Abstract
Prenatal exposure to chlorpyrifos (CPF), an organophosphorus insecticide, has long been associated with delayed neurocognitive development and most recently with decrements in working memory at age 7. In the current paper, we expanded the previous work on CPF to investigate how additional biological and social environmental factors might create or explain differential neurodevelopmental susceptibility, focusing on main and moderating effects of the quality of the home environment (HOME) and child sex. We evaluate how the quality of the home environment (specifically, parental nurturance and environmental stimulation) and child sex interact with the adverse effects of prenatal CPF exposure on working memory at child age 7 years. We did not observe a remediating effect of a high quality home environment (either parental nurturance or environmental stimulation) on the adverse effects of prenatal CPF exposure on working memory. However, we detected a borderline significant interaction between prenatal exposure to CPF and child sex (B (95% CI) for interaction term = −1.714 (−3.753 to 0.326)) suggesting males experience a greater decrement in working memory than females following prenatal CPF exposure. In addition, we detected a borderline interaction between parental nurturance and child sex (B (95% CI) for interaction term = 1.490 (−0.518 to 3.499)) suggesting that, in terms of working memory, males benefit more from a nurturing environment than females. To our knowledge, this is the first investigation into factors that may inform an intervention strategy to reduce or reverse the cognitive deficits resulting from prenatal CPF exposure.
Keywords: chlorpyrifos, neurodevelopment, working memory, HOME inventory, sex-specific
Background
Chlorpyrifos (CPF) is an organophosphorus (OP) insecticide widely recognized for its neurotoxic properties and effectiveness at eliminating household pests including cockroaches. Once the leading insecticide used throughout the Unites States in residential and agricultural settings, widespread use resulted in ubiquitous exposure (Landrigan, et al., 1999; Whitemore, et al., 1994) until the US EPA curtailed its residential use in 2001 (U.S. EPA, 2000). Previous studies have reported that prenatal and early childhood exposure to OP insecticides, including CPF, is associated with indicators of delayed neurodevelopment (Berkowitz, et al., 2004; Engel, et al., 2007; Eskenazi, et al., 2007; Guillette, et al., 1998; Lizardi, et al., 2008; Young, et al., 2005). Most recently, results from three separate longitudinal birth cohort studies demonstrate that prenatal exposure to OP insecticides is negatively associated with cognitive development at 7-years of age (Bouchard, et al., 2011; Engel, et al., 2011; Rauh, et al., 2011). Rauh et al (2011) reported evidence of deficits in 7-year working memory and full scale IQ scores as a function of prenatal CPF exposure (Rauh, et al., 2011). Working memory is one of the core processes of executive function. It encompasses the ability to memorize new information, hold it in short-term memory, concentrate, and manipulate information to produce results (Baddeley and Logie, 1999; Smith and Jonides, 1997). Insufficient development of executive functioning during early childhood has been associated with an array of adverse outcomes including psychopathology (Pennington and Ozonoff, 1996), increased physical aggression (Tremblay, et al., 2005), cortisol reactivity (Blair, et al., 2005), and lack of school readiness (Blair, 2002).
It is recognized that biologic and social factors interact to affect neurologic development in children (Escanola, 1982), including the development of executive functions such as working memory (Diamond, 2009). The quality of the home environment is a particularly important social factor that predicts child neurodevelopment. Numerous prior studies demonstrate associations between the home environment and child cognition, including assessment among groups that differed by ethnicity, socioeconomic status, child weight, child disabilities, maltreatment and/or exposures to neurotoxicants (Bradley, 1993). Intervention studies suggest that improving the quality of the home environment can improve child cognitive performance (Wasik, et al., 1990). The Home Observation for the Measurement of the Environment (HOME) (Caldwell and Bradley, 1984) is a well-validated instrument for assessing the quality of the home environment. It was designed to assess a child’s physical, intellectual, and emotional milieu through both direct observation and unstructured interview with the child’s primary caregiver. Notably, the HOME scale includes subscales measuring specific aspects of the child’s home life such as parental nurturance and environmental stimulation. Farah et al. (2008) used the HOME scale to demonstrate that these different aspects of the home environment affect different components of cognitive development in humans (Farah, et al., 2008). Parental nurturing was associated with improved working memory while environmental stimulation facilitated improved language development. In the current study, we evaluate whether specific aspects of the home environment can remediate the adverse effects of environmental toxicants on children’s cognitive development.
There is growing evidence that child sex is also an important determinant of behavior and cognition. Research has shown that developing males and females respond differently to the effects of chemical stressors (e.g. polychlorinated biphenyls) and nonchemical stressors (e.g. poverty) on child behaviors (Weiss, 2002; Werner and Ruth, 1992). In the guinea pig, prenatal social stress resulted in elevated cortisol levels in male offspring with no effect in female offspring (Kaiser and Sachser, 1998; Kaiser and Sachser, 2001). While clinical evidence suggests males are generally more susceptible to infectious disease, hypertension (cardiovascular disease), and aggressive behaviors (Wang, et al., 2007), it is less clear how sex may influence the impact of toxicant exposure on developmental outcomes. In a recent epidemiologic study examining the impact of prenatal exposure to phthalates, common plasticizers, on reproductive development, adverse effects were only observed in male children (Swan, et al., 2005). Conversely, in a study examining the impact of bisphenol A (BPA) exposure on behavior in children, effects were observed especially among female children (Braun, et al., 2011). Notably, many studies of the adverse effects of endocrine disrupting compounds on cognition and behavior do not investigate differential effects in males vs. females (Engel, et al., 2009; Whyatt, et al., 2012). In general, the evaluation of child sex as a potential effect modifier of the effects of environmental toxicants on child development has received little attention in epidemiologic studies to date (Schwartz, 2003; Vahter, et al., 2007; Vahter, et al., 2007).
In the current study, we built on our prior investigation of prenatal CPF exposure (Rauh, et al., 2011) to evaluate how the quality of the home environment (specifically, parental nurturance and environmental stimulation) and child sex interact with the adverse effects of prenatal CPF exposure on working memory at child age 7 years. We hypothesize that a nurturing home environment may moderate the adverse effects of prenatal CPF exposure on children’s working memory at age 7. Further, we hypothesize that the moderation effect may be stronger in males than in females.
2. METHODS
2.1 Participants
The sample consisted of 335 mother-child pairs selected from an ongoing prospective cohort study (Columbia Center for Children’s Environmental Health) of inner-city mothers and their children (Perera, et al., 2002). The larger parent cohort (N=725), enrolled between 1998–2006, comprised pregnant women age 18–35 years who self-identified as either African-American or Dominican, did not smoke, were low-risk pregnancies (classified as free of diabetes, hypertension, and known HIV infection), lived in the designated neighborhoods for at least one year, and had registered at the Obstetrics and Gynecology prenatal clinics at New York Presbyterian Medical Center or Harlem Hospital by the 20th week of pregnancy. All participants gave informed consent and the Institutional Review Board of Columbia University approved the study. For the current study, we selected all offspring from this cohort who had reached age 7 at the time of this analysis and had complete data in the following areas: maternal prenatal and 7-year interview; biomarkers of prenatal CPF exposure, HOME assessment completed at 3 years of age; and WISC-IV administered at 7 years of age. The characteristics of this subsample are presented in Table 1. In general, the 335 subjects selected for the current study did not differ from the full parent cohort with respect to demographic characteristics.
Table 1.
Demographic characteristics of the study population, New York City, 2009–2010 (N = 335)
| Characteristics | N | % |
|---|---|---|
| Family income | ||
| <$20,000 | 172 | 51.3 |
| ≥$20,000 | 163 | 48.7 |
| Maternal education (years) | ||
| <12 years | 230 | 68.7 |
| ≥12 years | 105 | 31.3 |
| Race/ethnicity | ||
| Dominican | 132 | 39.4 |
| African American | 203 | 60.6 |
| Median | Range | |
| Prenatal CPF (ng/g) | 0.36 | 0.25–32.1 |
| Mean | SE | |
| HOME score (total score) | 39.8 | 0.34 |
| WISC_IV* | ||
| Full Scale IQ | 99.3 | 0.70 |
| Working memory | 98.3 | 0.77 |
| Perceptual reasoning | 100.5 | 0.75 |
| Verbal comprehension | 96.97 | 0.65 |
| Processing speed | 102.0 | 0.87 |
Wechsler Intelligence Scale for Children, 4th edition, composite scores of We
2.2 Maternal interview and HOME assessment
Mothers were interviewed in the 3rd trimester of pregnancy and annually thereafter by a trained bilingual interviewer. Interviews included questions about demographics; residential history; living conditions; maternal education; maternal income and employment; illness, alcohol and drug use during pregnancy; and chemical exposures, including pesticides, polycyclic aromatic hydrocarbons (PAHs), lead and environmental tobacco smoke (ETS).
Children’s home environments were evaluated at 3 years of age (mean = 3.6 years; range = 1.1–6.3 years) using the HOME Inventory (Bradley, 1993; Caldwell and Bradley, 1984). The HOME Inventory is an unstructured 1-hour observational interview administered by a trained researcher and widely used as a predictor of child intelligence and achievement (Bradely, et al., 1989). The 55-item checklist is divided into eight subscales. Based on previous literature employing the HOME inventory to examine the association between childhood experience and cognitive development (Farah, et al., 2008), we divided the 8 subscales into two composite scales, Environmental Stimulation and Parental Nurturance. The Environmental Stimulation variable was created by summing the z-scores of the Learning Materials, Language Stimulation, Academic Stimulation, and Variety subscales, which measure the availability of intellectually stimulating materials in the home and the mother’s encouragement of learning. The Parental Nurturance variable was created by summing the z-scores of the Responsivity, Modeling, and Acceptance subscales, which measure such maternal behaviors as attentiveness, displays of physical affection, encouragement of delayed gratification, limit setting, and the ability of the mother to control her negative reactions.
In addition to the primary predictors included in the final models described below, we examined other maternal toxicant exposures with the potential to impact children’s cognitive development including prenatal exposure to PAHs (Perera, et al., 2006), ETS (Eskenazi and Castorina, 1999), and lead (Lanphear, et al., 2005). PAHs were measured in personal air during the 3rd trimester using a previously described method (Perera, et al., 2003). We computed a composite log-transformed PAH variable from eight correlated PAH air concentration measures (r values ranging from 0.34–0.94; all p-values < 0.001 by Spearman’s rank) (Perera, et al., 2003). The ETS variable was generated using maternal report of smoking and/or smokers in the home during pregnancy. The variable has been previously validated against maternal cotinine values and shown to accurately represent exposure to ETS (Rauh, et al., 2004). In this subset selected for analysis in the current paper, PAH was not an independent predictor of working memory (B (95% CI) = 0.10 (−0.31 to 0.52), p = 0.62). ETS weakly, though non-significantly, predicted working memory in unadjusted models (−2.72 (−0.59 to 0.51), p = 0.09), though it was not a significant predictor in models adjusted for income, maternal education and prenatal CPF (−1.956 (−5.203 to 1.290), p = 0.24). As inclusion of PAH or ETS did not change the effect size or the significance of the relationship between chlorpyrifos and working memory, or between the HOME inventory and working memory at 7 years of age, they were not included in the final models.
Blood lead was measured in only 91 blood samples collected when subjects were 7 years of age. Although in this small subset of 91, lead appeared to have an inverse relationship with working memory in models controlling for income, education, and HOME inventory (B (95% CI) = −3.62 (−5.98 to −1.26), CPF levels were below the limit of detection in all 91 blood samples thus we are unable to test the potential confounding effect of lead on the relationship between prenatal CPF and working memory.
2.3 Biological samples and pesticide exposure
Trained hospital staff collected a 30–60 ml sample of umbilical cord blood at delivery and a 30–35 ml sample of maternal blood within two days of delivery. Collection, processing, and storage of blood samples has been described elsewhere (Perera, et al., 2002; Whyatt, et al., 2003). Aliquots of blood samples were sent to the Centers for Disease Control and Prevention (Atlanta, Georgia) for analysis of levels of CPF, cotinine, and metals (Barr, et al., 2002). For 12% of the subjects included in the current analysis, the umbilical cord blood sample was unavailable and mother’s values were substituted using a standardized algorithm (Whyatt, et al., 2005). Within this subset, 40% of samples had CPF measurements less than the limit of detection (LOD). For these subjects, values below the limits of detection (0.5–1 pg/g) were assigned a value of one half the detection limit concentration.
2.4 Measures of neurodevelopment
To assess neurodevelopment at 7 years of age, trained research workers administered the Wechsler Scales of Infant Intelligence (WISC-IV) (Wechsler, 1991). The instrument consists of 4 indices designed to measure 4 different areas of mental functioning that are associated with, but distinct from, overall IQ, and is sensitive to cognitive deficits related to learning and working memory. The 4 indices include Verbal Comprehension, Perceptual Reasoning, Working Memory, and Processing Speed. The full scale IQ is a summary of the 4 separate indices. We examined the influence of the HOME inventory on all 4 indices of the WISC-IV as well as the full-scale score. However, due to the inverse relationship between CPF and working memory previously published in this cohort (Rauh, et al., 2011), we focused primarily on this index.
Conforming to the demographics of the study populations, subjects were offered the choice of completing the WISC-IV in English or Spanish. The Spanish version of the WISC-IV was designed to test the same constructs as the WISC-IV and results are compared to all U.S. children of the same age (Wechsler, 2005). 28/315 (8.4%) of the subjects in this cohort selected to test in Spanish. The scores for the working memory were not significantly different between subjects tested in English or Spanish (mean working memory score ± SE; English = 98.55 ± 0.81, Spanish = 95.54 ± 2.648, p = 0.279). Addition of language of administration (0=Spanish, 1=English) to the linear regression models did not alter the association between prenatal CPF exposure and working memory, nor the relationship between parental nurturance and working memory we therefore did not include this variable in the presented analyses.
2.5 Data analyses
These following analyses included observations from children with complete data on prenatal CPF exposure, HOME inventory administered at 3 years of age, and 7-year WISC-IV (n = 335). We conducted all analyses using SPSS (PAWS 18). CPF values were log transformed to an approximate normal distribution. We treated CPF (log pg/g), WISC-IV working memory scores, and HOME inventory variables including the total HOME score and the 2 composite scores as continuous variables.
Unadjusted analyses were used to explore the associations between prenatal CPF exposure, the quality of the home environment (Total HOME, as well as Parental Nurturance and Environmental Stimulation subscales), childhood IQ (WISC-IV) and demographic characteristics (Table 1).
Multivariable linear regression models examined the association between predictor variables and working memory scores at 7 years of age. Interaction terms were created to represent 1) prenatal CPF * Total HOME score 2) prenatal CPF * child sex and 3) Parental Nurturance * child sex.
Effect estimates, 95%CIs, and p-values were calculated for all analytic procedures. Results were considered significant at p < 0.05.
3. Results
3.1 Sample characteristics
Demographic characteristics of the families included in this study are presented in Table 1. Families were of low economic standing. Over half of the mothers enrolled in the cohort (51.3%) reported an annual family income <$20,000. Mothers also reported low educational status. 31% had not completed high school at the time of the child’s 7-year evaluation. Chlorpyrifos was detected in 60% of blood samples (cord or maternal), concentrations ranged from 0.25 to 32.14 pg/g. The mean WISC-IV Full Scale Composite IQ score for children at age 7 was 99.25 (range 48–133). The working memory composite score was 98.4 (range 54–135). The mean HOME Total score was 39.8 ± 0.34 (out of a maximum of 55).
3.2 Unadjusted relationships between CPF concentrations and working memory
Table 2 shows the unadjusted associations between family income, and maternal education and child sex with prenatal CPF levels, Total HOME scores and WISC-IV Working Memory scores. Although predictive analyses (presented later) considered the Parental Nurturance and Environmental Stimulation subscales of the HOME, in Table 1 we present the more familiar Total HOME score (Wasserman, et al., 2001). Family income and maternal years of education were positively associated with Total HOME score and WISC-IV working memory score. CPF levels and Total HOME scores did not differ significantly by child sex; however, WISC-IV working memory scores were higher among females (p = 0.034).
Table 2.
Unadjusted associationsa between prenatal chlorpyrifos measured in maternal blood samples collected during pregnancy (log pg/g), Total HOME score and WISC-IV working memory scores at age 7 (n = 335).
| Characteristics | All | Prenatal CPF (log pg/g) | Total HOME | WISC-IV Working Memory | |
|---|---|---|---|---|---|
| N | % | Mean ± SE | Mean ± SE | Mean ± SE | |
| Family Income | |||||
| <$20,000 | 172 | 51.3 | −0.068 ± 0.12 | 38.21 ± 0.45 | 96.5 ± 1.17 |
| ≥$20,000 | 163 | 48.7 | 0.031 ± 0.11 | 41.37 ± 0.46 | 100.2 ± 0.97 |
| p = 0.389 | p < 0.001 | p = 0.014 | |||
| Maternal education (years)b | |||||
| <12 years | 105 | 31.3 | 0.0002 ± 0.15 | 37.52 ± 0.59 | 95.9 ± 1.30 |
| ≥12 years | 230 | 68.7 | −0.029 ± 0.09 | 40.76 ± 0.38 | 14.27 ± 0.94 |
| p = 0.866 | p < 0.001 | p = 0.017 | |||
| Child Sex | |||||
| Female | 184 | 54.9 | −0.07 ± 0.11 | 40.2 ± 0.44 | 99.8 ± 1.00 |
| Male | 151 | 45.1 | 0.04 ± 0.12 | 39.3 ± 0.51 | 96.5 ± 1.20 |
| p = 0.519 | p = 0.162 | p = 0.034 | |||
Students t-test, p value
Maternal education was categorized for the table only, it was used as a continuous value in the regression analyses.
Univariate regression analyses examining prenatal CPF and WISC-IV working memory score confirmed the inverse relationship between CPF exposure and working memory (B (95%CI) = −1.479 (−2.52 to −0.44)) (Rauh, et al., 2011). Total HOME score was positively associated with WISC-IV working memory (B (95% CI) = 0.427 (0.18 to 0.67)). Of the two HOME subscales, Parental Nurturance was more strongly associated with working memory than Environmental Stimulation (B (95% CI) =1.68 (0.68 to 2.69) vs. 0.52 (0.06 to 0.98), respectively).
3.3 Adjusted relationships between CPF concentrations and working memory
Based on Farah (2008), we next sought to determine the specific aspects of the HOME environment that influenced working memory in the presence of prenatal CPF exposure. We applied linear regression models to predict the adjusted relationship between prenatal CPF exposure and WISC-IV working memory, controlling for family income, maternal education, child sex and the three different HOME scale scores (total HOME, Parental Nurturance and Environmental Stimulation). The three adjusted models are presented in Table 3. Chlorpyrifos is significantly predictive of working memory in all three models. Parental Nurturance more strongly predicts working memory than the Total HOME score or the Environmental Stimulation subscale: (B (95% CI)) Total HOME score = 0.283 (−0.02 to 0.55), p = 0.037; Parental Nurturance = 1.265 (0.27 to 2.28), p = 0.013; Environmental Stimulation 0.292 (−0.19 to 0.77), p = 0.230). Although the Total HOME Score did not differ by child sex, Parental Nurturance was significantly higher among females than males (Students T-test, p = 0.02).
Table 3.
Adjusted linear regression models predicting WISC-IV working memory at age 7 adjusting for Total HOME score, Parental Nurturance (z-score) or Environmental Stimulation (z-score).
| N=335 | WISC-IV Working Memory | ||
|---|---|---|---|
| B | 95% CI | p | |
| Total HOME score | |||
| CPF | −1.451 | −2.265 to –0.438 | 0.005 |
| Family income | 2.459 | −0.611 to 5.528 | 0.116 |
| Maternal education | 0.475 | −0.137 to 1.088 | 0.128 |
| Child sex | −3.032 | −6.003 to –0.062 | 0.072 |
| Total HOME score | 0.283 | −0.017 to 0.548 | 0.037 |
| Parental Nurturance | |||
| CPF | −1.355 | −2.368 to –0.341 | 0.009 |
| Family income | 2.870 | −0.136 to 5.875 | 0.061 |
| Maternal education | 0.617 | 0.036 to 1.199 | 0.037 |
| Child sex | −2.863 | −5.835 to 0.109 | 0.059 |
| Parental Nurturance | 1.275 | 0.273 to 2.278 | 0.013 |
| Environmental Stimulation | |||
| CPF | −1.478 | −2.496 to –0.459 | 0.005 |
| Family income | 2.817 | −0.243 to 5.876 | 0.071 |
| Maternal education | 0.581 | −0.025 to 1.188 | 0.060 |
| Child sex | −3.231 | −6.207 to –0.256 | 0.033 |
| Environmental Stimulation | 0.292 | −0.186 to 0.770 | 0.230 |
Variable/covariate definitions: CPF = log transformed prenatal chlorpyrifos exposure (log pg/g); Family income 0 (< $20,000), 1 (≥$20,000); Maternal education = years of maternal education at child age 7; Child sex 0 (female), 1 (male)
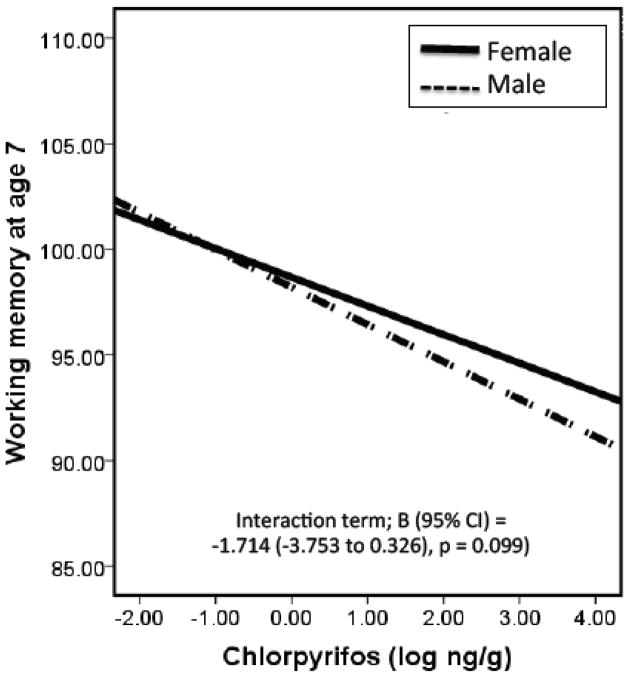
To determine the role of child sex on the relationship between prenatal CPF exposure and working memory, we stratified subjects based on child sex. In unadjusted stratified models, the impact of CPF on working memory was only observed in males (B (95% CI) males = −2.382 (−3.88 to −0.88) p = 0.002; B (95%CI) females = −0.524 (−1.90 to 0.85) p = 0.453). Figures 1 and 2 and Table 4 demonstrate the unadjusted and adjusted interactions between child sex, prenatal CPF, and Parental Nurturance on working memory at age 7. We detected a borderline significant interaction between prenatal exposure to CPF and child sex (B (95% CI) for interaction term = −1.714 (−3.753 to 0.326), p = 0.099) suggesting that males experience a greater decrement in working memory score following prenatal CPF exposure (Figure 1). In addition, we detected a borderline interaction between Parental Nurturance and child sex (B (95% CI) for interaction term = 1.490 (−0.518 to 3.499), p = 0.145) suggesting that, in terms of working memory, males benefit more from a nurturing environment than females (Figure 2). We did not detect an interaction between prenatal exposure to CPF and the quality of the home environment using either Total HOME score as a covariate (B (95% CI) for interaction term = −0.069 (−0.240 to 0.101), p = 0.424) or Parental Nurturance (B (95% CI) for interaction term = 0.024 (−0.069 to 0.738), p = 0.947), suggesting that the quality of the home environment does not modify the relationship between prenatal CPF exposure and working memory (Table 4).
Figure 1.
Interaction between prenatal CPF (log ng/g) and child sex on working memory at age 7. This figure depicts the interaction between prenatal CPF exposure and child sex as they effect working memory at child age 7. The solid line represents females, dashed line males. Males exposures to CPF seem to experience greater decrements in working memory scores than females exposed to CPF.
Figure 2.
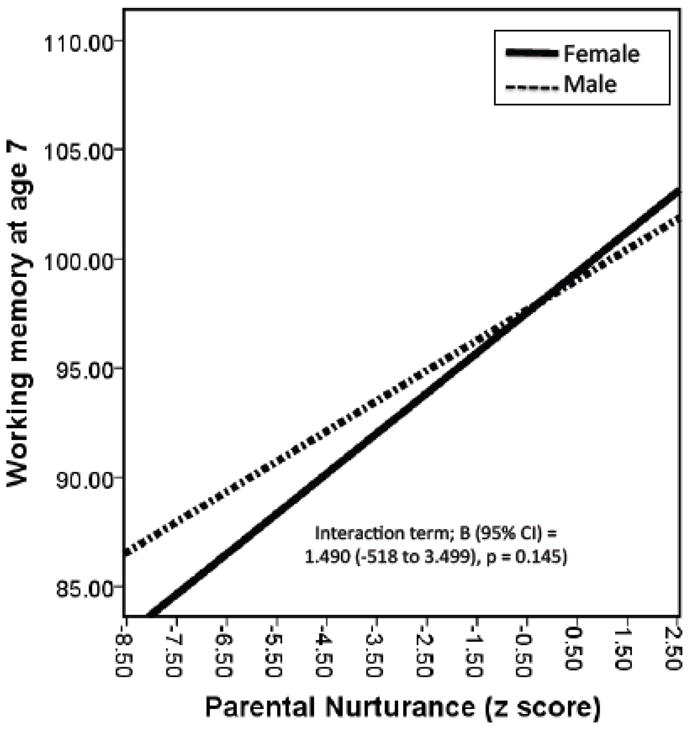
Interaction between Parental nurturance subset score of the HOME assessment (z-score) and child sex on working memory at age 7. The solid line represents females, dashed line males. In terms of working memory, males appear to benefit more from a nurturing home environment than females.
Table 4.
Unadjusted and adjusted linear regression relationships between prenatal CPF exposure and WISC-IV working memory at 7 years of age, including interactions between predictor variables.
| N=335 | WISC-IV Working Memory | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusteda | Adjustedb | Adjusted with interaction termc | |||||||
| B | 95% CI | p | B | 95% CI | p | B | 95% CI | p | |
| Prenatal CPF * Child sex | |||||||||
| Prenatal CPF | −1.479 | −2.516 to −0.442 | 0.005 | −1.355 | −2.368 to −0.341 | 0.009 | −0.553 | −1.943 to 0.836 | 0.434 |
| Family income | 2.870 | −0.136 to 1.199 | 0.061 | 2.862 | −0.136 to 5.860 | 0.061 | |||
| Maternal education | 0.617 | 0.036 to 1.199 | 0.037 | 0.615 | 0.035 to 1.195 | 0.038 | |||
| Parental Nurturance | 1.275 | 0.273 to 2.278 | 0.013 | 1.150 | 0.139 to 2.161 | 0.026 | |||
| Child sex | −2.863 | −5.835 to 0.109 | 0.059 | −2.930 | −5.895 to 0.036 | 0.053 | |||
| Prenatal CPF x child sex | −1.714 | −3.753 to 0.326 | 0.099 | ||||||
| Parental nurturance * Child sex | |||||||||
| Prenatal CPF | −1.479 | −2.516 to −0.442 | 0.005 | −1.355 | −2.368 to −0.341 | 0.009 | −1.248 | −2.270 to 0.227 | 0.017 |
| Family income | 2.870 | −0.136 to 1.199 | 0.061 | 2.793 | −2.09 to 5.795 | 0.068 | |||
| Maternal education | 0.617 | 0.036 to 1.199 | 0.037 | 0.621 | 0.040 to 1.201 | 0.036 | |||
| Parental Nurturance | 1.275 | 0.273 to 2.278 | 0.013 | 0.477 | −0.992 to 1.947 | 0.523 | |||
| Child sex | −2.863 | −5.835 to 0.109 | 0.059 | −2.969 | −5.940 to 0.002 | 0.050 | |||
| Parental nurturance x child sex | 1.490 | −0.518 to 3.499 | 0.145 | ||||||
| Prenatal CPF * Parental nurturance | |||||||||
| Prenatal CPF | −1.479 | −2.516 to −0.442 | 0.005 | −1.355 | −2.368 to −0.341 | 0.009 | −1.354 | −2.369 to − 0.339 | 0.009 |
| Family income | 2.870 | −0.136 to 1.199 | 0.061 | 2.864 | −0.151 to 5.879 | 0.063 | |||
| Maternal education | 0.617 | 0.036 to 1.199 | 0.037 | 0.618 | 0.035 to 1.200 | 0.038 | |||
| Parental Nurturance | 1.275 | 0.273 to 2.278 | 0.013 | 1.280 | 0.267 to 2.293 | 0.013 | |||
| Child sex | −2.863 | −5.835 to 0.109 | 0.059 | −2.846 | −5.865 to 0.173 | 0.013 | |||
| Prenatal CPF* Parental nurturance | 0.024 | −0.690 to 0.738 | 0.947 | ||||||
Variable/covariate definitions: CPF = log transformed prenatal chlorpyrifos exposure (log pg/g); Family income 0 (< $20,000), 1 (≥$20,000); Maternal education = years of maternal education at child age 7; Child sex 0 (female), 1 (male)
Unadjusted regression model
Adjusted models include family income, maternal education, parental nurturance, and child sex
Adjusted models include family income, maternal education, parental nurturance, child sex and interaction term
Discussion
This study presents results from analyses exploring the influence of social and biological factors on the inverse association between prenatal CPF exposure and working memory at child age 7. In 2011, Rauh et al reported a deficit in working memory and full scale IQ among 7 years olds following prenatal exposure to CPF using the WISC-IV instrument (Rauh, et al., 2011). We replicated the inverse association previously reported by Rauh (2011) between prenatal CPF exposure and neurodevelopment, focusing on the working memory component of IQ. We extended the previous model to determine how additional biological and social environmental factors might create or explain differential neurodevelopmental susceptibility, focusing on the main and moderating effects of the quality of the home environment (HOME) and child sex. Contrary to our hypothesis, we did not observe a buffering or remediating effect of a high quality home environment on the adverse effects of prenatal CPF exposure on working memory. To our knowledge, this is the first investigation into a potential intervention strategy to reduce or reverse the cognitive deficits resulting from prenatal CPF exposure.
Biologic and social factors may exacerbate the negative affects of toxic exposures, as is the case of stressful or impoverished conditions (Rauh, et al., 2008; Wright, 2009). Recently, the first population-based study demonstrated an association between a community level stressor (violence) and outdoor air pollution (NO2) with increasing risk for childhood asthma in an urban sample (Clougherty, et al., 2007). In this study, researchers observed an association between NO2 and risk for childhood asthma only among children who were also exposed to social stress. A similar epidemiologic study demonstrated that chronic traffic-related air pollution exposure and stress interacted in predicting increased asthma symptoms and heightened inflammatory profiles in adolescents with asthma (Chen, et al., 2008). However, biologic and social factors may also serve to buffer or remediate adverse cognitive effects, as has been demonstrated in the rodent literature. Over 30 years ago, it was suggested that environmental enrichment of rodent cages (i.e., addition of tunnels, ladders and toys as well as reduction of overcrowding) was shown to remediate the adverse effects of experimental cretinism induced in rats. Compared to rats living in impoverished conditions, enrichment remediated the adverse effects of hypothyroid-induced cretinism in rats; hypothyroid rats raised in enriched environments demonstrated reduced deficits in maze learning, maze retention, and resistance to extinction of bar-pressing (Davenport, et al., 1976). More recently, environmental enrichment has been shown to reverse the cognitive and molecular deficits induced by developmental lead exposure (Guilarte, et al., 2003). Maternal nurturing behavior (i.e. licking and grooming) following a brief stressor regulated pups’ later responses to stress and predicted better subsequent learning ability (Bredy, et al., 2003; Weaver, et al., 2005). In contrast to animal brain development, much less is known about the effect of childhood experience on the developing human brain (Rao, et al., 2010). In assessing the direct effects of the social environment, we know that children raised in “impoverished environments” exhibit impairments in cognitive and behavioral functioning whereas children raised in highly stimulating or enriched environments exhibit enhanced behavioral and cognitive outcomes (Joseph, 1999; Kaler and Freeman, 1994). Studies using the HOME Inventory suggest that children raised in environments with more cognitive stimulation and less socioeconomic adversity demonstrate better outcomes on global cognitive measures such as IQ and school achievement (Bradley, et al., 2001).
Historically, children’s environmental health studies have focused separately on how social, biological or environmental factors affect children’s development. In the past decade, recognition has grown that adverse outcomes of exposure to environmental pollutants are not simply due to the inherent properties of the chemical but derive from the joint action of psychosocial and biologic conditions that may exacerbate or alleviate the effects of toxic exposures (Weiss and Bellinger, 2006). Because of covariance across exposures and evidence that environmental and social stressors may influence common physiologic pathways, understanding the potentially synergistic effects promises to more completely inform children’s environmental health risk. This is all the more important because exposures to environmental hazards tend to co-occur with other forms of chronic psychosocial adversity, giving rise to environmental inequities whereby the most vulnerable members of society bear the greatest toxic burden (Krieger, et al., 1993; Zapata, et al., 1992) altering individual level and population level risk in systematic ways (Bellinger, 2000; Collins and Hammond, 1996; O’Campo, et al., 1997).
In our study, we detected a borderline significant interaction between prenatal exposure to CPF and child sex, suggesting that males experience a greater decrement in working memory score following prenatal CPF exposure. This is consistent with the literature suggesting sex selectivity of the neurotoxic effects of CPF (Garcia, et al., 2003; Levin, et al., 2002). Several factors may contribute to the differential vulnerability to CPF by sex. One potential explanation is CPF’s role as an endocrine disrupter. CPF has been shown to have anti-androgenic effects reducing serum testosterone levels in rats (Kang, et al., 2004). Male rats have a higher rate of hepatic activation of the CPF oxon, the metabolite that inhibits acetylcholinesterase AcTH, as well as more rapid detoxification of the CPF oxon. Inhibition of AcTH is noted as the mechanism of systemic toxicity for chlorpyrifos (Smegal, 2000). However, sexual differences in the activities of these enzymes that carry out the functions generally do not emerge until puberty. Further, the effects observed occur at levels well below those required to inhibit AcTH. Males have a slower rate of cortical development than females, making the male brain susceptible to insult for a longer period (Taylor, 1969).
We also observed a borderline interaction between Parental Nurturance and child sex suggesting that, in terms of working memory, males may benefit more from a nurturing environment than females. Our results support those of Farah et al (2008) suggesting a causal relationship between parental nurturance on memory ability. Nurturance has been widely associated with cognitive development (Bergman, et al., 2010; Farah, et al., 2008), as well as long-term health (Chen, et al., 2011). The effect of maternal nurturance appears to differ among males and females, with boys more strongly affected by the extremes at both ends of the spectrum. Attachment studies have shown that among children with an insecure or disorganized attachment style, boys are more likely than girls to manifest behavioral problems when they reach school age (Fearon, et al., 2010; Pasco Fearon and Belsky, 2011; Rauh, et al., 2008). In addition, a pregnancy cohort study demonstrated an association between breastfeeding duration and academic achievement at 10 years of age among boys, but not girls, suggesting that the mediator of this relationship could be increased mother bonding, attention, and interaction (Oddy, et al., 2011).
Generalizability of our study is limited, as the cohort comprises exclusively low-income, urban, Dominican and African American children. The majority of households are headed by single mothers who, because of their role as sole caregivers, may nurture their children differently than partnered women. Certain covariates such as prenatal stress, a possible contributor to working memory in children (Entringer, et al., 2010), were not included in the analysis. Further, because CPF exposure data were only collected at the time of delivery, it is impossible to draw any conclusions about the potentially differential effects of exposure at various stages of development. An additional limitation regarding the CPF measurements concerns the high frequency of subjects with levels of CPF below the limit of detection. A common practice in dealing with non-detects in to assign imputed values such as LOD/√2 (Arunajadai and Rauh, 2012), though it is possible that substitution of these values can lead to biased estimates (Lubin, et al., 2004).
In conclusion, these results do not support our main hypothesis that a high quality home environment modifies the adverse effects of prenatal CPF exposure on working memory at age 7. However, similar to other studies examining environmental toxicants such as lead, social circumstances and childhood IQ, these results suggest that the effects of social circumstances (i.e. quality of the home environment) on childhood IQ scores are substantially greater than the effects of environmental exposure (Wasserman, et al., 1997) and should be considered in studies examining the relationships between environmental exposures and child development. In addition to our main hypothesis examining the role of the home environment, we investigated the role of child sex in the relationship between prenatal CPF exposure and working memory. As child sex seems to interact with the relationship between the home environment and working memory, as well as between prenatal CPF exposure and working memory, we suggest that epidemiologic and laboratory based studies examining the relationship between prenatal and early childhood exposure to environmental toxicants and developmental outcomes include sex-specific analysis. Inclusion of both child sex and social circumstances in epidemiologic studies of environmental toxicants and neurodevelopment will greatly expand our understanding of these relationships and assist the development of studies to better identify vulnerable populations and perhaps direct interventions to protect them.
Highlights.
Prenatal exposure to chlorpyrifos (CPF) is associated with delayed neurocognitive development
We examine the impact of biological and social environmental factors on this association
A good quality home environment did not moderate the adverse effects of CPF on working memory
Males experience a greater deficit in working memory than females following prenatal CPF exposure
Male children benefit more from a nurturing home environment than females
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- U.S. EPA. US EPA: Chlorpyrifos Revised Risk Assessment and Agreement with Registrants. Washington, D.C: 2000. [Google Scholar]
- Arunajadai S, Rauh V. Handling covariates subject to limits of detection in regression. Environmental and Ecological Statistics. 2012 Apr 1;:1–23. [Google Scholar]
- Baddeley A, Logie R. Working memory: the multi-component model. In: AM, PS, editors. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. Cambridge University Press; New York: 1999. pp. 28–61. [Google Scholar]
- Barr DB, Barr JR, Maggio VL, Whitehead RD, Jr, Sadowski MA, Whyatt RM, et al. A multi-analyte method for the quantification of contemporary pesticides in human serum and plasma using high-resolution mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778(1–2):99–111. doi: 10.1016/s0378-4347(01)00444-3. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Effect modification in epidemiologic studies of low-level neurotoxicant exposures and health outcomes. Neurotoxicology and teratology. 2000;22(1):133–140. doi: 10.1016/s0892-0362(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, Glover V, O’Connor TG. Maternal prenatal cortisol and infant cognitive development: moderation by infant-mother attachment. Biological psychiatry. 2010;67(11):1026–1032. doi: 10.1016/j.biopsych.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz GS, Wetmur JG, Birman-Deych E, Obel J, Lapinski RH, Godbold JH, et al. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environ Health Perspect. 2004;112(3):388–391. doi: 10.1289/ehp.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. School readiness. Integrating cognition and emotion in a neurobiological conceptualization of children’s functioning at school entry. The American psychologist. 2002;57(2):111–127. doi: 10.1037//0003-066x.57.2.111. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger D, Peters Razza R. Cortisol reactivity is positively related to executive function in preschool children attending head start. Child development. 2005;76(3):554–567. doi: 10.1111/j.1467-8624.2005.00863.x. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environmental health perspectives. 2011;119(8):1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradely R, Caldwell B, Rock S, Barnard K, Gray C, Hammond M, et al. Home-environment and cognitive-development in the 1st 3 years of life - a collaborative study involving 6 sites and3 ethnic-groups in North America. Developmental Psychology. 1989;25(2):217–235. [Google Scholar]
- Bradley RH. Children’s home environments, health, behavior, and intervention efforts: a review using the HOME inventory as a marker measure. Genetic, social, and general psychology monographs. 1993;119(4):437–490. [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF, McAdoo HP, Coll CG. The home environments of children in the United States part I: variations by age, ethnicity, and poverty status. Child development. 2001;72(6):1844–1867. doi: 10.1111/1467-8624.t01-1-00382. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128(5):873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience. 2003;118(2):571–576. doi: 10.1016/s0306-4522(02)00918-1. [DOI] [PubMed] [Google Scholar]
- Caldwell B, Bradley RH. University of Arkansas: Little Rock. 1984. [Google Scholar]
- Chen E, Schreier HM, Strunk RC, Brauer M. Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environmental health perspectives. 2008;116(7):970–975. doi: 10.1289/ehp.11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular psychiatry. 2011;16(7):729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty JE, Levy JI, Kubzansky LD, Ryan PB, Suglia SF, Canner MJ, et al. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environmental health perspectives. 2007;115(8):1140–1146. doi: 10.1289/ehp.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JW, Jr, Hammond NA. Relation of maternal race to the risk of preterm, non-low birth weight infants: a population study. American journal of epidemiology. 1996;143(4):333–337. doi: 10.1093/oxfordjournals.aje.a008747. [DOI] [PubMed] [Google Scholar]
- Davenport JW, Gonzalez LM, Carey JC, Bishop SB, Hagquist WW. Environmental stimulation reduces learning deficits in experimental cretinism. Science. 1976;191(4227):578–579. doi: 10.1126/science.943127. [DOI] [PubMed] [Google Scholar]
- Diamond A. All or none hypothesis: a global-default mode that characterizes the brain and mind. Developmental Psychology. 2009;45(1):130–138. doi: 10.1037/a0014025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, et al. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am J Epidemiol. 2007;165(12):1397–1404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, Miodovnik A, et al. Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology. 2009;30(4):522–528. doi: 10.1016/j.neuro.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, et al. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environmental health perspectives. 2011;119(8):1182–1188. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Current opinion in endocrinology, diabetes, and obesity. 2010;17(6):507–516. doi: 10.1097/MED.0b013e3283405921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escanola S. Babies at double hazard: early development of infants at biologic and social risk. Pediatrics. 1982;70(5):670–676. [PubMed] [Google Scholar]
- Eskenazi B, Castorina R. Association of prenatal maternal or postnatal child environmental tobacco smoke exposure and neurodevelopmental and behavioral problems in children. Environmental health perspectives. 1999;107(12):991–1000. doi: 10.1289/ehp.99107991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115(5):792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ, Betancourt L, Shera DM, Savage JH, Giannetta JM, Brodsky NL, et al. Environmental stimulation, parental nurturance and cognitive development in humans. Developmental science. 2008;11(5):793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Fearon RP, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Lapsley AM, Roisman GI. The significance of insecure attachment and disorganization in the development of children’s externalizing behavior: a meta-analytic study. Child development. 2010;81(2):435–456. doi: 10.1111/j.1467-8624.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Slotkin TA. Developmental neurotoxicity elicited by prenatal or postnatal chlorpyrifos exposure: effects on neurospecific proteins indicate changing vulnerabilities. Environmental Health Perspectives. 2003;111(3):297–303. doi: 10.1289/ehp.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Toscano CD, McGlothan JL, Weaver SA. Environmental enrichment reverses cognitive and molecular deficits induced by developmental lead exposure. Annals of neurology. 2003;53(1):50–56. doi: 10.1002/ana.10399. [DOI] [PubMed] [Google Scholar]
- Guillette EA, Meza MM, Aquilar MG, Soto AD, Garcia IE. An anthropological approach to the evaluation of preschool children exposed to pesticides in Mexico. Environ Health Perspect. 1998;106(6):347–353. doi: 10.1289/ehp.98106347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R. Environmental influences on neural plasticity, the limbic system, emotional development and attachment: a review. Child psychiatry and human development. 1999;29(3):189–208. doi: 10.1023/a:1022660923605. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Sachser N. The social environment during pregnancy and lactation affects the female offsprings’ endocrine status and behaviour in guinea pigs. Physiology & behavior. 1998;63(3):361–366. doi: 10.1016/s0031-9384(97)00435-6. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Sachser N. Social stress during pregnancy and lactation affects in guinea pigs the male offsprings’ endocrine status and infantilizes their behaviour. Psychoneuroendocrinology. 2001;26(5):503–519. doi: 10.1016/s0306-4530(01)00009-9. [DOI] [PubMed] [Google Scholar]
- Kaler SR, Freeman BJ. Analysis of environmental deprivation: cognitive and social development in Romanian orphans. Journal of child psychology and psychiatry, and allied disciplines. 1994;35(4):769–781. doi: 10.1111/j.1469-7610.1994.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Kang HG, Jeong SH, Cho JH, Kim DG, Park JM, Cho MH. Chlropyrifos-methyl shows anti-androgenic activity without estrogenic activity in rats. Toxicology. 2004;199(2–3):219–230. doi: 10.1016/j.tox.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Krieger N, Rowley DL, Herman AA, Avery B, Phillips MT. Racism, sexism, and social class: implications for studies of health, disease, and well-being. American journal of preventive medicine. 1993;9(6 Suppl):82–122. [PubMed] [Google Scholar]
- Landrigan PJ, Claudio L, Markowitz SB, Berkowitz GS, Brenner BL, Romero H, et al. Pesticides and inner-city children: exposures, risks, and prevention. Environ Health Perspect. 1999;107(Suppl 3):431–437. doi: 10.1289/ehp.99107s3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environmental health perspectives. 2005;113(7):894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, et al. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicology and teratology. 2002;24(6):733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Lizardi PS, O’Rourke MK, Morris RJ. The effects of organophosphate pesticide exposure on Hispanic children’s cognitive and behavioral functioning. J Pediatr Psychol. 2008;33(1):91–101. doi: 10.1093/jpepsy/jsm047. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environmental health perspectives. 2004;112(17):1691–1696. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Campo P, Xue X, Wang MC, Caughy M. Neighborhood risk factors for low birthweight in Baltimore: a multilevel analysis. American journal of public health. 1997;87(7):1113–1118. doi: 10.2105/ajph.87.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddy WH, Li J, Whitehouse AJ, Zubrick SR, Malacova E. Breastfeeding duration and academic achievement at 10 years. Pediatrics. 2011;127(1):e137–145. doi: 10.1542/peds.2009-3489. [DOI] [PubMed] [Google Scholar]
- Pasco Fearon RM, Belsky J. Infant-mother attachment and the growth of externalizing problems across the primary-school years. Journal of child psychology and psychiatry, and allied disciplines. 2011;52(7):782–791. doi: 10.1111/j.1469-7610.2010.02350.x. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of child psychology and psychiatry, and allied disciplines. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Perera FP, Illman SM, Kinney PL, Whyatt RM, Kelvin EA, Shepard P, et al. The challenge of preventing environmentally related disease in young children: community-based research in New York City. Environ Health Perspect. 2002;110(2):197–204. doi: 10.1289/ehp.02110197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111(2):201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environmental Health Perspectives. 2006;114(8):1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Betancourt L, Giannetta JM, Brodsky NL, Korczykowski M, Avants BB, et al. Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. NeuroImage. 2010;49(1):1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, et al. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environmental health perspectives. 2011;119(8):1196–1201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Whyatt RM, Garfinkel R, Andrews H, Hoepner L, Reyes A, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicology and teratology. 2004;26(3):373–385. doi: 10.1016/j.ntt.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Landrigan PJ, Claudio L. Housing and health: intersection of poverty and environmental exposures. Annals of the New York Academy of Sciences. 2008;1136:276–288. doi: 10.1196/annals.1425.032. [DOI] [PubMed] [Google Scholar]
- Schwartz JB. The influence of sex on pharmacokinetics. Clinical Pharmacokinetics. 2003;42(2):107–121. doi: 10.2165/00003088-200342020-00001. [DOI] [PubMed] [Google Scholar]
- Smegal DC. US Environmental Protection Agency. Office of Pesticide Programs, Environmental Fate and Effects Division; Washington, D.C: 2000. Human Health Risk Assessment Chlorpyrifos; pp. 1–131. [Google Scholar]
- Smith EE, Jonides J. Working memory: a view from neuroimaging. Cognitive psychology. 1997;33(1):5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environmental Health Perspectives. 2005;113(8):1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DC. Differential rates of cerebral maturation between sexes and between hemispheres. Evidence from epilepsy. Lancet. 1969;2(7612):140–142. doi: 10.1016/s0140-6736(69)92445-3. [DOI] [PubMed] [Google Scholar]
- Tremblay RE, Nagin DS, Seguin JR, Zoccolillo M, Zelazo PD, Boivin M, et al. Physical aggression during early childhood: trajectories and predictors. The Canadian child and adolescent psychiatry review = La revue canadienne de psychiatrie de l’enfant et de l’adolescent. 2005;14(1):3–9. [PMC free article] [PubMed] [Google Scholar]
- Vahter M, Akesson A, Liden C, Ceccatelli S, Berglund M. Gender differences in the disposition and toxicity of metals. Environmental research. 2007;104(1):85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vahter M, Gochfeld M, Casati B, Thiruchelvam M, Falk-Filippson A, Kavlock R, et al. Implications of gender differences for human health risk assessment and toxicology. Environmental research. 2007;104(1):70–84. doi: 10.1016/j.envres.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, et al. Gender difference in neural response to psychological stress. Social cognitive and affective neuroscience. 2007;2(3):227–239. doi: 10.1093/scan/nsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasik BH, Ramey CT, Bryant DM, Sparling JJ. A longitudinal study of two early intervention strategies: Project CARE. Child development. 1990;61(6):1682–1696. [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Lolacono NJ, Factor-Litvak P, Kline JK, Popovac D, et al. Lead exposure and intelligence in 7-year-old children: the Yugoslavia Prospective Study. Environmental health perspectives. 1997;105(9):956–962. doi: 10.1289/ehp.97105956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Pine DS, Graziano JH. Contribution of maternal smoking during pregnancy and lead exposure to early child behavior problems. Neurotoxicology and teratology. 2001;23(1):13–21. doi: 10.1016/s0892-0362(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, et al. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(47):11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence--Revised. Psychological Corp; San Antonio, TX: 1991. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 4. Harcourt Assessment; San Antonio, TX: 2005. [Google Scholar]
- Weiss B, Bellinger DC. Social ecology of children’s vulnerability to environmental pollutants. Environmental Health Perspectives. 2006;114(10):1479–1485. doi: 10.1289/ehp.9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss MJ. Harrdiness and social support as predictors of stress in mothers of typical children, children with autism, and children with mental retardation. Autism : the international journal of research and practice. 2002;6(1):115–130. doi: 10.1177/1362361302006001009. [DOI] [PubMed] [Google Scholar]
- Werner E, Ruth S. Overcoming the Odds: High Risk Children from Birth to Adulthood. Cornell University; Ithica, New York: 1992. [Google Scholar]
- Whitemore RW, Immerman FW, Camann DE, Bond AE, Lewis RG, Schaum JL. Non-occupational exposures to pesticides for residents of two U.S. cities. Archives of environmental contamination and toxicology. 1994;26(1):47–59. doi: 10.1007/BF00212793. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, et al. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111(5):749–756. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Camann D, Perera FP, Rauh VA, Tang D, Kinney PL, et al. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicol Appl Pharmacol. 2005;206(2):246–254. doi: 10.1016/j.taap.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environmental health perspectives. 2012;120(2):290–295. doi: 10.1289/ehp.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ. Moving towards making social toxins mainstream in children’s environmental health. Current opinion in pediatrics. 2009;21(2):222–229. doi: 10.1097/MOP.0b013e3283292629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JG, Eskenazi B, Gladstone EA, Bradman A, Pedersen L, Johnson C, et al. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology. 2005;26(2):199–209. doi: 10.1016/j.neuro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Zapata BC, Rebolledo A, Atalah E, Newman B, King MC. The influence of social and political violence on the risk of pregnancy complications. American journal of public health. 1992;82(5):685–690. doi: 10.2105/ajph.82.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]