Abstract
Objective
Traumatic brain injury in contact sports has significant impact on short term neurological and neurosurgical function as well as longer term cognitive disability. In this study, we aim to demonstrate that contact sport participants exhibit differences in Diffusion Tensor Imaging (DTI) caused by repeated physical impact on the brain. We also aim to determine that impact incurred by the contact sports athletes during the season may result in differences between the pre- and post-season DTI scans.
Methods
DTI data was collected from 10 contact (mean age 20.4 +- 1.36) and 13 age-matched non-contact (mean age 19.5 +- 1.03) sport male athletes, on a 3T MRI scanner. A single shot echo-planar imaging sequence with b-value of 1000s/mm2 and 25 gradient directions was used. Eight of the athletes were again scanned post-season. The b0 non-diffusion weighted image was averaged 5 times. Voxel-wise 2-sample t-tests were run for all group comparisons, and in each case, the positive false discovery rate (pFDR) was computed to assess the whole map, multiple comparison corrected significance.
Results
There were significant differences in the FA values in the inferior fronto-occipital fasciculus, parts of the superior and posterior coronal radiate and the splenium of the corpus callosum (CC) as well as smaller clusters in the genu and parts of the body of the CC. In addition, the external capsule also shows some difference between the contact and non-contact athlete brains. Additionally, the pre-season and postseason show differences in these regions, however, the post-season p-values show significance in more areas of the CC.
Conclusions
There are significant DTI changes in the corpus callosum, the external capsule, the inferior fronto-occipital fasciculus as well as regions such as the superior/posterior corona radiata when comparing the pre-season contact versus the non-contact controls and also comparing the post-season contact athletes with the controls. There are also difference in the DTI between the post- vs. pre-season scans.
Keywords: Traumatic Brain Injury, Diffusion Tensor Imaging, Magnetic Resonance Imaging
Introduction
Brain trauma in contact sports, such as football, rugby, and boxing, has been the focus of recent media attention due to high profile cases involving professional athletes. Mild traumatic brain injury (mTBI), also known as concussive injury affects 1.7 million Americans annually, of which 300,000 are due to sports and recreational activities [5].
Concussions are associated with a host of symptoms including behavior changes, impairments of memory and attention, headache, unsteadiness, etc., leading to more permanent cognitive impairment [4]. Mechanical trauma to the brain triggers a complex cascade of structural, physiological, neuro-chemical and neuro-metabolic events due to the acceleration and deceleration forces, and this can cause further disruption of the neuronal cell membrane and axonal stretching [4].
Diffuse axonal injury (DAI), also known as traumatic axonal injury (TAI), is one of the consequences of traumatic brain injury, and is thought to be caused by shearing of nerve fibers due to the sudden acceleration and deceleration, sometimes combined with rotational and vibration forces [1,2,10,20]. Another possible consequence of TBI consists of local shearing of axons at the gray/white matter interface.
Both of these shearing processes disrupt axonal connections that are important to brain function [19]. A recent study on post-mortem brains, which included several American football players, showed that repeated mild TBI may progress to chronic traumatic encephalopathy (CTE) [11]. CTE, which has been most frequently associated with boxing, causes deteriorations in attention, concentration, and memory, as well as disorientation and confusion, and is occasionally accompanied by dizziness and headaches. As the disease progresses, further deterioration can occur, accompanied by lack of insight, poor judgment and even overt dementia [16].
Within the literature about traumatic brain injury in athletes, there exist several studies showing anatomical and cognitive differences before and after a concussion in sports such as hockey and football [5,9,7,23]. Additionally, the correlation between head trauma and cognitive decline has been shown in several studies in adult as well as adolescent populations [15,17,24].
In the last decade, diffusion tensor imaging (DTI) has become an important noninvasive tool to investigate white matter changes in TBI. DTI can reveal the white matter structures in the brain by quantifying the diffusion of water. DTI is sensitive to the diffusion of water molecules in the body, and is hindered by extracellular and restricted by intracellular components [3]. This diffusion is isotropic when it is not obstructed by tissues or barriers. However, when hampered by axons and their myelin sheath, diffusion becomes anisotropic. As a result, diffusion-based calculations enable quantification of the direction and integrity of axons. Common metrics derived from the diffusion tensors at each voxel of an image include for example the fractional anisotropy (FA) - a normalized measure of anisotropy -, the apparent diffusion coefficient (ADC) - a rotationally invariant measure of the magnitude of diffusion -, and the mean diffusivity (MD) - the average of the three eigenvalues.
Several regions of the brain have been shown to be affected by TBI consistently across DTI analyses. These regions include, but are not limited to the anterior corona radiata, the uncinate fasciculus, the genu of corpus callosum, the inferior longitudinal fasciculus, the cingulum bundle, the hippocampus/fornix, inferior fronto-occipital and cortico-spinal tracts, and the internal and external capsules [5,7,9,11,15,17,18,19,25]. FA, ADC, and MD have all been extensively investigated in population studies of TBI patients vs. controls. Some studies report an increase in FA and decrease in MD in some of the structures listed above [5,9,23], while others report an increase in MD [7] or decrease in FA instead [11, 15,17,18,25]. The apparent contradiction in these results however may be attributed to the dynamic nature of the axonal injury, and are thought to be due to the differences in the time course between the injury and the scan [5]. In this study, we hypothesize that contact sport participants may exhibit long term differences in DTI derived parameters caused by repeated physical impact. We utilize a voxel-based analysis to determine differences in FA, ADC and MD between male contact versus non-contact sport athletes scanned pre-season and post-season. We also hypothesized that impact incurred by the contact sports athletes during the season may result in differences between the pre- and post-season DTI scans.
Methods
DTI data was collected from 11 contact (mean age 20.4 +- 1.36) and 13 age-matched non-contact (mean age 19.5 +- 1.03) sport male athletes, on a 3T GE HDxT scanner. The athletes involved in our study participated in normal practice and competitions, but only one is known to have had a concussion. A single shot echo-planar imaging sequence with b-value of 1000s/mm2 and 25 gradient directions was used for data collection, with TR of 15.3ms, TE of 87ms, FOV of 256 mm2, and voxel size of 2×2×2mm3. Eight of the athletes were again scanned post-season. The b0 non-diffusion weighted image was averaged 5 times.
Images were corrected for motion and eddy current effects using FSL (www.fmrib.ox.ac.uk/fsl) [12]. The diffusion tensors were estimated from the data using the Tensor toolkit (https://gforge.inria.fr/projects/ttk). Next, MedINRIA [22] was used to linearly register all subjects to a standard template in ICBM space (IXI adult template). Non-linear registration was then performed using demon registration [8] algorithm to a single subject target. Finally, DTI parameters including fractional anisotropy (FA) and mean diffusivity (MD), were computed from this non-linearly registered tensor data. These two measures are complementary as the first measures the anisotropy of the diffusion, while the second measures its magnitude, and they may be sensitive to different aspects of injury. FA decrease has conventionally been associated with a loss of white matter integrity, however, new studies link FA increase with white matter damage in crossing areas. High MD is seen in unrestricted materials such as cerebrospinal fluid (CSF) and lower MD values would indicate areas with barriers to free diffusion, such as white matter.
The white matter region was classified as any voxel above 0.21 on the FA map. Voxel-wise 2-sample t-tests were run for all group comparisons, and in each case, the positive false discovery rate (pFDR) was computed to assess the whole map, multiple comparison corrected significance [21]. The results of the t-tests were thresholded to show only significant values (0.05 and lower, uncorrected) and were limited to clusters of 30 voxels or more. In this study, control data was not acquired post-season as the athletes were not involved in any sport that would contribute to head injury.
Results
Figures 1, 2, and 3 shows the uncorrected p-values computed using the two-tailed t-test from FA and MD respectively, superimposed on the IXI adult FA template. The images on the left are pre-season and the ones on the right are post-season. In Fig. 1, the p-values computed from the FA data show significance in the inferior fronto-occipital fasciculus, parts of the superior and posterior coronal radiata, and the splenium of the corpus callosum (CC) as well as smaller clusters in the genu and parts of the body of the CC. In addition, the external capsule also shows some difference. Both the pre-season and post-season show differences in these regions, however, the post-season p-values show significance in more areas of the CC.
Fig.1.
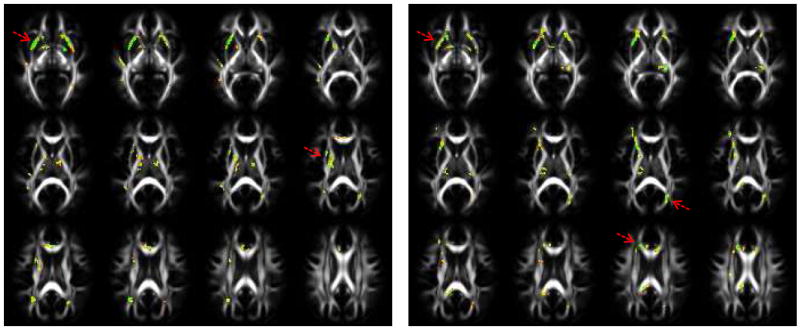
p-values computed using a two-tailed t-test from pre-season athlete and control data (left), and post-season athlete and control data (right) for the FA. The red arrows point to locations that show significant clusters.
Fig.2.
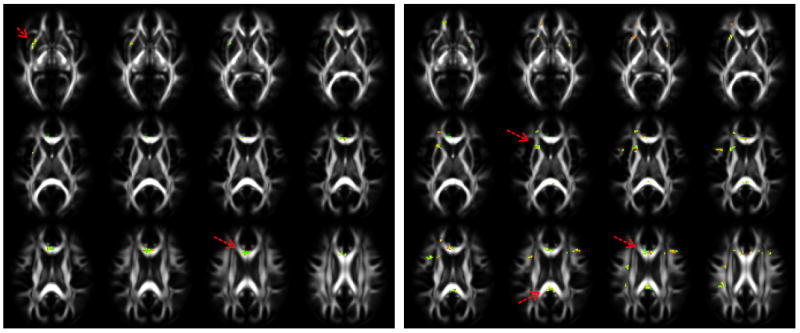
p-values computed using a two-tailed t-test from pre-season contact sport athlete and control data (left), and post-season athlete and control data (right) for the MD. The red arrows point to locations that show significant clusters.
A similar pattern is seen in figure 2 as well. The p-values computed from MD data show significance in the genu of the CC, the superior coronal radiata, and the inferior fronto-occipital fasciculus in both the pre-season and post-season data, but the post-season data is also significant in the splenium of the CC.
The pFDR acquired from the multiple comparisons testing had a range between of 0.0127 for the pre-season FA t-test, 0.0130 for the post-season FA t-test, 0.0154 for the MD pre-season t-test, 0.0152 for the MD post-season t-test, indicating significance between the athletes and controls for each case.
Discussion
In this data analysis, we statistically compared FA and MD of contact athletes to non-contact athletes, and found changes in the corpus callosum and the external capsule, the inferior fronto-occipital fasciculus as well as smaller regions such as the superior/posterior corona radiata. These differences were seen in both the pre-season and post-season data which infers long-term effects of high impact contact sports on brain white matter structure.
It is important to consider the possibility that even in the absence of clinically apparent traumatic brain injury, there may damage to the brain from the repeated impact sustained in contact sports. Repeated stress on the axons from collisions may lead to axonal damage over the course of time, but few studies to date have looked at the impact of repeated head collisions in non-concussed subjects. One exception is the work of Bazarian et al [6], who investigated differences in DTI derived parameters in individual subjects in nine high school athletes engaged in high impact sports, most of whom did not suffer from concussions, but received successive head blows through the season. The comparison between the athletes (one concussed subject, six subjects who suffered head blows) and six controls (who suffered no injuries or minor orthopedic injuries, in which X-rays revealed no fractures) showed significant changes in FA and MD values. However, some regions shown in previous literature to be key areas associated with TBI (corpus callosum, external capsule, etc.) had lesser FA and MD differences compared to regions such as the posterior thalamic radiata and the cingulum [5]. Given the popularity of contact sports and the potential long-term impact of brain injuries, it is crucial to understand the effects of repeated collisions on the brain.
Impacts incurred during the course of many contact sports include direct forces as well as rotational shearing forces. The regions shown in the figure 1 are consistent with areas detected as affected by injury in previous studies [5, 9,19]. There was no significant difference in the cortico-spinal tract, which was also mentioned in some post-concussion studies in the literature [9,19]; however, most of the participants in this study at both time-points had not been subjected to acute diagnosed concussions, even though they may have be subject to non-concussive head injuries.
It is interesting to note that while most of the locations indicating significance in the FA and MD data are similar, a few areas differ. This is not surprising, as these two values do not measure the same aspect of injury. The MD t-tests show significant change in the CC in both pre-season and post-season tests, although a larger section is shown to be significant in the post-season t-tests. This indicates a change in the way water diffuses in the CC and may be associated with diffusion in inflamed/injured tissue [5]. FA shows difference in the CC, but also shows a strong difference in the inferior fronto-occipital fasciculus, indicating that there is a larger change in restrictive movement of water in that region. These differences are thought to be due to the dynamic nature of the axonal injury [5,6] and the results of the t-tests above offer some insight into which areas to further focus analysis on.
The corpus callosum is the largest and one of the most important fiber tracts in the brain. Because it is the primary connecting fiber structure between the hemispheres, it is known to be highly affected by concussive impacts to the brain. Also, the corpus callosum is relatively tethered by the anterior and posterior inter-hemispheric fissure or falx, so direct or rotational force can result in direct or shearing injury of the CC.
In related work, we looked at the relative pose of the corpus callosum based on its point distribution model (PDM) to investigate the affect of the torque generated through collisions. The relative pose analysis is used to detect the relative translation, rotation and scale between the pre-season and postseason scans. For each CC, the relative pose was obtained by a full Procrustes fit of a template shape to the PDM. The template shape was selected as the mean shape that minimized the Procrustes distances, and it was computed iteratively. The results showed that the combined surface morphometry and relative pose analyses successfully detects differences in both the shape and post of the CC between pre-season and postseason contact sport participants [13]. The study described in this paper, along with the pose analysis described in the paragraph above, supports the concept that repeated physical impact may be related to changes in the brain.
Some of the limitations factors in our study are the b-value and the number of gradient directions. The bvalue is 1000s/mm2, which is commonly used in clinical settings. However, a higher b-value may provide more accurate information along with more gradient directions, as DTI is sensitive to slower water diffusion. With the b-value used in this study, it is expected that the extracellular water diffusion will dominate the signal [14].
A further limitation is our small sample size. Finding statistical significance in the DTI in a relatively small sample size does strengthen the significance of the findings. Longitudinal analysis of FA, MD, and other DTI parameters of contact and non-contact sport athletes would help us understand the progression or restoration patterns of these white matter tracts.
Finally, one potential confound in this study is that concussions are notoriously under-reported. Athletes often downplay their symptoms in spite of on-field psychometric and neurological examinations in order to be allowed to continue to play. Hence, it is possible that a few of the contact sports athletes in this study did suffer non-diagnosed concussions, though this is difficult to assess in this study.
In conclusion, we found significant DTI changes in the corpus callosum, the external capsule, the inferior fronto-occipital fasciculus as well as regions such as the superior/posterior corona radiata. These differences were seen in both comparing the pre-season contact versus the non-contact controls and also comparing the post-season contact athletes with the controls. There seems to be some difference in the p-values generated from post- vs. pre-season data which will be investigated in further analyses. We plan on recruiting further subjects and directly looking at individual pre-season to post-season differences in the near future.
Abbreviations and Acronyms
- DTI
Diffusion Tensor Imaging
- FA
Fractional Anisotropy
- MD
Mean Diffusivity
- CC
Corpus Callosum
- pFDR
positive False Discovery Rate
- TBI
Traumatic Brain Injury
- TR
Repetition time
- TE
Echo time
- CSF
cerebrospinal fluid
- FOV
Field of View
- CTE
chronic traumatic encephalopathy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Niharika Gajawelli, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA, gajawell@usc.edu
Yi Lao, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA, yilao1987@gmail.edu
Michael L.J. Apuzzo, Department of Neurosurgery, Keck School of Medicine, University of Southern California, Los Angeles, CA, apuzzo@usc.edu
Russ Romano, Department of Athletics, University of Southern California, Los Angeles, CA, rromano@usc.edu
Charles Liu, Department of Neurosurgery, Keck School of Medicine, University of Southern California, Los Angeles, CA, cliu@usc.edu
Sinchai Tsao, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA, stsao@usc.edu
Darryl Hwang, Department of Radiology, Keck School of Medicine, University of Southern California, Los Angeles, CA, darrylhw@usc.edu
Bryce Wilkins, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA, brycewil@usc.edu
Natasha Lepore, Department of Radiology, Children’s Hospital, Los Angeles, CA, nlepore@usc.edu.
Meng Law, Department of Radiology, Keck School of Medicine, University of Southern California, Los Angeles, CA, meng.law@med.usc.edu.
References
- 1.Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Annals of neurology. 1982;12(6):557–563. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- 2.Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. American Journal of Neuroradiology. 2002;23(5):794–802. [PMC free article] [PubMed] [Google Scholar]
- 3.Assaf Y, Freidlin RZ, Rohde GK, Basser PJ. New modeling and experimental framework to characterize hindered and restricted water diffusion in brain white matter. Magnetic Resonance in Medicine. 2004;52(5):965–978. doi: 10.1002/mrm.20274. [DOI] [PubMed] [Google Scholar]
- 4.Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clinics in sports medicine. 2011;30(1):33–48. doi: 10.1016/j.csm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. Journal of neurotrauma. 2007;24(9):1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- 6.Bazarian JJ, Zhu T, Blyth B, Borrino A, Zhong J. Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magnetic resonance imaging. 2012;30(2):171–180. doi: 10.1016/j.mri.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cubon VA, Putukian M, Boyer C, Dettwiler A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. Journal of neurotrauma. 2011;28(2):189–201. doi: 10.1089/neu.2010.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dru F, Vercauteren T. An ITK implementation of the symmetric log-domain diffeomorphic demons algorithm. 2009 [Google Scholar]
- 9.Henry LC, Tremblay J, Tremblay S, Lee A, Brun C, Lepore N, Theoret H, Ellemberg D, Lassonde M. Acute and chronic changes in diffusivity measures after sports concussion. Journal of neurotrauma. 2011;28(10):2049–2059. doi: 10.1089/neu.2011.1836. [DOI] [PubMed] [Google Scholar]
- 10.Huisman TA, Schwamm LH, Schaefer PW, Koroshetz WJ, Shetty-Alva N, Ozsunar Y, Wua O, Sorensen AG. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. American Journal of Neuroradiology. 2004;25(3):370–376. [PMC free article] [PubMed] [Google Scholar]
- 11.Inglese M, Makani S, Johnson G, Cohen BA, Silver JA, Gonen O, Grossman RI. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. Journal of neurosurgery. 2005;103(2):298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- 12.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–90. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Lao Y, Gajawelli N, Haas L, Wilkins B, Hwang D, Tsao S, Wang Y, Law M, Lepore N. 3D Pre- vs. Post-Season Comparisons of Surface and Relative Pose of the Corpus Callosum in Contact Sport Athletes. Submitted to SPIE Medical Imaging. 2013 [Google Scholar]
- 14.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. Journal of magnetic resonance imaging. 2001;13(4):534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 15.Lipton ML, Gellella E, Lo C, Gold T, Ardekani BA, Shifteh K, Bello JA, Branch CA. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. Journal of neurotrauma. 2008;25(11):1335–1342. doi: 10.1089/neu.2008.0547. [DOI] [PubMed] [Google Scholar]
- 16.McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: progressive tauopathy following repetitive head injury. Journal of neuropathology and experimental neurology. 2009;68(7):709. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niogi SN, Mukherjee P, Ghajar J, Johnson CE, Kolster R, Lee H, Sun M, Zimmerman RD, Manley GT, McCandliss BD. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008;131(12):3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- 18.Rutgers DR, Toulgoat F, Cazejust J, Fillard P, Lasjaunias P, Ducreux D. White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. American Journal of Neuroradiology. 2008;29(3):514–519. doi: 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh M, Jeong J, Hwang D, Sungkarat W, Gruen P. Novel diffusion tensor imaging methodology to detect and quantify injured regions and affected brain pathways in traumatic brain injury. Magnetic resonance imaging. 2010;28(1):22–40. doi: 10.1016/j.mri.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strich S. Shearing of nerve fibres as a cause of brain damage due to head injury: a pathological study of twenty cases. The Lancet. 1961;278(7200):443–448. [Google Scholar]
- 21.Storey JD. A direct approach to false discovery rates. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2002;64(3):479–498. [Google Scholar]
- 22.Toussaint N, Souplet JC, Fillard P. MedINRIA: Medical image navigation and research tool by INRIA. In Proc of MICCAI. 2007:7. [Google Scholar]
- 23.Virji-Babul N, Borich MR, Makan N, Moore T, Frew K, Emery CA, Boyd LA. Diffusion tensor imaging of sports-related concussion in adolescents. Pediatric neurology. 2013;48(1):24–29. doi: 10.1016/j.pediatrneurol.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, Hanten GR, Troyanskava M, Yallampalli R, Li X, Chia J, Levin H. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70(12):948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Rasmussen IA, Lagopoulos J, Håberg A. Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. Journal of neurotrauma. 2007;24(5):753–765. doi: 10.1089/neu.2006.0208. [DOI] [PubMed] [Google Scholar]


