., Kavanagh T., Baulcombe D. Potato virus X as a vector for gene expression in plants. Plant J. 1992 Jul;2(4):549–557. doi: 10.1046/j.1365-313x.1992.t01-24-00999.x. [DOI] [PubMed] [Google Scholar]
- Davies C., Hills G., Baulcombe D. C. Sub-cellular localization of the 25-kDa protein encoded in the triple gene block of potato virus X. Virology. 1993 Nov;197(1):166–175. doi: 10.1006/viro.1993.1577. [DOI] [PubMed] [Google Scholar]
- Dolja V. V., Haldeman R., Robertson N. L., Dougherty W. G., Carrington J. C. Distinct functions of capsid protein in assembly and movement of tobacco etch potyvirus in plants. EMBO J. 1994 Mar 15;13(6):1482–1491. doi: 10.1002/j.1460-2075.1994.tb06403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolja V. V., McBride H. J., Carrington J. C. Tagging of plant potyvirus replication and movement by insertion of beta-glucuronidase into the viral polyprotein. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10208–10212. doi: 10.1073/pnas.89.21.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donson J., Kearney C. M., Hilf M. E., Dawson W. O. Systemic expression of a bacterial gene by a tobacco mosaic virus-based vector. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7204–7208. doi: 10.1073/pnas.88.16.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto H., Sugiyama Y., Nakagawa N., Hashida E., Matsunaga Y., Takemoto S., Watanabe Y., Okada Y. A new tobacco mosaic virus vector and its use for the systemic production of angiotensin-I-converting enzyme inhibitor in transgenic tobacco and tomato. Biotechnology (N Y) 1993 Aug;11(8):930–932. doi: 10.1038/nbt0893-930. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman M. J., Linthorst H. J., Bol J. F., Cornelissen J. C. The complete nucleotide sequence of potato virus X and its homologies at the amino acid level with various plus-stranded RNA viruses. J Gen Virol. 1988 Aug;69(Pt 8):1789–1798. doi: 10.1099/0022-1317-69-8-1789. [DOI] [PubMed] [Google Scholar]
- Jones J. D., Shlumukov L., Carland F., English J., Scofield S. R., Bishop G. J., Harrison K. Effective vectors for transformation, expression of heterologous genes, and assaying transposon excision in transgenic plants. Transgenic Res. 1992 Nov;1(6):285–297. doi: 10.1007/BF02525170. [DOI] [PubMed] [Google Scholar]
- Kavanagh T., Goulden M., Santa Cruz S., Chapman S., Barker I., Baulcombe D. Molecular analysis of a resistance-breaking strain of potato virus X. Virology. 1992 Aug;189(2):609–617. doi: 10.1016/0042-6822(92)90584-c. [DOI] [PubMed] [Google Scholar]
- Kumagai M. H., Turpen T. H., Weinzettl N., della-Cioppa G., Turpen A. M., Donson J., Hilf M. E., Grantham G. L., Dawson W. O., Chow T. P. Rapid, high-level expression of biologically active alpha-trichosanthin in transfected plants by an RNA viral vector. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):427–430. doi: 10.1073/pnas.90.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonossoff G. P., Johnson J. E. The synthesis and structure of comovirus capsids. Prog Biophys Mol Biol. 1991;55(2):107–137. doi: 10.1016/0079-6107(91)90003-b. [DOI] [PubMed] [Google Scholar]
- Namba K., Pattanayek R., Stubbs G. Visualization of protein-nucleic acid interactions in a virus. Refined structure of intact tobacco mosaic virus at 2.9 A resolution by X-ray fiber diffraction. J Mol Biol. 1989 Jul 20;208(2):307–325. doi: 10.1016/0022-2836(89)90391-4. [DOI] [PubMed] [Google Scholar]
- Porta C., Spall V. E., Loveland J., Johnson J. E., Barker P. J., Lomonossoff G. P. Development of cowpea mosaic virus as a high-yielding system for the presentation of foreign peptides. Virology. 1994 Aug 1;202(2):949–955. doi: 10.1006/viro.1994.1417. [DOI] [PubMed] [Google Scholar]
- Prasher D. C., Eckenrode V. K., Ward W. W., Prendergast F. G., Cormier M. J. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992 Feb 15;111(2):229–233. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- Ryan M. D., Drew J. Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. EMBO J. 1994 Feb 15;13(4):928–933. doi: 10.1002/j.1460-2075.1994.tb06337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. D., King A. M., Thomas G. P. Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J Gen Virol. 1991 Nov;72(Pt 11):2727–2732. doi: 10.1099/0022-1317-72-11-2727. [DOI] [PubMed] [Google Scholar]
- Shen W. J., Forde B. G. Efficient transformation of Agrobacterium spp. by high voltage electroporation. Nucleic Acids Res. 1989 Oct 25;17(20):8385–8385. doi: 10.1093/nar/17.20.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuzeski J. M., Nichols L. M., Gesteland R. F., Atkins J. F. The signal for a leaky UAG stop codon in several plant viruses includes the two downstream codons. J Mol Biol. 1991 Mar 20;218(2):365–373. doi: 10.1016/0022-2836(91)90718-l. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y., Hamamoto H., Takemoto S., Watanabe Y., Okada Y. Systemic production of foreign peptides on the particle surface of tobacco mosaic virus. FEBS Lett. 1995 Feb 13;359(2-3):247–250. doi: 10.1016/0014-5793(95)00054-d. [DOI] [PubMed] [Google Scholar]
- Takamatsu N., Watanabe Y., Yanagi H., Meshi T., Shiba T., Okada Y. Production of enkephalin in tobacco protoplasts using tobacco mosaic virus RNA vector. FEBS Lett. 1990 Aug 20;269(1):73–76. doi: 10.1016/0014-5793(90)81121-4. [DOI] [PubMed] [Google Scholar]
- Turpen T. H., Reinl S. J., Charoenvit Y., Hoffman S. L., Fallarme V., Grill L. K. Malarial epitopes expressed on the surface of recombinant tobacco mosaic virus. Biotechnology (N Y) 1995 Jan;13(1):53–57. doi: 10.1038/nbt0195-53. [DOI] [PubMed] [Google Scholar]
- Usha R., Rohll J. B., Spall V. E., Shanks M., Maule A. J., Johnson J. E., Lomonossoff G. P. Expression of an animal virus antigenic site on the surface of a plant virus particle. Virology. 1993 Nov;197(1):366–374. doi: 10.1006/viro.1993.1598. [DOI] [PubMed] [Google Scholar]