Abstract
Some studies of nonhuman animals’ metacognitive capacity encourage competing low-level, behavioral descriptions of trial-decline responses by animals in uncertainty-monitoring tasks. To evaluate the force of these behavioral descriptions, six capuchin monkeys were presented with two density-discrimination tasks between sparse and dense stimuli. In one task, difficult trials with stimuli near the middle of the density continuum could be declined through an “uncertainty” response. In the other task, making a “middle” response to the same stimuli was rewarded. In Experiment 1, capuchins essentially did not use the uncertainty response, but they did use the middle response. In Experiment 2, we replicated this result with 5 of 6 monkeys while equating the overall pace and reinforcement structure of the two tasks, although one monkey also showed appropriate use of the uncertainty response. These results challenge a purely associative interpretation of some uncertainty-monitoring performances by animals, while sharpening the theoretical question concerning the nature of the psychological signal that occasions uncertainty responses.
Keywords: uncertainty monitoring, metacognition, primate cognition, comparative cognition, capuchin monkeys
Humans experience uncertainty and hesitation when facing ambiguous or incomplete information, and at these times they feel and report that they do not know or remember. These introspections are part of humans’ capacity for metacognition. They reflect a cognitive executive that monitors perception and memory, tracks available information, and evaluates the likelihood of positive outcomes from behavioral choices. These introspections can lead humans to decline to make behavioral choices and to seek help, hints, or further information instead. Thus, metacognition supports adaptive behavior in relation to feelings of doubt and uncertainty (Benjamin, Bjork, & Schwartz, 1998; Flavell, 1979; Koriat, 1995; in press; Koriat, Bjork, Sheffer, & Bar, 2004; Koriat, Ma’ayan, & Nussinson, 2006; Metcalfe, 2000; Nelson, 1992; Scheck & Nelson, 2005; Schwartz, 1994; Serra & Dunlosky, 2005).
Metacognition is a sophisticated cognitive capacity in humans that is linked to consciousness, introspection, and declarative reports about mental states. This raises the question of whether metacognition is uniquely human (Metcalfe & Kober, 2005). Researchers have reported abilities related to metacognition in nonhuman animals (hereafter animals), and experimental paradigms have been developed to explore these abilities (for a review see Smith, Shields, & Washburn, 2003). In these paradigms, researchers intermix easy and difficult trials. They give animals a response—beyond the primary discrimination responses—that lets them decline to complete the trials they choose. If animals can monitor cognition, they should decline difficult trials selectively because they recognize those trials’ difficulty and error potential. In fact, animals sometimes do so, producing isomorphic data patterns to those of humans (e.g., Shields, Smith, & Washburn, 1997). The trial-decline response is called the uncertainty response, and this article explores its appropriate psychological interpretation.
Investigations of primate metacognition have focused on family Cercopithecidae (e.g., Beran, Smith, Redford, & Washburn, 2006; Hampton, 2001; Kornell, Son, & Terrace, 2007; Shields et al., 1997, Smith, Beran, Redford, & Washburn, 2006; Smith, Smith, Shields, Allendoerfer, & Washburn, 1998; Smith, Shields, Schull, & Washburn, 1997; Washburn, Smith, & Shields, 2006). The first goal of the present research is to extend these investigations for the first time to the family Cebidae or New World monkeys. Capuchins sometimes show less cognitive sophistication than macaques (Beran, Klein, et al., 2008; Rumbaugh & Pate, 1984; Rumbaugh, Savage-Rumbaugh, & Washburn, 1996), including in an information-seeking task that might have assessed metacognition (Paukner, Anderson, & Fujita, 2006). However, capuchins also show cognitive sophistication in tool use, quantity judgment, and concept learning (e.g., Beran, 2008a; D’Amato & Colombo, 1988; Evans & Westergaard, 2004; Judge, Evans, & Vyas, 2005; McGonigle, Chalmers, & Dickinson, 2003; Wright & Katz, 2006). Therefore, either data pattern—with capuchins expressing an uncertainty-monitoring capacity or not—would be illuminating. A central issue in comparative psychology is to map the phylogenetic emergence of reflective mind within the order Primates (Gallup, 1982; Rumbaugh & Pate, 1984; Thompson & Oden, 2000). Studying metacognition in a new primate family would advance this larger enterprise.
The second goal of the present research is to explore the psychological organization of uncertainty responses: that is, their reward properties, stimulus bases, and motivational causes. Comparative psychologists are naturally cautious about interpreting uncertainty responses generously or about attributing metacognitive reflection to animals. Therefore, the psychological interpretation of animals’ uncertainty responses has been a source of ongoing theoretical discussion (Carruthers, 2008; Smith, Beran, Couchman, & Coutinho, in press; Staddon Jozefowiez, & Cerutti, 2007).
One concern is that researchers have rewarded animals for making uncertainty responses (e.g., Foote & Crystal, 2007; Kornell et al., 2007; Hampton, 2001). This might give the uncertainty response its own associative response strength independent of any role it plays in response to feelings of uncertainty. These rewards make it difficult to dismiss low-level interpretations of uncertainty responses or affirm metacognitive interpretations of them. Thus, it is important to understand the role that reward contingencies for uncertainty responses play in studies of animal metacognition.
Another concern is that difficult discriminative stimuli are usually associated with leaner rewards and negative outcomes such as timeouts. These outcomes might make difficult stimuli feel aversive, and the animal could be conditioned to avoid primary responses to those stimuli. The uncertainty response (even if not directly rewarded) could come by association to seem safest on those trials, with its use conditioned to those stimulus contexts. Response avoidance facing aversive stimuli and uncertainty awareness facing difficult trials are not the same thing psychologically. Thus, the metacognitive interpretation of existing experiments is challenged by this associative interpretation, too. It is a crucial question whether uncertainty responses are simply aversion-avoidance responses or are really uncertainty reports about difficult trials. We also consider in this article whether one can distinguish responses that have these two functional roles in a task.
Consider a discrimination task, in which organisms categorize stimuli that vary along a continuum (e.g., from being very sparsely pixilated to very densely pixilated) into one of two classes (e.g., Sparse or Dense). Stimuli that fall in the middle of that continuum are the most difficult to discriminate. If a third response option is offered, it could function in one of two ways. If it is used as an uncertainty response, the organisms should use it in the presence of those stimuli in the middle of the continuum because those stimuli produce the highest levels of uncertainty as to their correct classification into the other two (primary) categories. However, those same stimuli might also come to be perceived as belonging to a separate class, with that third response option acting functionally as a “Middle” response rather than an uncertainty response.
Interestingly, this theoretical contrast between true uncertainty responses and objectively defined middle responses has a 100-year-old history going back to the earliest research in human perception. Human observers in the early psychophysical studies often were allowed to respond uncertain or doubtful when they felt unable to assign a stimulus to one of the two stimulus categories (Angell, 1907; Fernberger, 1914, 1930; George, 1917; Watson, Kellogg, Kawanishi, & Lucas, 1973; Woodworth, 1938). Some researchers took these doubtful responses to be just middle responses on the same psychological level as the primary discrimination responses (see Watson et al., 1973). Other researchers noted the special psychological status of the uncertainty response, including its susceptibility to instructions (Brown, 1910; Fernberger, 1914, 1930; Woodworth, 1938), its personality/ temperament correlates (Angell, 1907; Fernberger, 1930; Thomson, 1920), and its reflective character (Angell, 1907; Fernberger, 1930; George, 1917). Accordingly, these theorists believed that uncertainty responses were meta- to the primary discrimination and were a comment on the participant’s failure to assign a stimulus to a stimulus category. For example, Boring (1920) suggested that doubt was an attitudinal seducer that took psychophysical observers away from the series of mental states that are a continuous function of the series of stimuli. George (1917) concluded that doubtful responses undermined the stimulus-based attitude required for psychophysical performance because they brought “extra-serial” attitudes to a task that depended on intra-serial, sensory attitudes. This issue was raised originally in relation to humans’ uncertainty responses. However, it is an intriguing possibility, explored in the present article, that this issue also could be explored comparatively.
The present research with capuchins also could provide an empirical and theoretical bridge to other cross-species metacognition research. In this growing field, researchers have used diverse approaches to test pigeons (Inman & Shettleworth, 1999; Sutton & Shettleworth, 2008), a dolphin (Smith et al., 1995), rats (Foote & Crystal, 2007), and apes (Call & Carpenter, 2001; Suda-King, 2007). The phylogenetic map of uncertainty responding is growing complex and difficult to interpret, because of cross-species methodological differences and because of the issues we have raised. There is a consensus among the researchers in this field that more needs to be learned about the balance between lower- and higher-level processes in producing uncertainty responses, about the functional meaning of uncertainty responses to animals of different species, and about whether uncertainty responses are functionally Middle responses or instead are commenting, meta- responses to uncertainty and difficulty.
In the present experiments, capuchin monkeys were given two discrimination tasks in which stimuli were classified as sparsely or densely pixilated. A third response was also available, but it functioned differently between tasks. In one task (the SUD task), making sparse-dense responses to the pixilated stimuli could be avoided through an uncertainty response that ended one trial and presented the next, without providing any feedback or reward. In the other task (the SMD task), the third response was to be used for pixilated stimuli in the middle of the density continuum. When that response was correctly chosen, food reward was given as with the sparse and dense responses (and likewise incorrect use of that response led to no food reward and a timeout). Thus, the third response differed across tasks in being an uncertainty response to manage sparse-dense indeterminacy or in being a middle response to manage middle stimuli.
Differential use of the third response between tasks would document the contrasting psychological organization of uncertainty and middle responses. It would functionally dissociate uncertainty and middle responses for the first time, informing a lasting debate about uncertainty responses by humans. It would sharpen the theoretical question concerning the psychological signal that occasions uncertainty responses.
Experiment 1
Methods
Participants
Six capuchin monkeys (Cebus apella) were tested: Logan (male, 2 years old), Liam (male, 3 years old), Wren (female, 4 years old), Nala (female, 4 years old), Gabe (male, 9 years old), and Griffin (male, 9 years old). All six monkeys had been trained to respond to computer-generated stimuli, and they had participated in previous experiments (e.g., Beran, 2008a, 2008b; Beran, Harris, et al., 2008). None of these experiments was related to uncertainty monitoring. The monkeys were housed socially in a building with indoor-outdoor access. Monkeys were physically but not visually isolated into test boxes that attached to the home cage for 2.5-hr morning test sessions. The monkeys then rejoined the larger social group for the remainder of the day. They were not food or water deprived, and they received a diet of fresh fruit and vegetables each day no matter their level of task participation (details in Evans et al., 2008).
Apparatus
Trials were presented on a Compaq DeskPro with an attached 17-inch color monitor. Joystick responses were made with a Gravis GamePad Pro digital joystick mounted vertically to the cage. The test program was written in Turbo Pascal. Food rewards for correct responses were automatically dispensed by the computer as single 45 mg Bio-Serv food pellets through a dispenser connected to the test cage. Consistent auditory feedback accompanied correct responses. Incorrect responses received a trial-less 20 s timeout period (except where otherwise specified) with associated auditory feedback (details of the testing system are in Evans et al., 2008).
Density continuum
Monkeys completed a psychophysical density-judgment task. On each trial, they saw a 201 × 101-pixel box in the top center of the computer screen. The box was filled with a variable number of randomly placed lit pixels (Figure 1). Forty-two stimulus levels could be presented (Levels 1 to 42). Each level’s pixel count was given by the formula pixels = round (base pixels × 1.018Level)—the base pixels were 1057. This formula gave the density continuum the appropriate logarithmic character, with density steps defined as a constant percentage increase in pixels and not as a constant absolute increase in pixels.
Figure 1.

A trial from the psychophysical discrimination task. The pixel box at the top center of the screen was colored yellow and was the stimulus to be classified sparse (“S”) or dense (“D”). The third response (“?”) cleared the screen and moved to the next trial—this was the uncertainty response. In the alternative version of the task, the third response option was the letter “M”, and it was the correct response for pixel boxes of an intermediate or middle density. This was the middle response. The cursor is the small circle in the bottom center—it was colored red.
Response modality
The capuchins always made an observing response to the pixel box: they used the joystick to move a cursor to touch the box. Then their task was to classify the pixel boxes as sparse or dense in one task, or as sparse, middle, or dense in the other task. In the former case, they touched an S and D icon on the screen to respond sparse or dense, respectively, and they touched ? to respond uncertain (Figure 1). In the latter case, they touched S, M, or D icons on the screen to respond sparse, middle, and dense, respectively. In this task, the M icon was located in the same place as the ? icon shown in Figure 1.
Psychophysical training
The capuchins first were trained to make a basic sparse-dense psychophysical discrimination. Beginning training, all stimuli were from the extremes of the stimulus continuum (Level 1 and Level 42), and monkeys were given only the two response icons S and D with which to respond to these clear Sparse and Dense trials. During the early phases of training the timeout period for errors was set at 10 s. Monkeys continued in this phase until they were correct on 85% of the most recent 20 trials. Then, all possible trials levels were allowed. The capuchins quickly learned to discriminate sparse and dense stimuli sensitively, and performance decreased for the stimuli that were nearer the sparse-dense breakpoint of the discrimination.
Fostering the use of the uncertainty response
At this same point in training, the uncertainty response was made available. The uncertainty response simply cleared the screen and ended the trial without providing food reward or a timeout period. Monkeys were tested extensively during this phase of training in the hope of establishing the uncertainty-monitoring data pattern that has been repeatedly demonstrated for macaques (e.g., Smith et al., 1997). However, despite this testing (weeks of testing and thousands of trials), none of the monkeys showed efficient or substantial use of the uncertainty response.
We proceeded to make several modifications to the task to foster the capuchins’ use of the uncertainty response, but none of these manipulations succeeded. First, we increased the penalty to 40 s, which put a premium on avoiding a primary response on difficult trials that might be incorrectly completed. Second, we increased the difficulty of the task by sampling more from the more difficult region of the continuum near the discrimination’s breakpoint. This could have fostered trial-decline responses because the most difficult trials became increasingly frequent. Third, we gave monkeys trials in which they were forced to use the uncertainty response so that its utility would be demonstrated to them. This manipulation occurred for randomly chosen box densities, so monkeys could not learn from these trials when they should most efficiently use the uncertainty response. Instead, they only experienced the general consequences of the response when it was used. This manipulation has apparently helped teach macaques the utility of the uncertainty response (e.g., Smith et al., 2006). However, it did not promote selection of the uncertainty response by any capuchin. Fourth, we tried stimulus continua that had slightly smaller density gradations (e.g., 1% per density step instead of 1.8% per density step). Fifth, we increased animals’ positive motivation to make uncertainty responses by having every uncertainty response be followed by a clear and easy sparse or dense trial. This also may have helped macaques learn to use the uncertainty response in the original studies of Smith et al. (1997). None of these manipulations fostered use of the uncertainty response beyond very low levels (less than 5% of trials overall, and spread broadly across the density continuum, not focused on the most difficult stimulus levels).
The final tasks in Experiment 1 that we describe now, and the results we report next, represent the final procedure and results from an extended effort to foster in capuchins an adaptive use of the uncertainty response. This was not an unbiased effort. To the contrary, we deliberately used every approach we knew—based on our long experience testing nonhuman primates—to coax from them an uncertainty-monitoring data pattern.
Sparse-Uncertainty-Dense (SUD) task
In this SUD task, the stimulus continuum was divided into sparse and dense regions. One of the two primary responses (Sparse or Dense) was appropriate for each level across that full continuum, and correct responses were rewarded immediately. The third response option, the uncertainty response, did not lead to food reward or a timeout period for its selection. Across a session, approximately half of the trials were sparse (Levels 1–21, 1076–1537 pixels) and dense (Levels 22–42 1565–2236 pixels). All of the trials were chosen randomly from across the whole stimulus continuum. All responses were available to the animals all the time. In this task, we continued the practice just described of having uncertainty responses release into a clear and easy Sparse or Dense next trial (Level 1 or Level 42).
Sparse-Middle-Dense (SMD) task
In this SMD task, the stimulus continuum was divided into sparse, middle, and dense regions. There were corresponding responses assigned to each region that produced immediate food reward when selected whereas incorrect responses led to a timeout. Across a session, approximately a third of the trials were sparse (Levels 1–14, 1076–1357 pixels), middle (Levels 15–28, 1381–1742 pixels), and dense (Levels 29–42, 1773–2236 pixels). All trials were chosen randomly from across the whole stimulus continuum. All responses were available to the animals all the time.
Counter-balancing of task order
Griffin, Liam, and Nala completed the tasks in the order SUD-SMD. Gabe, Logan, and Wren completed the tasks in the order SMD-SUD. These groups were roughly matched in age (Griffin/Gabe, Liam/Logan, and Nala/Wren were of similar ages). Monkeys participated in the task between the hours of 9:00–12:00 each morning and worked at their own pace. They were tested individually in test boxes, they rested as they chose, and they had continuous access to water. They naturally produced different numbers of trials. The result was trial counts of 1,000 to 4,000 trials per task per monkey depending on their performance speed.
Results and Discussion
Given the extensive training and testing just described, most capuchins required only two or three test sessions to provide a large corpus of data and produce stable individual performance curves like those reflected in the pooled data presented in the figures. Indeed, the shapes of their performance curves were evident even in the first session for almost all monkeys in both tasks. Variability in performance across time was minimal, and generally it reflected the growing sensitivity of the primary discrimination performance of the capuchins.
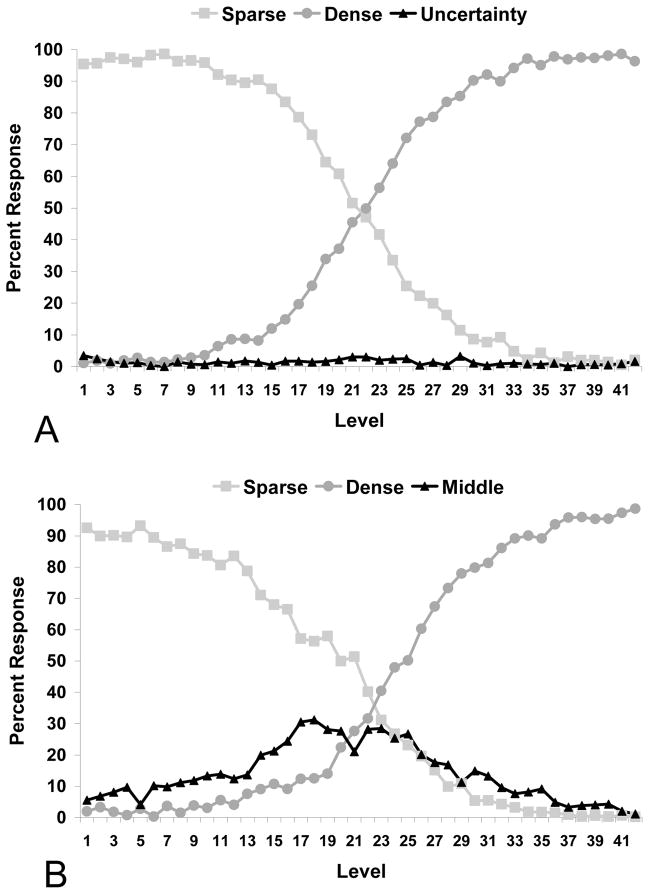
The mean percentages of each of the responses made for each stimulus level in the SUD and SMD tasks are shown in Figure 2A and 2B, respectively. For Task SUD, there was a significant effect of level on the percentage of dense responses, F (41, 205) = 272.41, p < .001, ηp2 = .98, and sparse responses, F (41, 205) = 273.25, p < .001, ηp2 = .98. There was no effect of level on the percentage of uncertainty responses, F (41, 205) < 1.09, p = .33, ηp2 = .18. That is, the monkeys did not use the uncertainty response reliably more often for some trial levels than others. For Task SMD, there was a significant effect of level on the percentage of dense responses, F (41, 205) = 242.54, p < .001, ηp2 = .98, and sparse responses, F (41, 205) = 107.74, p < .001, ηp2 = .96. There was also a significant effect of level on the percentage of middle responses, F (41, 205) = 5.51, p = .001, ηp2 = .53. That is, animals did use the middle response reliably more often for the middle range of stimulus levels. This conclusion was supported by a test of within-subject contrast—it indicated that the best fit to the middle response function was a quadratic fit, F = 9.43, p = .028, ηp2 = .65.
Figure 2.
Mean percentage of sparse, dense, and uncertainty or middle responses in the Uncertainty (A) and Middle (B) task of Experiment 1.
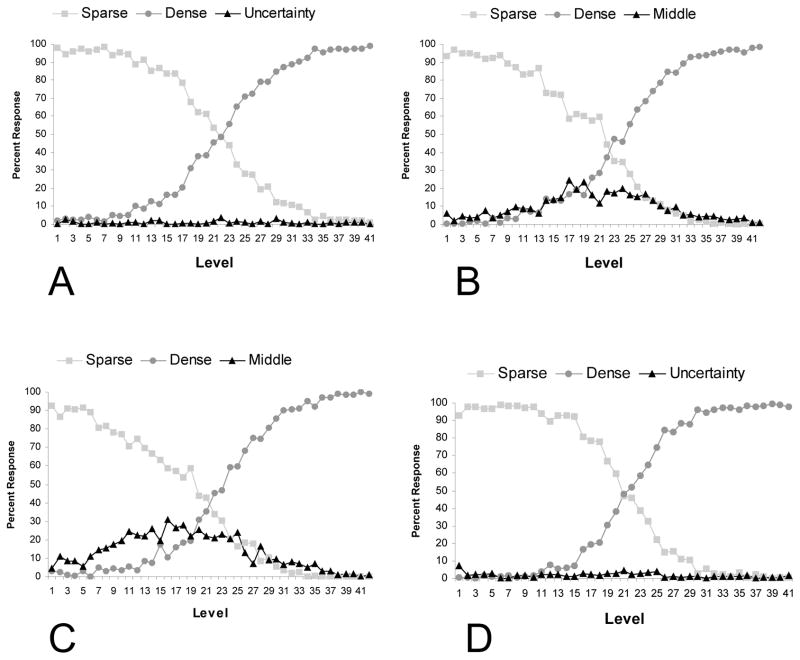
Despite the low levels of uncertainty responding, it was possible that task completion order might have had an effect on differential use of the third response (middle or uncertainty). Figure 3 presents the data separately for monkeys who did the SMD task before the SUD task from monkeys who did the tasks in the opposite order. As shown in the figure, there was no benefit in SUD performance from performing the SMD task first.
Figure 3.
Performance in Experiment 1 separated by task order completion. The top row shows performance for monkeys who completed the SUD task (A) followed by the SMD task (B). The bottom row shows performance for monkeys who completed the SMD task (C) followed by the SUD task (D).
Clearly, the capuchins adopted the stimulus-based, middle response category with ease and used it efficiently. However, despite extensive, targeted training efforts spanning thousands of trials for each monkey, they did not use an uncertainty response to manage sparse-dense indeterminacy regarding the same intermediate stimulus impressions. This is the first reported dissociation between an uncertainty response that manages indeterminacy and a response to a perceptually anchored, intermediate stimulus category. As we discussed above, the importance of this distinction has long been acknowledged in psychophysical research on humans’ responses at their perceptual limit. As we discuss below, this dissociation in capuchins has important theoretical implications regarding the appropriate psychological interpretation of uncertainty responses by macaques and other animals.
The Reinforcement Pace and Structure of Uncertainty and Middle Tasks
We conducted additional analyses comparing the reinforcement pace and structure of the SUD and SMD task. In the SUD task, capuchins used 75,344 s to earn 12,260 rewards and make 1,651 errors. Incorporating the approximate 3 s trial time and the 20 s timeout penalty, they received a reward every 6.15 s on average or 9.76 rewards per minute. We simulated the capuchins’ performance using formal models (see also Smith et al., 2003, 2006). Figure 4 (curve Uncertainty 1) shows the rewards per minute that capuchins could have earned if they had adopted uncertainty regions of increasing widths (i.e., increasingly liberal uncertainty responding). They could have earned about 20% more rewards if they had adopted an optimal uncertainty region that was 8 stimulus levels wide and that let them decline about 50% of the most difficult trials.
Figure 4.
Approximate rewards per minute that could be earned by different decisional strategies under the reinforcement and timeout contingencies used in Experiments 1 and 2. The simulations that produced these estimates are described in the text.
Nine rewards per minute is a good rate of return for these monkeys relative to their experience. The 20% possible improvement is not that great. The optimal level of uncertainty responding is not high. Therefore, the reinforcement rate of the task might have been sufficiently high to preclude behavioral changes that would have more fully optimized the level of reward in the SUD task.
In the SMD task, the capuchins used 130,664 s (more than in the SUD task) to earn 8,932 rewards (fewer than in the SUD task) and make 4,516 errors (more than in the SUD task)—even though they responded middle appropriately. Incorporating the approximate 3 s trial time and the 20 s timeout penalty, they received a reward every 14.63 s on average or 4.10 rewards per minute. Figure 4 (curve Middle) shows the monkeys’ potential reward rate had they adopted middle regions of increasing widths (or increasingly liberal middle responding). They could have earned about 60% more rewards if they had adopted a middle region that was 14 steps wide and that let them respond middle to about 75% of the clearest middle trials.
Four rewards per minute is a low rate relative to the monkeys’ experience. The 60% possible improvement is large. The optimal level of middle responding is high. Therefore, the reinforcement rate of the task might have been low enough to encourage behavioral change in using the middle response that increased the overall level of reward in the SUD task.
Therefore, monkeys may have felt strong pressure to optimize in the SMD task.
Therefore, we left Experiment 1 with an interpretative question. Did the capuchins not include the uncertainty response in their response set for interesting psychological reasons, or because they received enough food reward to preclude behavioral changes in their use of the uncertainty response with more experience in the task? We addressed this question by building a modified uncertainty task that had similar pace and reinforcement structure to the middle task, and that created as much or more pressure to optimize through uncertainty responding. We addressed the overall pace of the uncertainty task by using a 90 s timeout that more than quadrupled the time cost for each error and increased the advantage gained from declining difficult trials. Now the capuchins potentially gave up 30 trials for each penalty timeout. We also addressed the problem that the uncertainty task in Experiment 1 was easier than the middle task. The uncertainty task had only one difficult region near the sparse-dense boundary. The middle task had two difficult regions near the sparse-middle and middle-dense boundaries. In addition, some stimuli in the uncertainty task were trivially easy because they were up to 20 stimulus levels away from the discrimination boundary (i.e., Level 1 compared to the boundary at Levels 21–22). In the middle task, stimuli were at most 14 stimulus levels away from a boundary (e.g., Level 1 compared to the sparse-middle boundary at Levels 14–15), and middle stimuli were 7 steps at most away from a boundary. To equalize the tasks’ difficulty, we eliminated from the uncertainty task any presentations of the 9 easiest sparse trial types (Levels 1–9) and the 9 easiest dense trial types (Levels 34–42).
These changes produced an uncertainty task in which a monkey would receive about 2.34 rewards per minute if it could not incorporate uncertainty responding. It would receive about 4.74 rewards per minute—a 100% improvement—if it incorporated the uncertainty response optimally by choosing an uncertainty region 16 steps wide that allowed it to decline 80–85% of the most difficult trials. Figure 4 (curve Uncertainty 2) shows the predicted performance of monkeys on choosing different decision strategies. In every respect—low overall reward level, percentage reward gained by uncertainty responding, and the pressure for wide uncertainty regions and liberal uncertainty responding, this task exerted on monkeys strong associative pressure to incorporate the third response. The purpose of Experiment 2 was to ask if capuchins would now adopt the uncertainty response.
Experiment 2
Methods
Participants and apparatus
The same capuchin monkeys were tested using the same apparatus.
Design and procedure
The middle task was the same as in Experiment 1. The uncertainty task had the modified structure described in the last section. Each monkey completed both tasks two times: half of the monkeys in the order SUD-SMD-SUD-SMD and half in the order SMD-SUD-SMD-SUD.
Results
The reinforcement pace and structure of Experiment 2’s uncertainty and middle tasks
In the uncertainty task of Experiment 2, the capuchins used 669,999 s to earn 30,442 rewards and make 5,609 errors. Incorporating the approximate 3 s trial time and the 90 s timeout penalty, they received a reward every 22.01 s or 2.73 rewards per minute. In the middle task, the capuchins used 403,197 s to earn 29,910 rewards and make 13,629 errors. Incorporating the approximate 3 s trial time and the 20 s timeout penalty, they received a reward every 13.48 s on average or 4.45 rewards per minute.
Every criterion—low overall reward level, percentage reward gain from uncertainty responding, and the pressure for wide uncertainty regions and liberal uncertainty responding—favored the monkeys’ using the uncertainty response in the uncertainty task of Experiment 2.
First uncertainty-middle cycle
The mean percentage of each of the responses made for each stimulus level in the first uncertainty-middle cycle is shown in Figure 5A and Figure 5B. For the SMD task, there was a significant effect of level on the percentage of dense responses, F (41, 205) = 124.8, p < .001, ηp2 = .96, and sparse responses, F (41, 205) = 61.51, p < .001, ηp2 = .92. There was also a significant effect of level on the percentage of middle responses, F (41, 205) = 17.83, p < .001, ηp2 = .78. The capuchins used the middle response reliably more for the middle range of stimulus levels (a test of within-subject contrast indicated that the best fit to the “M” response function was a quadratic fit, F = 39.06, p = .002, ηp2 = .89).
Figure 5.
Mean percentage of sparse, dense, and uncertainty or middle responses in the two cycles of the Uncertainty (A,C) or Middle (B,D) tasks of Experiment 2. The top and bottom, graphs, respectively, come from the first and second Uncertainty-Middle cycle.
For the SUD task, there was a significant effect of level on the percentage of dense responses, F (23, 115) = 107.17, p < .001, ηp2 = .96, and sparse responses, F (23, 115) = 103.55, p < .001, ηp2 = .95. However, once again, there was no significant effect of level on the percentage of uncertainty responses, F (23, 115) = 1.33, p = .17, ηp2 = .21.
Second uncertainty-middle cycle
The mean percentage of each of the responses made for each stimulus level in the second uncertainty-middle cycle is shown in Figure 5C and Figure 5D. For the SMD task, there was a significant effect of level on the percentage of dense responses, F (41, 205) = 421.9, p < .001, ηp2 = .99, and sparse responses, F (41, 205) = 132.4, p < .001, ηp2 = .96. There was also a significant effect of level on the percentage of middle responses, F (41, 205) = 85.54, p = .001, ηp2 = .95. The capuchins used the middle response reliably more often for the middle range of stimulus levels (a test of within-subject contrast indicated that the best fit to the “M” response function was a quadratic fit, F = 579.7, p < .0019, ηp2 = .99).
For the SUD task, there was a significant effect of level on the percentage of dense responses, F (23, 115) = 237.27, p < .001, ηp2 = .98, and sparse responses, F (23, 115) = 189.5, p < .001, ηp2 = .97. In this one case, there was a significant effect of level on the percentage of uncertainty responses, F (23, 115) = 1.88, p = .016, ηp2 = .27. This effect was driven by the performance of one monkey, Logan, who learned to use the uncertainty response appropriately and frequently during his second cycle through the uncertainty task of Experiment 2. Figure 6 shows the performance of Logan on his second cycle, contrasted with the second-cycle performances of the remaining five animals that, with little variability, did not use the uncertainty response. To analyze his data in the SUD in isolation, we re-assigned each level as the distance from the center of the continuum (between levels 21 and 22). Level 21 and Level 22 were assigned the value 1, Level 20 and Level 23 were assigned the value 2, and so on. If Logan’s use the uncertainty response was related to the objective difficulty of the trial as measured by this distance from the center of the continuum, a negative correlation should be produced. The analysis showed such a correlation, r(22) = −.88, p < .001, R2 = .776. With Logan’s data removed, the remaining 5 monkeys still showed a significant effect of level on the percentage of dense responses, F (23, 92) = 220.75, p < .001, ηp2 = .98, and sparse responses, F (23, 92) = 245.35, p < .001, ηp2 = .98, but not uncertainty responses, F (23, 92) = 1.47, p = .11, ηp2 = .27.
Figure 6.
Mean percentage of sparse, dense, and uncertainty responses for Logan (A) compared to the five other capuchins (B) for the second Uncertainty-Middle cycle of Experiment 2.
General Discussion
This research is the first evaluation of uncertainty responding by a New World primate in a psychophysical task. All six capuchins failed to use an uncertainty response adaptively during intensive interventions to foster its use, during Experiment 1, and during the first half of Experiment 2. Five animals never used the uncertainty response to manage indeterminacy, even though that failure impoverished Experiment 2’s reinforcement landscape. Experiment 2 informed the interpretative question raised by Experiment 1. Animals were not just eschewing the uncertainty response because that task’s reinforcement landscape was rich enough without it. They barely engaged the uncertainty response even given a strong external motivation to do so. Apparently, they do not generally incorporate the uncertainty response for some cognitive-decisional reason, and we discuss presently the possible sources of this cognitive-decisional difficulty.
Thus, New World monkeys (capuchins) largely failed to exhibit the metacognitive data pattern that Old World monkeys (macaques) have commonly shown. This species difference matches that from a paradigm designed to assess search behavior and perhaps metacognition. Whereas great apes (Call & Carpenter, 2001) and rhesus monkeys (Hampton, Zivin, & Murray, 2004) search for information about hidden items more often when they have not seen the hiding event, capuchins (Paukner et al., 2006) failed to differentially search locations based on the food reward’s visibility. Paukner et al. suggested that this indicated an important phylogenetic difference between Old World and New World monkeys, and this difference appears to be reflected in our data.
Additional research has shown that macaques generalize their use of the uncertainty response to new tasks (e.g., Kornell et al., 2007; Washburn et al., 2006). They still use the uncertainty response when it affords no positive outcome other than removal of the current trial and presentation of a new trial (e.g., Beran et al., 2006). They use it when denied trial-by-trial feedback, demonstrating that uncertainty responses are not just low-level aversion-avoidance responses to stimuli that bring frequent timeouts and lean rewards (Smith et al., 2006). Finally, macaques also make uncertainty responses when their memories are indeterminate (Hampton, 2001; Smith et al., 1998). Their uncertainty-monitoring performances are advanced and sophisticated when compared to the nearly complete failure of capuchins to use an uncertainty response appropriately.
However, one capuchin, in Experiment 2’s last cycle, showed a pattern of results like that of macaques. Logan was the youngest animal we tested and an excellent performer in other cognitive tests (e.g., Beran, 2008a). His data suggest that Cebus apella does have a capacity for uncertainty monitoring. We have one reservation about Logan’s singular performance. He adopted the uncertainty response only at the very end of Experiment 2. Although he may have finally found access to an indeterminacy signal, it is also the case that by the time he did adopt the uncertainty response, he had experienced alternating tasks, and alternating middle and uncertainty response icons, several times over tens of thousands of trials. He could also have just come to construe the uncertainty response icon as an alternative, poorly rewarded but perceptually anchored middle response.
In any case, we do not conclude that there is a qualitative species difference in uncertainty monitoring between New World and Old World monkeys. The uncertainty results from five animals could be taken as null results with regard to the metacognitive ability of capuchins. However, this conclusion may be premature, and Logan’s data are a reminder of this. Capuchins might use uncertainty adaptively in some situations, and perhaps additional training and improved supports for uncertainty responses would promote them. In addition, although Logan was the only capuchin monkey to show this pattern, not all rhesus monkeys perform at the same robust level in uncertainty monitoring tasks (e.g., Smith et al., 2006), and this indicates some similarity across these two species. Nonetheless, we believe the present data suggest that capuchins’ uncertainty monitoring is more tenuous, more difficult for them to express, and more difficult for the experimenter to elicit. Shettleworth and her colleagues have shown similarly that pigeons have great difficulty expressing a capacity for uncertainty monitoring (Inman & Shettleworth, 1999; Sutton & Shettleworth, 2008).
The present results are certainly not null with regard to the dissociation between middle and uncertain responses. Taking the Uncertainty and Middle results together, one sees that each animal acted as its own control, in the sense that uncertainty responding and middle responding were manipulated within animals in an alternating and counterbalanced way. With only a single exception (Logan) in a single case (the last test series), capuchins used the middle response much more easily than they did the uncertainty response. This is the first dissociation of its kind: these two responses operationalize both sides of a long-lived debate within perceptual psychology. This is the key result of the present article. It shows that there are important differences in the psychological organization of uncertainty and middle responses.
How might middle responses and uncertainty responses differ, leading capuchins to use the one more than the other? Several possible differences could operate alone or in tandem to produce this dissociation. First, middle responses are directly rewarded and directly earn negative consequences, too. This gives them an associative / conditioning dimension grounded in the monkey’s appetitive motivational system. In contrast, the uncertainty response is never rewarded or punished in any stimulus context directly, and so its tie to the motivational system is more abstract and indirect. This distinction has a constructive parallel in the literature on perceptual categorization. In humans, there is an implicit system of categorization located in the tail of the caudate nucleus that maps responses to regions of perceptual space through the catalysis of immediate reinforcement signals (Ashby, Alfonso-Reese, Turken, & Waldron, 1998; Ashby & Waldron, 1999; Maddox & Ashby, 2004). The analogous system in monkeys could easily underlie the mastery of directly rewarded middle responses, but not the mastery of uncertainty responses that are only abstractly beneficial (i.e., rewards increase if one can strategically decline difficult trials). In fact, capuchins barely appreciated the abstract benefits of the uncertainty response. It would be interesting to correlate this abstract appreciation of the response’s benefits with other abstract appreciations of response strategies that animals show to varying degrees (e.g., delay of gratification and behavioral strategies to reduce temptation from the immediate reward—Beran & Evans, 2006; Evans & Beran, 2007). This would create links among different areas of executive and regulatory cognition that have not heretofore been connected.
Second, middle responses are strongly tied to and occasioned by a particular class of stimulus inputs, because the consequence of a middle response (reward or timeout) depends on what stimulus was presented. In contrast, uncertainty responses are not tied to the stimulus continuum in this way—they produce the same neutral consequence in every trial context. In a sense, the uncertainty response is a response without a specific appropriate stimulus class. This also might be part of why capuchins made middle but not uncertainty responses.
One can see from these differences that middle tasks and uncertainty tasks have different psychological organizations. Middle tasks have a single-level perceptual organization—there are three primary perceptual responses (sparse, middle, dense). Uncertainty tasks have a bi-level, more complex organization—there are two primary perceptual responses (sparse, dense) and one response with a different character.
The capuchin results join results from other species in supporting this description of the uncertainty task. Uncertainty responses are distinctive among discrimination responses because they are behaviorally fragile and changeable. In Smith et al. (2006), one macaque declined the difficult trials selectively; one macaque did not. Both macaques used the sparse and dense responses identically. Hampton (2001) found the same difference between macaques in his crucial third experiment. Smith et al. (1997) observed the same fragility of the uncertainty response in humans and monkeys.
This fragility has been observed in humans for a century. It helped convince the early psychophysicists that the uncertainty response was psychologically different from the primary discrimination responses. They agreed that primary discrimination responses (e.g., sparse, middle, dense) are tied to the stimulus continuum because they name or classify stimulus types, and that the uncertainty response is not so tied because it does not do so. Instead, they thought the uncertainty response was meta- to the ongoing discrimination, reporting the participant’s failure to assign a stimulus to one of the primary input classes. This description explains why capuchins made middle responses so easily but performed so differently on their uncertainty task. It explains the behavioral lability of the uncertainty response for humans and macaques, too. This interpretation makes the behavioral fragility of capuchins’ and macaques’ uncertainty responses intriguing—that response may be meta- to their primary discriminations, too, although this remains to be shown definitively.
Macaques, at least, cope well with the bi-level, decisional complexity of the uncertainty task whereas nearly all capuchins struggled with that complexity while having no difficulty with middle tasks. It remains to be determined what methodological variations might work to produce stronger behavioral evidence of uncertainty monitoring in capuchins. In addition, the present results suggest that capuchin monkeys can serve as valuable subjects in assessing the effects of varying the outcome for this third response. Between a true uncertainty response, that operates solely to clear a trial and move on to the next trial, and a true middle response with its direct reward and punishment contingencies, fall many additional variations (e.g., illustrating the correct response, removing the incorrect response, rewarding the use of the uncertainty response). How capuchins respond to such contingencies of the third response may illustrate the extent to which those contingencies support uncertainty monitoring versus middle responding. We also point out that a mature uncertainty-monitoring capacity could emerge phylogenetically as a gradual freeing of the indeterminacy response from its original, constricting, stimulus and reinforcement determinants. The same progression could be part of very young human children’s initial steps from reacting to bad stimuli to reacting to indeterminate mental states.
The present results show clearly that the psychological middle is theoretically complex and interesting, probably because it represents the confluence of different perceptual and cognitive representations at different cognitive and decisional levels. There is the stimulus middle, the rewarded middle, the aversive middle, the uncertain and metacognitive middle. The capuchins in the present article drew sharp attention to distinctions like these because they showed competence in responding to some but not all aspects of the psychological middle. Perhaps, by dissociating these different aspects, they hinted at some of their cognitive and decisional limits. However, in doing so, they also underscored the sophistication of the performances achieved by other primates in uncertainty-monitoring tasks. They also pointed to constructive ways to assess the many findings in this growing literature, and to integrate those findings into a richer understanding of the capacity called metacognition.
Acknowledgments
We thank Betty Chan, Ted Evans, and Emily Klein for assistance with data collection. The preparation of this article was supported by Grant BCS-0634662 from the National Science Foundation and by Grant HD-38051 from the National Institute of Child Health and Human Development.
Contributor Information
Michael J. Beran, Language Research Center, Georgia State University
J. David Smith, Department of Psychology and Center for Cognitive Science, SUNY Buffalo.
Mariana V. C. Coutinho, Department of Psychology, SUNY Buffalo
Justin J. Couchman, Department of Psychology, SUNY Buffalo
Joseph Boomer, Department of Psychology, SUNY Buffalo.
References
- Angell F. On judgments of “like” in discrimination experiments. American Journal of Psychology. 1907;18:253. [Google Scholar]
- Ashby FG, Alfonso-Reese LA, Turken AU, Waldron EM. A neuropsychological theory of multiple systems in category learning. Psychological Review. 1998;105:442–481. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Waldron EM. The nature of implicit categorization. Psychonomic Bulletin & Review. 1999;6:363–378. doi: 10.3758/bf03210826. [DOI] [PubMed] [Google Scholar]
- Benjamin AS, Bjork RA, Schwartz BL. The mismeasure of memory: When retrieval fluency is misleading as a metacognitive index. Journal of Experimental Psychology: General. 1998;127:55–68. doi: 10.1037//0096-3445.127.1.55. [DOI] [PubMed] [Google Scholar]
- Beran MJ. Monkeys (Macaca mulatta and Cebus apella) track, enumerate, and compare multiple sets of moving items. Journal of Experimental Psychology: Animal Behavior Processes. 2008;34:63–74. doi: 10.1037/0097-7403.34.1.63. [DOI] [PubMed] [Google Scholar]
- Beran MJ. Capuchin monkeys (Cebus apella) succeed in a test of quantity conservation. Animal Cognition. 2008 doi: 10.1007/s10071-007-0094-3. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Evans TA. Maintenance of delay of gratification by four chimpanzees (Pan troglodytes): The effects of delayed reward visibility, experimenter presence, and extended delay intervals. Behavioural Processes. 2006;73:315–324. doi: 10.1016/j.beproc.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Harris EH, Evans TA, Klein ED, Chan B, Flemming TM, Washburn DA. Ordinal judgments of symbolic stimuli by capuchin monkeys (Cebus apella) and rhesus monkeys (Macaca mulatta): The effects of differential and nondifferential reward. Journal of Comparative Psychology. 2008;1222:52–61. doi: 10.1037/0735-7036.122.1.52. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Klein ED, Evans TA, Chan B, Flemming TM, Harris EH, Washburn DA, Rumbaugh DM. Discrimination reversal learning in capuchin monkeys (Cebus apella) Psychological Record. 2008;58:3–14. [Google Scholar]
- Beran MJ, Smith JD, Redford JS, Washburn DA. Rhesus macaques (Macaca mulatta) monitor uncertainty during numerosity judgments. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:111–119. doi: 10.1037/0097-7403.32.2.111. [DOI] [PubMed] [Google Scholar]
- Boring EG. The control of attitude in psychophysical experiments. Psychological Review. 1920;27:440–452. [Google Scholar]
- Brown W. University of California Publications in Psychology. Vol. 1. Berkeley CA: The University Press; 1910. The judgment of difference; pp. 1–71. [Google Scholar]
- Call J, Carpenter M. Do apes and children know what they have seen? Animal Cognition. 2001;4:207–220. [Google Scholar]
- Carruthers P. Meta-cognition in Animals: a skeptical Look. Mind and Language. 2008;23(1):58–89. [Google Scholar]
- D’Amato MR, Colombo M. Representation of serial order in monkeys (Cebus apella) Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:131–139. [PubMed] [Google Scholar]
- Evans TA, Beran MJ. Chimpanzees use self-distraction to cope with impulsivity. Biology Letters. 2007;3:599–602. doi: 10.1098/rsbl.2007.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Beran MJ, Chan B, Klein ED, Menzel CR. An efficient computerized testing method for the capuchin monkey (Cebus apella): Adaptation of the LRC-CTS to a socially housed nonhuman primate species. Behavior Research Methods. 2008;40:590–596. doi: 10.3758/brm.40.2.590. [DOI] [PubMed] [Google Scholar]
- Evans TA, Westergaard GC. Discrimination of functionally appropriate and inappropriate throwing tools by captive tufted capuchins (Cebus apella) Animal Cognition. 2004;7:255–262. doi: 10.1007/s10071-004-0220-4. [DOI] [PubMed] [Google Scholar]
- Fernberger SW. The effect of the attitude of the subject upon the measure of sensitivity. American Journal of Psychology. 1914;25:538–543. [Google Scholar]
- Fernberger SW. The use of equality judgments in psychophysical procedures. Psychological Review. 1930;37:107–112. [Google Scholar]
- Flavell JH. Metacognition and cognitive monitoring: A new area of cognitive-developmental inquiry. American Psychologist. 1979;34:906–911. [Google Scholar]
- Foote A, Crystal J. Metacognition in the rat. Current Biology. 2007;17:551–555. doi: 10.1016/j.cub.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallup GG. Self-awareness and the emergence of mind in primates. American Journal of Primatology. 1982;2:237–48. doi: 10.1002/ajp.1350020302. [DOI] [PubMed] [Google Scholar]
- George SS. Attitude in relation to the psychophysical judgment. American Journal of Psychology. 1917;28:1–38. [Google Scholar]
- Hampton RR. Rhesus monkeys know when they remember. Proceedings of the National Academy of Sciences. 2001;98(9):5359–5362. doi: 10.1073/pnas.071600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR, Zivin A, Murray EA. Rhesus monkeys (Macaca mulatta) discriminate between knowing and not knowing and collect information as needed before acting. Animal Cognition. 2004;7:239–246. doi: 10.1007/s10071-004-0215-1. [DOI] [PubMed] [Google Scholar]
- Inman A, Shettleworth SJ. Detecting metamemory in nonverbal subjects: A test with pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1999;25:389–395. [Google Scholar]
- Judge PG, Evans TA, Vyas DK. Ordinal representation of numeric quantities by brown capuchin monkeys (Cebus apella) Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:79–94. doi: 10.1037/0097-7403.31.1.79. [DOI] [PubMed] [Google Scholar]
- Koriat A. Dissociating knowing and the feeling of knowing: Further evidence for the accessibility model. Journal of Experimental Psychology: General. 1995;124:311–333. [Google Scholar]
- Koriat A. Metacognition and consciousness. In: Zelazo PD, Moscovitch M, Thompson E, editors. Cambridge handbook of consciousness. New York: Cambridge University Press; in press. [Google Scholar]
- Koriat A, Bjork RA, Sheffer L, Bar SK. Predicting one’s own forgetting: The role of experience-based and theory-based processes. Journal of Experimental Psychology: General. 2004;133:643–656. doi: 10.1037/0096-3445.133.4.643. [DOI] [PubMed] [Google Scholar]
- Koriat A, Ma’ayan H, Nussinson R. The intricate relationships between monitoring and control in metacognition: Lessons for the cause-and-effect relation between subjective experience and behavior. Journal of Experimental Psychology: General. 2006;135:36–69. doi: 10.1037/0096-3445.135.1.36. [DOI] [PubMed] [Google Scholar]
- Kornell N, Son L, Terrace H. Transfer of metacognitive skills and hint seeking in monkeys. Psychological Science. 2007;18:64–71. doi: 10.1111/j.1467-9280.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- Maddox WT, Ashby FG. Dissociating explicit and procedural-learning based systems of perceptual category learning. Behavioural Processes. 2004;66:309–332. doi: 10.1016/j.beproc.2004.03.011. [DOI] [PubMed] [Google Scholar]
- McGonigle B, Chalmers M, Dickinson A. Concurrent disjoint and reciprocal classification by Cebus apella in seriation tasks: Evidence for hierarchical organization. Animal Cognition. 2003;6:185–197. doi: 10.1007/s10071-003-0174-y. [DOI] [PubMed] [Google Scholar]
- Metcalfe J. Metamemory: Theory and data. In: Tulving E, Craik FIM, editors. The Oxford handbook of memory. New York: Oxford University Press; 2000. pp. 197–211. [Google Scholar]
- Metcalfe J, Kober H. Self-reflective consciousness and the projectable self. In: Terrace HS, Metcalfe J, editors. The missing link in cognition: Origins of self-reflective consciousness. New York: Oxford University Press; 2005. pp. 57–83. [Google Scholar]
- Nelson TO, editor. Metacognition: Core readings. Toronto: Allyn and Bacon; 1992. [Google Scholar]
- Paukner A, Anderson JR, Fujita K. Redundant food searches by capuchin monkeys (Cebus apella): A failure of metacognition? Animal Cognition. 2006;9:110–117. doi: 10.1007/s10071-005-0007-2. [DOI] [PubMed] [Google Scholar]
- Rumbaugh DM, Pate JL. The evolution of cognition in primates: A comparative perspective. In: Roitblat HL, Bever TG, Terrace HS, editors. Animal cognition. Hillsdale, NJ: Erlbaum; 1984. pp. 569–587. [Google Scholar]
- Rumbaugh DM, Savage-Rumbaugh ES, Washburn DA. Toward a new outlook on primate learning and behavior: Complex learning and emergent processes in comparative perspective. Japanese Psychological Research. 1996;38:113–125. doi: 10.1111/j.1468-5884.1996.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Scheck P, Nelson TO. Lack of pervasiveness of the underconfidence-with-practice effect: Boundary conditions and an explanation via anchoring. Journal of Experimental Psychology: General. 2005;134:124–128. doi: 10.1037/0096-3445.134.1.124. [DOI] [PubMed] [Google Scholar]
- Schwartz BL. Sources of information in metamemory: Judgments of learning and feelings of knowing. Psychonomic Bulletin and Review. 1994;1:357–375. doi: 10.3758/BF03213977. [DOI] [PubMed] [Google Scholar]
- Serra MJ, Dunlosky J. Does retrieval fluency contribute to the underconfidence-with-practice effect? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:1258–1266. doi: 10.1037/0278-7393.31.6.1258. [DOI] [PubMed] [Google Scholar]
- Shields WE, Smith JD, Washburn DA. Uncertain responses by humans and rhesus monkeys (Macaca mulatta) in a psychophysical same-different task. Journal of Experimental Psychology: General. 1997;126:147–164. doi: 10.1037//0096-3445.126.2.147. [DOI] [PubMed] [Google Scholar]
- Smith JD, Beran MJ, Couchman JJ, Coutinho MVC. The comparative study of metacognition: Sharper paradigms, safer inferences. Psychonomic Bulletin & Review. doi: 10.3758/pbr.15.4.679. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Beran MJ, Redford JS, Washburn DA. Dissociating uncertainty states and reinforcement signals in the comparative study of metacognition. Journal of Experimental Psychology: General. 2006;135:282–297. doi: 10.1037/0096-3445.135.2.282. [DOI] [PubMed] [Google Scholar]
- Smith JD, Schull J, Strote J, McGee K, Egnor R, Erb L. The uncertain response in the bottlenosed dolphin (Tursiops truncatus) Journal of Experimental Psychology: General. 1995;124:391–408. doi: 10.1037//0096-3445.124.4.391. [DOI] [PubMed] [Google Scholar]
- Smith JD, Shields WE, Allendoerfer KR, Washburn WA. Memory monitoring by animals and humans. Journal of Experimental Psychology: General. 1998;127:227–250. doi: 10.1037//0096-3445.127.3.227. [DOI] [PubMed] [Google Scholar]
- Smith JD, Shields WE, Schull J, Washburn DA. The uncertain response in humans and animals. Cognition. 1997;62:75–97. doi: 10.1016/s0010-0277(96)00726-3. [DOI] [PubMed] [Google Scholar]
- Smith JD, Shields WE, Washburn DA. The comparative psychology of uncertainty monitoring and metacognition. The Behavioral and Brain Sciences. 2003;26:317–373. doi: 10.1017/s0140525x03000086. [DOI] [PubMed] [Google Scholar]
- Staddon JER, Jozefowiez J, Cerutti D. Metacognition: A problem not a process. PsyCrit. 2007 Apr 13;:1–5. [Google Scholar]
- Suda-King C. Do orangutans (Pongo pygmaeus) know when they do not remember? Animal Cognition. 2007 doi: 10.1007/s10071-007-0082-7. [DOI] [PubMed] [Google Scholar]
- Sutton JE, Shettleworth SJ. Memory without awareness: Pigeons do not show metamemory in delayed matching-to-sample. Journal of Experimental Psychology: Animal Behavior Processes. doi: 10.1037/0097-7403.34.2.266. in press. [DOI] [PubMed] [Google Scholar]
- Thomson GH. A new point of view in the interpretation of threshold measurements in psychophysics. Psychological Review. 1920;27:300–307. [Google Scholar]
- Thompson RKR, Oden DL. Categorical perception and conceptual judgments by nonhuman primates: The paleological monkey and the analogical ape. Cognitive Science. 2000;24:363–396. [Google Scholar]
- Washburn DA, Smith JD, Shields WE. Rhesus Monkeys (Macaca mulatta) immediately generalize the uncertain response. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:85–89. doi: 10.1037/0097-7403.32.2.185. [DOI] [PubMed] [Google Scholar]
- Watson CS, Kellogg SC, Kawanishi DT, Lucas PA. The uncertain response in detection-oriented psychophysics. Journal of Experimental Psychology. 1973;99:180–185. [Google Scholar]
- Woodworth RS. Experimental psychology. New York: Holt; 1938. [DOI] [PubMed] [Google Scholar]
- Wright AA, Katz JS. Mechanisms of same/different concept learning in primates and avians. Behavioural Processes. 2006;72:234–254. doi: 10.1016/j.beproc.2006.03.009. [DOI] [PubMed] [Google Scholar]