Abstract
Background
Chronic idiopathic urticaria (CU) has been associated with other autoimmune diseases and basophil-activating autoantibodies to FcεRI or IgE. It is unknown whether patients with systemic-autoimmune diseases have a similar prevalence of these autoantibodies.
Objective
To compare the prevalences of basophil-activating autoantibodies (elevated CU Index) in patients with CU, rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE). Clinical characteristics and laboratory studies were examined for an association with the CU Index.
Methods
Adult patients, 27 with CU, 27 with RA, and 26 with SLE, and 20 healthy controls were compared on the basis of the CU Index panel, anti-IgE, and antithyroid antibodies.
Results
The CU Index values were significantly higher in the CU group when compared with the RA group but not when compared with the SLE group. 33% of CU, 23% of SLE, 3.7% of RA, and 15% of controls had apositive CU Index. Elevated antithyroid antibody levels did not correlate with a positive CU Index in any of the groups. An elevated CU Index in the SLE group was not associated with age, sex, ethnicity, disease severity, or history of atopy.
Conclusion
The CU Index values were elevated in patients with CU and SLE. The presence of these autoantibodies did not correlate with disease activity or presence of thyroid antibodies. Functional autoantibodies may not be specific for chronic idiopathic urticaria, and their role in nonurticarial systemic autoimmune diseases requires further investigation.
Introduction
Chronic idiopathic urticaria (CU) is characterized by pruritic wheals lasting several hours and recurring during at least 6 weeks. It is a common and potentially debilitating skin condition that affects up to 1% of the general population with variable duration, typically several months, but occasionally decades.1
Although the pathogenesis of CU has not been fully elucidated, a proportion of patients with CU have been found to have functional autoantibodies.2–4 The first indication of the existence of such antibodies was the autologous serum skin test (ASST), which was initially described in 1986.5 The reported incidence of positive ASST results in patients with CU varies from 4% to 76%. One likely explanation for the wide range of prevalence is that the administration and interpretation of the test have not been standardized.6 In the past several years, in vitro tests have been developed using histamine release of basophils or basophil activation markers, such as CD63 and CD203c, to measure the function of these autoantibodies. In CU patients, IgG anti-FcεRIα autoantibodies are seen in 30% to 60% and IgG anti-IgE autoantibodies in 5% to 10%.2–4,7
CU has been associated with autoimmune conditions, such as the presence of elevated thyroid autoantibodies (prevalence of up to 30% in CU compared with 6% in the general population), type 1 diabetes mellitus, and HLA-DR4 and HLA-DR8 alleles.8–10 A recent large retrospective study demonstrated an association between chronic urticaria and the incidence of rheumatoid arthritis (RA), Sjögren syndrome, celiac disease, and systemic lupus erythematosus (SLE) diagnosed within 10 years after onset of urticaria.11 Increased prevalences of urticarial autoantibodies in SLE, psoriasis, bullous pemphigoid, and dermatomyositis have been described, but these antibodies were not pathogenic and did not activate basophils.12 Despite the known association of CU and autoimmunity, the prevalence and significance of basophil-activating autoantibodies in nonurticarial systemic autoimmune disease has not been clearly established.
This study sought to determine the prevalence of basophil-activating IgG anti-FcεRIα and IgG anti-IgE antibodies in patients with nonurticarial systemic autoimmune diseases, specifically RA and SLE, using commercially available testing (CU Index) and to determine whether there was a detectable difference in the prevalence of abnormal CU Index levels between patients with chronic urticaria and these autoimmune disorders. Further, we examined clinical characteristics among the CU, RA, and SLE groups, including disease activity and associated laboratory studies.
Methods
In this prospective study, 80 adult patients, 27 with CU, 27 with RA, and 26 with SLE, were recruited from an allergy clinic and a rheumatology clinic of a large academic center from March 2010 to May 2011. Patients who had a history of urticaria of 3 or more days per week for at least 6 weeks and did not have known systemic autoimmune disorders were included in the CU arm. Patients were diagnosed as having SLE and RA according to treating physician judgment guided by American College of Rheumatology classification criteria13–15 and were excluded if they had a history of urticaria within the past 6 months.
The primary aims of this study were to determine the prevalence of a positive CU Index in patients with CU, RA, or SLE and to evaluate whether there was a difference between these groups. Describing clinical or laboratory factors associated with an abnormal CU Index was a secondary goal. This study was approved by the institutional review board at the Ohio State University Wexner Medical Center.
Atopy history and disease activity scores were obtained from the patients. They were considered to have a history of atopy if they reported a history of allergic rhinitis (confirmed by skin prick testing or specific IgE testing if available), asthma, food allergy, or atopic dermatitis. Clinical confirmation of atopic disease in the RA and SLE patients was not performed. Standardized disease activity scores for the SLE (scores, 0–3) and RA (scores, 0–10) groups were scored by the patient’s rheumatologist. The CU disease activity was based on the patient’s estimate of weekly symptom frequency in the past month and scored on the day of recruitment, with 0 indicating 0 days; 1, 1 to 2 days; 2, 3 to 4 days; and 3, 5 to 7 days.
Serum from 20 healthy controls provided by Viracor-IBT Laboratories (Lee’s Summit, Missouri) were compared; their atopic status and other clinical characteristics were not obtained.
The CU Index was determined in each patient group by Viracor-IBT Laboratories. Sodium heparinized whole blood was collected from a healthy donor. Basophils were enriched by an initial slow 200g centrifugation; the plasma and buffy coat were removed and centrifuged at 500g. After the second centrifugation, the plasma was discarded. The enriched basophil preparation was diluted in stimulation buffer containing interleukin 3 (FLOW CAST kit; ALPCO, Salem, New Hampshire) and incubated with patient or control sera, buffer control, or anti-IgE (2μg/mL; Beckman Coulter, Brea, California) as a positive control for 30 minutes in a 37°C water bath. The cells were then centrifuged and the supernatants recovered. Aliquots of cells were lysed for determination of total histamine content. Using a quantitative enzyme immunoassay (Beckman Coulter), the histamine released into the supernatant was measured and compared with the total histamine in the basophils to generate a percentage of histamine release. The percentageof histamine was compared with the cutoff value for the donor and an index generated. CU Index values greater than 10 indicated that the serum caused basophil activation, suggesting the presence of autoantibodies. Levels of serum antibodies against thyroid peroxidase and thyroglobulin were measured on the Immulite 2000 (Siemens, Los Angeles, California). Reference values were based on the manufacturer’s recommendation. Reference values were less than 324 ng/mL for anti-IgE, less than 35 IU/mL for antithyroid peroxidase (TPO), and less than 40 IU/mL for antithyroglobulin. Thyrotropin levels in serum were measured using the rapid thyrotropin kit also on the Immulite 2000. For this method, the reference range was 0.4 to 4.0 mIU/mL. Levels of binding IgG antibodies against IgE and FcεRIα were quantified with solid-phase indirect enzyme-linked immunoabsorbent assays. Bound IgG antibodies were detected with an antihuman IgG antibody.
Statistical Analysis
A power calculation was performed on the study’s primary comparison to test the proportions that were categorized as having a high CU Index in the following disease groups: SLE, RA, and CU. The expected effect size of 0.399 was based on equal number of observations in each disease group (SLE, RA, and CU) with the following high CU index proportions: 5%, 10%, and 40%, respectively. A sample size of 27 per group has at least 80% power to detect this effect size with 2 df. This is based on a 2-sided χ2 test with an α = .0167. This α level is based on testing all pairwise comparisons; thus, 0.05/3 = .0167 preserves the overall type I error rate at 5%. There was a fourth group of healthy individuals who will determine whether the CU Index assay is working correctly. We expected this group to have a less than 5% high CU Index. This group was not formally tested because it is a negative control. We planned on accruing only 19 individuals in this group, so the total sample size will be 100 individuals, 27 from each of the tested groups and 19 healthy people.
Patient demographics and clinical characteristics are presented as either means and SDs or medians and interquartile ranges (IQRs) for continuous variables, depending on the distribution, and as frequencies and percentages for categorical variables. These characteristics were compared across disease groups using the Kruskal-Wallis test for the continuous variables and the Fisher exact test for categorical variables. A type I error of 0.0167 was used in the primary comparison of the CU Index across the disease status. This was done to conserve the overall type I error rate of 0.05 using a Bonferroni correction that divided 0.05 by 3 (number of multiple comparisons) to get 0.0167. Associations between continuous variables were determined using the Spearman rank correlation. All analyses were run using Stata 12.0 statistical software (Stata Corporation, College Station, Texas).
Results
The demographics and clinical characteristics of the study participants are given in Table 1. Most study participants were female and white in the RA, SLE, and CU groups. The patients in the RA group were significantly older, with a mean age of 52.4 years, compared with the SLE (mean age, 36.7 years) and CU (mean age, 39.2 years) groups. To assess disease activity of each group, we looked at duration of hives in the CU group and disease scores in the autoimmune diseases. The median duration of urticaria in the CU group was 12 months (IQR, 6–60 months). The disease scores for the RA and SLE groups were not normally distributed; the median disease scores were 3 (IQR, 2–4) for the RA group and 1 (IQR, 0–1) for the SLE group.
Table 1.
Demographics and clinical features
| Clinical Feature | RA (n=27) | SLE (n=26) | CU (n=27) | P valuea |
|---|---|---|---|---|
| Age, mean (SD), y | 52.4 (13.3) | 36.7 (11.0) | 39.2 (10.7) | <.001 |
| Female, No. (%) | 22 (81) | 19 (73) | 23 (85) | .54 |
| Race, No. (%) | .15 | |||
| White | 22 (81) | 18 (69) | 17 (63) | |
| Black | 4 (15) | 8 (31) | 6 (22) | |
| Other | 1 (4) | 0 (0) | 4 (15) | |
| Disease activity | ||||
| Median (IQR) | 3 (2–4) | 1 (0–1) | 0 (0–3) | |
| Score, mild to severe | 0–10 | 0–3 | 0–3 | |
| Duration of hives, median (IQR), mo | 12 (6–60) | |||
Abbreviations: CU, chronic idiopathic urticaria; IQR, interquartile range; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
P values are based on Fisher exact test where the overall P value (.01) was significant at the .05 level. Thus, the individual comparisons are tested against an α = .0167 (Bonferroni correction for 3 comparisons) for statistical significance to conserve the overall type I error rate at 0.05.
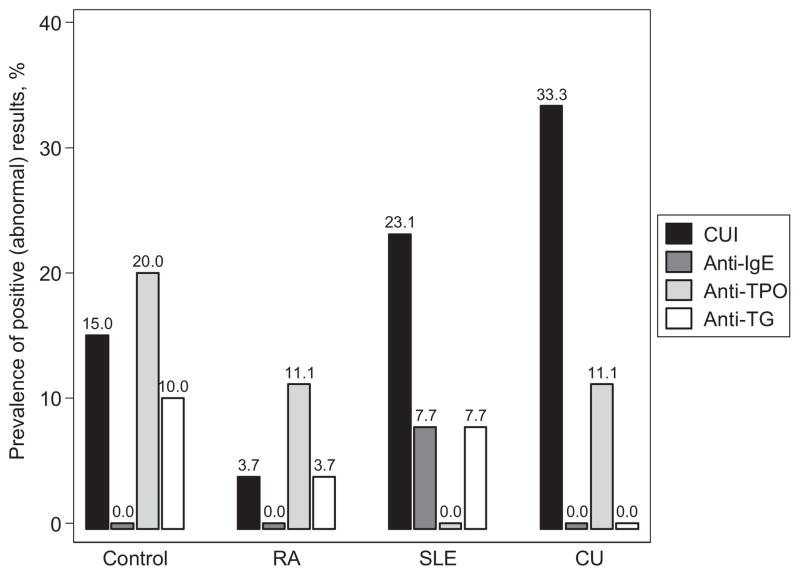
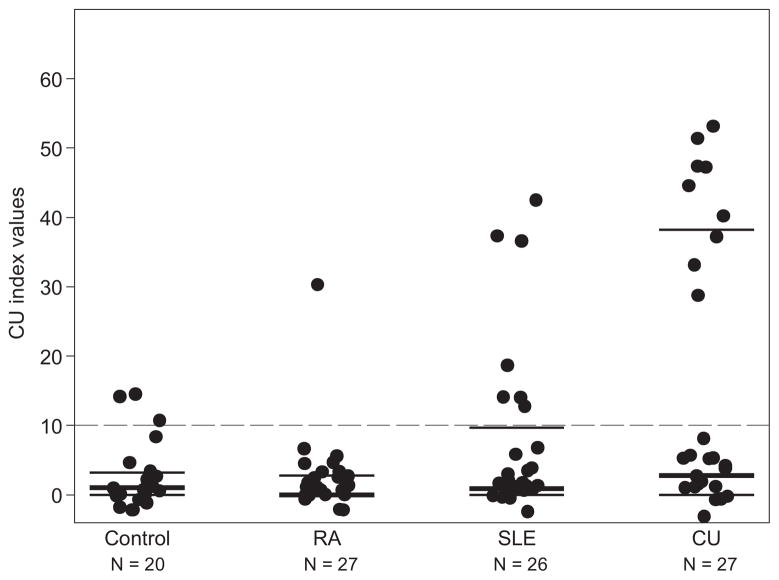
The primary endpoint of this study was to assess the prevalence of a positive CU Index in each patient group. We found that 33.3% of CU patients (95% confidence interval [CI], 16.5–54.0), 23.1% of SLE patients (95% CI, 9.0–43.6), and 3.7% of RA patients (95% CI, 0.09–19.0) had a CU Index greater than 10 (Fig 1). We found a significant difference in the prevalence of having a positive CU Index between the CU and RA groups (P = .01) but not between the CU and SLE groups (P = .55) or between the RA and the SLE groups (P = .05). A total of 15% of controls had a positive CU Index (95% CI, 3.2–37.9) (Fig 1). When looking at the actual levels of the CU Index, a significant difference was found among the 3 groups (P = .02, Table 2 and Fig 2).
Figure 1.
Prevalence of a positive (abnormal) result by disease status. CU indicates chronic idiopathic urticaria; CUI, CU Index; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; TG, thyroglobulin; TPO, thyroid peroxidase.
Table 2.
Levels of thyroid antibodies, CU Index, thyrotropin, and anti-IgE
| Test | Median (IQR)
|
P valuea | ||
|---|---|---|---|---|
| RA (n=27) | SLE (n=26) | CU (n=27) | ||
| Anti-TG, IU/mL | 0 (0–0) | 0 (0–0) | 0 (0–0) | .35 |
| Anti-TPO, IU/mL | 0 (0–15.0) | 0 (0–0) | 0 (0–14.0) | .20 |
| CU Index | 0 (0–2.8) | 0.9 (0–9.7) | 2.8 (0–38.3) | .02 |
| Thyrotropin, μIU/mL | 1.1 (0.7–1.9) | 1.4 (0.6–2.2) | 1 (0.9–1.5) | .92 |
| Anti-IgE, ng/mL | 0 (0–0) | 0 (0–0) | 0 (0–0) | .21 |
Abbreviations: CU, chronic idiopathic urticaria; IQR, interquartile range; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; TG, thyroglobulin; TPO, thyroid peroxidase.
P values based on the Kruskal-Wallis testcomparing the values across disease status.
Figure 2.
CU Index values by disease status. The medians and interquartile ranges are overlaid on the observed values. The dashed line is the cutoff where values above 10 indicate high values of the CU Index. CU indicates chronic idiopathic urticaria; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
The other laboratory studies performed in this study included anti-IgE antibody, thyrotropin, anti-TPO antibody, and antithyroglobulin antibody. No study participants demonstrated elevated anti-IgE antibodies. Patients in the control, RA, and CU groups were found to have elevated anti-TPO antibodies, and patients in the control, RA, and SLE groups were found to have elevated antithyroglobulin antibodies (Fig 1). However, we failed to find a significant difference in the levels of antithyroid antibodies or thyrotropin among the groups (Table 2).
Clinical characteristics were examined for associations with CU Index in the SLE and CU groups. Only 1 patient had a positive CU Index in the RA group, so this group was not compared. CU Index values were not statistically associated with race (P = .33), sex (P = .33), or age (P = .11). Because an elevated CU Index has been associated with disease severity of chronic urticaria in previous studies,17–19 we looked for an association of CU Index with disease severity in the CU and SLE groups. No statistically significant associations were found between the CU Index and hive duration in the CU group or disease activity score in the SLE group. Four RA patients and 8 SLE patients had a distant history of hives, which all lasted less than 6 weeks. A positive history of hives was not associated with elevated CU Index levels in these groups (RA: P = .32, SLE: P >.99). No statistical association was found between elevated CU Index and history of atopy.
Examining the CU group specifically, laboratory tests associated with autoimmune and allergic diseases were compared with CU Index levels (Table 3). CU Index levels were negatively associated with rheumatoid factor and total IgE (ρ = −0.791, −0.534; P = .03, .02, respectively) and positively associated with tryptase levels (ρ = 0.445, P = .04). No statistically significant relationship was found between CU Index and thyroid antibodies or antinuclear antibody.
Table 3.
Correlations between CUI and respective laboratory values for CU patients
| Test | No. of patients | ρ | P value |
|---|---|---|---|
| Thyroid antibodies | 27 | −0.115 | .57 |
| ANA | 18 | −0.345 | .16 |
| RF | 7 | −0.791 | .03 |
| Total IgE | 20 | −0.534 | .02 |
| Tryptase | 22 | 0.445 | .04 |
Abbreviations: ANA, antineutrophil antibody; RF, rheumatoid factor.
Discussion
In this study, 33.3% of CU patients had a positive CU Index, which is on the lower end of the prevalences previously reported (30%–60%).4,7 Increased prevalences of basophil-activating autoantibodies were found in the RA and SLE groups compared with previous studies. These results suggest that autoimmune diseases other than CU can be associated with increased levels of basophil-degranulating autoantibodies, contrary to previous findings. A separate prospective study reported increased anti-FcεRIα antibodies in 20% of patients with SLE (n = 15), but they did not cause histamine release from basophils in the 6 patients tested.12 Another study using basophil CD63 expression instead of histamine release found that serum samples from 16 SLE patients did not cause CD63 expression.16 On the basis of our findings, urticarial autoantibodies associated with histamine release may indeed be found in SLE patients. In this study, the CU Index differentiated CU patients from RA patients but not SLE patients. A possible clinical utility of these findings is that an elevated CU Index in a patient with SLE may be a false-positive result and may not be clinically helpful in detecting chronic idiopathic urticaria in this patient group.
Although the presence of various autoimmune diseases in patients with chronic idiopathic urticaria has been well described, it is not clear whether the presence of functional urticarial auto-antibodies reflects the general propensity toward autoimmunity or whether it is specific for patients with chronic idiopathic urticaria. The ASST result has been found to be positive in 30% to 50% of atopic controls, whereas other studies have demonstrated much lower prevalence in healthy individuals.6 The CU Index may have similar specificity.17,18 It is not known why functional autoantibodies can be found in patients without urticaria or angioedema. The causative role of these functional autoantibodies warrants future investigation.
In this study, the CU Index was not associated with race, sex, or history of atopy. However, the diagnosis of atopy was not confirmed by testing in all study patients, so the true prevalence of atopy in these patients is unknown. Interestingly, the CU Index was negatively associated with total IgE and positively associated with total tryptase. A positive association between functional autoantibodies (CD63) and total tryptase levels was also found (P = .04) in a prospective study where total tryptase levels in patients with CU were found to be significantly higher than atopic and healthy controls, with no elevation of mature β-tryptase levels.19 Those findings suggest increased mast cell burden in these patients; however, what causes this and how it relates to functional autoantibodies are not clear.
Variable results have been reported regarding correlation of CU Index levels and urticaria scores, although most studies report a positive correlation.20–22 Our study did not find a correlation between disease activity for urticaria and CU Index levels. However, a limitation was that a validated scale was not used. A large retrospective study reported that patients with CU had an increased risk of having RA (odds ratio, 13.25; P < .001) and SLE (odds ratio, 14.59;P < .001) compared with controls.11 In our study, 7 CU patients had their rheumatoid factor checked, and this was negatively associated with CU Index (Table 3). Although this finding was significant, the number of patients was low, and this should be examined with a larger group of patients. Among the patients with systemic nonurticarial autoimmune disease in our study, report of a distant history of hives was not correlated with CU Index levels. None of the RA or SLE patients had a history of chronic urticaria. Associations between CU Index and disease activity of the RA or SLE groups using validated scales were not found, but the study population was not powered to detect this relationship.
Antithyroid antibodies are the most common nonurticarial antibodies found in patients with chronic idiopathic urticaria, seen in 12% to 30%.8,9,23 Their presence in patients with CU is thought to demonstrate the propensity of these patients to develop functional autoantibodies.23 They are not thought to cause the disease independent of thyrotropin abnormalities, although there have been case reports of urticaria resolution with thyroid hormone supplementation. In our study, interestingly, only 3 CU patients (11.1%) demonstrated the presence of antithyroid antibodies, slightly below reported prevalences. These results were surprising because the CU patients reflected a typical CU patient population seen in an academic allergy practice. This may raise concern that the patients in the CU group may have had another underlying disease process causing urticaria; however, we do not believe this is the case. Diagnostic studies, including screening for allergic, infectious, malignant, and autoimmune causes, were conducted by a board-certified allergist for each CU patient based on clinical history and examination before assigning a diagnosis of CU. Previous studies have found mixed results regarding an association between functional urticarial autoantibodies and antithyroid antibodies.23,24 An explanation for this discrepancy among different studies may be that the development of thyroid antibodies in patients with CU could be associated with duration of disease. The median duration of urticaria in our study’s patients was 12 months, which may be significantly less than the duration of urticaria in previous studies, which have found a higher prevalence of antithyroid antibodies in patients with CU. Finally, antithyroid antibodies were detected in the RA, SLE, and control groups, but were not associated with elevated CU Index levels.
Other unexpected findings in this study were the elevated prevalence of autoantibodies in the control group and the decreased prevalence in the RA group, with the exception of anti-TPO, which was 11%. There may have been a selection bias with the control group because the serum was provided by Viracor-IBT Laboratories, and they did not report randomization with their selection process. No clinical characteristics were known about the controls except that they did not report active urticaria. The reported prevalence of antithyroid antibodies in RA range from 7% to 37%, with anti-TPO being more common than antithyroglobulin.25–27 Studies reporting increased incidences have recruited at least 70 RA patients. Our study was not powered to determine the prevalence of these antithyroid antibodies in patients with RA. Most studies have demonstrated that the CU Index can predict severity of CU.20–22 This was not found to be true in this study. Our study was not designed to predict treatment response in CU patients.
Our findings suggest that functional autoantibodies are indeed present in diseases other than CU, and may reflect a population that can be distinguished from RA patients but not SLE patients. This population of patients was not found to be atopic but may have an increased mast cell burden that could predispose them to urticaria. It appears from our results that a positive CU Index may not be specific to only CU patients, may not correlate with disease severity, and is present in only a subset of CU patients. It may be of clinical benefit to continue to study the subpopulation of CU patients who demonstrate a positive CU Index. Furthermore, why some SLE patients demonstrate these autoantibodies and do not exhibit urticaria should be studied to learn about the clinical significance of functional urticarial antibodies in systemic nonurticarial autoimmune disorders.
Acknowledgments
Funding Sources: This study was funded by the Columbus Medical Research Foundation and Viracor-IBT Laboratories.
We acknowledge Eric Oliver, MD, for his help in collecting data from the study participants.
Footnotes
Disclosures: Dr. Stutes has received speaker fees from Dey Pharmaceuticals. Dr. Altrich is currently employed at Viracor-IBT Laboratories and has volunteered at CBLI. Dr. Ardoin is a consultant for Johnson & Johnson.
References
- 1.Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664–672. doi: 10.1067/mai.2000.105706. [DOI] [PubMed] [Google Scholar]
- 2.Sabroe RA, Grattan CE, Francis DM, Barr RM, Kobza Black A, Greaves MW. The autologous serum skin test: a screening test for autoantibodies in chronic idiopathic urticaria. Br J Dermatol. 1999;140:446–452. doi: 10.1046/j.1365-2133.1999.02707.x. [DOI] [PubMed] [Google Scholar]
- 3.Hide M, Francis DM, Grattan CE, Hakimi J, Kochan JP, Greaves MW. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N Engl J Med. 1993;328:1599–1604. doi: 10.1056/NEJM199306033282204. [DOI] [PubMed] [Google Scholar]
- 4.Ferrer M, Kinet JP, Kaplan AP. Comparative studies of functional and binding assays for IgG anti-Fc(epsilon)RIalpha (alpha-subunit) in chronic urticaria. J Allergy Clin Immunol. 1998;101:672–676. doi: 10.1016/s0091-6749(98)70176-9. [DOI] [PubMed] [Google Scholar]
- 5.Grattan CE, Wallington TB, Warin RP, Kennedy CT, Bradfield JW. A serological mediator in chronic idiopathic urticaria: a clinical, immunological and histological evaluation. Br J Dermatol. 1986;114:583–590. doi: 10.1111/j.1365-2133.1986.tb04065.x. [DOI] [PubMed] [Google Scholar]
- 6.Konstantinou GN, Asero R, Maurer M, Sabroe RA, Schmid-Grendelmeier P, Grattan CE. EAACI/GA(2)LEN task force consensus report: the autologous serum skin test in urticaria. Allergy. 2009;64:1256–1268. doi: 10.1111/j.1398-9995.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- 7.Gruber BL, Baeza ML, Marchese MJ, Agnello V, Kaplan AP. Prevalence and functional role of anti-IgE autoantibodies in urticarial syndromes. J Invest Dermatol. 1988;90:213–217. doi: 10.1111/1523-1747.ep12462239. [DOI] [PubMed] [Google Scholar]
- 8.Leznoff A, Sussman GL. Syndrome of idiopathic chronic urticaria and angioedema with thyroid autoimmunity: a study of 90 patients. J Allergy Clin Immunol. 1989;84:66–71. doi: 10.1016/0091-6749(89)90180-2. [DOI] [PubMed] [Google Scholar]
- 9.Verneuil L, Leconte C, Ballet JJ, et al. Association between chronic urticaria and thyroid autoimmunity: a prospective study involving 99 patients. Dermatology. 2004;208:98–103. doi: 10.1159/000076480. [DOI] [PubMed] [Google Scholar]
- 10.Asero R, Orsatti A, Tedeschi A, Lorini M. Autoimmune chronic urticaria associated with type 1 diabetes and Graves’ disease. J Allergy Clin Immunol. 2005;115:1088–1089. doi: 10.1016/j.jaci.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Confino-Cohen R, Chodick G, Shalev V, Leshno M, Kimhi O, Goldberg A. Chronic urticaria and autoimmunity: associations found in a large population study. J Allergy Clin Immunol. 2012;129:1307–1313. doi: 10.1016/j.jaci.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 12.Fiebiger E, Hammerschmid F, Stingl G, Maurer D. Anti-FcepsilonRIalpha autoantibodies in autoimmune-mediated disorders: identification of a structure-function relationship. J Clin Invest. 1998;101:243–251. doi: 10.1172/JCI511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 14.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 16.Gyimesi E, Sipka S, Danko K, et al. Basophil CD63 expression assay on highly sensitized atopic donor leucocytes: a useful method in chronic autoimmune urticaria. Br J Dermatol. 2004;151:388–396. doi: 10.1111/j.1365-2133.2004.06042.x. [DOI] [PubMed] [Google Scholar]
- 17.Platzer MH, Grattan CE, Poulsen LK, Skov PS. Validation of basophil histamine release against the autologous serum skin test and outcome of serum-induced basophil histamine release studies in a large population of chronic urticaria patients. Allergy. 2005;60:1152–1156. doi: 10.1111/j.1398-9995.2005.00841.x. [DOI] [PubMed] [Google Scholar]
- 18.Altrich ML, Halsey JF, Altman LC. Comparison of the in vivo autologous skin test with in vitro diagnostic tests for diagnosis of chronic autoimmune urticaria. Allergy Asthma Proc. 2009;30:28–34. doi: 10.2500/aap.2009.30.3185. [DOI] [PubMed] [Google Scholar]
- 19.Ferrer M, Nunez-Cordoba JM, Luquin E, et al. Serum total tryptase levels are increased in patients with active chronic urticaria. Clin Exp Allergy. 2010;40:1760–1766. doi: 10.1111/j.1365-2222.2010.03582.x. [DOI] [PubMed] [Google Scholar]
- 20.Biagtan MJ, Viswanathan RK, Evans MD, Mathur SK. Clinical utility of the Chronic Urticaria Index. J Allergy Clin Immunol. 2011;127:1626–1627. doi: 10.1016/j.jaci.2011.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lapolla W, Desai N, English JC., III Clinical utility of testing for autoimmunity in chronic idiopathic urticaria. J Am Acad Dermatol. 2012;66:e83–e88. doi: 10.1016/j.jaad.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Sabroe RA, Fiebiger E, Francis DM, et al. Classification of anti-FcepsilonRI and anti-IgE autoantibodies in chronic idiopathic urticaria and correlation with disease severity. J Allergy Clin Immunol. 2002;110:492–499. doi: 10.1067/mai.2002.126782. [DOI] [PubMed] [Google Scholar]
- 23.Najib U, Bajwa ZH, Ostro MG, Sheikh J. A retrospective review of clinical presentation, thyroid autoimmunity, laboratory characteristics, and therapies used in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2009;103:496–501. doi: 10.1016/S1081-1206(10)60266-9. [DOI] [PubMed] [Google Scholar]
- 24.Kikuchi Y, Fann T, Kaplan AP. Antithyroid antibodies in chronic urticaria and angioedema. J Allergy Clin Immunol. 2003;112:218. doi: 10.1067/mai.2003.1605. [DOI] [PubMed] [Google Scholar]
- 25.Charles PJPD, Chowdhury M, Worthington J, Venables P. Antibodies to thyroglobulin and thyroid peroxidase in rheumatic arthritis: environmental and genetic associations. Ann Rheum Dis. 2011;70:A88–A89. [Google Scholar]
- 26.Yavasoglu I, Senturk T, Coskun A, Bolaman Z. Rheumatoid arthritis and anti-thyroid antibodies. Autoimmunity. 2009;42:168–169. doi: 10.1080/08916930802428114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atzeni F, Doria A, Ghirardello A, et al. Anti-thyroid antibodies and thyroid dysfunction in rheumatoid arthritis: prevalence and clinical value. Autoimmunity. 2008;41:111–115. doi: 10.1080/08916930701620100. [DOI] [PubMed] [Google Scholar]