Abstract
We have assessed the ability of bispecific fusion proteins to improve adenovirus-mediated transfer of therapeutic and marker transgenes. We constructed an expression vector that can be easily modified to synthesize a variety of fusion proteins for retargeting adenoviral gene therapy vectors to cell surface markers, which are differentially expressed between normal and cancer cells. Adenoviral transduction can be improved in a number of tumour cell lines which overexpress EGFR (epidermal growth factor receptor) or uPAR (urokinase-type plasminogen activator receptor), but which have only low levels of endogenous hCAR (human coxsackie B and adenovirus receptor) expression. Up to 40-fold improvement in β-galactosidase transgene expression was seen using an EGFR retargeting protein, and up to 16-fold using a second fusion protein targeting uPAR. In vitro, our uPAR retargeting fusion protein improved the sensitivity to adenoviral herpes simplex virus thymidine kinase/ganciclovir by an order of magnitude, whereas in vivo, our EGFR retargeting protein is able to significantly delay tumour growth in rodent animal models in a dose-dependent manner. The ‘cassette’ design of our fusion protein constructs offers a flexible method for the straightforward synthesis of multiple adenoviral retargeting proteins, directed against a variety of tumour-associated antigens, for use in clinical trials.
Keywords: adenovirus, hCAR, retargeting, bladder cancer, EGFR, uPAR
Introduction
Adenovirus-mediated cancer gene therapy is yet to fulfil its clinical potential. A variety of factors may account for this, including vector losses when delivered systemically, immune clearance, incomplete spread through the tumour bulk, limited cell entry due to lack of appropriate cell surface receptors, inefficient transgene expression, limited cytotoxicity of existing therapeutic genes and inappropriate expression in non-tumour tissues.1 Much attention has focussed on improving the entry of viral vectors into tumour cells, including ‘retargeting’ of adenoviral gene therapy vectors by modifying viral tropism,2 such that the vector preferentially binds to and enters tumour cells rather than normal cells. In this way, there is potential for improving therapeutic index and enhancing clinical efficacy.
Although adenoviral vectors are able to interact directly with αv-integrins,3 major histocompatibility complex-1,4 heparan sulphate glycosaminoglycans5 and coagulation factor X,6 it is widely accepted that the primary human adenovirus receptor is the coxsackie B and adenovirus receptor (hCAR).1,7 Transient transfection of hCAR into a panel of hCAR-deficient ovarian cancer cell lines increased adenoviral transduction.8,9 Similar findings were reported in bladder cancer cell lines, in which stable transfection of hCAR into hCAR-negative cells and antisense hCAR into hCAR-positive cells modified adenoviral gene transfer.10 hCAR is expressed on the surface of a wide variety of normal cells, but its expression is reduced in bladder cancer specimens in a stage- and grade-dependent manner.11 hCAR expression is also reduced/lost in many cancer cell types including bladder11,12 and ovarian9 cancers. Although gene therapy aimed at reversing this relative hCAR underexpression by introducing exogenous hCAR might improve transduction efficiency, a more elegant strategy is to modify adenoviral tropism towards a non-hCAR cell surface target.2,13 These targeting strategies aimed at achieving selective hCAR-independent transduction of tumour cells can be sub-divided into ‘genetic’ and ‘conjugate-based’ targeting strategies. The genetic targeting approach involves modification of the viral genome, for example, the introduction of integrin-binding RGD motifs,14–16 CD4017 or the construction of chimeric adenoviruses, in which a heterologous fibre knob gene is incorporated into a serotype 5 genome.18 The conjugate-based approach involves the production of antibody conjugates,19 or bispecific fusion proteins.20 Polymer-coating of viruses has also been used successfully to retarget adenoviruses selectively to different cell types such as ovarian cancer.21 These ‘adaptor molecule’ methods do not involve genetic modification of the viral genome, thereby permitting rapid production of bulk quantities in vitro and, in principle, allowing retargeting of any adenovirus vector by any characterized cell surface protein.
The epidermal growth factor receptor (EGFR; h-erbB1) is a potential target for tumour-selective retargeted delivery of adenoviral gene therapy vectors for a variety of tumour types. EGFR is overexpressed in many tumour types such as breast, bladder, colorectal, lung, prostate and ovarian cancers.22–24 In transgenic mice, this relative overexpression has been shown to promote bladder tumour growth and urothelial hyperplasia,25 and in human bladder cancer specimens is associated with poor prognosis.26,27 In addition to overexpression, point mutations within the kinase domain of EGFR have proved to be useful in predicting the response to anti-EGFR-targeted therapies, particularly, in non-small cell lung cancer.28 A predictor of response to EGFR-targeted therapies has also emerged in colorectal cancer, the Kirsten Ras (K-ras) status. Patients with wild-type K-ras status demonstrate a better clinical response to cetuximab compared with mutant K-ras status.29 Alternative, clinically relevant targets for tumour-selective retargeting of adenoviral vectors include the urokinase-type plasminogen activator (uPAR), a key regulator of cancer cell invasion and metastasis.30,31 Overexpression has been described as a prognostic indicator in a variety of cancers including breast,32 colorectal, and upper gastrointestinal cancers.33,34 It is also a prognostic indicator for bladder cancer35,36 and is known to be upregulated in human bladder tumour specimens.37
We have extended the ‘conjugate’ approach of Dmitriev et al.20 to investigate whether bispecific fusion proteins can be used to improve adenoviral transduction of a panel of bladder tumour cell lines and/or reduce transduction of normal urothelial cells. We describe the production of two fusion proteins, in which the extracellular domain of hCAR is fused with polypeptide sequences, which bind to the cell surface receptors EGFR and uPAR, respectively. As a prelude to potential clinical trials of adenoviral cancer gene therapy, we have tested their ability to modify adenoviral transduction of a panel of human cancer cell lines in vitro and in vivo.
Results
Design, production and purification of the fusion protein constructs
Fusion proteins in which the extracellular domain of hCAR is fused with polypeptide sequences that bind to the cell surface receptors EGFR and uPAR were constructed (Figure 1a). Protein elution from HeLa cells transiently transfected with pQE-sCAR-L-EGF53 and pQE-sCAR-L-Pro-uPA resulted in the generation of His-tagged fusion proteins at the predicted molecular weights (35 and 75 kDa, respectively) (Figure 1b). Although purified sCAR-L-EGF53 was found predominantly in the elution fragment, most of the sCAR-L-Pro-uPA fusion protein remained in the supernatant after bead incubation. This inefficient purification may be due to the His-tag being folded within the protein, and therefore inaccessible to the Ni-beads. The 60 kDa band seen in both eluates is considered to be a nonspecific protein, as it was seen in all purifications performed in the HeLa cell system with this expression plasmid construct. Design of our plasmid expression vector includes the ability to express protein in bacterial, insect or mammalian cells. Better yields of protein, required for subsequent in vivo studies, were therefore obtained from insect cells by incorporating our construct into a baculovirus expression vector. Baculovirus expression of the sCAR-L-EGF53 fusion protein resulted in a product of the same size as that observed in the HeLa/plasmid expression system and was able to produce ~10 times more recombinant protein (Figure 1c). Furthermore, the nonspecific protein of 60 kDa was not observed in the baculovirus-derived protein preparations.
Figure 1.
Design, production and purification of retargeting fusion proteins. (a) Schematic representation of the fusion proteins constructs. A series of plasmid expression vectors were constructed to create a series of retargeting proteins with various ligands placed C terminal to the extracellular portion of the hCAR protein (sCAR), and a short synthetic linker peptide. An 8xHis epitope was included to facilitate subsequent protein purification. The positions of restriction sites used in the cloning strategy are shown. ‘X’ represents any alternative ligand of choice. (b) Western blot analysis of protein purified from transiently transfected HeLa cells using an anti-His antibody. Both supernatant (S/N) remaining after bead extraction of protein, and protein released from beads are shown for sCAR-L-EGF53 and sCAR-L-Pro-uPA. Also shown are molecular weight markers (kDa) (c) Coomassie-stained gel of baculovirus and mammalian-expressed sCAR-L-EGF53 protein. Similar volumes from a 100 µl preparation of each were run on a 12.5% SDS–polyacrylamide gel electrophoresis.
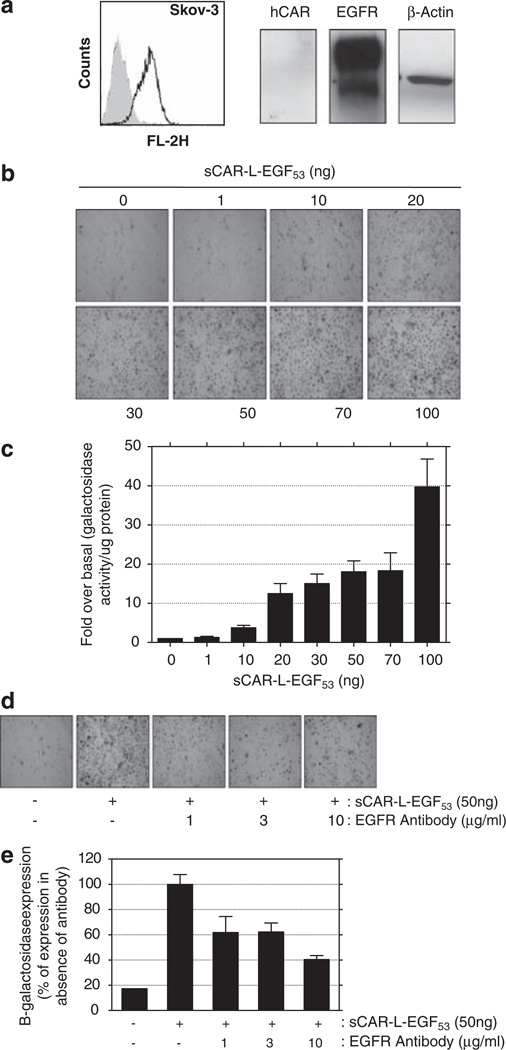
Retargeting Ad-CMV-lacZ to SKOV3 cells using sCAR-L-EGF53
The SKOV3 ovarian cancer cell line was chosen as an appropriate model for testing sCAR-L-EGF53, as it has very low/undetectable hCAR expression, but overex-presses EGFR to an extent greater than any other cell line we have investigated (Figure 2a). In the absence of fusion protein, there was minimal viral transgene expression in SKOV3 cells. However, mixing virus with increasing amounts of sCAR-L-EGF53 caused a corresponding increase in adenoviral transduction, culminating in 95–100% of cells transduced using 50 ng of fusion protein (Figure 2b). Quantitative assessment of the β-galactosidase activity of infected cells revealed that the improvement of adenoviral transduction seen at 50 ng protein corresponded to an 18-fold over basal increase in transgene expression compared with untargeted adenovirus (Figure 2c). Increasing the amount of sCAR-L-EGF53 to 100 ng protein improved transduction still further to 40-fold over basal, as compared with control cells. This extra increase above that seen with 50 ng of fusion protein is likely to reflect an increase in β-galactosidase expression per cell rather than an increase in the number of cells transduced. The recombinant protein obtained from insect cells was functionally equivalent to that derived from mammalian cells in in vitro retargeting experiments using Ad-CMV-lacz in SKOV3 cells with a plateau in increasing transduction seen above 75 ng of retargeting protein (data not shown).
Figure 2.
Retargeting of Ad-CMV-lacZ to SKOV3 (ovarian cancer) cells. (a) FACS and western blot analysis of hCAR and EGFR protein expression in SKOV3 cells. hCAR and EGFR expressions, as assessed by FACS, are represented by shaded grey areas and black lines, respectively. (b and c) Effects of increasing amounts of sCAR-L-EGF53 on the efficiency of viral transduction of SKOV3 cells by Ad-CMV-lacZ (multiplicity of infection = 1). Cells were transduced in the absence of retargeting protein (0) or after preincubation of the adenovirus with increasing amounts of sCAR-L-EGF53 fusion protein (1–100 ng). Cells were stained with Xgal for lacZ gene expression (b) or assayed for β-galactosidase activity (c). Histograms show the ratio of β-galactosidase activity in the absence and presence of varying amounts of fusion protein. All values are the means of four to six independent experiments, and errors bars represent 1 s.d. (d and e) Effects of EGFR neutralizing antibody on adenoviral EGFR retargeting of Ad-CMV-lacZ in SKOV3 cells. Cells were infected with adenovirus alone (−) or adenovirus preincubated with 50 ng sCAR-L-EGF53 (+), and increasing concentrations of neutralizing antibody. Cells were stained with Xgal for lacZ gene expression (d) or assayed for β-galactosidase activity (e). Histograms show percentage β-galactosidase activity with respect to Ad-CMV-lacZ retargeted with 50 ng sCAR-L-EGF53 in the absence of neutralizing antibody (100%). Values are the means of three independent experiments and errors bars represent 1 s.d.
Preincubation of SKOV3 cells with an EGFR neutralizing antibody considerably reduced the retargeting of Ad-CMV-lacZ (Figures 2d and e), suggesting that adenoviral entry mediated by sCAR-L-EGF53 depends predominantly on the expression of EGFR. An ‘irrelevant’ neutralizing antibody was also used as a control without any reduction in adenoviral retargeting (data not shown). The observation that reversal of retargeting with neutralizing antibody was incomplete is consistent with the possibility that additional nonspecific interactions may be contributing to adenoviral entry, in addition to specific receptor-mediated mechanisms.
sCAR-L-EGF53 improves adenoviral gene transfer in a panel of bladder cancer cells
Fluorescence-activated cell sorting (FACS) analysis of a panel of bladder tumour cell lines showed wide variation in expression of hCAR and EGFR between the different cell lines (Figure 3a). Although some cell lines have no detectable levels of hCAR (T24, J82), others have either low-to-moderate (5637, JON) or strong (RT112, RT4, UMUC3, VMCUB1) expression of the protein. Transduction of the cell lines with Ad-CMV-lacZ alone revealed that, as expected, adenoviral entry and subsequent expression of a marker transgene correlates with cellular hCAR expression (Figure 3b). However, both JON and 5637 cell lines are transduced to a greater extent than was predicted from the hCAR expression data, perhaps suggesting the involvement of alternative hCAR-independent methods of entry. Adenoviral transduction of a number of the bladder cancer cell lines is improved with addition of sCAR-L-EGF53. For example, the T24 cell line is naturally refractory to adenoviral transduction, but is converted into a relatively passive cell line after retargeting using the fusion protein sCAR-L-EGF53. The enhancement of T24 cell transduction corresponds to an 11-fold improvement in β-galactosidase activity compared with untargeted cells (Figure 3c). In order to assess any correlation between retargeting and protein expression, quantitation of the FACS plots was performed by comparing the geometric mean fluorescence index for each curve (Figure 3c) and calculating an EGFR/hCAR ratio. In six of the eight cell lines tested, a good relationship between the retargeting achieved (dark bars) and the EGFR/hCAR expression ratio (light bars) was found. The four cell lines with the highest EGFR/hCAR ratios showed the greatest retargeting and the four with the lowest ratios were retargeted least effectively.
Figure 3.
Retargeting of Ad-CMV-lacZ in a panel of bladder cancer cell lines using sCAR-L-EGF53. (a) Flow cytometry analysis of hCAR and EGFR expression in bladder cancer cell lines. Expression was assessed using an anti-CAR antibody followed by a phycoerythrin (PE)-conjugated secondary antibody (shaded grey) and a direct conjugate EGFR-PE antibody (black line). Negative controls were included to determine background fluorescence. As both control histograms occupied the same region on the FL-2H axis, only one control (open histogram) is shown for clarity. (b) Effects of 50 ng sCAR-L-EGF53 on transduction of a panel of bladder cancer cell lines with Ad-CMV-lacZ (multiplicity of infection=1). Cells were infected with Ad-CMV-lacZ in the absence (minus) or presence (plus) of 50 ng sCAR-L-EGF53 fusion protein and stained with Xgal for lacZ gene expression. (c) Cells were assayed for β-galactosidase activity (left y axis), and this was compared with the ratio of EGFR/hCAR expression as calculated from the geometric MFI for each curve of the FACs histograms (right y axis). β-galactosidase data shown represents fold-over-basal activity for each cell line compared to its individual control. Values are the means from four to six independent experiments and errors represent 1 s.d.
Effects of EGFR retargeting in normal urothelial cells
Although the sCAR-L-EGF53 fusion protein is useful in improving adenoviral gene transfer in cells with low hCAR/high EGFR expression, a second potential benefit of adenoviral retargeting would be to reduce/restrict adenoviral entry into cells with a high hCAR/low EGFR expression profile—the expected profile of a normal cell. In order to test this hypothesis, three normal human urothelial (NHU) cell lines were investigated. Figure 4a demonstrates that the three NHU cell lines express hCAR at varying but relatively high levels, and also have low but detectable levels of EGFR. As seen in our previous work,38 all three were permissive to viral entry and showed similar levels of transduction in the absence of retargeting protein to those seen in tumour cell lines in the presence of sCAR-L-EGF53. However, after addition of 50 ng sCAR-L-EGF53, adenoviral entry was not hindered, but rather was slightly increased in each of three NHU cell lines (Figure 4b). We hypothesized that the low levels of EGFR seen in NHU cells might provide an additional binding opportunity, permitting entry of the virus into NHU cells by either CAR and/or EGFR. In support of this, incubation of NHU cells with antibodies against CAR and/or EGFR, before the addition of the Ad-CMV-lacz/sCAR-L-EGF53 mix, reduced adenoviral transduction of NHU cells to the levels seen with virus alone (Figure 4c). A similar pattern was seen with two other NHU cell lines (data not shown).
Figure 4.
Retargeting of Ad-CMV-lacZ to NHU cell lines using sCAR-L-EGF53. (a) Western blot analysis of hCAR and EGFR expression in three NHU cell lines (420, 816 and 919). Molecular weight markers represent protein size in kDa. FACS analysis of the hCAR (shaded grey) and EGFR (black line) expression in the NHU816 cell line for comparison. Expression was assessed as described for previous hCAR and EGFR FACS analysis in other cell lines. (b) Effects of 50 ng sCAR-L-EGF53 on transduction of cultured NHU cells by Ad-CMV-lacZ (multiplicity of infection = 1) Cells were treated with Ad-CMV-lacZ alone (minus) or after preincubation of virus with 50 ng sCAR-L-EGF53 fusion protein (plus). Cells were quantitatively assayed for β-galactosidase activity. Histograms represent ratio of enzyme activity in the presence of retargeting protein compared to in its absence for each NHU cell line. Values are the mean of three independent experiments and error bars represent 1 s.d. (c) Effect of anti-EGFR and/or anti-CAR antibodies on adenoviral transduction of NHU cells. NHU919 cells were incubated with anti-EGFR and/or anti-CAR antibody before the addition of virus +/− sCAR-L-EGF53 fusion protein, as in (b). Representative data are shown for duplicate experiments with NHU919 and with two other NHU cell lines.
UPAR as an alternative receptor for adenoviral retargeting
Although many clinical bladder tumour specimens overexpress EGFR,23 the heterogeneity of tumour tissue makes it unlikely that targeting EGFR alone will be sufficient for all bladder tumours, and additional or alternative cell surface targets are likely to be required for clinically useful therapy. We therefore investigated uPAR as a potential alternative target using a second fusion protein, sCAR-L-Pro-uPA. The majority of bladder cancer cell lines investigated express uPAR as assessed by western blot (Figure 5a, lower band). An additional nonspecific (upper) band was visible in all cell lines tested. Increasing amounts of sCAR-L-Pro-uPA caused an increase in adenoviral transduction and expression of a marker transgene in T24 cells and in J82 cells (Figure 5b). In T24 cells, 100 ng sCAR-L-Pro-uPA fusion protein caused a similar improvement in β-galactosidase activity to that seen using sCAR-L-EGF53 (Figure 5c). However, in J82 cells, although sCAR-L-EGF53 produced only a 4-fold improvement, sCAR-L-Pro-uPA caused a greater, 16-fold improvement.
Figure 5.
Retargeting of Ad-CMV-lacZ to bladder cancer and NHU cell lines using sCAR-L-Pro-uPA. (a) Western blot analysis of uPAR expression in a panel of bladder cancer cell lines. Molecular weight markers represent protein size in kDa. (b) Effects of transducing T24 and J82 bladder cancer cell lines with Ad-CMV-lacZ (multiplicity of infection = 1) in the absence of sCAR-L-Pro-uPA protein or after preincubation with increasing amounts of the retargeting protein (20–100 ng). Cells were stained with Xgal for lacZ gene expression. (c) Cells were assayed for β-galactosidase activity. The histogram shown represents the ratio of enzyme activity in the presence of 50 ng sCAR-L-EGF53 (dark grey bars) and 100 ng sCAR-L-Pro-uPA (light grey bars) compared with Ad-CMV-lacZ alone. Values are the mean of four independent experiments and error bars represent 1 s.d. (d) Effect of sCAR-L-Pro-uPA protein on transduction of NHU cell lines. Ad-CMV-lacz virus was preincubated with 50 ng scar-L-Pro-uPA. To investigate the relative contributions of CAR and uPAR to viral entry, cells were also preincubated with 10 µg ml−1 of anti-uPAR and/or anti-CAR neutralizing antibodies before the addition of virus +/− sCAR-L-Pro-uPA fusion protein. Representative data are shown for duplicate experiments with NHU919 and with two other NHU cell lines.
In analogous experiments to those with sCAR-L-EGF53 above, the ability of sCAR-L-Pro-uPA to retarget adenovirus to NHU cells was investigated (Figure 5d). As with sCAR-L-EGF53, the uPA retargeting protein slightly increased the binding of Ad-CMV-lacZ to the NHU cell line, rather than hindering viral entry. Again, preincubation of the NHU cells with antibodies against uPAR and/ or CAR, separately or together, reduced the levels of β-galactosidase activity to those seen with virus alone, suggesting that virus can enter NHU cells by CAR and/or uPAR. A similar pattern was seen with two other NHU cell lines (data not shown).
Retargeting of a therapeutic adenovirus using sCAR-L-Pro-uPA
In (hCAR-negative, uPAR-positive) J82 cells, addition of 100 ng sCAR-L-Pro-uPA resulted in a 10-fold improvement in IC50 using Ad-CMV-HSVtk (herpes simplex virus thymidine kinase) and ganciclovir (GCV) (Figure 6), suggesting a marked increase in the sensitivity of these tumour cells to GCV in the presence of our fusion protein. This significant improvement in transgene-mediated cell-kill emphasizes the potential of this targeting strategy, transforming J82 cells from a GCV-resistant line when treated with adenovirus alone into a cell line that is highly susceptible when retargeting fusion protein is added.
Figure 6.
Effects of the sCAR-L-Pro-uPA retargeting protein on viability of J82 cells treated with Ad-CMV-HSVtk/GCV. Cells (1 × 105) J82 cells were transduced with Ad-CMV-HSVtk (multiplicity of infection = 1) in the absence of sCAR-L-Pro-uPA fusion protein or after preincubation of virus with 50 ng of fusion protein. After 24 h, virally transduced cells were lifted, counted, and plated in a 96-well plate. Following a further 24 h, increasing concentrations of GCV was added, and after 5 days a cell viability assay was performed. Data shown are representative of three independent experiments and errors bars represent 1 s.d.
In vivo enhancement of adenoviral gene therapy using sCAR-L-EGF53
The ability of Ad-CMV-HSVtk/GCV to delay tumour growth in the absence or presence of sCAR-L-EGF53 protein was examined in a SKOV3 xenograft model in nude mice. To maintain similar ratios of virus to protein to those used in vitro, 1 × 108 plaque-forming units (PFU) of Ad-CMV-HSVtk was pre-mixed with 20 or 50 µg of protein and injected intratumourally. No significant impact on tumour growth was seen for protein alone, saline or untreated control groups. Although virus preincubated with 20 µg of protein administered to mice showed no effect on tumour growth compared with virus alone, with or without GCV (data not shown), statistically significant effects were seen when 50 µg of protein was used. A significant delay in tumour growth (P⩽.05) was seen when tumours were treated with naked virus and GCV, compared with virus alone, but an even greater effect (P⩽.03) was seen when virus was pre-mixed with 50 µg of sCAR-L-EGF53 protein (Figure 7). These results reflect those obtained for the in vitro studies, in which 50 ng of protein was sufficient to demonstrate significant retargeting of the adenovirus to high EGFR/low CAR expressing cells with the same ratio of virus to protein, and importantly demonstrate that the strategy is effective in more complex in vivo models.
Figure 7.
Retargeting of Ad-CMV-HSVtk to SKOV3 Xenografts using sCAR-L-EGF53. Ad-CMV-HSVtk preincubated with 50 µg EGF53 protein or virus alone was administered as a single intratumoural injection into SKOV3 tumours established in the flanks of mice. Mice in the GCV-treatment groups were given intraperitoneal GCV 100 mg kg−1 daily for 7 days and tumour growth was measured daily. Mean relative tumour volume was calculated relative to tumour size on the day of tumour injection (day 0).
Discussion
We have examined the effectiveness of bispecific fusion proteins in improving adenoviral transduction of tumour cell lines that are intrinsically refractory to adenoviral transduction. We demonstrate in vitro that the sCAR-L-EGF53 protein increases marker gene expression in human bladder and ovarian tumour cell lines and that the improvement broadly correlates with EGFR/hCAR expression status of the cells. In vivo the sCAR-L-EGF53 protein delayed tumour growth by retargeting of Ad-CMV-HSVtk specifically in the presence of GCV. We also show that a second novel fusion protein, designed to target the alternative cell surface molecule, uPAR, effectively improves gene transfer in bladder cancer cell lines, including one cell line that was not enhanced using the EGFR-targeting protein. Furthermore, we demonstrate that a therapeutic adenovirus, Ad-CMV-HSVtk, can be retargeted to cells overexpressing uPAR, thereby increasing their sensitivity to the prodrug GCV. Our fusion protein strategy therefore allows selective targeting of cancer cells depending on their cell surface marker expression profiles.
Despite recent advances in virus-mediated cancer gene therapy, major hurdles still remain. Efficient gene therapy relies heavily on selective transduction of the cancer cell. A number of cancer-specific targets, such as integrins,16,39 MUC1,40 CD4041,42 fibroblast growth factor-2,43 transferrin,44 the G250 protein45 and the melanoma-associated antigen,46,47 have been utilized in a number of different cancer types, but very little ‘retargeting’ has been attempted in the context of bladder cancer. Van Der Poel et al.48 demonstrated a similar 12-fold increase in adenoviral entry to that seen here, by using an EGFR-targeting bispecific single chain antibody in multiple bladder cancer cell lines. A second study, also using bladder cancer cell lines, demonstrated an improvement in adenoviral infection using a retargeting strategy directed to the RGD-mediated integrins and Ad3 receptors.18
A number of other approaches to modifying the natural tropism of adenoviral vectors for hCAR have been reported. For example, Martin et al.49 also exploited overexpression of uPAR to improve adenoviral infectivity of liver cells by incorporating a uPAR-binding ligand into the H1 loop of the adenoviral fibre knob. However, we believe that our system has a number of advantages. First, the ‘cassette’ design of our fusion protein expression construct allows both flexibility and rapid, efficient production of a variety of fusion proteins. In principle, any cell surface molecule that is overexpressed in cancer cells, and for which a known ligand has been characterized, can be targeted. Second, the genetic modification approach to altering adenoviral tropism requires time-consuming modification of the adenoviral fibre knob domain and lengthy bulking-up of each virus that is to be retargeted; whereas a single fusion protein can be used to retarget almost any adenoviral vector, whether they are replication-defective or conditionally replicating, and whether they express marker or therapeutic transgenes. Third, the ease with which these fusion proteins can be expressed and purified may allow simultaneous use of multiple retargeting fusion proteins to target a range of heterogeneous tumours without detailed characterization of their cell surface expression pattern.
Previous studies in our laboratory have demonstrated that NHU cells are readily infectable using adenoviruses.38 Vectors expressing a therapeutic transgene may therefore produce an undesirable cell kill in normal cells. On the basis of our hCAR/EGFR expression data, we predicted that the addition of our fusion proteins to NHU cells (high hCAR, low EGFR and low uPAR) would interfere with adenoviral entry, thus reducing NHU cytotoxicity and providing an extra therapeutic advantage in a mixed population of tumour and normal cells, such as would be seen in a clinical setting. In fact, addition of the fusion proteins caused a modest increase in adenoviral transduction of the NHU cells, rather than a decrease. Our data suggest that, unlike the situation in hCAR-poor tumour cells, adenoviral entry to NHU cells may be possible by both the retargeted receptor and hCAR. Nevertheless, the increase in transduction seen with retargeting protein in NHUs is clearly less than that seen in tumour cells, suggesting that a differential effect could still be achieved.
Although a good correlation between the relative expression of EGFR/hCAR and the potential for adenoviral retargeting has been shown for the majority of cell lines tested, correlation was not perfect. A possible explanation is the presence of an EGFR- and hCAR-independent mechanism of viral entry. Alternatively, a rate-limiting step other than viral attachment may explain the variability observed. Certainly, caution must be exercised in predicting the success of retargeting experiments on the basis of protein expression profiles alone.
Our observation that the use of uPAR retargeting protein can increase the sensitivity of tumour cells to adenovirus-mediated HSVtk/GCV in vitro, and especially to cells not rendered passive by EGFR retargeting, confirms the flexibility of our system and implies that different tumours may require alternative retargeting approaches. Using a similar sCAR-EGF fusion protein to that used here, Liang et al.50 successfully retargeted an adenovirus containing a marker gene to tumour cell line xenografts expressing EGFR, and showed a good increase in adenoviral targeting, demonstrating that the adenovirus/protein complex is stable in vivo. However, they did not test viruses expressing a therapeutic gene.
Our demonstration that retargeting fusion proteins can enhance transduction of tumour cells by an adenoviral vector expressing the HSVtk transgene, and delay the growth of tumour xenografts in vivo suggests that this strategy would be amenable to use in clinical trials of cancer gene therapy. Future clinical use might be in one of two scenarios. The first would be an individualized therapeutic approach. Patient biopsies would be taken and tumour expression profiles obtained. A decision could then be made as to whether a patient was suitable for use of a particular fusion protein, and if so, which one was appropriate for treatment. This ‘individualized therapy’ would be subject to the above limitation of the imperfect correlation between expression patterns and transduction efficiency. The second approach would be to use a ‘cocktail’ of multiple fusion proteins to target a single adenovirus to multiple cell surface markers commonly overexpressed in tumours. The plasmid-based ‘cassette’ design of our basic vector allows the synthesis of such a cocktail, more easily and more rapidly than multiple genetic modifications of the adenoviral genome. This approach is attractive as, using tumour array analysis from a large number of patients, a panel of ‘optimum targets’ could be established. A mixture of retargeting proteins would then direct adenoviral vector entry towards one or more of these, thereby efficiently targeting a large proportion of patients’ tumours without any prior knowledge of the characteristics of an individual tumour.
In conclusion, this approach represents an opportunity to improve adenoviral gene therapy strategies, in multiple cancer types, and can be utilized in combination with existing and future adenovirus-mediated gene therapy strategies to further enhance tumour/tissue-selective expression of therapeutic genes. Our in vivo data suggest that this strategy is amenable to testing in clinical trials of hCAR-poor tumours.
Materials and methods
Cell culture
Established human tumour cell lines (transitional cell carcinoma cell lines, RT112, 5637, JON, VMCUB1, RT4, T24, UMUC3, J82; ovarian cancer cell line, SKOV3; cervical carcinoma cell line, HeLa) were grown in monolayer culture at 37 °C in a humidified atmosphere containing 5% CO2. NHU cells were cultured from surgical specimens of ureter and renal pelvis as described previously,51 and maintained in keratinocyte serum-free medium supplemented with bovine pituitary extract and recombinant EGF, which were added according to the manufacturers’ instructions to give final concentrations of 50 and 5 ng ml−1, respectively. Cholera toxin was included at 30 ng ml−1.
Fusion protein construct production
pQE-sCAR-LINKER Intermediate
RNA was isolated from RT4 cells using RNeasy (Qiagen, West Sussex, UK), according to the manufacturer’s protocols. First-strand cDNA was produced using Omniscript (Qiagen) from 1 mg of isolated RNA using oligo-dT as a primer. The full-length coding sequence of hCAR was PCR amplified from the RT4 cDNA using sequence-specific primers, 5′-a ggagcgagagccgcctac-3′ and 5′-acggagagcacagatgagaca-3′. The PCR product was gel extracted, TA-cloned into pGEM-Teasy (Promega, Southampton, UK), and nested PCR primers were used to amplify the extracellular domain of hCAR (sCAR), 5′-ccatggcgctcctgctgtgc-3′ and 5′-ctgcagagctttatttgaaggagggacaacg-3′. The nested primers contained additional 5′-NcoI (forward) and 5′-PstI (reverse) restriction-site recognition sequences for subsequent cloning steps. The resulting sCAR PCR product was TA-cloned into pGEM-Teasy, excised using an NcoI/ PstI digest and inserted into the expression plasmid pQE-TriSystems (Qiagen), which contains an 8 × His-tag epitope to facilitate protein purification steps. The resulting intermediate construct was designated pQE-sCAR. To produce the linker peptide composed of the amino-acid sequence (SASASASAP), two oligonucleotides 5′-gtccgcaagtgcctcagcgtctgctccaggtac-3′ and 5′-c tggagcagacgctgaggcacttgcggactgca-3′ containing PstI and KpnI restriction-site overhangs were annealed and inserted into pQE-sCAR. The resulting plasmid was named pQE-sCAR-LINKER.
pQE-sCAR-L-EGF53
The full-length EGF coding sequence was PCR amplified from lambda EGF116 phage lysate (ATCC(R); 93-12; 59956) using sequence-specific primers, 5′-gtcaatcatactcaccttgcc-3′ and 5′-caggcattgagt aggtgattagtcg-3′. The PCR product was gel extracted, TA-cloned into pGEM-Teasy, and nested primers were used to PCR amplify a 53 amino-acid peptide encoding the mature form of human EGF (EGF53), 5′-ggtaccaatagtg actctgaatgtcc-3′ and 5′-ctcgaggcgcagttcccaccacttcag-3′. The nested primers contained additional 5′-KpnI (forward) and 5′-XhoI (reverse) restriction-site recognition sequences necessary for further cloning steps. The PCR product was TA-cloned, producing pGEM-EGF53, and excised using KpnI and XhoI. EGF53 was then inserted into pQE-sCAR-LINKER, producing pQE-sCAR-L-EGF53.
pQE-sCAR-L-Pro-uPA
RNA was isolated from T24 cells and reverse transcribed to produce cDNA, as above. The cDNA was used as a template to PCR amplify the entire Pro-uPA coding sequence using sequence-specific primers, 5′-ctcctgccgcaggccaccgag-3′ and 5′-gctgatgga gatgactctactgc-3′. The PCR product was gel extracted and TA-cloned. The complete coding sequence was PCR amplified using sequence-specific primers including 5′-KpnI (forward) and 5′-XhoI (reverse) restriction-site overhangs, 5′-ggtaccatgagagccctgctggcgcg-3′ and 5′-ctcgaggagggccaggccattctcttc-3′. The PCR product was TA-cloned, gel extracted and excised using KpnI and XhoI. The KpnI/XhoI fragment was ligated into pQE-sCAR-LINKER, producing pQE-sCAR-L-Pro-uPA.
Protein purification and detection
Two micrograms of expression plasmid DNA was transiently transfected into 1 × 106 HeLa cells in each of four 10-cm dishes using Effectene (Qiagen) according to the manufacturer’s instructions. After 48 h, cells were lifted using phosphate-buffered saline (PBS)/0.1% EDTA, and resuspended in 1 ml of lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 0.05% Tween-20,10 mM imidazole, pH 8.0). Cell lysate was then sonicated (six 15 s bursts at room temperature with 10 s gaps on ice), and centrifuged (13 000 r.p.m., 10 min, 4 °C). Supernatant was collected and 65 ml of magnetic nickel beads (Qiagen) were added. The supernatant/bead mixture was incubated on an end-over roller at 4 °C for 1 h, before beads were collected and protein was eluted. Beads were washed three times in wash buffer 1 (as above, but 25 mM imidazole), once in wash buffer 2 (as above but 100 mM imidazole), and eluted using 60 µl elution buffer (as above but 250 mM imidazole). A volume of 10 and 2 µl of purified protein was then analysed on a Coomassie-stained gel, and an anti-His western blot, respectively, to assess purity and quantity.
For large-scale production of the sCAR-L-EGF53 fusion protein, the same pQE construct was recombined with BacPAK6 baculoviral DNA by co-transfection into Spodoptera frugiperda (Sf9) insect cells according to the manufacturer’s protocol (Clontech, Saint-Germainen-Laye, France). Plaques were screened for protein expression and specificity by western blot analysis and a recombinant virus stock was prepared. For preparation of recombinant protein, four to six 150 cm2 flasks were seeded with 2 × 107 Sf9 cells and infected at an multiplicity of infection of 5–10 for 3 days at 27 °C. The cells were then harvested using a cell scraper and pelleted at 1300 r.p.m. for 10 mins at 4 °C. The pellet was resuspended in 3 ml of buffer 1 (10 mM Tris-HCl, pH 7.5, 10 mM NaCl, 1.5 mM MgCl2, 10 mM 2-ME) with protease inhibitors and incubated on ice for 30 mins. The cells were then pelleted at 13 000 r.p.m. for 10 mins at 4 °C and the pellet resuspended in 3 ml of buffer 2 (20 mM Tris-HCl, pH 7.5, 300 mM NaCl, 10 mM MgCl2, 0.5% Triton X-100, 20% glycerol, 10 mM 2-ME) with protease inhibitors and sonicated with four bursts of 30 s each on ice. The lysate was centrifuged again at 13 000 r.p.m. for 10 mins at 4 °C and the supernatant rotated overnight at 4 °C with 200 µl of magnetic nickel beads (Qiagen). The beads were washed with 3 ml of buffer A (20 mM Tris-HCl, pH 8, 500 mM KCl, 10% glycerol, 10 mM 2-ME, 20 mM imidazole) with protease inhibitors and then the protein was eluted in 200 ml of buffer B (20 mM Tris-HCl, pH 8, 100 mM KCl, 10% glycerol, 10 mM 2-ME, 250 mM imidazole). The protein concentration was measured using the Nanodrop spectrophotometer (Thermo Fisher Scientific, Loughborough, UK) and the purity was examined by Coomassie-stained SDS-polyacrylamide gel electrophoresis.
Western blotting
Proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred on to a nitrocellulose membrane overnight at 40 V in transfer buffer (20 mM Tris pH 7.4,150 mM glycine, 20% (v/v) methanol). Membranes were blocked for 1 h at 18–22 °C with 5% (w/v) non-fat milk powder in PBS-Tween (PBS plus 0.5% Tween-20) and further incubated for 1 h at 18–22 °C with primary antibody: anti-EGFR (1005: sc-03), 1:1000; anti-uPAR (FL-290: sc-10815, Santa Cruz Biotechnology, Heidelberg, Germany), 1:200, anti-CAR (ab9891-1, Abcam, Cambridge, UK), 1:160, performed under non-reducing conditions, anti-β-actin (A4551: clone AC-15, Sigma Aldrich, Dorset, UK), 1:10000, and anti-tetra-His (34670, Qiagen), 1:1000, in PBS-Tween. After washing in PBS-Tween, blots were incubated for 1 h at 18–22 °C with a 1:3000 dilution (in 3% (w/v) non-fat milk powder in PBS-Tween) of anti-(mouse IgG) (BioRad, Hemel Hempstead, UK) or anti-(rabbit immunoglobulin-G) (Southern Biotechnology Associates from Cambridge Biosciences, Cambridge, UK) antibodies coupled to peroxidase. Immunoblots were developed by enhanced chemiluminescence (Amersham Biosciences, Little Chalfont, UK).
Flow cytometry analysis
Cells (1 × 105) from each cell line were aliquoted into FACS tubes and washed in FACS buffer (PBS + 1% (v/v) fetal calf serum + 0.01% (v/v) sodium azide). Cells were labelled with a directly conjugated EGFR-phycoerythrin antibody (BD Biosciences, Oxford, UK) and CAR antibody (Clone RmcB) (Millipore, Watford, UK) followed by a secondary phycoerythrin-conjugated goat anti-mouse immunoglobulin (F[ab]’2 fragment) antibody (Southern Biotechnology Associates). Irrelevant isotype-matched controls were included for all experiments. Cells were analysed on a FACScalibur instrument using CellQuest Pro software (Becton Dickinson, Hertfordshire, UK) acquiring 5000 events per sample.
Replication-defective adenovirus production
Ad-CMV-lacZ was purchased from Invitrogen (Paisley, UK), and bulked-up, purified and titred as previously described38. Ad-CMV-HSVtk was produced using pAdEasy (Stratagene, Agilent Technologies, La Jolla, CA, USA). The full-length CMV (cytomegalovirus) promoter was PCR-amplified from Ad-CMV-lacZ genomic DNA using the sequence-specific primers, 5′-tcaatattggccat-tagcca-3′ and 5′-gctagcctatagtgagtcgtatta-3′. The PCR product was TA-cloned into pGEMTeasy, excised using a NotI digest and ligated into pGL3-basic to produce pGL3-CMV. pBS-SK/MEEP-tk was a generous gift from Georges Vassaux (CRUK, Imperial College, London). HSVtk was excised using HindIII/XbaI, and inserted into pGL3-CMV downstream of the cloned CMV promoter. CMV-HSVtk was then excised using a NotI/XbaI partial digest, and inserted into pSHUTTLE. Progression to a primary viral lysate was performed according to the manufacturer’s protocols (Stratagene). Adenoviral bulk-up, CsCl purification, and quantitation was performed by ViraQuest (North Liberty, IA, USA). All multiplicities of infection shown are stated in terms of PFU per cell.
In vitro retargeting of marker transgene vectors
Cells were plated out at 1 × 105 cells per well in a 24-well plate, and left overnight. A volume of 1 × 105 PFU ml−1 of Ad-CMV-lacZ was added to varying amounts of retargeting fusion protein (pQE-sCAR-L-EGF53 or pQE-sCAR-L-Pro-uPA, 1–100 ng) to produce a final volume of 35 µl. The adenovirus/fusion protein mixture was pipette-mixed and incubated at room temperature for 1 h. Cells were washed in medium containing 2% fetal calf serum, and 165 ml of medium was added to each well. The 35 ml adenovirus/protein mix was then added to the cells and gently mixed to produce a final volume of 200 µl. The mixture was incubated at room temperature for 1 h with occasional gentle agitation. Cells were washed once with medium, and then 1 ml complete medium was added. After overnight incubation, cells were either stained for β-galactosidase activity as previously described38 or assayed for β-galactosidase activity using GalactoStar (Applied Biosystems, Foster City, CA, USA), according to the manufacturers’ instructions. Briefly, cells were lysed in 200 µl lysis buffer, and 10 µl of cleared lysate was added to 100 µl of 50:1 buffer dilution solution/reagent in a 96-well plate. The plate was incubated for 30 min at room temperature with continual agitation, and luminescence measured in a 1450 Micro-β Liquid Scintillation and Luminescence Counter (Wallac, Perkin Elmer, Cambridge, UK). For tumour cell line experiments involving EGFR neutralizing antibodies, cells were preincubated with varying concentrations of anti-EGFR antibody Ab-1 (EMD Biosciences, San Diego, CA, USA) for 1 h at 37 °C/5% CO2 in medium containing 2% fetal calf serum, prior to addition of virus +/− retargeting fusion protein. For NHU-blocking experiments, the anti-EGFR antibodies Ab-1 or cetuximab (Merck Serono, Geneva, Switzerland) or anti-uPAR antibody FL-290: (sc-10815, Santa Cruz Biotechnology) and/or anti-CAR antibody (Upstate Biotechnology, Lake Placid, NY, USA) were added at 10 mg ml−1 to the cells for 1 h, prior to addition of virus +/− retargeting fusion protein.
In vitro cytotoxicity assays
Cells were plated at 1 × 105 cells per well in a 24-well plate, and left overnight. 1 × 105 PFU ml−1 of Ad-CMV-HSVtk was added to 50 ng retargeting fusion protein to produce a final volume of 35 µl. Adenoviral retargeting was performed as described above. After overnight incubation, cells were trypsinized, replated at 5000 cells per well in a 96-well plate, and left overnight. The appropriate concentration of GCV was added to the cells in complete medium, at varying concentrations. Cells were incubated for 5 days before cell viability was assessed using WST-1 (Roche Diagnostics, Burgess Hill, UK). Briefly, 100 µl of a 1:10 dilution of WST-1/medium was added per well of the 96-well plate, and incubated at 37 °C for 45 min. Absorbance was then measured at 420 and 650 nm on a Fluostar Galaxy 96-well plate reader (MTX Lab Systems, Vienna, VA, USA).
Animals
Female Balb/C immunodeficient nude mice (Harlan UK, Blackthorn, UK) aged 6–8 weeks were used. Mice received CRM diet (SDS, Witham, UK) and water ad libitum. Mice were kept in cages in an air-conditioned room with regular alternating cycle of light and darkness. All animal procedures were carried out under a project licence issued by the UK Home Office, and UKCCCR guidelines52 were followed throughout.
SKOV3 xenografts
Tumours were excised from a donor animal, placed in sterile physiological saline containing antibiotics and cut into small fragments of ~2 mm3. Under brief general inhalation anaesthesia, SKOV3 tumour fragments were implanted into the left and right flank of each mouse using a trocar. Once the tumours had reached a tumour volume of 100–200 mm3, as measured by calipers, the mice were allocated into cages of eight (treatment groups) or four (control groups) by restricted randomization to keep group mean tumour size variation to a minimum.
In vivo retargeting of Ad-CMV-HSVtk using the pQE-sCAR-L-EGF53 fusion protein
Prior to injection, 1 × 108 PFU of Ad-CMV-HSVtk was mixed with 20 or 50 µg of pQE-sCAR-L-EGF53 protein per injection for 1 h at room temperature and then stored on ice until injection. The mice were then injected intratumourally with virus/protein and virus alone, in the right and left flank, respectively, in 50 µl volumes at two sites on the tumour with the day of injection designated as day 0. Twenty mg of protein alone and saline injections were also included in this study. GCV was administered intraperitoneally as a single injection on days 0–7 with 100 mg kg−1 given on days 0, 1, 5, 6 and 7, and 50 mg kg−1 given on days 2, 3 and 4. The effect of therapy was assessed as previously described.53 Briefly, two-dimensional caliper measurements of the tumours were taken daily, with volumes calculated using the formula (a2 × b)/2, where a is the smaller and b the larger diameter of the tumour. Tumour volume was then normalized to the respective volume on day 0, and log plots of relative tumour volume (RTV) versus time were determined. Unpaired t-tests were performed to determine the statistical significance of any differences in growth rate (based on tumour volume doubling time, RTV2) between control and treated groups, and for each virus/protein group with GCV compared with and without GCV.
Acknowledgements
This work was supported by Cancer Research UK and a grant from the National Institutes of Health (R01 CA107082). Thanks to Katie Hasler and Karen King for secretarial support.
Abbreviations
- EGFR
epidermal growth factor receptor
- GCV
ganciclovir
- hCAR
human coxsackie B and adenovirus receptor
- HSVtk
herpes simplex virus thymidine kinase
- 2-ME
2-mercaptoethanol
- RGD
Arginine-Glycine-Aspartate
- sCAR
extracellular domain of hCAR
- uPAR
urokinase-type plasminogen activator receptor
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Lundstrom K. Latest development in viral vectors for gene therapy. Trends Biotechnol. 2003;21:117–122. doi: 10.1016/S0167-7799(02)00042-2. [DOI] [PubMed] [Google Scholar]
- 2.Everts M, Curiel DT. Transductional targeting of adenoviral cancer gene therapy. Curr Gene Ther. 2004;4:337–346. doi: 10.2174/1566523043346372. [DOI] [PubMed] [Google Scholar]
- 3.Mathias P, Galleno M, Nemerow GR. Interactions of soluble recombinant integrin alphav beta5 with human adenoviruses. J Virol. 1998;72:8669–8675. doi: 10.1128/jvi.72.11.8669-8675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong SS, Karayan L, Tournier J, Curiel DT, Boulanger PA. Adenovirus type 5 fiber knob binds to MHC class I alpha2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dechecchi MC, Tamanini A, Bonizzato A, Cabrini G. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology. 2000;268:382–390. doi: 10.1006/viro.1999.0171. [DOI] [PubMed] [Google Scholar]
- 6.Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 8.Kim JS, Lee SH, Cho YS, Choi JJ, Kim YH, Lee JH. Enhancement of the adenoviral sensitivity of human ovarian cancer cells by transient expression of coxsackievirus and adenovirus receptor (CAR) Gynecol Oncol. 2002;85:260–265. doi: 10.1006/gyno.2002.6607. [DOI] [PubMed] [Google Scholar]
- 9.Kim M, Zinn KR, Barnett BG, Sumerel LA, Krasnykh V, Curiel DT, et al. The therapeutic efficacy of adenoviral vectors for cancer gene therapy is limited by a low level of primary adenovirus receptors on tumour cells. Eur J Cancer. 2002;38:1917–1926. doi: 10.1016/s0959-8049(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 10.Okegawa T, Pong RC, Li Y, Bergelson JM, Sagalowsky AI, Hsieh JT. The mechanism of the growth-inhibitory effect of coxsackie and adenovirus receptor (CAR) on human bladder cancer: a functional analysis of car protein structure. Cancer Res. 2001;61:6592–6600. [PubMed] [Google Scholar]
- 11.Sachs MD, Rauen KA, Ramamurthy M, Dodson JL, De Marzo AM, Putzi MJ, et al. Integrin alpha(v) and coxsackie adenovirus receptor expression in clinical bladder cancer. Urology. 2002;60:531–536. doi: 10.1016/s0090-4295(02)01748-x. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Pong RC, Bergelson JM, Hall MC, Sagalowsky AI, Tseng CP, et al. Loss of adenoviral receptor expression in human bladder cancer cells: a potential impact on the efficacy of gene therapy. Cancer Res. 1999;59:325–330. [PubMed] [Google Scholar]
- 13.Mizuguchi H, Hayakawa T. Targeted adenovirus vectors. Hum Gene Ther. 2004;15:1034–1044. doi: 10.1089/hum.2004.15.1034. [DOI] [PubMed] [Google Scholar]
- 14.Wesseling JG, Bosma PJ, Krasnykh V, Kashentseva EA, Blackwell JL, Reynolds PN, et al. Improved gene transfer efficiency to primary and established human pancreatic carcinoma target cells via epidermal growth factor receptor and integrin-targeted adenoviral vectors. Gene Therapy. 2001;8:969–976. doi: 10.1038/sj.gt.3301473. [DOI] [PubMed] [Google Scholar]
- 15.Kanerva A, Wang M, Bauerschmitz GJ, Lam JT, Desmond RA, Bhoola SM, et al. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol Ther. 2002;5:695–704. doi: 10.1006/mthe.2002.0599. [DOI] [PubMed] [Google Scholar]
- 16.Haviv YS, Blackwell JL, Kanerva A, Nagi P, Krasnykh V, Dmitriev I, et al. Adenoviral gene therapy for renal cancer requires retargeting to alternative cellular receptors. Cancer Res. 2002;62:4273–4281. [PubMed] [Google Scholar]
- 17.Belousova N, Korokhov N, Krendelshchikova V, Simonenko V, Mikheeva G, Triozzi PL, et al. Genetically targeted adenovirus vector directed to CD40-expressing cells. J Virol. 2003;77:11367–11377. doi: 10.1128/JVI.77.21.11367-11377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borovjagin AV, Krendelchtchikov A, Ramesh N, Yu DC, Douglas JT, Curiel DT. Complex mosaicism is a novel approach to infectivity enhancement of adenovirus type 5-based vectors. Cancer Gene Ther. 2005;12:475–486. doi: 10.1038/sj.cgt.7700806. [DOI] [PubMed] [Google Scholar]
- 19.Kashentseva EA, Douglas JT, Zinn KR, Curiel DT, Dmitriev IP. Targeting of adenovirus serotype 5 pseudotyped with short fiber from serotype 41 to c-erbB2-positive cells using bispecific singlechain diabody. J Mol Biol. 2009;388:443–461. doi: 10.1016/j.jmb.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dmitriev I, Kashentseva E, Rogers BE, Krasnykh V, Curiel DT. Ectodomain of coxsackievirus and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor-positive cells. J Virol. 2000;74:6875–6884. doi: 10.1128/jvi.74.15.6875-6884.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison J, Briggs SS, Green N, Fisher K, Subr V, Ulbrich K, et al. Virotherapy of ovarian cancer with polymer-cloaked adenovirus retargeted to the epidermal growth factor receptor. Mol Ther. 2008;16:244–251. doi: 10.1038/sj.mt.6300363. [DOI] [PubMed] [Google Scholar]
- 22.Grandis JR, Sok JC. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol Ther. 2004;102:37–46. doi: 10.1016/j.pharmthera.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Colquhoun AJ, Mellon JK. Epidermal growth factor receptor and bladder cancer. Postgrad Med J. 2002;78:584–589. doi: 10.1136/pmj.78.924.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villares GJ, Zigler M, Blehm K, Bogdan C, McConkey D, Colin D, et al. Targeting EGFR in bladder cancer. World J Urol. 2007;25:573–579. doi: 10.1007/s00345-007-0202-7. [DOI] [PubMed] [Google Scholar]
- 25.Cheng J, Huang H, Zhang ZT, Shapiro E, Pellicer A, Sun TT, et al. Overexpression of epidermal growth factor receptor in urothelium elicits urothelial hyperplasia and promotes bladder tumor growth. Cancer Res. 2002;62:4157–4163. [PubMed] [Google Scholar]
- 26.Van Brussel JP, Mickisch GH. Prognostic factors in renal cell and bladder cancer. BJU Int. 1999;83:902–908. doi: 10.1046/j.1464-410x.1999.00120.x. quiz 908–909. [DOI] [PubMed] [Google Scholar]
- 27.Black PC, Dinney CP. Growth factors and receptors as prognostic markers in urothelial carcinoma. Curr Urol Rep. 2008;9:55–61. doi: 10.1007/s11934-008-0011-6. [DOI] [PubMed] [Google Scholar]
- 28.Heist RS, Christiani D. EGFR-targeted therapies in lung cancer: predictors of response and toxicity. Pharmacogenomics. 2009;10:59–68. doi: 10.2217/14622416.10.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 30.Andreasen PA, Kjoller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer. 1997;72:1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 31.Mazar AP. Urokinase plasminogen activator receptor choreographs multiple ligand interactions: implications for tumor progression and therapy. Clin Cancer Res. 2008;14:5649–5655. doi: 10.1158/1078-0432.CCR-07-4863. [DOI] [PubMed] [Google Scholar]
- 32.Ulisse S, Baldini E, Sorrenti S, D’Armiento M. The urokinase plasminogen activator system: a target for anti-cancer therapy. Curr Cancer Drug Targets. 2009;9:32–71. doi: 10.2174/156800909787314002. [DOI] [PubMed] [Google Scholar]
- 33.Duffy MJ, Duggan C. The urokinase plasminogen activator system: a rich source of tumour markers for the individualised management of patients with cancer. Clin Biochem. 2004;37:541–548. doi: 10.1016/j.clinbiochem.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Pillay V, Dass CR, Choong PF. The urokinase plasminogen activator receptor as a gene therapy target for cancer. Trends Biotechnol. 2007;25:33–39. doi: 10.1016/j.tibtech.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Shariat SF, Monoski MA, Andrews B, Wheeler TM, Lerner SP, Slawin KM. Association of plasma urokinase-type plasminogen activator and its receptor with clinical outcome in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder. Urology. 2003;61:1053–1058. doi: 10.1016/s0090-4295(02)02522-0. [DOI] [PubMed] [Google Scholar]
- 36.Champelovier P, Boucard N, Levacher G, Simon A, Seigneurin D, Praloran V. Plasminogen- and colony-stimulating factor-1-associated markers in bladder carcinoma: diagnostic value of urokinase plasminogen activator receptor and plasminogen activator inhibitor type-2 using immunocytochemical analysis. Urol Res. 2002;30:301–309. doi: 10.1007/s00240-002-0270-5. [DOI] [PubMed] [Google Scholar]
- 37.Seddighzadeh M, Steineck G, Larsson P, Wijkstrom H, Norming U, Onelov E, et al. Expression of UPA and UPAR is associated with the clinical course of urinary bladder neoplasms. Int J Cancer. 2002;99:721–726. doi: 10.1002/ijc.10426. [DOI] [PubMed] [Google Scholar]
- 38.Chester JD, Kennedy W, Hall GD, Selby PJ, Knowles MA. Adenovirus-mediated gene therapy for bladder cancer: efficient gene delivery to normal and malignant human urothelial cells in vitro and ex vivo. Gene Therapy. 2003;10:172–179. doi: 10.1038/sj.gt.3301851. [DOI] [PubMed] [Google Scholar]
- 39.Coughlan L, Vallath S, Saha A, Flak M, McNeish IA, Vassaux G, et al. In vivo retargeting of Ad5 to {alpha}v{beta}6 integrin results in reduced hepatotoxicity and improved tumor uptake following systemic delivery. J Virol. 2009;83:6416–6428. doi: 10.1128/JVI.00445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkie S, Picco G, Foster J, Davies DM, Julien S, Cooper L, et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol. 2008;180:4901–4909. doi: 10.4049/jimmunol.180.7.4901. [DOI] [PubMed] [Google Scholar]
- 41.Hakkarainen T, Hemminki A, Pereboev AV, Barker SD, Asiedu CK, Strong TV, et al. CD40 is expressed on ovarian cancer cells and can be utilized for targeting adenoviruses. Clin Cancer Res. 2003;9:619–624. [PubMed] [Google Scholar]
- 42.Izumi M, Kawakami Y, Glasgow JN, Belousova N, Everts M, Kim-Park S, et al. In vivo analysis of a genetically modified adenoviral vector targeted to human CD40 using a novel transient transgenic model. J Gene Med. 2005;7:1517–1525. doi: 10.1002/jgm.806. [DOI] [PubMed] [Google Scholar]
- 43.Huch M, Abate-Daga D, Roig JM, Gonzalez JR, Fabregat J, Sosnowski B, et al. Targeting the CYP2B 1/cyclophosphamide suicide system to fibroblast growth factor receptors results in a potent antitumoral response in pancreatic cancer models. Hum Gene Ther. 2006;17:1187–1200. doi: 10.1089/hum.2006.17.1187. [DOI] [PubMed] [Google Scholar]
- 44.Zhu ZB, Makhija SK, Lu B, Wang M, Rivera AA, Preuss M, et al. Transport across a polarized monolayer of Caco-2 cells by transferrin receptor-mediated adenovirus transcytosis. Virology. 2004;325:116–128. doi: 10.1016/j.virol.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 45.Jongmans W, van den Oudenalder K, Tiemessen DM, Molkenboer J, Willemsen R, Mulders PF, et al. Targeting of adenovirus to human renal cell carcinoma cells. Urology. 2003;62:559–565. doi: 10.1016/s0090-4295(03)00378-9. [DOI] [PubMed] [Google Scholar]
- 46.Nettelbeck DM, Rivera AA, Kupsch J, Dieckmann D, Douglas JT, Kontermann RE, et al. Retargeting of adenoviral infection to melanoma: combining genetic ablation of native tropism with a recombinant bispecific singlechain diabody (scDb) adapter that binds to fiber knob and HMWMAA. Int J Cancer. 2004;108:136–145. doi: 10.1002/ijc.11563. [DOI] [PubMed] [Google Scholar]
- 47.Sebestyen Z, de Vrij J, Magnusson M, Debets R, Willemsen R. An oncolytic adenovirus redirected with a tumor-specific T-cell receptor. Cancer Res. 2007;67:11309–11316. doi: 10.1158/0008-5472.CAN-07-0739. [DOI] [PubMed] [Google Scholar]
- 48.Van Der Poel HG, Molenaar B, Van Beusechem VW, Haisma HJ, Rodriguez R, Curiel DT, et al. Epidermal growth factor receptor targeting of replication competent adenovirus enhances cytotoxicity in bladder cancer. J Urol. 2002;168:266–272. doi: 10.1097/00005392-200207000-00089. [DOI] [PubMed] [Google Scholar]
- 49.Martin K, Brie A, Saulnier P, Perricaudet M, Yeh P, Vigne E. Simultaneous CAR- and alpha V integrin-binding ablation fails to reduce Ad5 liver tropism. Mol Ther. 2003;8:485–494. doi: 10.1016/s1525-0016(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 50.Liang Q, Dmitriev I, Kashentseva E, Curiel DT, Herschman HR. Noninvasive of adenovirus tumor retargeting in living subjects by a soluble adenovirus receptor-epidermal growth factor (sCAR-EGF) fusion protein. Mol Imaging Biol. 2004;6:385–394. doi: 10.1016/j.mibio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Hutton KA, Trejdosiewicz LK, Thomas DF, Southgate J. Urothelial tissue culture for bladder reconstruction: an experimental study. J Urol. 1993;150:721–725. doi: 10.1016/s0022-5347(17)35597-0. [DOI] [PubMed] [Google Scholar]
- 52.Workman P, Balmain A, Hickman JA, McNally NJ, Rohas AM, Mitchison NA, et al. UKCCCR guidelines for the welfare of animals in experimental neoplasia. Lab Anim. 1988;22:195–201. doi: 10.1258/002367788780746467. [DOI] [PubMed] [Google Scholar]
- 53.Shnyder SD, Cooper PA, Pettit GR, Lippert JW, 3rd, Bibby MC. Combretastatin A-1 phosphate potentiates the antitumour activity of cisplatin in a murine adenocarcinoma model. Anticancer Res. 2003;23:1619–1623. [PubMed] [Google Scholar]