Abstract
Objective To examine whether first trimester fetal growth restriction correlates with cardiovascular outcomes in childhood.
Design Population based prospective cohort study.
Setting City of Rotterdam, the Netherlands.
Participants 1184 children with first trimester fetal crown to rump length measurements, whose mothers had a reliable first day of their last menstrual period and a regular menstrual cycle.
Main outcomes measures Body mass index, total and abdominal fat distribution, blood pressure, and blood concentrations of cholesterol, triglycerides, insulin, and C peptide at the median age of 6.0 (90% range 5.7-6.8) years. Clustering of cardiovascular risk factors was defined as having three or more of: high android fat mass; high systolic or diastolic blood pressure; low high density lipoprotein cholesterol or high triglycerides concentrations; and high insulin concentrations.
Results One standard deviation score greater first trimester fetal crown to rump length was associated with a lower total fat mass (−0.30%, 95% confidence interval −0.57% to −0.03%), android fat mass (−0.07%, −0.12% to −0.02%), android/gynoid fat mass ratio (−0.53, −0.89 to −0.17), diastolic blood pressure (−0.43, −0.84 to −0.01, mm Hg), total cholesterol (−0.05, −0.10 to 0, mmol/L), low density lipoprotein cholesterol (−0.04, −0.09 to 0, mmol/L), and risk of clustering of cardiovascular risk factors (relative risk 0.81, 0.66 to 1.00) in childhood. Additional adjustment for gestational age and weight at birth changed these effect estimates only slightly. Childhood body mass index fully explained the associations of first trimester fetal crown to rump length with childhood total fat mass. First trimester fetal growth was not associated with other cardiovascular outcomes. Longitudinal growth analyses showed that compared with school age children without clustering of cardiovascular risk factors, those with clustering had a smaller first trimester fetal crown to rump length and lower second and third trimester estimated fetal weight but higher weight growth from the age of 6 months onwards.
Conclusions Impaired first trimester fetal growth is associated with an adverse cardiovascular risk profile in school age children. Early fetal life might be a critical period for cardiovascular health in later life.
Introduction
Fetal developmental adaptations in response to adverse environmental exposures may permanently affect the structure and function of cardiovascular organs.1 These adaptations may lead to increased risks of cardiovascular disease in adulthood.1 Human development rates are highest during the first trimester of pregnancy.2 This period includes the embryonic phase and is essential for development of fetal cardiovascular and metabolic organs.3 Therefore, the first trimester of pregnancy may be a critical period for cardiovascular health in childhood and adulthood.
In obstetric care practice, first trimester fetal crown to rump length is commonly used for dating pregnancy, assuming no growth variation.3 However, among pregnant women with a known first day of the last menstrual period and a regular cycle, fetal crown to rump length can be used as a first trimester growth outcome.4 First trimester fetal growth seems to be influenced by maternal age, ethnicity, parity, blood pressure, haemoglobin concentrations, smoking, and folic acid supplement use and is associated with increased risks of adverse birth outcomes.4 5 6 7 8 Whether first trimester fetal growth restriction is associated with risk factors for cardiovascular disease in later life remains unknown.
In a population based prospective cohort study among 1184 mothers with a known first day of the last menstrual period and a regular cycle, and their children, we examined the associations of first trimester fetal crown to rump length with cardiovascular risk factors in childhood. Cardiovascular outcomes of interest included body mass index, body fat distribution, blood pressure, lipid concentrations, and insulin measures, which are known risk factors for cardiovascular disease in adulthood and track from childhood to adulthood.9 10
Methods
Design and population
This study was nested in the Generation R Study, a population based prospective cohort study from early pregnancy onwards in Rotterdam, the Netherlands.11 Participating mothers gave written consent.12 Enrolment in the full Generation R Study was aimed at early pregnancy but allowed until birth. In total, 8880 mothers were enrolled in the full study during pregnancy. Of these mothers, 4685 did not have a fetal crown to rump length measurement, mainly because of a later enrolment in the study.4 10 Of all 4195 mothers with a fetal crown to rump length measurement, 2576 were not eligible for the nested study because their fetal crown to rump length measurements were not within the range of 10 weeks 0 days to 13 weeks 6 days or they had an unknown first day of last menstrual period or an irregular menstrual cycle.4 Of the remaining 1619 eligible mothers who had a first trimester crown to rump length measurement, had a known gestational age based on the last menstrual period, and gave birth to a singleton liveborn child, 1184 participated with their children in detailed follow-up measurements at the age 6 years (supplementary figure S1).
First trimester fetal crown to rump length
We measured first trimester fetal crown to rump length in the gestational age range of 10 weeks 0 days to 13 weeks 6 days in a true mid-sagittal plane with the genital tubercle and the fetal spine longitudinally in view.4 13 First day of the last menstrual period came from the referring letter from the community midwife or hospital.4 We confirmed this date with the mother at the ultrasound visit and obtained additional information on the regularity and duration of the menstrual cycle. Intra-class correlation coefficients for intra-observer and inter-observer reproducibility of crown to rump length measurements were 0.998 and 0.995.14 As previously described, we constructed gestational age adjusted standard deviation scores for first trimester fetal crown to rump length.4
Fetal and childhood growth
We measured second and third trimester fetal head circumference, abdominal circumference, and femur length to the nearest millimetre by using standardised ultrasound procedures.15 We used the Hadlock formula to calculate estimated fetal weight.16 Sex, date of birth, and birth anthropometrics (length, weight) came from registries. Well trained staff in community health centres measured childhood growth characteristics (weight, length) by using standardised procedures at the ages of 6, 12, 24, 36, and 48 months.9 For all fetal, birth, and childhood growth characteristics, we used reference growth charts to construct standard deviation score values with a commercially available package (Growth Analyser 3.0, Dutch Growth Research Foundation, Rotterdam, Netherlands).15 17
Childhood cardiovascular outcomes
We invited all children to a dedicated research facility in the Erasmus University Medical Center, Sophia Children’s Hospital for detailed measurements at the age of 6 years. We measured height and weight and calculated body mass index. We measured body fat by dual energy x ray absorptiometry (iDXA, General Electrics, 2008, Madison, WI, USA). We calculated total fat mass as a percentage of total body weight measured by absorptiometry. We calculated android and gynoid fat mass as a percentage of total fat mass, as well as their ratio.18 We used the android/gynoid fat mass ratio as a measure of body fat distribution, as we did not measure waist/hip ratio. Higher waist/hip ratio and android/gynoid fat mass ratio reflect an adverse body fat distribution and are associated with mortality in adults and insulin resistance in children, respectively.19 20 We measured systolic and diastolic blood pressure at the right brachial artery, four times at one minute intervals, by using the validated automatic sphygmomanometer Datascope Accutor Plus (Paramus, NJ, USA).21 We selected a cuff with a width approximately 40% of the arm circumference and long enough to cover 90% of the arm circumference. We obtained venous blood samples after 30 minutes’ fasting from the children and measured total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglycerides, insulin, and C peptide concentrations.
We used the previously described definition of childhood metabolic syndrome phenotype to define children with clustering of cardiovascular risk factors.22 We defined children with clustering of cardiovascular risk factors as those with three or more of the following components: android fat mass percentage 75th centile or above, systolic or diastolic blood pressure 75th centile or above; high density lipoprotein cholesterol 25th centile or below or triglycerides 75th centile or above, and insulin concentration 75th centile or above. We used android fat mass percentage as proxy for waist circumference, as waist circumference was not available.20
Covariates
We obtained information on maternal age, ethnicity, educational level, parity, folic acid supplementation, and smoking by questionnaire at enrolment.11 Maternal height and weight were measured and body mass index was calculated at enrolment. We measured maternal blood pressure with the validated oscillometric sphygmomanometer (OMRON Healthcare Europe B V, Hoofddorp, Netherlands) and documented the mean value of two blood pressure readings.23
Statistical analysis
Firstly, we used first trimester fetal crown to rump length standard deviation scores as a continuous variable, to analyse the linear associations of first trimester fetal crown to rump length with childhood outcomes. Using mean plots and one way analysis of variance tests, we observed that the best fitting trend lines for these associations were linear. The model fit and explained variance did not improve with addition of a quadratic term to the multivariate regression models. To further explore non-linearity and for presentation purposes, we also categorised first trimester fetal crown to rump length in fifths and examined the associations of fifths of first trimester fetal crown to rump length standard deviation score with childhood outcomes by using multivariate regression models. For these analyses, we constructed standard deviation score values ((observed value−mean)/SD) for the childhood outcome measures to enable comparison of effect estimates for the different outcomes. We did not create age adjusted standard deviation scores, as the childhood outcomes were measured in a small age range without changes in standard deviation.
Secondly, we used different linear regression models to examine the associations of first trimester fetal crown to rump length standard deviation score with childhood outcomes and the role of fetal and childhood growth in these associations. We used four different models. The basic model was adjusted for duration of last menstrual cycle and child’s sex and age at outcome measurement. Childhood height was included in all models on fat mass outcomes to take account of skeletal growth.24 The confounder model was additionally adjusted for maternal and childhood covariates including maternal age, educational level, ethnicity, parity, pre-pregnancy body mass index, diastolic blood pressure, smoking during pregnancy, folic acid supplement use, and duration of breast feeding. We selected these confounders on the basis of their associations with the outcomes of interest or a change in effect estimate of more than 10%. Supplementary tables S1-S3 show the associations of each confounder with the outcomes of interest. We considered the confounder models to be the main models. Next, these models were additionally adjusted for gestational age at birth and birth weight to explore whether any association was explained by later fetal growth (fetal pathway model) and for child’s current body mass index to explore whether any association was explained by current childhood size (childhood pathway model).
Thirdly, we examined the association of first trimester fetal crown to rump length standard deviation scores continuously and in fifths with the risk of childhood clustering of cardiovascular risk factors. We used a multivariate generalised linear model with a Poisson assumption, log linear link function, and robust standard errors estimation to calculate adjusted relative risks.25 Subsequently, we explored the longitudinal length and weight growth patterns from first trimester onwards until the age of 6 years for children with and without clustering of cardiovascular risk factors. For this analysis, we used repeated measurement regression models, which take into account the correlation between repeated growth measurements of the same participant.26 27
For all analyses, the percentages of missing values of covariates were lower than 20%. We imputed missing data of the covariates by using multiple imputations.28 Five datasets were created and analysed together. We used SAS version 9.2 for the repeated measurement analysis and SPSS 17.0 for other analyses.
Results
Participants’ characteristics and non-response
Table 1 shows the maternal, fetal, and childhood characteristics. The specific fetal and childhood growth characteristics are shown in supplementary table S4. As only mothers with fetal crown to rump length measurement between 10 and14 weeks of gestation and a known and reliable first day of last menstrual period were eligible for this analysis, we did several non-response analyses. Supplementary tables S5-S7 show results from these analyses. Compared with mothers with a first trimester fetal crown to rump length measurement (n=4195), those without this measurement (n=4685) were on average younger, shorter, and heavier; had a lower blood pressure; and were less frequently highly educated and European. We found no difference in birth weight (table S5). Among mothers with a first trimester fetal crown to rump length measurement, we found similar differences between those without (n=2576) and with (n=1619) information about their known last menstruation (table S6). Of the eligible group of 1619 mothers and children, 1184 participated in the follow-up studies at the age of 6 years. Mothers of children not participating in these follow-up studies (n=435) were on average younger, were less frequently higher educated and European, and less frequently used folic acid supplements. Their children were more frequently breast fed. We found no differences in gestational age and weight at birth (table S7).
Table 1.
Maternal, fetal, and childhood characteristics (n=1184). Values are numbers (percentages) unless stated otherwise
| Characteristics | Value |
|---|---|
| Maternal | |
| Median (90% range) age, years | 31.3 (22.7-38.1) |
| Mean (SD) height, cm | 168.8 (7.0) |
| Mean (SD) pre-pregnancy weight, kg | 66.9 (11.8) |
| Mean (SD) pre-pregnancy body mass index, kg/m2 | 23.4 (3.9) |
| Median (90% range) gestational age at intake, weeks | 12.4 (10.5-13.9) |
| Mean (SD) systolic blood pressure, mm Hg | 116.7 (12.4) |
| Mean (SD) diastolic blood pressure, mm Hg | 69.1 (9.4) |
| Nulliparous | 717/1179 |
| Education: | |
| Primary or secondary school | 507/1155 (43.9) |
| Higher education | 648/1155 (56.1) |
| Race/ethnicity: | |
| Dutch, other European | 855/1175 (72.8) |
| Non-European | 320/1175 (27.2) |
| Smoking habits: | |
| Non-smoker | 820/1060 (77.4) |
| Smoker | 240/1060 (22.6) |
| Folic acid supplement use: | |
| None | 119/949 (12.5) |
| First 10 weeks | 294/949 (31.0) |
| Preconception | 536/949 (56.5) |
| Fetal | |
| Median (90% range) gestational age at fetal crown to rump length measurement, weeks | 12.4 (11.0-13.9) |
| Mean (SD) first trimester fetal crown to rump length, cm | 61 (11) |
| Birth and infant | |
| Male sex | 575 (48.6) |
| Median (90% range) gestational age at birth, weeks | 40.1 (37.0-42.0) |
| Mean (SD) birth weight, g | 3456 (551) |
| Ever breast feeding: | |
| No | 80/1046 (7.6) |
| Yes | 966/1046 (92.4) |
| Mean (SD) breastfeeding duration, months | 5.3 (3.8) |
| Childhood | |
| Median (90% range) age at follow-up, years | 6.0 (5.7-6.8) |
| Mean (SD) height, cm | 119.0 (5.5) |
| Mean (SD) weight, kg | 22.8 (3.7) |
| Mean (SD) body mass index, kg/m2 | 16.1 (1.7) |
| Mean (SD) total fat mass, % | 24.6 (5.2) |
| Mean (SD) android fat mass, % | 3.8 (0.9) |
| Mean (SD) gynoid fat mass, % | 15.3 (1.6) |
| Mean (SD) android/gynoid fat mass ratio | 0.25 (0.1) |
| Mean (SD) systolic blood pressure, mm Hg | 102.6 (8.1) |
| Mean (SD) diastolic blood pressure, mm Hg | 60.7 (6.8) |
| Mean (SD) cholesterol, mmol/L | 4.2 (0.7) |
| Mean (SD) low density lipoprotein cholesterol, mmol/L | 2.4 (0.6) |
| Mean (SD) high density lipoprotein cholesterol, mmol/L | 1.3 (0.3) |
| Mean (SD) high density/low density lipoprotein cholesterol ratio | 0.6 (0.2) |
| Median (90% range) triglycerides, mmol/L | 1.0 (0.4-2.1) |
| Median (90% range) insulin, pmol/L | 118.2 (25.9-342.4) |
| Median (90% range) C peptide, nmol/L | 1.0 (0.4-1.9) |
| Cardiovascular risk factor clustering | 81/745 (10.9) |
First trimester fetal crown to rump length and cardiovascular risk factors
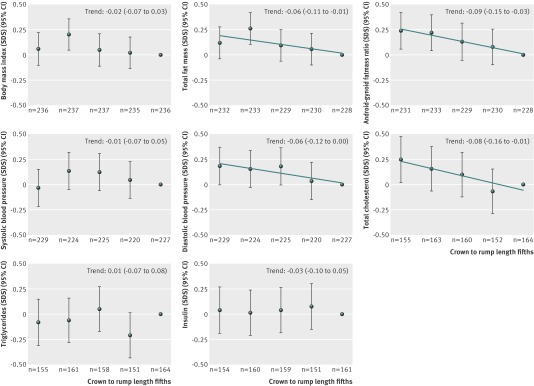
Figure 1 shows that compared with children in the highest fifth of first trimester fetal crown to rump length, those in the lowest fifth tended to have higher total fat mass percentage, android/gynoid fat mass ratio, diastolic blood pressure, and total cholesterol (all P for trend <0.05). First trimester fetal crown to rump length was not associated with insulin or C peptide concentrations. Results for C-peptide are not shown. Table 2 shows that in the confounder models, one standard deviation score greater first trimester fetal crown to rump length was associated with a lower total fat mass (−0.30%, 95% confidence interval −0.57% to −0.03%), android fat mass (−0.07%, −0.12% to −0.02%), android/gynoid fat mass ratio (−0.53, −0.89 to −0.17), diastolic blood pressure (−0.43, −0.84 to −0.01, mm Hg), total cholesterol (−0.05, −0.10 to 0, mmol/L), and low density lipoprotein cholesterol (−0.04, −0.09 to 0, mmol/L) in childhood. Additional adjustment for gestational age and weight at birth only slightly changed these effect estimates. Childhood body mass index fully explained the associations of first trimester fetal crown to rump length with childhood total fat mass. First trimester fetal crown to rump length was not associated with childhood body mass index, systolic blood pressure, or concentrations of triglycerides or insulin.
Fig 1 First trimester fetal growth and cardiovascular risk factors in childhood (n=1184). Values are linear regression coefficients (95% CI) that reflect the difference in childhood outcomes, expressed as standard deviation scores (SDS) between first trimester fetal crown to rump length fifths, and reference group (highest fifth). Estimates are based on multiple imputed data. Models were adjusted for child’s sex and age at measurement and for maternal duration of last menstrual cycle, age, educational level, ethnicity, parity, pre-pregnancy body mass index, diastolic blood pressure, smoking during pregnancy, folic acid supplement use, and duration of breast feeding. Models for total fat mass and android/gynoid fat mass ratio were additionally adjusted for current childhood height. Trend lines are given only when P for linear trend <0.05
Table 2.
First trimester fetal growth and childhood cardiovascular risk factors (n=1184)*
| Cardiovascular risk factor | No | Difference (95% CI) in cardiovascular risk factors per SDS change in first trimester fetal crown to rump length | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Basic model | P value | Confounder model† | P value | Fetal pathway model‡ | P value | Childhood pathway model§ | P value | ||
| Body mass index (kg/m2) | 1181 | −0.06 (−0.16 to 0.04) | 0.23 | −0.04 (−0.14 to 0.05) | 0.38 | −0.08 (−0.18 to 0.02) | 0.10 | — | — |
| Total fat mass (%) | 1152 | −0.41 (−0.70 to −0.13) | 0.01 | −0.30 (−0.57 to −0.03) | 0.03 | −0.31 (−0.59 to −0.04) | 0.03 | −0.16 (−0.36 to 0.04) | 0.11 |
| Android fat mass (%) | 1151 | −0.08 (−0.14 to −0.03) | <0.01 | −0.07 (−0.12 to −0.02) | 0.01 | −0.07 (−0.12 to −0.01) | 0.02 | −0.04 (−0.08 to 0) | 0.07 |
| Gynoid fat mass (%) | 1151 | 0.05 (−0.04 to 0.14) | 0.27 | 0.05 (−0.04 to 0.14) | 0.32 | 0.05 (−0.04 to 0.14) | 0.25 | 0.06 (−0.03 to 0.15) | 0.17 |
| Android/gynoid fat mass ratio | 1151 | −0.62 (−0.98 to −0.26) | <0.01 | −0.53 (−0.89 to −0.17) | <0.01 | −0.52 (−0.88 to −0.16) | <0.01 | −0.38 (−0.68 to −0.08) | 0.01 |
| Systolic blood pressure (mm Hg) | 1125 | −0.22 (−0.70 to 0.27) | 0.39 | −0.10 (−0.59 to 0.39) | 0.69 | −0.09 (−0.58 to 0.41) | 0.74 | −0.01 (−0.50 to 0.47) | 0.96 |
| Diastolic blood pressure (mm Hg) | 1125 | −0.51 (−0.92 to −0.10) | 0.02 | −0.43 (−0.84 to −0.01) | 0.04 | −0.42 (−0.84 to 0) | 0.05 | −0.40 (−0.81 to 0.02) | 0.06 |
| Total cholesterol (mmol/L) | 794 | −0.05 (−0.10 to 0) | 0.04 | −0.05 (−0.10 to 0) | 0.04 | −0.06 (−0.10 to −0.01) | 0.03 | −0.05 (−0.10 to 0) | 0.04 |
| HDL cholesterol (mmol/L) | 794 | −0.01 (−0.04 to 0.01) | 0.29 | −0.01 (−0.04 to 0.01) | 0.27 | −0.01 (−0.04 to 0.01) | 0.25 | −0.01 (−0.04 to 0.01) | 0.23 |
| LDL cholesterol (mmol/L) | 793 | −0.04 (−0.08 to 0) | 0.06 | −0.04 (−0.09 to 0) | 0.06 | −0.04 (−0.09 to 0) | 0.06 | −0.04 (−0.09 to 0) | 0.06 |
| HDL/LDL cholesterol ratio | 786 | 0.01 (−0.01 to 0.02) | 0.20 | 0.01 (−0.01 to 0.03) | 0.21 | 0.01 (−0.01 to 0.03) | 0.19 | 0.01 (−0.01 to 0.03) | 0.22 |
| Triglyceride (mmol/L)¶ | 789 | 0.01 (−0.03 to 0.04) | 0.71 | 0.00 (−0.03 to 0.04) | 0.86 | 0.01 (−0.03 to 0.04) | 0.76 | 0.01 (−0.03 to 0.04) | 0.69 |
| Insulin (pmol/L)¶ | 785 | −0.01 (−0.07 to 0.05) | 0.66 | −0.02 (−0.08 to 0.04) | 0.49 | −0.02 (−0.08 to 0.04) | 0.54 | −0.01 (−0.07 to 0.05) | 0.63 |
HDL=high density lipoprotein; LDL=low density lipoprotein; SDS=standard deviation score.
*Values are regression coefficients (95% confidence interval) that reflect the difference in childhood outcomes per SDS first trimester crown to rump length. Basic model was adjusted for duration of last menstrual cycle and child’s sex and age at outcome measurements. Models for fat mass outcomes were additionally adjusted for current childhood height.
†Confounders include maternal age, educational level, ethnicity, parity, pre-pregnancy body mass index, diastolic blood pressure, smoking during pregnancy, folic acid supplement use, and breastfeeding duration.
‡Model additionally adjusted for gestational age and weight at birth.
§Model additionally adjusted for childhood current body mass index.
¶Variables were log transformed.
First trimester fetal crown to rump length and clustering of cardiovascular risk factors
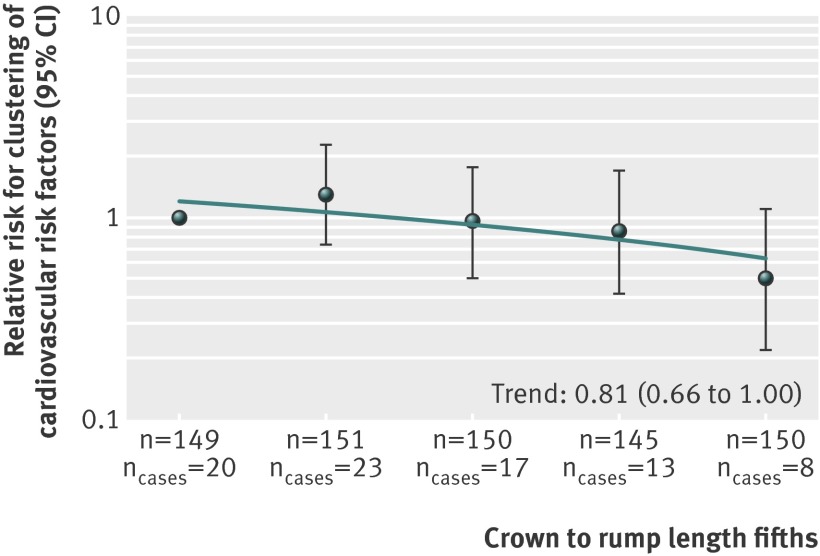
One standard deviation score greater first trimester fetal crown to rump length was associated with a lower risk of clustering of cardiovascular risk factors (relative risk 0.81, 95% confidence interval 0.66 to 1.00) in childhood (fig 2). When we compared fifths, we observed that compared with children in the lowest fifth of first trimester fetal crown to rump length, those in the highest fifth tended to have lower risks of clustering of cardiovascular risk factors (15.5% v 5.6% for lowest and highest fifth; relative risk 0.50, 0.22 to 1.10) (fig 2). Adjustment for gestational age and weight at birth changed these effect estimates only slightly (supplementary figure S2).
Fig 2 First trimester fetal growth and clustering of cardiovascular risk factors (n=745). Values are relative risks (95% CI) from generalised linear models that reflect risk of childhood clustering of cardiovascular risk factors for fifths of first trimester fetal crown to rump length, compared with reference group (lowest fifth). Estimates are based on multiple imputed data. Clustering of cardiovascular risk factors was defined as having three or more of android fat mass percentage ≥75th centile, systolic or diastolic blood pressure ≥75th centile, high density lipoprotein cholesterol ≤25th centile or triglycerides ≥75th centile, and insulin concentration ≥75th centile.22 Model was adjusted for child’s sex and age at measurement and for maternal duration of last menstrual cycle, age, educational level, ethnicity, parity, pre-pregnancy body mass index, diastolic blood pressure, smoking during pregnancy, folic acid supplement use, and duration of breast feeding
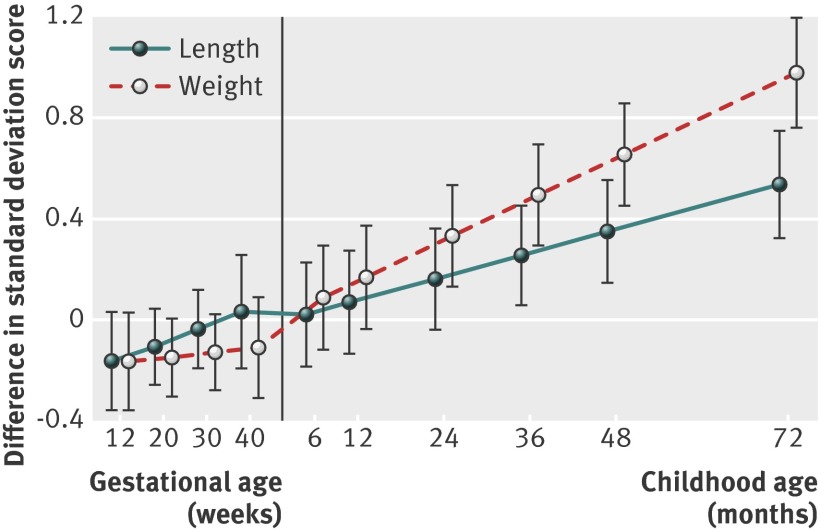
Figure 3 shows the longitudinal growth in fetal and childhood length and weight from first trimester fetal crown to rump length onwards in children with clustering of cardiovascular risk factors, compared with those without clustering of cardiovascular risk factors. First trimester fetal crown to rump length tended to be smaller in children with clustering of cardiovascular risk factors (difference −0.16, 95% confidence interval −0.36 to 0.03 standard deviation scores). Estimated fetal weight, but not femur length, until birth tended to be smaller in children with clustering of cardiovascular risk factors. From the age of 6 months onwards, children with clustering of cardiovascular risk factors at age 6 years had a higher length and weight, with larger effect estimates for weight. The effect estimates were not materially affected by additional adjustment for potential confounders (supplementary table S8).
Fig 3 Fetal and childhood length and weight growth from first trimester onwards in children with clustering of cardiovascular risk factors (n=745). Values are regression coefficients (95% confidence interval) that reflect the difference in length and weight standard deviation score from first trimester onwards for children with clustering of cardiovascular risk factors, compared with children without such clustering. Models were adjusted for maternal duration of last menstrual cycle and child’s sex and age at outcome measurements. Length and weight growth characteristics used in the models were: fetal period—first trimester crown to rump length as both length and weight measure (starting point), second and third trimester femur length and estimated fetal weight; at birth—length and weight; during childhood—length and weight. Clustering of cardiovascular risk factors was defined as having three or more of android fat mass percentage ≥75th centile, systolic or diastolic blood pressure ≥75th centile, high density lipoprotein cholesterol ≤25th centile or triglycerides ≥75th centile, and insulin concentration ≥75th centile22
Discussion
We observed that smaller first trimester fetal size was associated with an adverse body fat distribution, higher diastolic blood pressure, and an adverse blood cholesterol profile in childhood. First trimester fetal growth restriction was also associated with an increased risk of clustering of these cardiovascular risk factors in childhood. These associations were not explained by maternal, birth, and childhood characteristics.
Interpretation of main findings
Adverse fetal exposures may lead to early developmental adaptations, including changes in the anatomy, physiology, and metabolism of various organ systems.1 These adaptations may be beneficial for short term survival but may have adverse consequences at birth and in later life, such as increased risks of low birth weight and common diseases in adulthood.1 Studies showing consistent associations of low birth weight with increased risks of cardiovascular disease strongly support this hypothesis.29 30 Clearly, low birth weight is not the causal factor leading to diseases in later life. Birth weight is merely an endpoint of different fetal exposures and growth patterns and the starting point of childhood growth. Most children with a low birth weight have a catch-up growth leading to a normal weight from the age of 2 years onwards.31 Longitudinal studies also showed that the risk of cardiovascular disease is highest among adults born with a low birth weight who had a high postnatal weight gain.32 33 These results suggest that a low birth weight as a result of restricted fetal environment may specifically lead to cardiovascular disease in later life, when postnatal life is characterised by a relatively high body mass index as a result of an affluent environment.1 Not much is known about the specific fetal growth patterns leading to cardiovascular disease in later life.
Rates of growth and development are much higher in fetal life than in childhood. The highest development rates are in the first trimester of pregnancy, which includes the embryonic phase.2 Studies in spontaneously conceived pregnancies and in pregnancies resulting from assisted reproductive technology observed that first trimester fetal growth restriction was associated with increased risks of prematurity and small size for gestational age at birth.4 7 8 We also observed that smaller first trimester fetal crown to rump length led to compensatory accelerated childhood growth.4 High rates of childhood weight gain may subsequently lead to development of cardiovascular risk factors in later life.
The study reported here shows for the first time that first trimester fetal crown to rump length is also associated with an adverse cardiovascular risk profile in childhood. Smaller first trimester fetal crown to rump length was associated with higher total fat mass percentage, android/gynoid fat mass ratio, diastolic blood pressure, and total cholesterol concentration in childhood. These associations were observed across the full range of first trimester fetal crown to rump length and not in the extremes only. Also, these associations were independent of potential maternal and childhood confounders and were changed only slightly by adjustment for gestational age and weight at birth and childhood body mass index. First trimester fetal growth was not associated with childhood body mass index, systolic blood pressure, or concentrations of triglycerides, insulin, or C peptide. The observed associations suggest that the first trimester of pregnancy is a critical period for cardiovascular health in later life. Previous studies have shown that risk factors for cardiovascular disease in childhood track into adulthood and are related to development of cardiovascular disease in later life.9 10 34 Thus, cardiovascular disease may have at least part of its origins in the first trimester of pregnancy or even the preconception period. The developmental mechanisms that explain the associations of first trimester fetal growth and risk factors for cardiovascular disease are not known, but they may include changes in methylation of DNA and expression of RNA in response to a suboptimal fetal environment.1 More detailed first trimester ultrasound studies are needed to assess early cardiovascular and metabolic developmental adaptations.
The results from this study are important from an aetiological perspective. They suggest that the first trimester might be a critical period for cardiovascular and metabolic function. However, we acknowledge that the observed effect estimates were small and reflect subclinical changes in cardiovascular and metabolic function in school age children. None of the children had known cardiovascular disease. Previous longitudinal studies have shown tracking of cardiovascular and metabolic risk factors from childhood to adulthood.9 10 Also, adiposity in school age children is related to cardiovascular disease in later life.34 Further follow-up studies are needed to explore whether suboptimal first trimester development really is a risk factor for clinically manifest cardiovascular and metabolic disease in adulthood.
Strengths and limitations
This study was nested in a large population based prospective cohort study. In the full study, enrolment was aimed at early pregnancy but allowed until birth.11 As this study was specifically focused on the long term effects of variation in first trimester fetal growth, only a subgroup of mothers with a first trimester fetal crown to rump length measurement between 10 and 14 weeks of gestation and a known and reliable first day of last menstrual period was eligible. As a result of these necessary selection criteria, the eligible mothers reflect a small fraction of the full study population. Of all eligible mothers, 73% participated with their children in the follow-up studies at the age of 6 years. The non-response analyses showed that mothers not included in the analyses were on average younger, shorter, and heavier; had a lower blood pressure; were less frequently high educated and European; and less frequently used folic acid supplements. Their children were more frequently breast fed. Our effect estimates would be biased if the associations differ between participants included and not included in the analysis. Although this seems unlikely, we cannot exclude it. We found no differences in first trimester fetal crown to rump length or birth weight between children with and without participation in the follow-up studies. More importantly, the selection of the study sample might have affected the generalisability of the results. The study population is a rather healthy and relatively highly educated population. Whether the observed associations are similar in high risk populations should be studied further.
We tested the associations of first trimester fetal crown to rump length with several cardiovascular and metabolic outcomes that track from childhood to adulthood and are risk factors for cardiovascular disease in adulthood. The large number of statistical tests that we did may have led to false positive associations. However, because of the correlations between the cardiovascular and metabolic outcomes, we did not adjust the analyses for multiple testing. We measured first trimester fetal growth by fetal crown to rump length and used the first day of the last menstrual period to determine gestational age. Misclassification of gestational age might still be a problem, as the post-conception age depends on the timing of ovulation and implantation, which we were unable to measure.35 Several maternal factors, such as maternal age and smoking, are associated with the duration of the follicular phase, after which ovulation occurs. Recall bias may also affect the dating of the last menstrual period.36 However, all analyses were adjusted for the duration of last menstrual cycle, which is strongly associated with the timing of ovulation. Even with a known and reliable date of last menstrual period, a certain fraction of women with regular cycles have early or delayed ovulation. We did a sensitivity analysis with a restriction to participants who had a gestational age based on last menstruation within seven days of a gestational age based on crown to rump length (93%). This analysis did not materially change our effect estimates for the childhood outcomes. The analyses were adjusted for several maternal and childhood confounders. Although we observed that stepwise adjustment for various different potential maternal and childhood confounders did not strongly change the effect estimates, residual confounding may still be a concern, as in any observational study.
Conclusions
These results suggest that the first trimester of pregnancy may be a critical period for development of cardiovascular risk factors in later life. The observed associations are primarily important from an aetiological perspective. Further studies are needed to identify the underlying causal biological mechanisms and long term consequences. Future strategies to improve cardiovascular health may start from early pregnancy onwards or even before conception.
What is already known on this topic
Low birth weight is associated with cardiovascular disease in adulthood, but not much is known about the specific critical periods in fetal life or infancy
The first trimester of pregnancy includes the embryonic phase and is essential for fetal development of cardiovascular and metabolic organs
The first trimester of pregnancy may be a critical period for cardiovascular health in childhood and adulthood
What this study adds
Impaired first trimester fetal growth is associated with an adverse cardiovascular risk profile in school age children
These associations are independent of growth during the second half of pregnancy and in childhood
First trimester fetal development might be critical for cardiovascular health in later life
Contributors: VWVJ obtained funds, designed the study, had overall responsibility for managing the study, completed the background literature search, contributed to the analysis protocol, contributed to data management, wrote the first draft of the paper, and collated comments from other authors. RG contributed to the analysis protocol and completed analyses. LLdJ, AH, OHF, and EAS obtained funds, designed the study, and contributed to the manuscript. All authors had access to all of the data and approved the final version of the submitted manuscript. VWVJ and RG are the guarantors.
Funding: VWVJ received funding from the Netherlands Organization of Scientific Research (NWO), Netherlands Organization of Health Research and Development (ZonMw), VIDI 016.136.361. OHF works in ErasmusAGE, a centre for ageing research across the life course funded by Nestle Nutrition, Metagenics, and AXA. RG received funding from the European Union’s Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition under grant agreement No 289346.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmj.org/cio_disclosure.pdf (available on request from the corresponding author) and declare: no financial support from any organisation for the submitted work other than those listed above; no financial relationship with any companies that might have an interest in the submitted work in the previous three years; no non-financial interests or relationships that may be relevant to the submitted work.
Ethical approval: Written informed consent was obtained from the parents to include their children in the Generation R Study. The Medical Ethical Committee of the Erasmus Medical Center in Rotterdam approved the study.
Data sharing: The protocol is available on request.
Transparency: The lead author (VWVJ) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Cite this as: BMJ 2014;348:g14
Web Extra. Extra material supplied by the author
References
- 1.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008;359:61-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingstone C, ed. Human embryology. 3rd ed. Elsevier, 2001.
- 3.Robinson HP. Sonar measurement of fetal crown-rump length as means of assessing maturity in first trimester of pregnancy. Br Med J 1973;4:28-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mook-Kanamori DO, Steegers EA, Eilers PH, Raat H, Hofman A, Jaddoe VW. Risk factors and outcomes associated with first-trimester fetal growth restriction. JAMA 2010;303:527-34. [DOI] [PubMed] [Google Scholar]
- 5.Bukowski R, Smith GC, Malone FD, Ball RH, Nyberg DA, Comstock CH, et al. Human sexual size dimorphism in early pregnancy. Am J Epidemiol 2007;165:1216-8. [DOI] [PubMed] [Google Scholar]
- 6.Bottomley C, Daemen A, Mukri F, Papageorghiou AT, Kirk E, Pexsters A, et al. Assessing first trimester growth: the influence of ethnic background and maternal age. Hum Reprod 2009;24:284-90. [DOI] [PubMed] [Google Scholar]
- 7.Smith GC, Smith MF, McNay MB, Fleming JE. First-trimester growth and the risk of low birth weight. N Engl J Med 1998;339:1817-22. [DOI] [PubMed] [Google Scholar]
- 8.Bukowski R, Smith GC, Malone FD, Ball RH, Nyberg DA, Comstock CH, et al. Fetal growth in early pregnancy and risk of delivering low birth weight infant: prospective cohort study. BMJ 2007;334:836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams CL, Hayman LL, Daniels SR, Robinson TN, Steinberger J, Paridon S, et al. Cardiovascular health in childhood: a statement for health professionals from the Committee on Atherosclerosis, Hypertension, and Obesity in the Young (AHOY) of the Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 2002;106:143-60. [DOI] [PubMed] [Google Scholar]
- 10.Hayman LL, Meininger JC, Daniels SR, McCrindle BW, Helden L, Ross J, et al. Primary prevention of cardiovascular disease in nursing practice: focus on children and youth: a scientific statement from the American Heart Association Committee on Atherosclerosis, Hypertension, and Obesity in Youth of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, Council on Epidemiology and Prevention, and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2007;116:344-57. [DOI] [PubMed] [Google Scholar]
- 11.Jaddoe VW, van Duijn CM, Franco OH, van der Heijden AJ, van Iizendoorn MH, de Jongste JC, et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol 2012;27:739-56. [DOI] [PubMed] [Google Scholar]
- 12.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2000;284:3043-5. [PubMed] [Google Scholar]
- 13.Royal College of Obstetricians and Gynaecologists. Routine ultrasound screening in pregnancy: protocol, standards and training. RCOG, 2000.
- 14.Verburg BO, Mulder PG, Hofman A, Jaddoe VW, Witteman JC, Steegers EA. Intra- and interobserver reproducibility study of early fetal growth parameters. Prenat Diagn 2008;28:323-31. [DOI] [PubMed] [Google Scholar]
- 15.Verburg BO, Steegers EA, De Ridder M, Snijders RJ, Smith E, Hofman A, et al. New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population-based cohort study. Ultrasound Obstet Gynecol 2008;31:388-96. [DOI] [PubMed] [Google Scholar]
- 16.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements—a prospective study. Am J Obstet Gynecol 1985;151:333-7. [DOI] [PubMed] [Google Scholar]
- 17.Niklasson A, Ericson A, Fryer JG, Karlberg J, Lawrence C, Karlberg P. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977-1981). Acta Paediatr Scand 1991;80:756-62. [DOI] [PubMed] [Google Scholar]
- 18.Helba M, Binkovitz LA. Pediatric body composition analysis with dual-energy X-ray absorptiometry. Pediatr Radiol 2009;39:647-56. [DOI] [PubMed] [Google Scholar]
- 19.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008;359:2105-20. [DOI] [PubMed] [Google Scholar]
- 20.Aucouturier J, Meyer M, Thivel D, Taillardat M, Duché P. Effect of android to gynoid fat ratio on insulin resistance in obese youth. Arch Pediatr Adolesc Med 2009;163:826-31. [DOI] [PubMed] [Google Scholar]
- 21.Wong SN, Tz Sung RY, Leung LC. Validation of three oscillometric blood pressure devices against auscultatory mercury sphygmomanometer in children. Blood Press Monit 2006;11:281-91. [DOI] [PubMed] [Google Scholar]
- 22.Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2009;119:628-47. [DOI] [PubMed] [Google Scholar]
- 23.Gaillard R, Bakker R, Willemsen SP, Hofman A, Steegers EA, Jaddoe VW. Blood pressure tracking during pregnancy and the risk of gestational hypertensive disorders: the Generation R Study. Eur Heart J 2011;32:3088-97. [DOI] [PubMed] [Google Scholar]
- 24.Wells JC. A critique of the expression of paediatric body composition data. Arch Dis Child 2001;85:67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 162:199-200. [DOI] [PubMed]
- 26.Goldstein H. Multilevel statistical methods. 2nd ed. Edward Arnold, 1995.
- 27.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 1999;28:964-74. [DOI] [PubMed] [Google Scholar]
- 28.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huxley R, Owen CG, Whincup PH, Cook DG, Rich-Edwards J, Smith GD, et al. Is birth weight a risk factor for ischemic heart disease in later life? Am J Clin Nutr 2007;85:1244-50. [DOI] [PubMed] [Google Scholar]
- 30.Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, el al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA 2008;300:2886-97. [DOI] [PubMed] [Google Scholar]
- 31.Taal HR, van der Heijden AJ, Steegers EA, Hofman A , Jaddoe VW. Small and large size for gestational age at birth, infant growth and childhood overweight. Obesity (Silver Spring) 2013;21:1261-8 [DOI] [PubMed] [Google Scholar]
- 32.Barker DJ, Osmond C, Forsén TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med 2005;353:1802-9. [DOI] [PubMed] [Google Scholar]
- 33.Bhargava SK, Sachdev HS, Fall CH, Osmond C, Lakshmy R, Barker DJ, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med 2004;350:865-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med 2010;362:485-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Gold EB, Lasley BL, Johnson WO. Factors affecting menstrual cycle characteristics. Am J Epidemiol 2004;160:131-40. [DOI] [PubMed] [Google Scholar]
- 36.Savitz DA, Terry JW Jr, Dole N, Thorp JM Jr, Siega-Riz AM, Herring AH. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol 2002;187:1660-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.