Abstract
Purpose.
Age-related macular degeneration (AMD) is a leading cause of visual impairment worldwide. Genetics and diet contribute to the relative risk for developing AMD, but their interactions are poorly understood. Genetic variations in Complement Factor H (CFH), and dietary glycemic index (GI) are major risk factors for AMD. We explored the effects of GI on development of early AMD-like features and changes to central nervous system (CNS) inflammation in Cfh-null mice.
Methods.
Aged 11-week-old wild type (WT) C57Bl/6J or Cfh-null mice were group pair-fed high or low GI diets for 33 weeks. At 10 months of age, mice were evaluated for early AMD-like features in the neural retina and RPE by light and electron microscopy. Brains were analyzed for Iba1 macrophage/microglia immunostaining, an indicator of inflammation.
Results.
The 10-month-old WT mice showed no retinal abnormalities on either diet. The Cfh-null mice, however, showed distinct early AMD-like features in the RPE when fed a low GI diet, including vacuolation, disruption of basal infoldings, and increased basal laminar deposits. The Cfh-null mice also showed thinning of the RPE, hypopigmentation, and increased numbers of Iba1-expressing macrophages in the brain, irrespective of diet.
Conclusions.
The presence of early AMD-like features by 10 months of age in Cfh-null mice fed a low GI diet is surprising, given the apparent protection from the development of such features in aged WT mice or humans consuming lower GI diets. Our findings highlight the need to consider gene–diet interactions when developing animal models and therapeutic approaches to treat AMD.
Keywords: glycemic index, complement, gene-diet interaction, inflammation, aging
Risk for and progression of AMD are enhanced in all cohorts of humans and mice that consumed higher glycemic index diets. In contrast, in Cfh-null mice, RPE showed AMD-like features only when fed a low glycemic index diet, emphasizing the importance of gene–diet interactions.
Introduction
Age-related macular degeneration (AMD) causes blindness or severe visual impairment in over 14 million individuals worldwide,1 and is on a trajectory to increase 50% in North America by 2020.2 Although our understanding of the etiology of disease initiation and progression has increased in the last decade, to date, no effective treatments for the dry form of the disease exist to our knowledge. Means to prevent the onset or progress of AMD clearly are of critical interest for personal and public health.
The majority of adults presenting with early or intermediate stage AMD generally are given nutritional supplements, including copper, zinc, vitamins A and C, β-carotene, macular pigments, and ω-3-long-chain polyunsaturated acids, as a therapeutic intervention. These treatments emerged from the Age-Related Eye Disease Study (AREDS) interventional trial, which showed that progress from intermediate to advanced AMD is slowed in users of such supplements and/or epidemiologic studies that demonstrated the value of these nutrients for diminishing the risk for AMD.3,4 Recent epidemiologic investigations indicate that people who consume higher glycemic index (GI) diets are at increased risk for developing or showing progression of AMD.5,6 The GI is a kinetic parameter that measures the rate at which glucose is released into the blood from 50 g of dietary carbohydrate relative to glucose or white bread. Diets with the same percentage of carbohydrates may have very different GIs. Whole grain foods release carbohydrates more slowly than food with more refined composition.
Animal models are essential for obtaining mechanistic insights into disease pathology, and for developing new drugs and treatments in a preclinical setting. This has been a challenge, as there is the lack of robust mouse models for AMD.7–9 primarily because mice lack a macula and have a dramatically shortened lifespan relative to humans. Nevertheless, in response to known causes of AMD in humans, including blue light exposure, oxidative damage, cigarette smoke, high-fat diet, and aging, the RPE of mice shows similar phenotypic features of damage to those observed in human AMD.10–13 We recently demonstrated that mice that consumed high GI diets sustained more RPE damage, mimicking that observed in early AMD, including vacuole formation, lipofuscin accumulation, thickening of Bruch's membrane, loss of basal infoldings, and formation of basal laminar deposits.14 Biochemical studies indicated that the RPE damage that was observed in the high GI-fed animals was associated with accumulation of advanced glycation end-products, which compromised the protein quality control systems necessary for RPE health and function.15
Additional animal models have been created in efforts to mimic some of the genetic alterations common in human AMD. In particular, genetic variants in Complement Factor H (CFH), a negative regulator of the alternate complement pathway, are associated with increased or decreased risks of developing AMD.16–19 The risk-associated alleles of CFH generally are associated with decreased function, suggesting they represent partial loss-of-function mutations.20,21 Identification of a rare, highly penetrant CFH mutation that abrogates C-terminal activity of the protein further supports this hypothesis.22 Mice have been generated that are deficient in Cfh gene function (Cfh−/−), and evaluated for visual function after aging.23 By 2 years, Cfh−/− mice showed visual dysfunction associated with changes in the retinal rod photoreceptor outer segments. However, typical hallmarks of AMD normally observed in the RPE, such as thickening of Bruch's membrane, were not observed, even though other ultrastructural features of the RPE were abnormal.23
To extend our understanding of the Cfh−/− mouse and possibly improve its ability to model AMD, we sought to determine the impact of nutritional modulation of GI on early AMD-like phenotypes in Cfh−/− mice. We found that relatively young Cfh−/− mice developed many early features of AMD, including loss of basal infoldings, increased numbers of basal laminar deposits, and vacuolation. To our surprise, these lesions were observed in Cfh−/− mice fed a low GI diet, whereas age-matched wild type (WT) mice or Cfh−/− mice fed a high GI diet showed minimal changes in the RPE. This seemingly paradoxical finding was independent of other genotype-specific, but diet-independent alterations in Cfh−/− mice, including increased inflammation, and thinning and hypopigmentation of the RPE. Our findings established critical functions for Cfh in maintaining RPE health under specific dietary conditions and compelled greater consideration of gene-diet interactions.
Materials and Methods
Animals and Diets
The Cfh−/− gene-targeted mice24 were maintained as homozygotes on a C57Bl/6J background. The C57Bl/6J WT mice were purchased from Jackson Laboratories (Bar Harbor, ME). Animals were fed standard chow ad libitum (Teklad 7012; Harlan Laboratories, Madison, WI) until 11 weeks of age, at which time they were placed on diet. Groups of 9 to 12 male mice were fed high or low GI diets containing identical macronutrient compositions that differed only in their starch components.14 The high GI starch was composed of 100% amylopectin (Amioca starch; Ingredion, Inc., Bridgewater, NJ), while the low GI starch was composed of 70% amylose/30% amylopectin (Hylon VII starch; Ingredion, Inc.). All diets were formulated by Bio-Serv (Frenchtown, NJ). Mice were group pair-fed to ensure equal consumption between diet groups. At 44 weeks of age, mice were anesthetized with ketamine and xylazine, and euthanized. All animal work was performed at the Human Nutrition Research Center on Aging (HNRCA) and approved by the HNRCA Institutional Animal Care and Use Committee (IACUC) in adherence with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Statistical Analyses
Data were evaluated using either SPSS (IBM, SPSS, Inc., Chicago, IL) or Microsoft Excel (Microsoft Corporation, Redmond, WA). First, data were evaluated as to whether they fit in a normal distribution or not, based on kurtosis and skewness. Pairwise data that fit in a normal distribution were analyzed by a 2-tailed Student's t-test followed by an F-test to determine if the samples have equal variance. For group comparisons, 1-way ANOVA was performed followed by Tukey's HSD test. If the data did not fit a normal distribution, they were evaluated for pairwise comparisons using the Wilcoxon Mann-Whitney U test or for group comparisons using the Kruskal-Wallis test followed by Mann-Whitney post hoc testing. Indications of test choice and sample size are elaborated in Figure legends.
Blood Measurements
Mice were fasted for 6 hours before intraperitoneal glucose or insulin tolerance tests. A clean razor blade was used to make a horizontal cut in the lateral tail vein, releasing approximately 5 μL of blood that was applied to a OneTouch Ultra test strip in a OneTouch Ultra2 glucometer (Lifescan, Inc., Milpitas, CA) to obtain fasting glucose levels. For the glucose tolerance test, each mouse was injected intraperitoneally with 1 g/kg body weight D-(+)-glucose (≥99.5%; Sigma, St. Louis, MO) via a #25 5/8-gauge syringe. At 30, 60, and 120 minutes after injection, the tail vein cut in each mouse was moistened to remove the clot and dried before release of another 5 μL of blood. This blood was applied to the test strip in the glucometer to measure the blood glucose level. For the insulin tolerance test, each mouse was injected intraperitoneally with 0.75 U/kg body weight Novolin R human insulin (Novo Nordisk Pharmaceutical, Princeton, NJ) via a #25 5/8-gauge syringe, and blood glucose levels were measured at 15, 30, 45, 60, and 90 minutes after injection.
Transmission Electron Microscopy (TEM) and Light Microscopy Analysis
Mice were either (1) perfused with normal saline and 4% paraformaldehyde, then eyes removed and further fixed in EM fixative (2.5% glutaraldehyde, 2% paraformaldehyde, with 0.025% (wt/vol) CaCl2 in 0.1 M sodium cacodylate buffer, pH 7.4), or (2) perfused with normal saline, and eyes removed and further fixed in EM fixative. Eyes were washed 2 × 10 minutes in 0.1 M sodium cacodylate buffer containing 5% sucrose, postfixed 3.5 hours in 1% osmium tetroxide in 0.1 M cacodylate buffer containing 2% sucrose, washed 1 × 10 minutes in buffer and 1 × 10 minutes in distilled water (DW), and held in 4% uranyl acetate 1 hour in the dark. Samples then were washed 1 × 10 minutes in DW, dehydrated 2 × 15 minutes each in graded ethanols (30%–100%) and propylene oxide, infiltrated in Embed-812 resin (Electron Microscopy Sciences, Hatfield, PA) for 24 hours on a rotator, and polymerized at 70°C for 48 hours. Sectioning was performed on a Leica EM UC7 Ultracut microtome using diamond knives (Diatome, Hatfield, PA). Semi-thin (0.5 μm) sections were stained with 1% toluidine blue in 1% sodium borate. Thin (silver) sections were collected on copper 135 hex grids, poststained with 4% uranyl acetate in 50% (aq.) methanol and Reynold's lead citrate, and then viewed and photographed using a JEOL 1200 transmission electron microscope (JEOL Ltd., Tokyo, Japan). Sections were photographed over a 1.5 to 2 mm area on the side of the optic nerve head that corresponded to the best alignment of photoreceptor outer segments, generally halfway between the optic nerve head and the ciliary body.
For quantitative analysis of EM features, 24 to 36 images were evaluated from 4 to 6 different eyes per group, taken from either fixation condition. Scoring was done as described previously,14 with the exception of the scoring of basal infoldings, which was modified due to the range of phenotypes observed in these younger animals (see legend in Fig. 4 for details). A severity score was assigned to each image. Bruch's membrane thickness was determined by averaging the thickest and thinnest parts of the membrane for each section, as described previously.14
Figure 4.
Quantification of TEM features. (A) Basal infolding severity scores across diet and genotype indicate significant differences in Cfh−/− mice fed low GI diet. Scoring is as follows: 1, normal length and organization; 2, shortened length, but normal organization; 3, disorganized; 4, loss of infoldings. (B–F) Frequency of basal laminar deposits (B), lipofuscin granules (C), autophagosomes in basal infoldings (D), and total numbers of autophagosomes (E) across diet and genotype. (F) Bruch's membrane thickness across genotype and diet indicates no significant differences. Bar graphs indicate mean + SEM: n = 4, WT low GI; n = 5, WT high GI; n = 5, Cfh−/− low GI; n = 6, Cfh−/− high GI. Statistical significance is indicated as P < 0.05 (*), or substatistical significance (#), as determined by Kruskal-Wallis test ([A] H = 9.13; [B] H = 8.75; [C] H = 6.05, P = 0.11; [D] H = 6.6, P = 0.086; [E] H = 2.8, P = 0.42) or 1-way ANOVA (F).
For light microscopy, semi-thin toluidine blue–stained sections were photographed on an Olympus photomicroscope (Olympus Corporation, Tokyo, Japan) equipped with a digital camera.
For morphometric analysis of RPE, ×60 magnification images were taken from three independent sections of each eye; four to six different eyes per group. Images were taken approximately 1 mm from the optic nerve head, on the same side as where TEM images were photographed. Quantification was performed using ImageJ software (National Institutes of Health [NIH], Bethesda, MD), setting thresholds for pigmentation based on the red channel or for vacuolation on the green channel against a dark background. Each section had a contiguous RPE stretch of 291 μm. For quantification of photoreceptor rows, measurements were averaged for images taken approximately 250, 1000, or 1500 μm from the optic nerve head.
Immunohistochemistry
Brains from 5 Cfh−/− mice and 4 WT mice that were fixed via perfusion with 4% paraformaldehyde were cryosectioned coronally (30 μm thickness sections). Tissue sections were incubated with an antibody monospecific for ionized calcium-binding adapter molecule 1 (Iba1; Wako Pure Chemical Industries Ltd., Osaka, Japan) at 1:200 dilution, washed in PBS containing 0.1% (vol/vol) Triton X-100 and incubated with appropriate secondary antibodies conjugated with Alexa Fluor 594 (Invitrogen-Molecular Probes, Carlsbad, CA). Images were acquired using a Leica microscope (Diatome) and digital camera at the level of the paraventricular nucleus (PVN). Each side of the third ventricle was photographed using a ×10 objective, including regions of the lateral hypothalamus that border the PVN. The number of Iba1-positive cells was quantified in two to three serial sections using ImageJ software (NIH).
Results
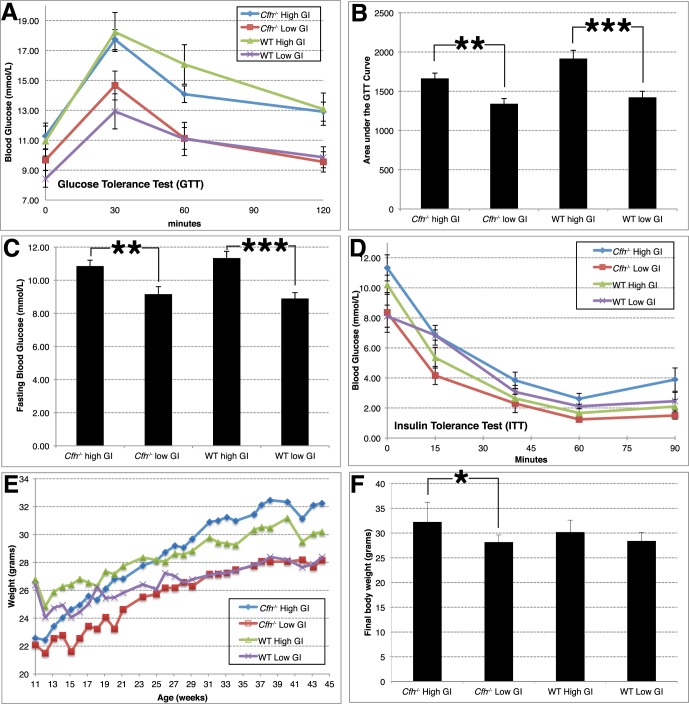
To test for gene–diet interactions, we set up four cohorts of animals: mice that were WT or Cfh−/−, and within those groups, mice that were fed high or low GI diets. We tested identical diets to those used in our previous GI studies.14 However, we started with significantly younger mice and pair-fed them beginning at 11 weeks of age. Attesting to the efficacy of the diets,15 mice of both genotypes that were fed low GI diets showed an attenuated response to the glucose tolerance test (Figs. 1A, 1B). Quantification of the area under the curve of the glucose tolerance test demonstrated statistically significant lower levels of glucose in the bloodstream of mice of both genotypes that consumed the low GI diet compared to the high GI diet (Fig. 1B). The chronic feeding of mice with high GI diets resulted in an approximately 20% statistically significant increase in fasting blood glucose levels relative to mice fed a low GI diet (Fig. 1C). Nevertheless, mice on a high GI diet showed similar kinetics of clearance of blood glucose when challenged with insulin, indicating that these were nondiabetic mice (Fig. 1D). Mice of both genotypes that were fed low GI diets gained less weight than those fed high GI diets (Figs. 1E, 1F), the difference being statistically significant in Cfh−/− mice (Fig. 1F).
Figure 1.
Weight and metabolic profiles of mice fed high or low GI diets. (A, B) Mice fed low GI diets show attenuated blood glucose responses in glucose tolerance test (A), as quantified by analysis of area-under-the-curve (B). (C) Fasting blood glucose levels were lower in mice fed a low GI diet. Results were averaged from four independent measurements. (D) Mice fed either diet showed similar rates of glucose clearance in insulin tolerance test, indicating they are nondiabetic. (E, F) Average body weight for mice in different diet and genotypes across time (E), or at termination (F) indicate increased weight in mice fed a high GI diet. n = 11, Cfh−/− High GI (blue); n = 10, Cfh−/− Low GI (red); n = 9, WT High GI (green); n = 10, WT Low GI (magenta). Bar graphs show mean + SEM. Statistical significance is indicated as P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), using Student's t-test.
To evaluate retinas in “young-middle aged” Cfh−/− or WT animals, mice were euthanized at 44 weeks of age and eyes were evaluated histologically using light microscopy (Fig. 2). The WT mice did not show gross abnormalities when fed either high or low GI diets, with regard to retinal layering and size (Fig. 2A, and data not shown). The Cfh−/− eyes on either diet similarly showed proper retinal layering (Figs. 2B, 2C, and data not shown) and did not show loss of photoreceptor nuclei (outer nuclear layer, ONL), as evaluated by counting rows of photoreceptor nuclei in three ONL locations across the retina (WT, 10.6 ± 0.17, n = 9; Cfh−/−, 10.4 ± 0.19, n = 11; P = 0.37). Photoreceptor inner and outer segments appeared to be shortened in Cfh−/− retinas fed either diet, but due to histologic artefacts, we were unable to determine whether this was a quantitatively meaningful difference.
Figure 2.
Evaluation of 44-week WT and Cfh−/− retinas by light microscopy. (A–C) Representative semi-thin toluidine blue stained retinal sections from WT mice fed a low GI diet (A), or Cfh−/− mice fed a high (B) or low (C) GI diet show normal layering and comparable thickness of photoreceptor nuclei in the outer nuclear layer. (D–F) Higher magnification images of the RPE from the boxed areas show thinning of the Cfh−/− RPE (E, F) and vacuoles in Cfh−/− RPE fed a low GI diet (F). (G, H) High magnification images of RPE over a 300 μm area show alterations to pigmentation and RPE thickness in Cfh−/− RPE fed a low GI diet (H). The arrow in (H) indicates a region of RPE demarcating a thinned region of RPE (left of arrow) from nonthinned RPE (right of arrow). The unthinned region constituted a minority of RPE in this retina and vacuolation was observed in both regions. (I–L) Quantification of total RPE area (I), pigmentation area (J), vacuolation area (K), and number of vacuoles (cutoff 0.25 μm2 area [L]). Cfh−/− RPE from mice fed either diet showed reduced RPE area (I) and reduced pigment area (J), while only Cfh−/− RPE from low GI diets showed increased vacuolar area (K) and numbers of vacuoles (L). Scale bars shown in (C, F, H) indicate 10 μm. Bar graph values are mean + SEM; n = 9 WT (n = 5 high GI, n = 4 low GI), n = 11 Cfh−/− (n = 6 high GI, n = 5 low GI). Statistical significance is indicated as P < 0.05 (*), P < 0.01 (**), or P < 0.0001 (***) as determined by Student's t-test (I, J) or 1-way ANOVA (K, L). is, photoreceptor inner segments; os, photoreceptor outer segments; bm, Bruch's membrane.
The RPE of WT mouse eyes on either diet appeared normal, with a well-defined Bruch's membrane and even distribution of pigment granules (Figs. 2A, 2D, 2G, and data not shown). The RPE of Cfh−/− mice on a high GI diet also appeared grossly normal, although it appeared thinner (along the apical–basal axis) and had irregular pigmentation (Figs. 2B, 2E). These differences in RPE width and pigmentation also were observed in Cfh−/− mice fed a low GI diet (Figs. 2C, 2F, 2H). In one Cfh−/− retina, we observed a small region containing RPE of an approximately normal thickness juxtaposed next to an area that was highly thinned (Fig. 2H, arrow). Careful quantification of the total RPE area and total pigment area from matched regions of the retina approximately 1 mm from the optic nerve head revealed an approximately 15% increased RPE area and 40% decreased area of pigmentation between WT and Cfh−/− mice fed either diet (Figs. 2I, 2J).
Surprisingly, Cfh−/− mice fed a low GI diet presented distinct abnormalities in the RPE, principally vacuolation (Figs. 2C, 2F, 2H). Larger vacuoles appeared apically, while smaller vacuoles overlaid Bruch's membrane (Fig. 2F). Quantification of either vacuolation area or number of vacuoles across diet and genotype showed a dramatic increase in the area and number of vacuoles present in Cfh−/− mice fed a low GI diet. Therefore, in contrast with WT aged C57Bl/6 mice, which showed a protective effect of a low GI diet,14 the low GI diet had a detrimental effect on Cfh−/− retinas.
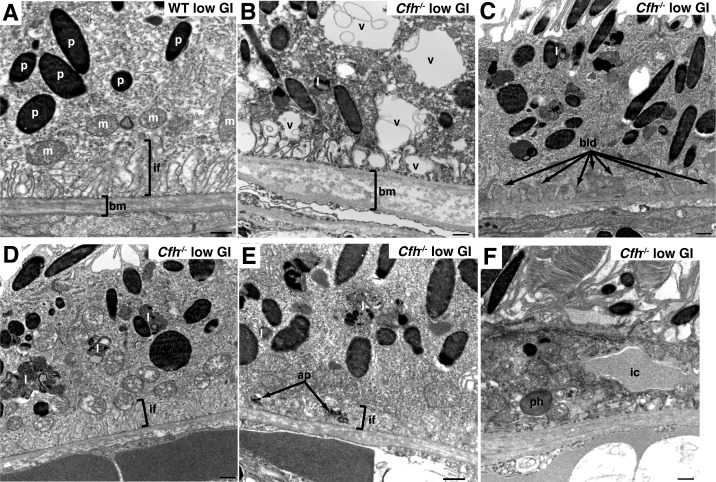
To evaluate the nature of RPE dystrophy in greater detail, we analyzed the RPE using TEM with regard to typical pathology-related features, including basal infoldings, basal laminar deposits, lipofuscin granules, and the thickness of Bruch's membrane (Figs. 3, 4). The WT mice that consumed the low GI diet showed a structurally normal RPE, with well-defined and large vertically aligned basal infoldings, an even pentalaminated Bruch's membrane, and normal distribution of pigment granules and mitochondria (Fig. 3A). These features also were observed in WT mice fed a high GI diet (data not shown), indicating that these mice were too young to show age-related pathology that would be expected to be impacted by diet.14 In comparison, Cfh−/− mice on low GI diets showed a highly aberrant RPE organization (Figs. 3B–F); the RPE contained cells that were highly vacuolated, with large vacuoles dispersed centrally and apically, and smaller vacuoles distributing along the basal membrane and disrupting basal infoldings (Fig. 3B).
Figure 3.
Evaluation of RPE in 44-week low GI fed animals by TEM. (A) TEM micrograph of RPE from WT mouse fed low GI diet showing normal thickness of bm and normal basal infoldings (if) as indicated by brackets. Pigment granules (p) and mitochondria (m) also were normal. (B–F) TEM micrographs of RPE from Cfh−/− mice fed low GI diet showing altered basal infoldings, increased vacuolation (v), increased basal laminar deposits (bld), increased lipofuscin granules (l), autophagosomes (ap) in basal infoldings. Phagosomes (ph) and infiltrating cells (ic) also were observed. Note the loss of basal infoldings where significant vacuoles (B), basal laminar deposits (C), autophagosomes (E), or infiltrating cells (F) were observed. Scale bar: 500 nm.
To quantify further the effect of diet and genotype on basal infoldings, we created a severity scoring system to evaluate basal infoldings (Fig. 4A). Basal infolding severity scores were low in all groups of animals except for the Cfh−/− mice fed the low GI diet (Fig. 4A). The most severe loss of basal infoldings was associated with vacuolation (Fig. 3B), but disorganization or shortening of basal infoldings also was associated with formation of basal laminar deposits (Fig. 3C), the presence of autophagosomes (Fig. 3E), or infiltrating cells (Fig. 3F). Basal laminar deposits (BLamD) were observed in all groups of animals, but were significantly increased in Cfh−/− animals fed the low GI diet (Figs. 3C, 4B). The BLamD are consistent with “early” deposits, because they lacked features, such as wide-spaced collagen fibrils, observed in AMD and some more severe mouse AMD models.25,26
Accumulation of lipofuscin granules is observed frequently in damaged RPE along with loss of basal infoldings and the presence of BLamD. We observed increased numbers of lipofuscin granules in Cfh−/− animals fed a low GI diet (Figs. 3D, 4C). In Nrf2-deficient mice (also known as Nfe2l2), autophagosomes, intermediates of autophagy, were observed alongside other AMD-like features.26 We observed increased numbers of autophagosomes found within the basal infoldings (Figs. 3E, 4D). Interestingly, the number of total autophagosomes was not significantly different between the diet or genotype groups (Fig. 4E), indicating that enhanced localization of autophagosomes within the basal infoldings was not due to overall increased formation of autophagosomes.
The thickness of Bruch's membrane often is used as a metric of retinal change upon aging or stress. In Cfh−/− mice fed low GI diets, Bruch's membrane was variable, with some sections showing thickening and disorganization (Fig. 3B), while other sections looked overall normal (Figs. 3D–F). Quantification of the thickness of Bruch's membrane in the four groups of animals revealed no statistically significant thickening or thinning in Cfh−/− mice (Fig. 4F). These results are in contrast with prior reports regarding Cfh−/− mice aged to 2 years, which showed thinning of Bruch's membrane, rather than the expected thickening.23
To determine whether the high GI diet was protective or whether the low GI diet was detrimental for Cfh−/− mice, we compared the retinal features and RPE of Cfh−/− mice that were aged to 50 weeks on a standard diet with our high or low GI-fed Cfh−/− mice. With the exception of Bruch's membrane, which was slightly thicker than 44-week Cfh−/− RPE from high GI diet-fed mice, basal infoldings, frequency of BlamD, and frequency of lipofuscin granules were similar to Cfh−/− mice fed a high GI diet and statistically significant differences were observed across genotypes (Supplementary Fig. S1). Other recent publications describing 1-year-old Cfh−/− retinas fed a standard diet also did not report significant phenotypes in the RPE.27,28
Macrophage/microglia infiltration frequently is used as an indicator of inflammatory stress and we were interested in determining whether there was increased systemic inflammation in Cfh−/− animals, and if the diet might modulate the inflammatory status of mice. However, evaluation of macrophage/microglia numbers in Cfh−/− is complicated by the fact that Cfh is involved in maintaining retinal perfusion,29 Indeed, numbers of retinal macrophages are decreased in Cfh−/− mice,28 despite other evidence of possibly increased inflammation23,27 and evidence of infiltrating cells possibly macrophages, in the RPE of Cfh−/− mice on a low GI diet (Fig. 3F). Therefore, we decided to look elsewhere in the animal for evidence of inflammatory stress.
We chose to analyze the hypothalamus at the level of the paraventricular nucleus as this region regulates release of inflammatory cells from the bone marrow along with other sympathetic brain nuclei.30 Our hypothesis was that inflammation in these regions produces neuronal excitation that drives the release of peripheral inflammatory cells from the bone marrow promoting inflammation at other sites, such as that seen in the retina in AMD.31,32 More recently, inflammation in the hypothalamus was shown to regulate the aging process and lifespan in mice.33 To assay the extent and frequency of macrophage/microglia infiltration independent of retinal changes, we analyzed the numbers of Iba1-positive (also known as AIF-1) cells (Fig. 5A), and evaluated levels of Iba1, since Iba1 expression is increased during macrophage activation following injury. The shape of cells and levels of Iba1 immunoreactivity were indistinguishable between genotypes and diet groups. However, Cfh−/− tissues showed a statistically significant increase in Iba1-positive cells relative to WT tissue (Fig. 5B). There were slightly higher numbers of Iba1-positive cells in high GI-fed animals compared to low GI-fed animals in both genotypes; however, this difference was smaller than the effect of genotype and was not statistically significant. These data indicated that Cfh−/− animals have increased inflammatory cells in key regions that regulate systemic inflammation and aging compared to WT mice. However, although increased inflammation in Cfh−/− animals may cooperate with low GI to induce AMD-like features, it is not sufficient, since Cfh−/− animals fed a high GI diet did not share these features, despite increased inflammation in the brain.
Figure 5.

Increased macrophages are observed in 44-week Cfh−/− brains. (A) Representative sections through the brain of WT and Cfh−/− animals fed low (upper panels) or high (lower panels) GI diets, stained with the macrophage/microglial marker Iba1 (intense white staining), showing increased Iba1 staining in Cfh−/− brains. Location of the ventricle (v) is indicated. (B) Quantitation of Iba1 staining shows statistically significantly increased Iba1-positive cells in Cfh−/− animals. Bar graph values are mean + SEM; n = 4 WT, n = 5 Cfh−/−. Statistical significance is indicated as P < 0.05 (*) by Mann-Whitney U test.
Discussion
Few, if any, studies published to date have examined interactions between dietary GI and genetics with regard to retinal health. Our investigations into the interaction of dietary GI with Cfh deficiency revealed several surprising findings. First, we found an early AMD-like phenotype in Cfh−/− mice, in contrast with a previous investigation of Cfh−/− mice that did not show AMD-like changes in the RPE.23 Second, we observed the early AMD-like phenotype under the conditions of a low GI diet rather than, as expected, in the high GI diet group. The low GI diet was shown to be protective against AMD in WT mice as well as in human clinical epidemiologic investigations.4,14 We also observed thinning and hypopigmentation of the RPE and increased inflammation in the brains of Cfh−/− mice that were diet-independent. It is provocative to consider that increased hypothalamic inflammation in Cfh−/− mice also may accelerate their aging.33 In total, our findings have implications pertinent to understanding the role of Cfh in retinal health and consideration of gene-diet interactions in humans.
Given the relatively young age of the mice used in our study, we were able to detect some of the earliest changes in the RPE that precede the more severe, progressive AMD-related retinal phenotypes. These events include cytoplasmic vacuolization, increased accumulation of lipofuscin granules, disorganization of the basal infoldings, and early appearance of BLamD. The presence of areas showing loss of basal infoldings, but without BLamD, suggests that these events are progressive and possibly functionally linked.11,14 We also evaluated the number of autophagosomes in the RPE, as increased autophagy has been observed in Nrf2−/− mice, which, similar to mice fed high GI diets,15 also show increased oxidative damage,26 and have been linked to AMD pathogenesis.34–36 Although we did not observe a gross increase in autophagosome numbers, we did observe an increase in numbers of autophagosomes located specifically in and proximal to the basal infoldings, a finding that has not been reported previously to our knowledge. It is tempting to speculate that autophagy might be involved in the loss of basal infoldings that precedes formation of BLamD. At this relatively young age, we did not observe significant thickening of Bruch's membrane in either diet or genetic background. This would suggest that the thinning of Bruch's membrane reported in Cfh−/− mice by Coffey et al.23 may represent a later-onset phenotype.
It is surprising that, in the context of the Cfh−/− mouse, a low GI diet was detrimental, whereas in WT aged C57Bl/6 mice and in human epidemiologic studies, a low GI diet has been shown to be salutary with regard to AMD development. Several explanations are possible. The low GI diet may have led to a slightly energy-deprived condition, relative to normal or high GI diets. Indeed, RPE cells have enormous energy requirements, and experimental lowering of ATP in RPE cells has been shown to result in compromised phagocytosis, autophagy, and increased oxidative stress.37 Combined with the fact that Cfh appears to function, in part, to lower oxidative stress, either by binding to oxidized phospholipids20 or to malondialdehyde,21 any putative diminution in energetics in the low GI diet may exacerbate and more fully reveal the Cfh−/− phenotype. A very different possibility may relate to changes in the gut microbiome caused by consuming the low GI diet, containing high amounts of the resistant starch, amylose, relative to amylopectin alone in the high GI diet. Consumption of large amounts of resistant starch is associated with a different composition and increased absorption of short-chain fatty acids.38,39 These biochemical changes lead to changes in the gut microbiota, which may interact negatively with a Cfh−/− genotype.40 Downstream changes in metabolic responses to diet, such as altered glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) secretion, or decreased glucose absorption, may mediate the gene–diet interaction as well.38
The link between metabolism, inflammation, and the innate immune system has been well characterized, including the interaction between the complement pathway and metabolism. Activation of the complement pathway is associated with development of diabetes and insulin resistance,41 whereas mutants in the complement effector pathways render animals resistant to diet-induced obesity.41,42 Complement activation is associated with macrophage infiltration in adipose tissue and subsequent inflammatory pathways leading to insulin resistance.41 Although our diets and feeding regimes did not induce obesity or insulin resistance in Cfh−/− animals, we nevertheless saw increased hypothalamic inflammation. The connection between Cfh and metabolic syndrome is likely to be complex, as in humans CFH levels were associated positively with insulin resistance,43 suggesting that complement activation and dysregulation of complement regulators contribute to metabolic syndrome.
Taken together, the results of our study indicated that prediction of genetic interactions across broad conditions must be done cautiously. A retrospective study evaluating 876 participants in the AREDS found that patients carrying the CFH Y402H risk allele showed a treatment interaction with zinc, but not with antioxidants.44 Similarly, a case-control study showed changing genetic associations between CFH genotype and AMD depending on the age group.45 In light of our current data, it would be interesting to evaluate retrospectively the impact of low GI diets with regard to CFH genotype, and determine if and how these results relate to humans. As the cost of genotyping individuals continues to decrease and larger numbers of epidemiologic studies are carried out, it would be prudent to consider individualized pharmacogenetic treatments for AMD.
Acknowledgments
The authors thank Fu Shang and Elizabeth Whitcomb for providing helpful discussions and comments on this manuscript; Charlotte Peltier, Jun Zhong, and Aurelie LeFeuvre for assistance with feeding and tissue collection; and Thomas Bowman for helpful assistance with metabolic assays. The Cfh−/− mice were a generous gift from Joe G. Hollyfield (Cole Eye Institute, Cleveland Clinic Foundation, Cleveland, OH). The starches used in this study were donated by Rhonda Witwer and Christine Pelkman (Ingredion, Inc., Westchester, IL).
Supported by intramural funds from the HNRCA (AT); by NEI RO1 EY021212 (AT), NEI RO1 EY13250 (AT), NEI EY007361 (SJF), USDA 1950-510000-060-01A (AT), Johnson and Johnson Focused Giving (AT); an unrestricted gift from Alcon Laboratories (Elkridge, MD; AT), an RPB Unrestricted Grant (SJF), and by facilities and resources provided by the Veterans Administration Western New York Healthcare System (SJF). The authors alone are responsible for the content and writing of the paper.
Disclosure: S. Rowan, None; K. Weikel, None; M.-L. Chang, None; B.A. Nagel, None; J.S. Thinschmidt, None; A. Carey, None; M.B. Grant, None; S.J. Fliesler, None; D. Smith, None; A. Taylor, Johnson and Johnson Focused Giving (F), Alcon (F)
References
- 1. Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004; 82: 844–851 [PMC free article] [PubMed] [Google Scholar]
- 2. Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004; 122: 564–572 [DOI] [PubMed] [Google Scholar]
- 3. Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001; 119: 1417–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weikel KA, Chiu CJ, Taylor A. Nutritional modulation of age-related macular degeneration. Mol Aspects Med. 2012; 33: 318–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiu CJ, Milton RC, Gensler G, Taylor A. Association between dietary glycemic index and age-related macular degeneration in nondiabetic participants in the Age-Related Eye Disease Study. Am J Clin Nutr. 2007; 86: 180–188 [DOI] [PubMed] [Google Scholar]
- 6. Chiu CJ, Milton RC, Klein R, Gensler G, Taylor A. Dietary carbohydrate and the progression of age-related macular degeneration: a prospective study from the Age-Related Eye Disease Study. Am J Clin Nutr. 2007; 86: 1210–1218 [DOI] [PubMed] [Google Scholar]
- 7. Pennesi ME, Neuringer M, Courtney RJ. Animal models of age related macular degeneration. Mol Aspects Med. 2012; 33: 487–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rakoczy EP, Yu MJ, Nusinowitz S, Chang B, Heckenlively JR. Mouse models of age-related macular degeneration. Exp Eye Res. 2006; 82: 741–752 [DOI] [PubMed] [Google Scholar]
- 9. Zeiss CJ. Animals as models of age-related macular degeneration: an imperfect measure of the truth. Vet Pathol. 2010; 47: 396–413 [DOI] [PubMed] [Google Scholar]
- 10. Cousins SW, Espinosa-Heidmann DG, Alexandridou A, Sall J, Dubovy S, Csaky K. The role of aging, high fat diet and blue light exposure in an experimental mouse model for basal laminar deposit formation. Exp Eye Res. 2002; 75: 543–553 [DOI] [PubMed] [Google Scholar]
- 11. Fujihara M, Nagai N, Sussan TE, Biswal S, Handa JT. Chronic cigarette smoke causes oxidative damage and apoptosis to retinal pigmented epithelial cells in mice. PLoS One. 2008; 3: e3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Espinosa-Heidmann DG, Suner IJ, Catanuto P, Hernandez EP, Marin-Castano ME, Cousins SW. Cigarette smoke-related oxidants and the development of sub-RPE deposits in an experimental animal model of dry AMD. Invest Ophthalmol Vis Sci. 2006; 47: 729–737 [DOI] [PubMed] [Google Scholar]
- 13. Hollyfield JG, Bonilha VL, Rayborn ME, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008; 14: 194–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weikel KA, Fitzgerald P, Shang F, et al. Natural history of age-related retinal lesions that precede AMD in mice fed high or low glycemic index diets. Invest Ophthalmol Vis Sci. 2012; 53: 622–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uchiki T, Weikel KA, Jiao W, et al. Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in non diabetics). Aging Cell. 2012; 11: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005; 308: 385–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edwards AO, Ritter R III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005; 308: 421–424 [DOI] [PubMed] [Google Scholar]
- 18. Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005; 308: 419–421 [DOI] [PubMed] [Google Scholar]
- 19. Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005; 102: 7227–7232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaw PX, Zhang L, Zhang M, et al. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc Natl Acad Sci U S A. 2012; 109: 13757–13762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weismann D, Hartvigsen K, Lauer N, et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011; 478: 76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raychaudhuri S, Iartchouk O, Chin K, et al. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat Genet. 2011; 43: 1232–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coffey PJ, Gias C, McDermott CJ, et al. Complement factor H deficiency in aged mice causes retinal abnormalities and visual dysfunction. Proc Natl Acad Sci U S A. 2007; 104: 16651–16656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pickering MC, Cook HT, Warren J, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002; 31: 424–428 [DOI] [PubMed] [Google Scholar]
- 25. Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999; 117: 329–339 [DOI] [PubMed] [Google Scholar]
- 26. Zhao Z, Chen Y, Wang J, et al. Age-related retinopathy in NRF2-deficient mice. PLoS One. 2011; 6: e19456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williams JA, Greenwood J, Moss SE. Retinal changes precede visual dysfunction in the complement factor h knockout mouse. PLoS One. 2013; 8: e68616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoh Kam J, Lenassi E, Malik TH, Pickering MC, Jeffery G. Complement component c3 plays a critical role in protecting the aging retina in a murine model of age-related macular degeneration. Am J Pathol. 2013; 183: 480–492 [DOI] [PubMed] [Google Scholar]
- 29. Lundh von Leithner P, Kam JH, Bainbridge J, et al. Complement factor h is critical in the maintenance of retinal perfusion. Am J Pathol. 2009; 175: 412–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Card JP, Kobiler O, Ludmir EB, Desai V, Sved AF, Enquist LW. A dual infection pseudorabies virus conditional reporter approach to identify projections to collateralized neurons in complex neural circuits. PLoS One. 2011; 6: e21141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008; 452: 442–447 [DOI] [PubMed] [Google Scholar]
- 32. Yellowlees Douglas J, Bhatwadekar AD, Li Calzi S, et al. Bone marrow-CNS connections: implications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2012; 31: 481–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang G, Li J, Purkayastha S, et al. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 2013; 497: 211–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS One. 2009; 4: e4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Handa JT. How does the macula protect itself from oxidative stress? Mol Aspects Med. 2012; 33: 418–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mitter SK, Rao HV, Qi X, et al. Autophagy in the retina: a potential role in age-related macular degeneration. Adv Exp Med Biol. 2012; 723: 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schutt F, Aretz S, Auffarth GU, Kopitz J. Moderately reduced ATP levels promote oxidative stress and debilitate autophagic and phagocytic capacities in human RPE cells. Invest Ophthalmol Vis Sci. 2012; 53: 5354–5361 [DOI] [PubMed] [Google Scholar]
- 38. Keenan MJ, Zhou J, McCutcheon KL, et al. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity (Silver Spring). 2006; 14: 1523–1534 [DOI] [PubMed] [Google Scholar]
- 39. Regmi PR, van Kempen TA, Matte JJ, Zijlstra RT. Starch with high amylose and low in vitro digestibility increases short-chain fatty acid absorption, reduces peak insulin secretion, and modulates incretin secretion in pigs. J Nutr. 2011; 141: 398–405 [DOI] [PubMed] [Google Scholar]
- 40. Regmi PR, Metzler-Zebeli BU, Ganzle MG, van Kempen TA, Zijlstra RT. Starch with high amylose content and low in vitro digestibility increases intestinal nutrient flow and microbial fermentation and selectively promotes bifidobacteria in pigs. J Nutr. 2011; 141: 1273–1280 [DOI] [PubMed] [Google Scholar]
- 41. Mamane Y, Chung Chan C, Lavallee G, et al. The C3a anaphylatoxin receptor is a key mediator of insulin resistance and functions by modulating adipose tissue macrophage infiltration and activation. Diabetes. 2009; 58: 2006–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hillian AD, McMullen MR, Sebastian BM, et al. Mice lacking C1q are protected from high fat diet-induced hepatic insulin resistance and impaired glucose homeostasis. J Biol Chem. 2013; 288: 22565–22575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moreno-Navarrete JM, Martinez-Barricarte R, Catalan V, et al. Complement factor H is expressed in adipose tissue in association with insulin resistance. Diabetes. 2010; 59: 200–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Klein ML, Francis PJ, Rosner B, et al. CFH and LOC387715/ARMS2 genotypes and treatment with antioxidants and zinc for age-related macular degeneration. Ophthalmology. 2008; 115: 1019–1025 [DOI] [PubMed] [Google Scholar]
- 45. Adams MK, Simpson JA, Richardson AJ, et al. Can genetic associations change with age? CFH and age-related macular degeneration. Hum Mol Genet. 2012; 21: 5229–5236 [DOI] [PubMed] [Google Scholar]