Abstract
Purpose.
We assessed the in vivo release profile of bevacizumab from and biocompatibility of poly(ethylene glycol)-poly-(serinol hexamethylene urethane), or ESHU, a thermoresponsive hydrogel administered intravitreally for drug delivery.
Methods.
The technical feasibility of injection was assessed quantitatively via mechanical testing. For in vivo studies, New Zealand White rabbit eyes were injected intravitreally with 0.05 mL of either: ESHU dissolved in 25 mg/mL bevacizumab, ESHU dissolved in PBS, or 25 mg/mL bevacizumab. Clinical examination included IOP measurements and examination with indirect ophthalmoscopy for signs of inflammation. Additionally, eyes were examined histologically following euthanasia. To quantify bevacizumab release, aqueous humor samples were obtained via anterior chamber paracentesis and ELISA was used to determine the concentration of drug weekly. In vitro cytotoxicity testing also was performed using bovine corneal endothelial cells.
Results.
The ESHU was injected easily through a 31-gauge needle, was well tolerated in vivo, and caused minimal cell death in vitro when compared to other common materials, such as silicone oil. The long-term presence of the gel did not affect IOP, and there was no evidence of inflammation histologically or through indirect observation. The ESHU sustained the release of bevacizumab for over 9 weeks and maintained a drug concentration that averaged 4.7 times higher than eyes receiving bolus bevacizumab injections.
Conclusions.
To our knowledge, this is the first report demonstrating sustained bevacizumab release in vivo from an intravitreally injected hydrogel formulation, suggesting that this delivery system may be a promising candidate for ocular drug delivery.
Keywords: thermally responsive hydrogel, ocular drug delivery, sustained release, biocompatibility, injectable gel
In this study, we studied the sustained release of bevacizumab from an injectable, thermally responsive hydrogel in vivo, and assessed the biocompatibility of the gel in rabbit eyes.
Introduction
Choroidal neovascularization (CNV) is the hallmark of many blinding disorders, most notably wet age-related macular degeneration (AMD) and diabetic retinopathy. It is characterized by pathologic blood vessel growth, which originates in the choroid and progresses through the Bruch's membrane into the subretinal space.1 These vessels are fragile and permeable, causing hemorrhage, retinal detachment, scarring, and ultimately, loss of central vision. Elevated levels of VEGF is a central cause of CNV.2–4 Thus, intravitreal injection of anti-VEGF medications, such as bevacizumab (Avastin) or ranibizumab (Lucentis), has emerged as a leading treatment strategy.5–7 The efficacy of these drugs, however, is limited severely by rapid clearance from the eye; their half-lives are on the order of 7 days.8,9 This necessitates frequent injections, imposing a significant burden on patients and increasing healthcare costs as well as procedure-related complications, such as endophthalmitis, retinal detachment, cataract, and uveitis.10–13 Therefore, a delivery system that extends the presence of intravitreal drugs in the eye is highly desirable for reducing injection frequency and adverse effects, while maximizing therapeutic outcomes.
A number of polymeric delivery systems have been considered by researchers for intravitreal drug delivery, including microparticles, which are well tolerated in the eye and are capable of delivering drugs over a longer period of time.14,15 For example, microparticle-encapsulated or PEGylated bevacizumab is more effective than bevacizumab alone in treating CNV in rats.15 However, direct intravitreal injection of such microparticle formulations may result in rapid particle dispersion and cause turbidity of the vitreous humor, affecting vision. Thus, in situ–forming, injectable hydrogels are an attractive alternative for intravitreal delivery as they can be formulated to encapsulate drugs and sustain release locally. Studies with nondegradable, thermally-responsive hydrogels have demonstrated excellent protein encapsulation, minimal toxicity, and easy injectability with no long-term effects on retinal function in vivo.16,17 These studies established that thermoresponsive hydrogels are excellent candidates for intraocular drug delivery.
Ideally, hydrogels for intravitreal administration should be biodegradable so as to avoid material accumulation in the vitreous with repeated injections over time. To this end, we investigated the utility of a biodegradable, thermally responsive hydrogel, poly(ethylene glycol)-poly-(serinol hexamethylene urethane), or ESHU, to deliver bevacizumab in rabbit eyes. Previously, we have demonstrated that an aqueous solution of ESHU will undergo a sol-to-gel phase transition when the temperature is increased from room to body temperature, making it attractive for minimally invasive applications.18 We hypothesized that ESHU would be a suitable platform for intraocular drug delivery and demonstrated that it is compatible with cells from ocular sources, and capable of releasing bevacizumab over a 17-week period in vitro.19 The aim of the present study was to demonstrate sustained bevacizumab release in vivo when compared to a standard, bolus injection. Additionally, we compared the cytocompatibility of ESHU to other commonly-used ocular biomaterials and assessed the in vivo biocompatibility of the gel.
Materials and Methods
Materials
N-Boc-serinol, hexamethylene diisocyanate (HDI), tetramethyl benzidine, and BSA were obtained from Sigma-Aldrich (St. Louis, MO). Polyethylene glycol (PEG) was obtained from Alfa Aesar (Ward Hill, MA). Anhydrous diethyl ether and 10% formalin were obtained from Fisher Scientific (Pittsburgh, PA), and anhydrous N,N-dimethylformamide was purchased from EMD (Gibbstown, NJ). The Spectra/Por dialysis membrane (MWCO, 3500–5000) was purchased from Spectrum Laboratories (Rancho Domingues, CA). The Live/Dead cytotoxicity assay kit was purchased from Molecular Probes (Carlsbad, CA). Recombinant human VEGF165 was obtained from Peprotech (Rocky Hill, NJ). Horseradish peroxidase-goat anti-human IgG was purchased from Invitrogen (Carlsbad, CA). Bevacizumab was obtained from Genentech (South San Francisco, CA).
Preparation of Bevacizumab-Loaded ESHU
The ESHU was prepared as described previously.18,19 To prepare bevacizumab-loaded ESHU, the desired amount of polymer was measured out and sterilized via ethylene oxide (12-hour cycle, 20°C, >35% relative humidity). The sterilized ESHU polymer then was dissolved in a solution of 25 mg/mL bevacizumab, or PBS for control gels, at 4°C in the dark at a polymer concentration of 15% wt/vol.
Injection Force Measurements
The injection force of 15% wt/vol ESHU in bevacizumab was measured using a material testing system (Insight; MTS Systems, Eden Prairie, MN). Water and 1% hyaluronic acid were used as controls. Samples were loaded into syringes containing a 31-gauge needle and the plunger was fixed to the instrument. The syringe was attached to a vial to collect the injected liquid, and compression tests were performed at speeds of 0.5, 1, and 3 mm/s. The force measurement at steady-state was considered to be the required injection force. Three separate runs were averaged for each sample at every speed.
In Vitro Cytotoxicity
Bovine corneal endothelial cells were isolated as described previously.19 Cells were suspended in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), penicillin/streptomycin, gentamicin, and amphotericin B, then incubated at 37°C in 5% CO2 until cells were 90% confluent. For in vitro cytotoxicity assays, cells were exposed to one of four experimental conditions: serum-free media (control), 15% wt/vol ESHU in DMEM, perfluorooctane (PFO), or 5000 cs silicone oil. As commonly utilized ocular materials, PFO and silicone oil served as control groups, and all conditions were run in triplicate. At 1, 12, and 24 hours, cell viability was assessed using the Live/Dead assay. Cells were stained with 50 μg/mL Calcein AM (in DMEM) at 37°C for 20 minutes, and during the last 5 minutes of incubation, 5 μg/mL propidium iodide (PI) was added. After washing in DMEM, nuclei were stained with 1 μg/mL Hoechst 33342 for 5 minutes. Representative images were taken of each culture using a fluorescence microscope. Cell viability was expressed as the ratio of PI-positive cells–to–Hoechst-stained cells in each sample.
Intravitreal Injection
All animal experiments were conducted in compliance with protocols approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh, with strict adherence to guidelines of the National Institutes of Health (NIH), United States Department of Agriculture, and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Male New Zealand White rabbits (8 weeks old; Charles River Laboratories, Boston, MA) were used for this study. Before injection, animals were anesthetized by intramuscular administration of ketamine/xylazine (40 mg/kg). Topical antibiotic (Vigamox; Alcon Laboratories, Fort Worth, TX) and anesthetic (proparacaine; Falcon Pharmaceuticals, Fort Worth, TX) eye drops were applied before injection. Intravitreal injections were conducted through the pars plana (2.5 mm posterior to the limbus). Eyes received one of three 50 μL injections: 1.25 mg bevacizumab in saline (n = 3), 1.25 mg bevacizumab in 15% ESHU (n = 4), or 15% ESHU in saline (n = 2). Uninjected eyes served as healthy controls (n = 2).
Clinical Examination
Clinical evaluations were conducted before injection, immediately after injection, 15 minutes after injection, and at 1, 3, and 7 days, and weekly thereafter for 10 weeks. Clinical examinations included analysis of inflammatory response via indirect ophthalmoscopy and IOP measurements.
Indirect Ophthalmoscopic Observation
To observe the gel in situ and identify potential gross inflammation in the anterior or posterior segments, eyes were dilated using tropicamide 0.5% (Bausch and Lomb, Tampa, FL) and phenylephrine 2.5% (Alcon Laboratories), and examined using an indirect ophthalmoscope with a 20-diopter lens (Nikon, Tokyo, Japan).
Intraocular Pressure
The IOP was monitored using a rebound tonometer (TONOVET; Icare, Helsinki, Finland). Baseline IOP measurements were obtained following anesthesia, before injection. Measurements were taken again immediately following injection, 15 minutes later, and at each sampling time point thereafter. Three measurements per eye were averaged.
In Vivo Release Study
Aqueous humor samples were obtained via anterior chamber paracentesis with a 30-gauge syringe (approximately 100 μL sample volume) at 1, 4, and 7 days after injection, and weekly thereafter until euthanization. The concentration of bevacizumab present in samples was determined using ELISA. Wells of 96-well plates were coated with 100 μL of 1-μg/mL recombinant human VEGF165 overnight at 4°C. Following ×3 washing with PBS containing 0.05% Tween-20, wells were blocked for 1 hour at room temperature with 1% BSA in PBS. Samples were diluted with 0.1% BSA and added to the plates following ×5 washing. After 1 hour at room temperature, wells were washed three times and horseradish peroxidase-goat anti-human IgG (diluted 1:2000 in 0.1% BSA) was added to each well for 1 hour at room temperature. Wells then were washed five times and color development was performed using 100 μL of tetramethyl benzidine. The reaction was stopped by addition of 100 μL of 1-M hydrogen chloride. A standard curve of bevacizumab (linear region, 0.05–1 ng/mL) was used to determine the concentration of bevacizumab in samples.
Histologic Analysis
At 9 weeks, four of five animals were sacrificed and the eyes were explanted for histologic analysis. One animal was euthanized at 18 weeks to assess long-term compatibility. Eyes were fixed in 10% formalin, embedded in paraffin, and sectioned at 5-μm thickness. Following de-paraffinization, sections then were stained with hematoxylin and eosin (H&E), Masson's Trichrome, and PAS stain to assess eye morphology.
Statistical Analysis
Data are expressed as mean ± SEM. One-way ANOVA with Tukey post hoc testing was used to determine statistical differences between groups for IOP and injection force data. Two-way ANOVA was used for the live/dead assay. Differences were considered significant at P < 0.05. For in vivo studies, the observed bevacizumab concentrations in anterior chamber samples were modeled as a nonlinear function of time (day) and treatment group using a first order compartment model for power transformed bevacizumab concentrations. To account appropriately for the longitudinal repeated measurements, a nonlinear mixed effects model was used. The R language and environment for statistical computing and graphics (Version 2.15.1)20 with the nonlinear mixed effects model (nlme, Version 3.1-108) R package21 were used to compute the maximum likelihood estimates and confidence intervals via the nlme and self-starting first order compartment model (SSfol) functions. A detailed description of the model and parameter estimates can be found in the Supplementary Material.
Results
ESHU Is Easily Injectable Through a 31-Gauge Needle and Forms an Ovoid-Shaped Gel Upon Injection Into a Solution at Body Temperature
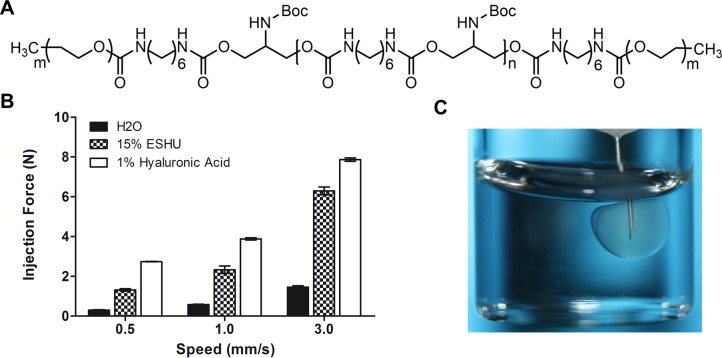
The small needle commonly used for intravitreal injection makes viscous materials difficult to inject; therefore, the force required to push ESHU through a 31-gauge needle was quantified using compression testing. Water and 1% hyaluronic acid (HA) were used as controls to relate the injection force to common materials (Fig. 1). Intuitively, the force required to inject ESHU (15% wt/vol dissolved in 25 mg/mL bevacizumab) increased in a speed-dependent manner. At all three speeds tested, the injection force for ESHU was larger than that of water, but less than 1% HA (P < 0.05 in all conditions tested). A swift 3 mm/s required an easily manageable injection force of 6.291 ± 0.197 N, compared to 7.865 ± 0.083 N for HA. We next observed the phase transition behavior of ESHU following injection to a solution of HA heated to 37°C, to mimic intravitreal administration. The ESHU immediately undergoes the sol-gel phase transition and forms a spherical hydrogel (Fig. 1C).
Figure 1.
(A) The chemical structure of ESHU. (B) The force required to inject a 15% wt/vol solution of ESHU through a 31-gauge needle is less than for a 1% solution of hyaluronic acid, a commonly-injected ophthalmic material. (C) Upon injection to a solution of hyaluronic acid at 37°C, ESHU forms a spherical hydrogel.
The ESHU Does Not Cause Significant Toxicity to Bovine Corneal Endothelial Cells
Extensive cytotoxicity testing was performed using primary bovine corneal endothelial cells. Cells were exposed to either serum-free media (control), ESHU (15% wt/vol in DMEM), silicone oil, or PFO. The PFO is used intraoperatively during vitreoretinal surgery, and silicone oil is used commonly as a vitreous substitute for the repair of complex retinal detachments.22,23 At 1, 12, and 24 hours, cells were stained with Calcein AM, PI, and Hoechst, and toxicity was quantified as the number of PI+ cells compared to the total number of Hoechst+ cells. In cultures treated with ESHU and PFO, no significant cell death occurred throughout the 24-hour culture period (Fig. 2, P > 0.05). Silicone oil–treated cultures underwent substantial cell death at each time point compared to the control and ESHU groups, with approximately 76% death at 24 hours. Qualitatively, cells exposed to ESHU showed little to no evidence of cellular damage and appeared morphologically identical to control groups.
Figure 2.
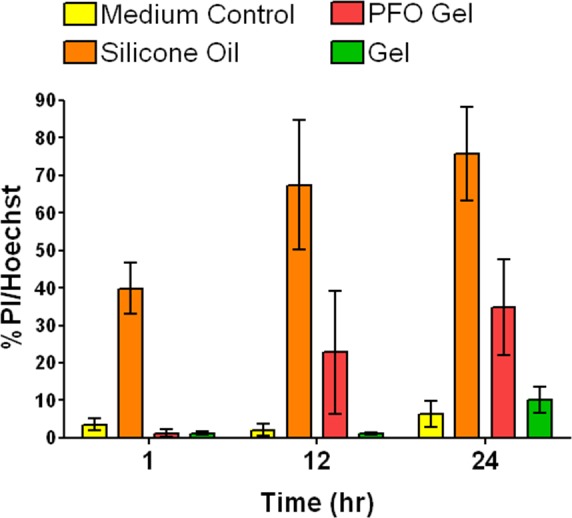
Comparison of cytocompatibilty of ESHU to common vitreous materials. Bovine CE cells survive well over a 24-hour period following exposure to ESHU gels, with no significant difference when compared to TCPS controls. In contrast, silicone oil caused significant cell death compared to all other experimental groups (P < 0.05).
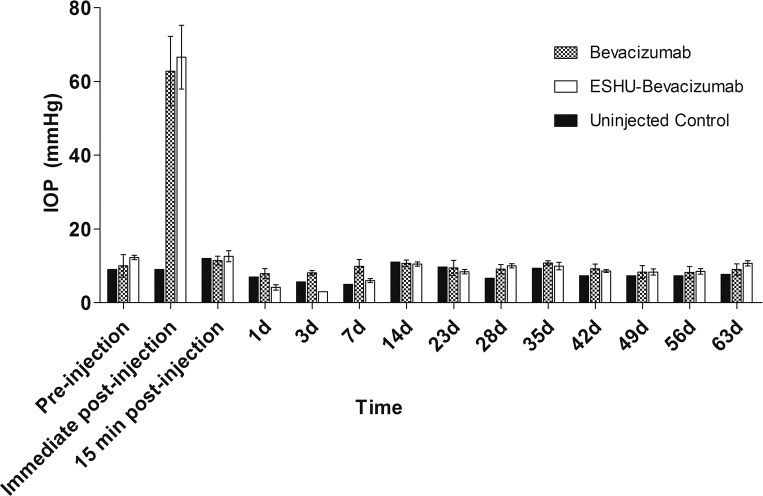
The Presence of Intravitreal ESHU Does Not Increase Long-Term IOP
The IOP measurements were taken immediately before and after injection, and before each sampling time point throughout the course of the study. Measurements showed that IOP spiked sharply following injection of ESHU and bevacizumab solutions, and returned to baseline levels within 15 minutes (Fig. 3). Throughout the remainder of the study, IOP remained at baseline and was not significantly different from control eyes.
Figure 3.
Presence of ESHU does not affect IOP. Pressure measurements return to baseline values 15 minutes following intravitreal injection. Throughout the study period, IOP was not significantly different between ESHU and control groups (P > 0.05).
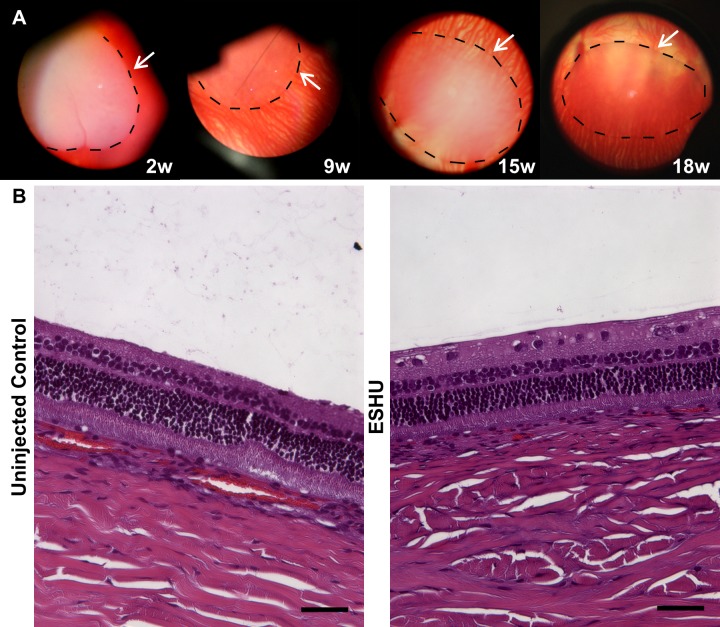
ESHU Does Not Elicit a Significant Inflammatory Response Over a 9-Week Period In Vivo
In addition to IOP measurements, gels were observed and photographed in situ through indirect ophthalmoscopy. At 24 hours after injection, injected hydrogels had sunk to the bottom of the vitreous space. Animals displayed no signs of discomfort or external signs of inflammation (redness or tearing). Throughout the study period, the gels remained translucent and ovoid in shape, with no evidence of inflammation or cellular accumulation on the gel surface (Fig. 4A). In the animal that was euthanized at 18 weeks, gels appeared to become more transparent as degradation occurred. Additionally, the long-term presence of the gel did not seem to affect the eye based on indirect observation, or histologically. Though ESHU appears to fill a large portion of the vitreous from the indirect, it should be noted that the view includes only part of the vitreous space. The gels typically are 3 to 4 mm in diameter and occupy approximately 3% of the vitreous volume. Following euthanasia, eyes were processed for histologic analysis and representative H&E images are shown in Figure 4B. The retinal layers are intact with no evidence of inflammation, corroborating the indirect observations.
Figure 4.
The ESHU is biocompatible in the eye. (A) Indirect images of the hydrogel in the vitreous space. The surface of the gel (traced in black) is free of inflammatory cells and the vitreous humor is clear. (B) H&E stains comparing the retinal structure of ESHU and control groups. The two groups are morphologically indistinguishable, suggesting that the presence of ESHU did not adversely affect retinal health. Scale bar: 100 μm.
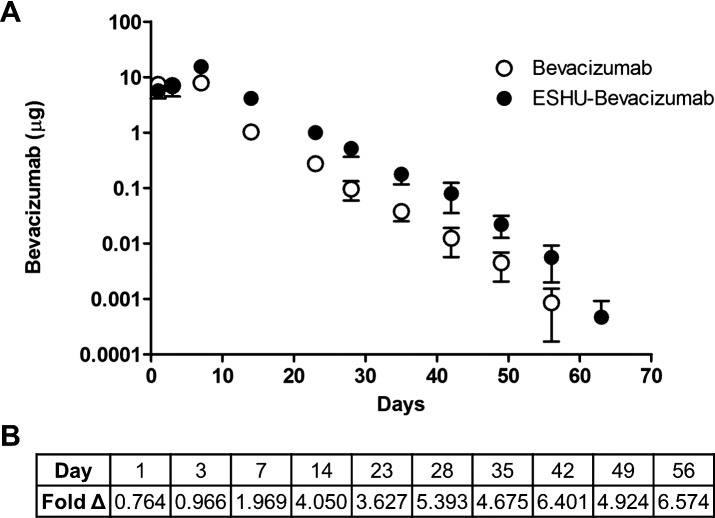
ESHU Sustains Bevacizumab Release for 9 Weeks in Rabbit Eyes
We sought to compare the concentration of bevacizumab in rabbit eyes receiving the ESHU delivery system to a single bolus intravitreal injection. We injected 1.25 mg of bevacizumab (50 μL) in both experimental groups to allow for direct comparison to the clinically administered dose. Anterior chamber (AC) paracentesis was performed to obtain aqueous humor samples, and ELISA was used to quantify the amount of bevacizumab present (Fig. 5). A first-order compartment model was used to model the bevacizumab concentration in experimental groups over time. The fixed effect parameters were found to be well-estimated by the model (Supplementary Fig. S1, Supplementary Tables S1, S2). The concentration in bevacizumab-only groups peaks earlier and is lower than for ESHU-bevacizumab. The parameters that characterize these differences are highly statistically significant (P < 0.05). On average, the ESHU maintained a bevacizumab concentration that was 4.7-fold higher than eyes which received a bolus injection. It should be noted that any bevacizumab present in control eyes was below the limit of detection for the ELISA assay and, therefore, was considered to be negligible. Thus, these data are not shown in Figure 5.
Figure 5.
In vivo bevacizumab release. (A) The concentration of bevacizumab in AC samples over time. Data are plotted on a semi-log scale. (B) Fold change demonstrating the increased bevacizumab concentration in animals receiving the delivery system.
Discussion
The present study demonstrated that ESHU, a thermally responsive hydrogel, is feasible for intravitreal injection, is biocompatible in vitro and in vivo, and can maintain bevacizumab concentrations at levels approximately five times higher than in controls. The force required to inject ESHU is less than that for HA, a highly viscous glycosaminoglycan and major component of the vitreous humor. The HA is used extensively in ophthalmic surgery, and cataract surgery in particular as an injectable vitreous substitute.24,25 The injection force data indicated that ESHU formulations are suitable for intravitreal injection through small gauge needles without requiring significant effort to administer. Upon injection into a solution of HA heated to 37°C, ESHU formulations undergo a rapid sol-gel phase transition and form spherical-to-ovoid–shaped hydrogels. This transition occurs rapidly, yet not instantaneously, enabling time for injection without gelation occurring within the needle. The ESHU does not cause significant cell death when cultured with bovine corneal endothelial cells; in previous work, we demonstrated ESHU had good cytocompatibility with bovine corneal endothelial and retinal pigment epithelial cells.19 The experiments in this study elaborated upon these results by comparing ESHU to other synthetic materials that are used commonly in retinal surgery – silicone oil and perfluorooctane. The substantial cell death caused by silicone oil treatment, which is used commonly clinically, suggests that ESHU will be well-tolerated in vivo. While ESHU appeared to cause less cell death than PFO at 12 and 24 hours, this difference was not statistically significant. Taken together, these results indicated that ESHU is less toxic than other Food and Drug Administration–approved materials for ocular applications, and should be biocompatible in the eye.
Next, intravitreal injections of bevacizumab, ESHU dissolved in bevacizumab, or ESHU dissolved in PBS were performed in rabbits. The IOP was measured throughout the study as one metric of ocular health. The initial spike following injection is typical and secondary to a small increase in volume of the eye, and is a function of the ocular rigidity.23 As IOP variations can be indicative of pathologies, such as trabeculitis and retinal detachment,26,27 the normal measurements observed throughout the study suggested that the presence of ESHU did not cause significant damage to the eye. Indirect and histologic observation revealed that the gel remained spherical-to-ovoid in shape throughout the course of the study, sunk to the bottom of the eye within one day of injection, caused no significant inflammatory response to the presence of ESHU, and did not affect retinal structures. In one animal that remained under observation for 18 weeks, the gel became more transparent over time, suggesting occurrence of degradation. Previously, it was demonstrated that ESHU undergoes approximately 10% and 20% degradation after 45 days in vitro in PBS in the absence and presence of cholesterol esterase, respectively.18 It was hypothesized that in vivo degradation would occur more rapidly; however, the immune privileged state of the eye may have protected ESHU from enzyme- and cell-mediated breakdown, resulting in minimal degradation. Current studies are focused on introducing more rapidly degrading bonds to the ESHU backbone to control its degradation rate.
To our knowledge, this is the first study to date that compares the long-term in vivo release of bevacizumab from thermoresponsive biodegradable hydrogels to bolus intravitreal administration. The suitability of thermoresponsive hydrogels for intravitreal applications has been explored previously using nondegradable poly(N-isopropylacrylamide) (PNIPAAM)–based hydrogels, as well as biodegradable poly(2-ethyl-2-oxazoline)-b-poly(ε-caprolactone)-b-poly(2-ethyl-2-oxazoline) (PEOz-PCL-PEOz), or ECE gels.16,17,28,29 These studies demonstrated that intravitreal injection of synthetic hydrogels caused no long-term (up to 2 months) changes to retinal function, IOP, or histomorphology in rabbits, corroborating our observations. However, PNIPAAM gels are nondegradable and, therefore, would require surgical removal. Additionally, their synthesis protocol requires free radical polymerization and involves the use of potentially toxic initiators, whereas ESHU synthesis requires no catalysts. The ESHU polymer is biodegradable and, thus, would disappear with time. Compared to the biodegradable ECE gel, we demonstrated previously that ESHU releases bevacizumab for approximately 17 weeks in vitro, compared to less than 3 weeks in the ECE gel.19 A potential reason for this is hydrogen bond formation between the ESHU gel backbone and bevacizumab, which would sustain the release of the drug. These prior studies have demonstrated elegantly the benefit that minimally invasive hydrogels may provide; however, they did not demonstrate sustained release in an animal model. We built upon their work to show that intravitreal injection of a clinically-relevant volume of bevacizumab-containing hydrogel sustains bevacizumab release in vivo and does not elicit a chronic inflammatory response. Though the eye displays immune privilege and generally is less susceptible to foreign body responses,30 chronic inflammation can manifest itself as alterations in retinal morphology, which was not observed in this study. The nearly 5-fold increase in bevacizumab concentration in ESHU-injected eyes suggested that the polymer functions to protect bevacizumab from degradation and, therefore, should be more effective in treating CNV. Future work will focus on optimizing the drug release kinetics by varying drug dose, polymer concentration, molecular weight, and degradation, as well as confirming the efficacy of the drug delivery system in a nonhuman primate model of CNV. According to some researchers, such efficacy studies should be done in primates, as bevacizumab is humanized and its effect on CNV can be species-specific.31
Conclusions
We have studied the feasibility of a unique thermally-responsive hydrogel, ESHU, as an intraocular drug delivery vehicle. The ESHU is easily injectable and, from a clinical standpoint, its administration would be no different than the current practice of injecting bevacizumab. In vivo, ESHU gels are well tolerated with little to no evidence of inflammation, and are capable of sustaining bevacizumab release over 9 weeks. Because the polymer is an injectable solution, it can be used to sustain the release of many different medications, and can be useful for other ocular diseases as well as diseases in other tissues.
Acknowledgments
The authors thank Deanna Rhodes for histologic processing.
Supported by the Center for Medical Innovation at the University of Pittsburgh, The Louis J. Fox Center for Vision Restoration, and NIH Grant 2T32EB003392-07.
Disclosure: B.M. Rauck, None; T.R. Friberg, None; C.A. Medina Mendez, None; D. Park, None; V. Shah, None; R.A. Bilonick, None; Y. Wang, None
References
- 1. Kinnunen K, Yla-Herttuala S. Vascular endothelial growth factors in retinal and choroidal neovascular diseases. Ann Med. 2012; 44: 1–17 [DOI] [PubMed] [Google Scholar]
- 2. Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996; 37: 1929–1934 [PubMed] [Google Scholar]
- 3. Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, deJong P. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997; 81: 154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kwak N, Okamoto N, Wood JM, Campochiaro PA. VEGF is major stimulator in model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000; 41: 3158–3164 [PubMed] [Google Scholar]
- 5. Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006; 113: 1695.e1–1695.e15 [DOI] [PubMed] [Google Scholar]
- 6. Lynch SS, Cheng CM. Bevacizumab for neovascular ocular diseases. Ann Pharmacother. 2007; 41: 614–625 [DOI] [PubMed] [Google Scholar]
- 7. Kleinman ME, Ambati J. Pathophysiology of retinal neovascularization. In: Pine JW. ed Therapy for Ocular Angiogenesis: Principles and Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 8. Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol. 2008; 146: 508–512 [DOI] [PubMed] [Google Scholar]
- 9. Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007; 114: 2179–2182 [DOI] [PubMed] [Google Scholar]
- 10. D'Amico DJ. Pegaptanib sodium for neovascular age-related macular degeneration: two-year safety results of the two prospective, multicenter, controlled clinical trials. Ophthalmology. 2006; 113: 992–1001 [DOI] [PubMed] [Google Scholar]
- 11. Fintak DR, Shah GK, Blinder KJ, et al. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina. 2008; 28: 1395–1399 [DOI] [PubMed] [Google Scholar]
- 12. Holz FG, Amoaku W, Donate J, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology. 2011; 118: 663–671 [DOI] [PubMed] [Google Scholar]
- 13. Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008; 145: 239–248 [DOI] [PubMed] [Google Scholar]
- 14. Li F, Hurley B, Liu Y, Leonard B, Griffith M. Controlled release of bevacizumab through nanospheres for extended treatment of age-related macular degeneration. Open Ophthalmol J. 2012; 6: 54–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pan CK, Durairaj C, Kompella UB, et al. Comparison of long-acting bevacizumab formulations in the treatment of choroidal neovascularization in a rat model. J Ocul Pharmacol Ther. 2011; 27: 219–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang Derwent JJ, Mieler WF. . Thermoresponsive hydrogels as a new ocular drug delivery platform to the posterior segment of the eye. Trans Am Ophthalmol Soc. 2008; 106: 206–213, discussion 213–204 [PMC free article] [PubMed] [Google Scholar]
- 17. Turturro SB, Guthrie MJ, Appel AA, et al. The effects of cross-linked thermo-responsive PNIPAAm-based hydrogel injection on retinal function. Biomaterials. 2011; 32: 3620–3626 [DOI] [PubMed] [Google Scholar]
- 18. Park D, Wu W, Wang Y. A functionalizable reverse thermal gel based on a polyurethane/PEG block copolymer. Biomaterials. 2011; 32: 777–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park D, Shah V, Rauck BM, Friberg TR, Wang Y. An anti-angiogenic reverse thermal gel as a drug-delivery system for age-related wet macular degeneration. Macromol Biosci. 2013; 13: 464–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The R Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 21. Pinheiro J, Bates D, Debroy S, Sarkar D. and the R Development Core Team nlme: Linear and Nonlinear Mixed Effects Models. R Package version 3.1–108, 2013. [Google Scholar]
- 22. Friberg TR, Siska PE, Somayajula K, Williams J, Eller AW. Interactions of perfluorocarbon liquids and silicone oil as characterized by mass spectrometry. Graefe's Arch Clin Exp Ophthalmol. 2003; 241: 809–815 [DOI] [PubMed] [Google Scholar]
- 23. Friberg TR, Verstraeten TC, Wilcox DK. Effects of emulsification, purity, and fluorination of silicone oil on human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1991; 32: 2030–2034 [PubMed] [Google Scholar]
- 24. Pruett RC, Schepens CL, Swann DA. Hyaluronic acid vitreous substitute. A six-year clinical evaluation. Arch Ophthalmol. 1979; 97: 2325–2330 [DOI] [PubMed] [Google Scholar]
- 25. Schramm C, Spitzer MS, Henke-Fahle S, et al. The cross-linked biopolymer hyaluronic acid as an artificial vitreous substitute. Invest Ophthalmol Vis Sci. 2012; 53: 613–621 [DOI] [PubMed] [Google Scholar]
- 26. Sniegowski M, Mandava N, Kahook MY. Sustained intraocular pressure elevation after intravitreal injection of bevacizumab and ranibizumab associated with trabeculitis. Open Ophthalmol J. 2010; 4: 28–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sampat KM, Garg SJ. Complications of intravitreal injections. Curr Opin Ophthalmol. 2010; 21: 178–183 [DOI] [PubMed] [Google Scholar]
- 28. Wang CH, Hwang YS, Chiang PR, Shen CR, Hong WH, Hsiue GH. Extended release of bevacizumab by thermosensitive biodegradable and biocompatible hydrogel. Biomacromolecules. 2011; 13: 40–48 [DOI] [PubMed] [Google Scholar]
- 29. Hwang YS, Chiang PR, Hong WH, et al. Study in vivo intraocular biocompatibility of in situ gelation hydrogels: poly(2-ethyl oxazoline)-block-poly(epsilon-caprolactone)-block-poly(2-ethyl oxazoline) copolymer, matrigel and pluronic F127. PLoS One. 2013; 8: e67495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Streilein JW, Takeuchi M, Taylor AW. Immune privilege, T-cell tolerance, and tissue-restricted autoimmunity. Hum Immunol. 1997; 52: 138–143 [DOI] [PubMed] [Google Scholar]
- 31. Lu F, Adelman RA. Are intravitreal bevacizumab and ranibizumab effective in a rat model of choroidal neovascularization? Graefe's Arch Clin Exp Ophthalmol. 2009; 247: 171–177 [DOI] [PubMed] [Google Scholar]