Abstract
Apnea, the cessation of breathing, is a common physiological and pathophysiological phenomenon with many basic scientific and clinical implications. Among the different forms of apnea, obstructive sleep apnea (OSA) is clinically the most prominent manifestation. OSA is characterized by repetitive airway occlusions that are typically associated with peripheral airway obstructions. However, it would be a gross oversimplification to conclude that OSA is caused by peripheral obstructions. OSA is the result of a dynamic interplay between chemo- and mechanosensory reflexes, neuromodulation, behavioral state and the differential activation of the central respiratory network and its motor outputs. This interplay has numerous neuronal and cardiovascular consequences that are initially adaptive but in the long-term become major contributors to the morbidity and mortality associated with OSA. However, not only OSA, but all forms of apnea have multiple, and partly overlapping mechanisms. In all cases the underlying mechanisms are neither “exclusively peripheral” nor “exclusively central” in origin. While the emphasis has long been on the role of peripheral reflex pathways in the case of OSA, and central mechanisms in the case of central apneas, we are learning that such a separation is inconsistent with the integration of these mechanisms in all cases of apneas. This review discusses the complex interplay of peripheral and central nervous components that characterizes the cessation of breathing.
1. Introduction
Obstructive Sleep Apnea (OSA) is a major health issue worldwide affecting 3–7% of adult men and 2–5% of adult women (Young et al., 2002) with the incidence increasing because of the dramatic rise in obesity (Bhattacharjee et al., 2012). Weight change predicts the incidence of OSA, and a 10% increase in weight is associated with a 32% increase in the apnea/hypopnea index (Peppard et al., 2000a). Furthermore, OSA is an important contributor to the morbidity and mortality associated with obesity (Gozal and Kheirandish-Gozal, 2009; Tuomilehto et al., 2012). OSA is defined as the cessation of breathing caused by the repetitive, episodic collapse of the pharyngeal airway due to an obstruction or increased airway resistance. The first modern description of OSA was by Burwell and colleagues (1956) but was documented much earlier (Bickelmann et al., 1956; Bray, 1994; Lavie, 1984). OSA is distinguished from central apnea (CA), which is primarily caused by the cessation of the central respiratory network. CA is highly prevalent in congestive heart failure but is also present in normal subjects (Eckert et al., 2009a).
The distinction between each form of apnea, however, is not straightforward. OSA (Figure 1) as well as CA is the result of complex interactions between the peripheral and central nervous system (Eckert et al., 2009a). These interactions lead to short-term and long-term changes that contribute to the evolution of OSA and CA. Consequences of these disorders include excessive daytime somnolence, neurocognitive impairment, and increased risk for accidents related to sleep deprivation (Gozal et al., 2012; Gozal and Kheirandish-Gozal, 2012; Jordan and White, 2008; Kim et al., 1997; Young et al., 1997). In addition to these neurological consequences, the number of apneas that patients experience is positively correlated with an increased risk of hypertension (Peppard et al., 2000b). Other serious cardiovascular morbidities include increased risk for stroke, coronary artery disease, and heart failure (Phillips, 2005).
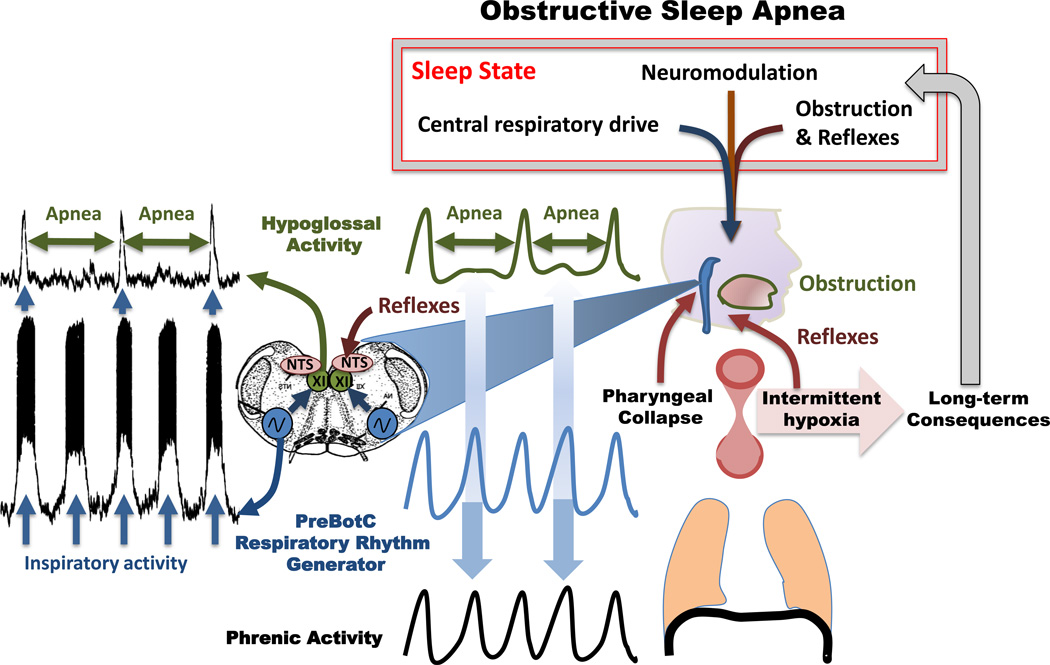
Figure 1.
Mechanisms of obstructive apneas. Left panel: Upper trace: integrated extracellularly recorded hypoglossus activity obtained simultaneously with an intracellular recording from an inspiratory neuron located within the pre-Bötzinger complex (lower trace). The recordings were obtained in an isolated transverse slice preparation shown in the schematic. The preparation contains the functionally active pre-Bötzinger complex (blue), the nucleus tractus solitaries (NTS, pink), which in the intact animal would receive reflex inputs. Contained within the slice is also the hypoglossal nucleus (XII, green), which in the intact animal would innervate the genioglossus muscle. Note the XII activity is not always phase-locked with the pre-Bötzinger complex resulting in XII apneas that are uncoupled from the respiratory rhythm generator located within the pre-Bötzinger complex. (Figure modified from Ramirez et al. 1997). The actual data shown on the left panel inspired the middle panel, which schematically illustrates how the rhythmically active pre-Bötzinger complex (blue trace) could continue to activate the phrenic activity (black trace) resulting in diaphragmatic activity, while activity in the XII becomes uncoupled resulting in a cessation of XII activity (green trace, apnea). The right panel illustrates the anatomical components contributing to an airway occlusion: A decreased activity in the genioglossus (tongue in the schematic) and continued activity in the diaphragm (schematically drawn beneath the lung) will lead to the negative pressure that results in a pharyngeal collapse. The bilaterally organized pre-Bötzinger complex isolated in the transverse slice preparation, which was obtained from the lower brainstem (medulla – schematically shown in blue). The airway occlusion is the result of sleep state, neuromodulation, central respiratory drive, reflexes and the airway obstruction. These contributors are altered by long-term consequences of the intermittent hypoxia that is caused by repetitive pharyngeal collapses. For more details see text.
Mechanistically, increased sympathetic activity, endothelial dysfunction, and systemic inflammation as well as oxidative stress are all contributors to myocardial damage and hypertension (Baguet et al., 2012). Thus, the airway obstruction in OSA as well as CA is the beginning of a complex series of events that affect numerous central and peripheral neuronal and cardiovascular mechanisms (Eckert et al., 2009a; Gozal et al., 2013; Jordan and White, 2008; Leung and Bradley, 2001; Meier and Andreas, 2012; Susarla et al., 2010). Some of the long-term consequences of OSA, such as hypertension, often persist even after obstructions are eliminated or prevented through surgery or continuous positive airway pressure (CPAP) (Alchanatis et al., 2001; Vanderveken et al., 2011). Moreover, after surgical removal of the anatomical obstructions, or after treatment with CPAP, patients often remain refractory and shift towards the generation of central apneas (Boyd, 2009; Eckert et al., 2009b; Susarla et al., 2010).
In this review we use OSA as a template to discuss the complex interactions between factors that contribute to apnea pathogenesis. The first key concept we hope to convey is that OSA results from the convergence of multiple peripheral and central nervous system factors, not a single factor in isolation. The second concept is that many of the peripheral and central nervous system changes associated with OSA are initially reversible, and possibly even adaptive, but they become detrimental and irreversible during disease progression.
2. Airway obstruction and the importance of hypoglossal activity
Various anatomical abnormalities can contribute to the airway obstructions associated with OSA. Thus surgical procedures to remove these obstructions need to be adapted to the individual pattern and type of airway obstruction (Bhattacharjee et al., 2010; Sher et al., 1996). Obstructions can include macroglossia, adenotonsillar hypertrophy, increased nasal resistance, pharyngeal edema, and craniofacial abnormalities such as micrognathia and retrognathia (Bhattacharjee et al., 2010; Enoz, 2007; Lam et al., 2010; Prabhat et al., 2012; Shott and Cunningham, 1992; Verbraecken and De Backer, 2009; White, 2005; Won et al., 2008). Craniofacial factors are particularly important for pediatric OSA (Gozal, 2000). However, alone none of these anatomical determinants is sufficient to cause an airway occlusion.
Under normal conditions airflow is facilitated by a central respiratory drive to the upper airways (Figure 1). Of critical importance are the hypoglossal (XII) motoneurons that innervate the genioglossus muscle via the medial branch of the hypoglossal nerve. The genioglossus muscle is the largest extrinsic muscle of the human tongue (Abd-El-Malek, 1938; Saboisky et al., 2007; Takemoto, 2001). Innervation of the genioglossus is very complex and many types of phasic and tonic motor units originating in the hypoglossal motor nucleus contribute to the genioglossus contraction (Haxhiu et al., 1992; Hwang et al., 1983; Saboisky et al., 2007). The XII motoneurons phasically activate the genioglossus muscle during each inspiration (Figure 1), and some activity is maintained during expiration (Akahoshi et al., 2001; Fogel et al., 2001; Otsuka et al., 2000; Saboisky et al., 2010; Sauerland and Harper, 1976).
Overall, however, respiratory drive increases genioglossus muscle tone preferentially during inhalation, resulting in a contraction that pulls the tongue forward (Brouillette and Thach, 1979) and enlarges the upper airways (Bailey and Fregosi, 2004; Fuller et al., 1999; Mann et al., 2002; Oliven et al., 2001; Sokoloff, 2000). This mechanism largely prevents airway collapse during wakefulness. Indeed during wakefulness, electromyography (EMG) activity of the genioglossus is enhanced in OSA patients when compared to controls (Fogel et al., 2001; Mezzanotte et al., 1992), an adaptation that seems to compensate for the increased upper airway resistance and compliance that characterizes OSA patients (Malhotra and White, 2002; Randerath, 2007; Saboisky et al., 2007). However, during sleep or while anesthetized, the central respiratory drive to the genioglossus muscle weakens, and, as a consequence, anatomical obstructions can occlude the airway during inhalation (Eastwood et al., 2002; Remmers et al., 1978; Sauerland and Harper, 1976). Because a decreased central drive during sleep is necessary for the occlusion to occur during inhalation, OSA must be considered as a neuronal issue.
Indeed, airway obstructions are promoted by multiple central and peripheral nervous systems factors. These factors include sleep state-dependent pathologies and respiratory instabilities that are caused by loop gain changes as has been discussed in great detail (Thomas et al., 2004; White, 2005). Yet, whether and how an obstruction causes the cessation of breathing, i.e. the actual apnea, are not trivial questions. It is safe to conclude that the mechanisms and events leading to apneas are not fully understood, and that multiple factors must come together. In the following section we will discuss some of the potential mechanisms that contribute to the apnea.
3. Biomechanical and modulatory contributions to airway occlusion in OSA
3.1. The pharyngeal collapse
Cessation of airflow with continued respiratory effort is the hallmark of OSA (Praud et al., 1988; Remmers et al., 1978; Zucconi et al., 1996). Figure 2 illustrates two example traces from OSA patients (A from (Praud et al., 1988); B from (Remmers et al., 1978)). In both examples oro-nasal flow is blocked, while respiratory efforts continue in the abdomen. From a biomechanical perspective, continued respiratory effort in the thorax/abdomen increases thoracic volume and decreases pressure at the level of the pharynx, which would normally enable air to flow into the lungs. But, due to the increased oro-nasal resistance, the decreased pharyngeal pressure results in negative intraluminal pressure within the upper airways and leads to pharyngeal collapse (Figure 1). Considering the mismatch between negative intraluminal pressure and the decreased airflow arriving through the upper airways, OSA may not only result from an upper airway obstruction, but it could also be caused by an imbalance in lung volume compared to upper airway size. Thus, various anatomical causes together with decreased XII activation are important contributors to the pharyngeal collapse and thus to the airway occlusion in OSA (Figures 1, 2).
Figure 2.
Airway occlusion and genioglossus activity. Obstructive sleep apnea is characterized by airway occlusions that block nasal airflow (A, upper trace), while abdominal respiratory movements continue as illustrated by the abdominal movements (A, second trace) and esophageal pressure changes (B, upper trace). Continuous respiratory activity is also characterized by respiratory rhythmic diaphragmatic EMG (A, third trace, EMG D). While respiratory rhythmic activity continues in the abdomen, genioglossus activity either decreases during the airway occlusion (B, lower trace, EMG G), or the phasic genioglossus EMG becomes increasingly tonic (A, lower trace, EMG G). The termination of the airway occlusion is characterized by a burst of genioglossus activity (A,B). Figure modified from: A: Praud et al. 1988, B: Remmers et al. 1978.
3.2. Neuromodulators and transmitters
Multiple neuronal mechanisms contribute to a sleep-related decrease in XII activation as both neurotransmitter and neuromodulatory systems undergo drastic state dependent changes. As demonstrated in intracellular recordings, glutamatergic and GABAergic mechanisms (Chase et al., 1989; Funk et al., 1997; Soja et al., 1991; Soja et al., 1987) as well as a powerful glycinergic premotor inhibitory system likely contribute to the REM specific decrease in XII motoneuron activity (Yamuy et al., 1999). However, the degree of inhibition may only be detectable in intracellular recordings, while active inhibition is difficult to demonstrate in EMG recordings (Funk et al., 2011). This difficulty may partly explain why the relative importance of fast neurotransmission remains a matter of discussion (Chan et al., 2006; Morrison et al., 2003a; Morrison et al., 2003b).
In addition to increased active inhibition by fast synaptic transmitters, there is also a pronounced sleep related decrease in the activity of noradrenergic (Aston-Jones and Bloom, 1981) and serotonergic neurons (Jacobs and Fornal, 1991; Leung and Mason, 1999) suggesting that the loss of noradrenergic and serotonergic neuromodulatory inputs play critical roles (Fenik et al., 2005a; Funk et al., 2011; Horner, 2008, 2009; Kubin et al., 1998; Ladewig et al., 2004). This hypothesis is consistent across various manipulations in unrestrained animals (Chan et al., 2006; Morrison et al., 2003a; Sood et al., 2007; Sood et al., 2005), slice preparations (Funk et al., 1994; Viemari and Ramirez, 2006), and with research in the so-called carbachol model for rapid eye movement (REM) sleep (Fenik et al., 2004; Fenik et al., 2005a, b; Fenik et al., 2005c; Fenik et al., 2008). The noradrenergic neurons from the A5 and A7 regions converge at the level of the XII motoneurons (Aldes et al., 1992) and seem to have their effect through α1 adrenergic receptor activation (Parkis et al., 1995; Selvaratnam et al., 1998; Volgin et al., 2001). Interestingly, the pre-Bötzinger complex (preBötC), an area critical for breathing also receives noradrenergic and serotonergic inputs and is activated by a variety of serotonergic and adrenergic receptors (Doi and Ramirez, 2008, 2010; Lalley et al., 1995; Pena and Ramirez, 2002; Ptak et al., 2009; Tryba et al., 2006; Viemari et al., 2011; Viemari and Ramirez, 2006). These neuromodulators are particularly important during hypoxic conditions since endogenous noradrenergic and serotonergic drive within the ventrolateral medulla is essential for the activation of bursting neurons that depend on the persistent sodium current (Pena and Ramirez, 2002; Viemari et al., 2011). It is likely that a decrease in noradrenergic and serotonergic drive during sleep will weaken respiratory network activity and thus may contribute to or exaggerate the instabilities associated with OSA. Thus, noradrenergic and serotonergic excitatory inputs may play a role in the modulatory effects on both the central respiratory network and the XII motor output.
Other neuromodulators will also play important roles. Acetylcholine could be a key modulator involved in modulating respiratory activity (Shao and Feldman, 2009; Tryba et al., 2008) and suppressing genioglossus activity during REM sleep (Bellingham and Berger, 1996; Bellingham and Funk, 2000; Grace et al., 2013; Liu et al., 2005; Robinson et al., 2002). The recent study by Grace et al. 2013 demonstrated that REM specific suppression can be overcome by injecting muscarinic antagonists into the XII motoneuron pool (Grace et al., 2013). At the cellular level, this inhibitory effect appears to involve the activation of G protein-coupled inward rectifying potassium (GIRK) channels. These modulatory mechanisms appear to suppress XII motor activity by acting on the motoneurons themselves (Grace et al., 2013). This cholinergic drive could come from XII premotor neurons, a subpopulation of which is cholinergic (Volgin et al., 2008).
It is important to note, that the neuromodulatory mechanisms contributing to OSA and CA are likely very different. The number of apneas significantly increases during REM sleep in OSA patients, and some patients show apneas exclusively during REM sleep (Eckert et al., 2009b; Findley et al., 1985; Kass et al., 1996). By contrast, the number of central apneas is lowest during REM sleep (Eckert et al., 2007a). Thus, further research will need to explain how the modulatory and activity characteristics associated with the different sleep states relate to the different forms of apnea. REM sleep is characterized by decreased firing of noradrenergic and serotonergic neurons, which could lead to decreased activation of respiratory neurons within the preBötzC (Funk et al., 2011; Pena and Ramirez, 2002; Viemari et al., 2011)Such a decreased activation could contribute to a weakened central drive to the hypoglossal nucleus that could suffice to predispose the upper airways to a pharyngeal collapse. However, it is more difficult to understand why the incidence of CA should decrease under these conditions. One possibility is that CAs occur less often during REM sleep because excitatory cholinergic inputs are capable of compensating for decreased levels of norepinephrine and serotonin. This compensatory mechanism is not sufficient to prevent pharyngeal collapse but is sufficient to prevent CAs in the absence of an airway obstruction.
With regards to OSA the modulatory mechanisms during REM sleep could indeed explain not only decreased activation of the respiratory network but also a decrease in airway tone (Remmers et al., 1978; Sauerland and Harper, 1976). Yet either mechanism can only partly explain how decreased XII motoneuronal activation predisposes the upper airways to a pharyngeal collapse. Thus, it remains uncertain how the apneas themselves are generated.
Indeed, the possibility that modulators are causing the decreased tone but not the apnea itself is consistent with the well-known inefficiency of aminergic therapies that have largely failed to alleviate OSA (Dempsey et al., 2010; Funk et al., 2011). Moreover, noradrenergic and serotonergic innervation is strengthened following exposure to chronic intermittent hypoxia which may oppose the decreased muscle tone during sleep (Rukhadze et al., 2010), and OSA patients show a variety of neurogenic changes in the upper airways that could potentially compensate for decreased muscle tone. These adaptations include increased activation, earlier firing, and increased sprouting of the XII motoneurons (Saboisky et al., 2007; Saboisky et al., 2012). As illustrated in Figure 2 there is not a general suppression of the upper airways, but instead the airflow is “suddenly” disturbed for a few cycles and then the oral-nasal flow reappears and re-synchronizes with the respiratory abdominal muscles. Thus, while a persistently decreased drive to the XII motoneurons may predispose the pharynx to sudden collapse, the sudden failure in XII motor activity cannot be entirely explained by altered modulatory tone generating persistent atonia during a specific sleep state.
As illustrated in Figure 2, genioglossus EMG activity is specifically weakened and less phasic during the airway occlusion but not before or after the occlusion. Thus, in addition to a neuromodulator- and transmitter-driven decrease in muscle tone, one needs to consider additional central nervous and reflex mechanisms that contribute to the disconnect between the ongoing phasic respiratory activity that drives the diaphragmatic activity and the decrease in phasic respiratory drive to the XII motoneurons which is specifically associated with the airway occlusion.
4. Reflex regulation
In OSA, airway occlusion also involves reflex mechanisms (Figure 1) that are characterized by pathological gain changes in the mechano- and chemosensory reflex loops regulating ventilation. These reflex pathways become specifically dysregulated during sleep and could therefore destabilize the respiratory response to an airway obstruction resulting in pharyngeal collapse during sleep and not wakefulness (Douglas et al., 1982; White, 2005).
4.1. Mechano-sensory reflexes
To prevent pharyngeal collapse, mechanoreceptors located within the pharyngeal walls specifically regulate the XII motoneurons (Figure 1). These receptors are activated by negative intraluminal pressure generated during inspiration. They transmit this afferent information via the superior branch of the internal laryngeal nerve, and genioglossus premotoneurons located near the obex mediate the reflex (Chamberlin et al., 2007). This is an important reflex, as activation of the hypoglossal muscles caused by a pressure drop should counteract a pharyngeal collapse (Eckert et al., 2007b; Horner et al., 1991; Malhotra et al., 2000). Under physiological conditions this mechano-sensory pathway, as well as central nervous system components that are not involved in the reflex, contribute to the phasic genioglossus contraction during inspiration (Chamberlin et al., 2007; Fogel et al., 2001; Horner, 2000; Susarla et al., 2010; van Lunteren, 1993). Importantly, the reflex activation of the genioglossus during these pressure drops is dramatically reduced or even suppressed during sleep, a finding that is of great significance in understanding OSA because a reduced activation could promote a pharyngeal collapse (Wheatley et al., 1993).
4.2. Arterial chemosensory mechanisms
Hypoxia and hypercapnia initiated chemoreflexes are known to contribute to the regulation of ventilation (Figure 1), and a high gain in any of these chemosensory loops could contribute to breathing instabilities (White, 2005). The following lines of evidence suggest that the arterial chemoreflex is augmented in OSA subjects: a) brief hyperoxic exposure, which inhibits chemoreceptor activity, reduces blood pressure in OSA patients but not in control subjects (Narkiewicz et al., 1998), b) the hypoxic ventilatory response, a hallmark response of the chemoreflex, is augmented in OSA subjects compared to controls (Hedner et al., 1992), and c) activation of muscle sympathetic nerve activity by apneas is more pronounced in OSA subjects compared to controls (Smith et al., 1996). Development of altered chemosensory reflexes in OSA is further supported by studies using intermittent hypoxia (IH), the hallmark manifestation of recurrent apnea. Rodents exposed to chronic IH showed: a) enhanced carotid body sensitivity to hypoxia, and b) a progressive increase in baseline carotid body sensory activity, a phenomenon termed sensory long-term facilitation (sLTF) (Pawar et al., 2008; Peng et al., 2009; Peng et al., 2003; Peng and Prabhakar, 2004; Peng et al., 2006; Rey et al., 2004). The subnuclei of the nucleus tractus solitarius (NTS, Figure 1), especially the commissural part of the NTS (cNTS), receive inputs from the carotid body (Chitravanshi and Sapru, 1995; Zhang and Mifflin, 1993). Neuronal activity in cNTS is regulated by various neurotransmitters, including glutamate, an excitatory amino acid transmitter, and dopamine, an inhibitory biogenic amine. Chronic IH up regulates GluR2/3 glutamate receptor subunit expression in cNTS (Costa-Silva et al., 2012) and down regulates tyrosine hydroxylase (TH) expression, the rate-limiting enzyme in dopamine (DA) synthesis (Gozal et al., 2005; Kline et al., 2002). It is likely that chronic IH, by down regulating the synthesis of DA, enhances glutamatergic excitatory transmission in NTS (Chen et al., 1999) resulting in the enhanced hypoxic ventilatory response (HVR). Collectively, these studies indicate that the augmented chemoreflex by chronic IH involves reconfiguration of neurotransmitter profiles in the central nervous system.
Does an augmented chemoreflex contribute to pathogenesis of apnea? It was proposed that the increased carotid body sensitivity to hypoxia can lead to a greater magnitude of hyperventilation during each episode of apnea, thus driving the respiratory controller below the apneic threshold for CO2, leading to greater number of apneas (Prabhakar, 2001). In other words, the heightened hypoxic sensitivity of the carotid body might act as a “positive feedback,” thereby exacerbating the occurrence of apneas. Supporting such a possibility is the finding that chronic IH exposed rats with intact carotid bodies exhibit greater incidence of spontaneous apneas. This effect was absent in carotid body sectioned rats exposed to chronic IH (Prabhakar, 2013). Since peripheral chemoreceptors regulate hypoglossal motoneuron activity (Bruce et al., 1982), it remains to be established whether the chemoreflex directly or indirectly contributes to the hypoglossal motoneuron dysfunction leading to OSA.
5. Airway occlusion and the central respiratory network
Chemo- and mechanosensory afferents and modulatory inputs converge via the NTS on the XII motoneurons where they closely interact with the central respiratory drive acting on the XII motoneurons. As shown in Figure 1, an apnea generated at the level of the XII motoneurons could involve a temporary drop-out of central XII activity while respiratory rhythmic activity continues to be generated within the central respiratory network. Neuronal mechanisms that could lead to such a drop out could occur locally within the medulla. Located within the same transverse plane as the XII nucleus is the pre-Bötzinger complex (preBötC; Figure 1). The preBötC is a well-defined neuronal network that is essential for breathing. Selective lesion of the preBötC in intact animals abolishes breathing (Gray et al., 2010; Ramirez et al., 1998; Tan et al., 2008). Moreover, isolated in medullary slice preparations that encompass the preBötC (Figure 1, preBötC, blue), this neuronal network continues to spontaneously generate inspiratory rhythmic activity (Figure 1, left panel). Inspiratory activity generated within the preBötC is transmitted to the XII nucleus and leads to the phasic activation of an inspiratory population burst within the hypoglossal nucleus (Figure 1, left panel). Located within this slice preparation are premotor neurons that transmit the respiratory signal from the preBötC to the XII motoneurons (Chamberlin et al., 2007; Dobbins and Feldman, 1995; Luo et al., 2006; Peever et al., 2002; Sebe and Berger, 2008). These premotor neurons are not simple followers, but they seem to play a major role in generating synchronous oscillations within the XII motor nucleus (Sebe and Berger, 2008). Interestingly, not every burst in the preBötC is transmitted to the XII and in some slice preparations the XII can fail to burst in phase with the respiratory cycle generated within the preBötC (Figure 1; (Ramirez et al., 1996)). It is conceivable that such an activation failure could provide a mechanistic explanation for XII inactivity during continued inspiratory respiratory rhythm generation from the preBötC. The inspiratory rhythm generated in the preBötC would then continue to be transmitted to the phrenic nucleus (Figure 1). Continued activation of the diaphragm is an important aspect of OSA, as it is the activated diaphragm that produces the expansion of the thorax which together with a lack of genioglossus activation creates negative pressure and pharyngeal collapse. At this point, we do not know how the respiratory drive from the preBötC is transmitted under conditions that mimic sleep states or conditions that mimic sleep apnea. During hypoxia, however, transmission failure from the preBötC to the XII motoneurons is increased (Pena et al., 2008). Thus, an important avenue for future research will be to understand how chronic intermittent hypoxia or certain neuromodulatory conditions associated with sleep can cause such transmission failures between the respiratory rhythm generator and the XII motor output. Investigating this issue could provide important and much needed clues into the pathology of OSA.
6. The recovery from airway occlusion
In addition to the onset and maintenance of airway occlusion, recovery from an airway obstruction has been the subject of intense discussions (Figure 2). One notion is that reflex recruitment of pharyngeal dilator muscles is insufficient to open the airway once it is occluded and that arousal is required for the termination. This is an important consideration, since breathing instabilities that promote OSA likely involve pathological changes in arousal threshold (Younes, 2004). Arousal is stimulated by increased negative pharyngeal pressure and increasingly hypoxic and hypercapnic conditions that in turn increase respiratory drive (Berry and Gleeson, 1997; Gleeson et al., 1990; Kimoff et al., 1994). The stimulation of arousal then activates dilator activity and opens the airways (Remmers et al., 1978). Yet, arousal is not required for apnea termination (Younes et al., 2012). Figure 2 illustrates the recovery from an airway occlusion which is typically abrupt and associated with a sudden burst of genioglossus EMG (Berry and Gleeson, 1997; Rees et al., 1995; Remmers et al., 1978; Wulbrand et al., 1998, 2008). The abrupt increase in genioglossus muscle activity seems to be due to the recruitment of phasic inspiratory motor units (Wilkinson et al., 2010). Wulbrand and coworkers proposed that this burst is synchronized with the generation of sigh or sigh-like neuronal mechanisms (Wulbrand et al., 1998). The induction of sighs by airway occlusion has also been reported by Alvarez et al. (1993) (Alvarez et al., 1993). Sighs are large amplitude inspiratory efforts that are mechanistically different from normal “eupneic” breaths (Figure 3) (Lieske and Ramirez, 2006a, b; Lieske et al., 2000). Although, it was initially believed that sighs are exclusively dependent on lung stretch receptor stimulation (Bartlett, 1971; Reynolds, 1962; Wulbrand et al., 2008); there is ample evidence to the contrary – i.e. sighs are generated within the central nervous system and do not require afferent input (Orem and Trotter, 1993). For example sighs can be generated following deafferentation in vivo (Cherniack et al., 1981), and humans continue to generate sighs following lung transplantation (Shea et al., 1988).
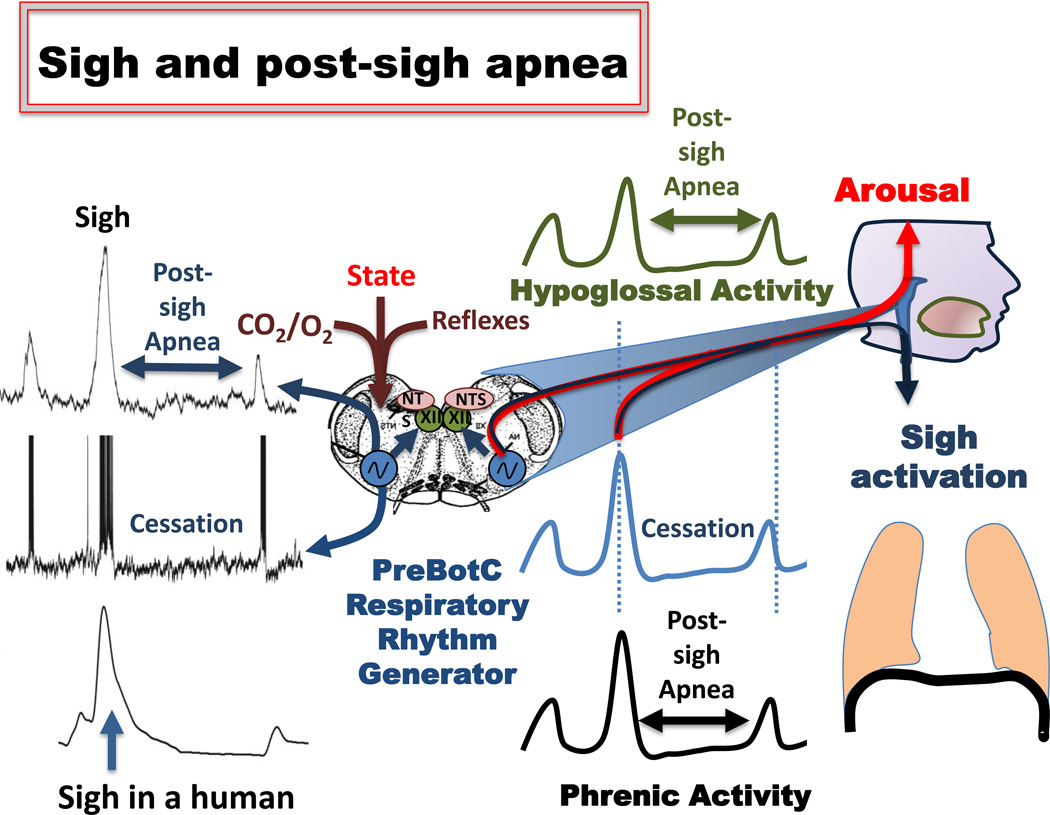
Figure 3.
Mechanisms of sigh and post-sigh apnea. Left panel: Upper trace: integrated extracellularly recorded pre-Bötzinger complex population activity obtained simultaneously with an intracellular recordings from an inspiratory neuron located within the pre-Bötzinger complex (middle trace). The lower trace represents a recording from a human subject. While the lower trace was obtained using induction plethysmographic recording (for more details see Weese-Mayer et al. 2006), the upper and middle traces were obtained in an isolated transverse slice preparation shown in the schematic. The preparation contains the functionally active pre-Bötzinger complex (blue), the nucleus tractus solitaries (NTS, pink), which in the intact animal would receive reflex inputs. Contained within the slice is also the hypoglossal nucleus (XII, green), which in the intact animal would innervate the genioglossus muscle. The actual data shown on the left panel inspired the middle panel, which schematically illustrates how the rhythmically active pre-Bötzinger complex (blue trace) could activate a sigh and apnea in the phrenic nucleus (black trace) resulting in diaphragmatic activity, and sigh activity and apnea in the XII (green trace, apnea). The right panel illustrates the anatomical components contributing to the central apnea associated with the sigh, as well as the illustration of arousal that is associated with the generation of the sigh. The bilaterally organized pre-Bötzinger complex isolated in the transverse slice preparation was obtained from the lower brainstem (medulla – schematically shown in blue). The sigh is activated by changes in the state, oxygenation and CO2 levels. For more details see text.
Moreover, sighs are even generated in the in situ working heart preparation, a fully deafferented brainstem preparation, (Ramirez and Viemari, 2005), as well as transverse slice preparations that contain the preBötC (Figure 3) (Hill et al., 2011; Lieske et al., 2000; Pena et al., 2004). Important for the discussion of OSA, this centrally generated mechanism is specifically facilitated under hypoxic conditions (Bartlett, 1971; Bell et al., 2009; Bell and Haouzi, 2010; Cherniack et al., 1981; Hill et al., 2011; Lieske et al., 2000; Schwenke and Cragg, 2000). Although peripheral chemoreceptors certainly play a facilitatory role (Cherniack et al., 1981; Glogowska et al., 1972; Matsumoto et al., 1997), even in the absence of peripheral chemoreceptors, hypoxic conditions within the preBötC are sufficient to centrally activate the generation of sighs (Hill et al., 2011; Koch et al., 2013; Pena et al., 2004; Telgkamp et al., 2002). Thus, the hypoxic conditions associated with OSA will likely play a role in activating sighs. As characterized in infants, sighs triggered by an airway occlusion are coordinated with a sleep startle, that marks the beginning of arousal (Figures 3,4), and accompanying changes in electroencephalogram (EEG) and EMG activity (Wulbrand et al., 2008). Although cortical arousal is not always observed, sighs consistently coincide with a sudden rise in limb EMG activity and a distinct neck extension, an adaptive response that can contribute to the termination of an airway occlusion (Wulbrand et al., 2008). Not only in infants, but also in adults, sighs are linked to EMG activation and EEG changes (Perez-Padilla et al., 1983).
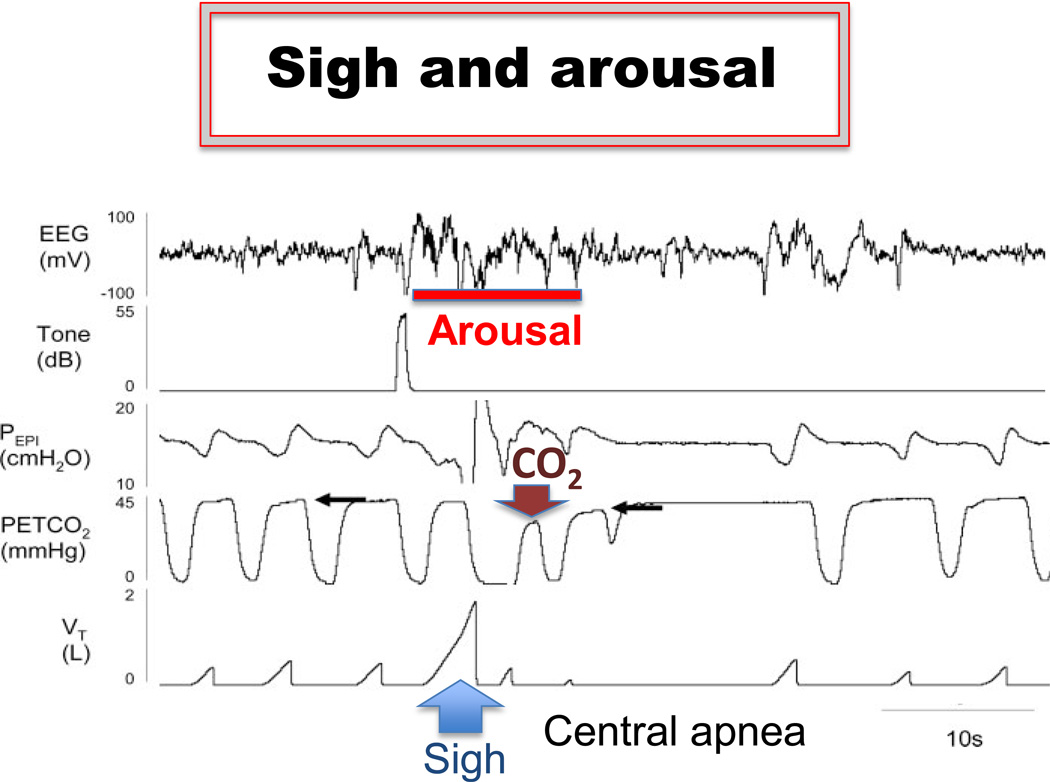
Figure 4.
The generation of the sigh is associated with arousal. The example was obtained during the transition from sleep to wakefulness. Note, the sigh (lower trace) is associated with a prolonged central apnea that follows a decrease in the level of CO2, which was caused by the sigh, suggesting that the hypocapnia may contribute to the generation of the apnea that followed the sigh. Figure modified from Eckert et al. 2007
Sighs are also associated with a heart rate increase followed by a heart rate decrease (Haupt et al., 2012; McNamara et al., 1998; Porges et al., 2000; Weese-Mayer et al., 2008; Wulbrand et al., 2008). The heart rate changes associated with the sigh are often altered in human diseases such as familial dysautonomia, sickle cell anemia, and SIDS (Franco et al., 2003; Sangkatumvong et al., 2011; Weese-Mayer et al., 2008). From the organismic perspective, the hypoxic sensitivity of the sigh becomes a very important central nervous system mechanism that allows the organism to detect and respond to hypoxic conditions. Hypoxia-induced sighs activate important muscles and can lead to subcortical and cortical arousal (Figure 3). Once aroused, an organism can avoid the hypoxic condition by for example changing its sleeping position. The sigh may therefore link the hypoxic condition caused by OSA to arousal, which eventually results in sleep deprivation, one of the detrimental consequences of OSA. Interestingly sighs may also play an important role in the generation of periodic breathing as postulated by (Guntheroth, 2011).
This centrally generated mechanism is very sensitive to state changes (Orem and Trotter, 1993). The transition from sleep to wakefulness is often characterized by the activation of a sigh and arousal (Figure 4) (Eckert et al., 2007a). Note, in Figure 4, the sigh seems to contribute to a decrease in CO2 level. This decrease in CO2 may be involved in the generation of the apnea that typically follows the sigh. Indeed, during an “augmented” breath simulated by a ventilator, a decreased CO2 drive can generate a brief apnea as elegantly demonstrated by Remmers et al. (1978). However, these simulated augmented breaths evoked brief apneas only under certain conditions such as hypoxia. Moreover, we know that the post-sigh apnea can be generated centrally within the preBötC under conditions in which oxygen and CO2 are not altered (Figure 3). Thus, the post-sigh apnea is indeed a “central apnea” generated within the ventrolateral medulla. Interestingly, a “post-sigh-like apnea” can be simulated centrally, by maximally stimulating isolated medullary respiratory pacemaker neurons. This purely central electrical stimulation is followed by a prolonged pause in the rhythmic bursting of these respiratory neurons (Tryba et al., 2008).
The post-sigh apnea is an important manifestation of a central apnea (Eckert et al., 2007a; Radulovacki et al., 2001; Saponjic et al., 2007). Post-sigh apneas are very common in children (Haupt et al., 2012; O'Driscoll et al., 2009) but are also present in adults (Vlemincx et al., 2010). Post-sigh apneas can be exaggerated in neurological disorders such as Leigh Syndrome (Quintana et al., 2012; Saito, 2009; Yasaki et al., 2001), Familial Dysautonomia (Weese-Mayer et al., 2008), and Rett Syndrome (Voituron et al., 2010). Although it is clearly generated centrally, it must be emphasized that in the intact organism, additional chemosensory mechanisms will contribute and potentially exaggerate the post-sigh apnea because the post-sigh apnea is associated with significant changes in blood gases.
7. Conclusion
Apneas emerge through a complex interplay between peripheral and central nervous system factors that affect all levels of integration: from the molecular to the cellular and organismic level. This interplay affects many aspects of respiratory control making it difficult to clearly separate central versus peripheral contributions to the generation of the apnea. Indeed, several central and peripheral factors must come together to cause pharyngeal collapse and apneas in case of OSA. Moreover, OSA as well as central apneas have numerous long-term consequences that include changes in the neuromodulatory milieu, mechano- and chemosensory reflex loops, cardiorespiratory integration and neurotransmitter systems. These changes may be partly adaptive during wakefulness but they often fail to adequately adapt the organism during the night. Indeed, many of the consequences become critical contributors to the morbidity of the apneas. While traditionally, much emphasis has been placed on understanding the contributions of chemo- and mechanosensory reflexes, the changes in blood gases, and the biomechanics of the apneas, we have only recently begun to understand how these contributors interact with the central respiratory network, an integration that still raises many unanswered questions. Future research will elucidate many of these questions and may inspire novel avenues for therapies that could target the most detrimental and persisting consequences of sleep apnea, a health issue that affects an increasing proportion of the pediatric and adult populations.
Highlights.
We review the central and peripheral contributions to obstructive sleep apnea.
We examine the dynamic interactions between central pre-motor elements and motor output that give rise to obstructive sleep apnea.
We discuss the complex interplay between both peripheral and central components that cause apneas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abd-El-Malek S. A contribution to the study of the movements of the tongue in animals, with special reference to the cat. J Anat. 1938;73:15–30. 11. [PMC free article] [PubMed] [Google Scholar]
- Akahoshi T, White DP, Edwards JK, Beauregard J, Shea SA. Phasic mechanoreceptor stimuli can induce phasic activation of upper airway muscles in humans. The Journal of physiology. 2001;531:677–691. doi: 10.1111/j.1469-7793.2001.0677h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alchanatis M, Tourkohoriti G, Kakouros S, Kosmas E, Podaras S, Jordanoglou JB. Daytime pulmonary hypertension in patients with obstructive sleep apnea: the effect of continuous positive airway pressure on pulmonary hemodynamics. Respiration. 2001;68:566–572. doi: 10.1159/000050574. [DOI] [PubMed] [Google Scholar]
- Aldes LD, Chapman ME, Chronister RB, Haycock JW. Sources of noradrenergic afferents to the hypoglossal nucleus in the rat. Brain Res Bull. 1992;29:931–942. doi: 10.1016/0361-9230(92)90168-w. [DOI] [PubMed] [Google Scholar]
- Alvarez JE, Bodani J, Fajardo CA, Kwiatkowski K, Cates DB, Rigatto H. Sighs and their relationship to apnea in the newborn infant. Biol Neonate. 1993;63:139–146. doi: 10.1159/000243923. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguet JP, Barone-Rochette G, Tamisier R, Levy P, Pepin JL. Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nat Rev Cardiol. 2012;9:679–688. doi: 10.1038/nrcardio.2012.141. [DOI] [PubMed] [Google Scholar]
- Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol. 2004;96:440–449. doi: 10.1152/japplphysiol.00733.2003. [DOI] [PubMed] [Google Scholar]
- Bartlett D., Jr Origin and regulation of spontaneous deep breaths. Respir Physiol. 1971;12:230–238. doi: 10.1016/0034-5687(71)90055-7. [DOI] [PubMed] [Google Scholar]
- Bell HJ, Ferguson C, Kehoe V, Haouzi P. Hypocapnia increases the prevalence of hypoxia-induced augmented breaths. Am J Physiol Regul Integr Comp Physiol. 2009;296:R334–R344. doi: 10.1152/ajpregu.90680.2008. [DOI] [PubMed] [Google Scholar]
- Bell HJ, Haouzi P. The hypoxia-induced facilitation of augmented breaths is suppressed by the common effect of carbonic anhydrase inhibition. Respiratory physiology & neurobiology. 2010;171:201–211. doi: 10.1016/j.resp.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Berger AJ. Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneurons by muscarinic M2 receptors. J Neurophysiol. 1996;76:3758–3770. doi: 10.1152/jn.1996.76.6.3758. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Funk GD. Cholinergic modulation of respiratory brain-stem neurons and its function in sleep-wake state determination. Clin Exp Pharmacol Physiol. 2000;27:132–137. doi: 10.1046/j.1440-1681.2000.03192.x. [DOI] [PubMed] [Google Scholar]
- Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep. 1997;20:654–675. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, Mitchell RB, Promchiarak J, Simakajornboon N, Kaditis AG, Splaingard D, Splaingard M, Brooks LJ, Marcus CL, Sin S, Arens R, Verhulst SL, Gozal D. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182:676–683. doi: 10.1164/rccm.200912-1930OC. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee R, Kim J, Alotaibi WH, Kheirandish-Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in children without hypertension: potential contributions of obesity and obstructive sleep apnea. Chest. 2012;141:682–691. doi: 10.1378/chest.11-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickelmann AG, Burwell CS, Robin ED, Whaley RD. Extreme obesity associated with alveolar hypoventilation; a Pickwickian syndrome. The American journal of medicine. 1956;21:811–818. doi: 10.1016/0002-9343(56)90094-8. [DOI] [PubMed] [Google Scholar]
- Boyd SB. Management of obstructive sleep apnea by maxillomandibular advancement. Oral Maxillofac Surg Clin North Am. 2009;21:447–457. doi: 10.1016/j.coms.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Bray GA. What's in a name? Mr. Dickens' "Pickwickian" fat boy syndrome. Obesity research. 1994;2:380–383. doi: 10.1002/j.1550-8528.1994.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Brouillette RT, Thach BT. A neuromuscular mechanism maintaining extrathoracic airway patency. J Appl Physiol. 1979;46:772–779. doi: 10.1152/jappl.1979.46.4.772. [DOI] [PubMed] [Google Scholar]
- Bruce EN, Mitra J, Cherniack NS. Central and peripheral chemoreceptor inputs to phrenic and hypoglossal motoneurons. J Appl Physiol. 1982;53:1504–1511. doi: 10.1152/jappl.1982.53.6.1504. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. The Journal of physiology. 2007;579:515–526. doi: 10.1113/jphysiol.2006.121889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med. 2006;174:1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- Chase MH, Soja PJ, Morales FR. Evidence that glycine mediates the postsynaptic potentials that inhibit lumbar motoneurons during the atonia of active sleep. J Neurosci. 1989;9:743–751. doi: 10.1523/JNEUROSCI.09-03-00743.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Kombian SB, Zidichouski JA, Pittman QJ. Dopamine depresses glutamatergic synaptic transmission in the rat parabrachial nucleus in vitro. Neuroscience. 1999;90:457–468. doi: 10.1016/s0306-4522(98)00594-6. [DOI] [PubMed] [Google Scholar]
- Cherniack NS, von Euler C, Glogowska M, Homma I. Characteristics and rate of occurrence of spontaneous and provoked augmented breaths. Acta Physiol Scand. 1981;111:349–360. doi: 10.1111/j.1748-1716.1981.tb06747.x. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN. Chemoreceptor-sensitive neurons in commissural subnucleus of nucleus tractus solitarius of the rat. Am J Physiol. 1995;268:R851–R858. doi: 10.1152/ajpregu.1995.268.4.R851. [DOI] [PubMed] [Google Scholar]
- Costa-Silva JH, Zoccal DB, Machado BH. Chronic intermittent hypoxia alters glutamatergic control of sympathetic and respiratory activities in the commissural NTS of rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:R785–R793. doi: 10.1152/ajpregu.00363.2011. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. J Comp Neurol. 1995;357:376–394. doi: 10.1002/cne.903570305. [DOI] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respiratory physiology & neurobiology. 2008;164:96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci. 2010;30:8251–8262. doi: 10.1523/JNEUROSCI.5361-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas NJ, White DP, Weil JV, Pickett CK, Zwillich CW. Hypercapnic ventilatory response in sleeping adults. Am Rev Respir Dis. 1982;126:758–762. doi: 10.1164/arrd.1982.126.5.758. [DOI] [PubMed] [Google Scholar]
- Eastwood PR, Szollosi I, Platt PR, Hillman DR. Comparison of upper airway collapse during general anaesthesia and sleep. Lancet. 2002;359:1207–1209. doi: 10.1016/S0140-6736(02)08224-7. [DOI] [PubMed] [Google Scholar]
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: Pathophysiology and treatment. Chest. 2007a;131:595–607. doi: 10.1378/chest.06.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert DJ, Malhotra A, Jordan AS. Mechanisms of apnea. Prog Cardiovasc Dis. 2009a;51:313–323. doi: 10.1016/j.pcad.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert DJ, Malhotra A, Lo YL, White DP, Jordan AS. The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest. 2009b;135:957–964. doi: 10.1378/chest.08-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert DJ, McEvoy RD, George KE, Thomson KJ, Catcheside PG. Genioglossus reflex inhibition to upper-airway negative-pressure stimuli during wakefulness and sleep in healthy males. The Journal of physiology. 2007b;581:1193–1205. doi: 10.1113/jphysiol.2007.132332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoz M. Effects of nasal pathologies on obstructive sleep apnea. Acta Medica (Hradec Kralove) 2007;50:167–170. doi: 10.14712/18059694.2017.77. [DOI] [PubMed] [Google Scholar]
- Fenik V, Davies RO, Kubin L. Combined antagonism of aminergic excitatory and amino acid inhibitory receptors in the XII nucleus abolishes REM sleep-like depression of hypoglossal motoneuronal activity. Arch Ital Biol. 2004;142:237–249. [PubMed] [Google Scholar]
- Fenik VB, Davies RO, Kubin L. Noradrenergic, serotonergic and GABAergic antagonists injected together into the XII nucleus abolish the REM sleep-like depression of hypoglossal motoneuronal activity. J Sleep Res. 2005a;14:419–429. doi: 10.1111/j.1365-2869.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005b;172:1322–1330. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik VB, Ogawa H, Davies RO, Kubin L. Carbachol injections into the ventral pontine reticular formation activate locus coeruleus cells in urethane-anesthetized rats. Sleep. 2005c;28:551–559. doi: 10.1093/sleep/28.5.551. [DOI] [PubMed] [Google Scholar]
- Fenik VB, Rukhadze I, Kubin L. Inhibition of pontine noradrenergic A7 cells reduces hypoglossal nerve activity in rats. Neuroscience. 2008;157:473–482. doi: 10.1016/j.neuroscience.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley LJ, Wilhoit SC, Suratt PM. Apnea duration and hypoxemia during REM sleep in patients with obstructive sleep apnea. Chest. 1985;87:432–436. doi: 10.1378/chest.87.4.432. [DOI] [PubMed] [Google Scholar]
- Fogel RB, Malhotra A, Pillar G, Edwards JK, Beauregard J, Shea SA, White DP. Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. Am J Respir Crit Care Med. 2001;164:2025–2030. doi: 10.1164/ajrccm.164.11.2102048. [DOI] [PubMed] [Google Scholar]
- Franco P, Verheulpen D, Valente F, Kelmanson I, de Broca A, Scaillet S, Groswasser J, Kahn A. Autonomic responses to sighs in healthy infants and in victims of sudden infant death. Sleep Med. 2003;4:569–577. doi: 10.1016/s1389-9457(03)00107-2. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. The Journal of physiology. 1999;519(Pt 2):601–613. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GD, Parkis MA, Selvaratnam SR, Walsh C. Developmental modulation of glutamatergic inspiratory drive to hypoglossal motoneurons. Respir Physiol. 1997;110:125–137. doi: 10.1016/s0034-5687(97)00078-9. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Development of thyrotropin-releasing hormone and norepinephrine potentiation of inspiratory-related hypoglossal motoneuron discharge in neonatal and juvenile mice in vitro. J Neurophysiol. 1994;72:2538–2541. doi: 10.1152/jn.1994.72.5.2538. [DOI] [PubMed] [Google Scholar]
- Funk GD, Zwicker JD, Selvaratnam R, Robinson DM. Noradrenergic modulation of hypoglossal motoneuron excitability: developmental and putative state-dependent mechanisms. Arch Ital Biol. 2011;149:426–453. doi: 10.4449/aib.v149i4.1271. [DOI] [PubMed] [Google Scholar]
- Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis. 1990;142:295–300. doi: 10.1164/ajrccm/142.2.295. [DOI] [PubMed] [Google Scholar]
- Glogowska M, Richardson PS, Widdicombe JG, Winning AJ. The role of the vagus nerves, peripheral chemoreceptors and other afferent pathways in the genesis of augmented breaths in cats and rabbits. Respir Physiol. 1972;16:179–196. doi: 10.1016/0034-5687(72)90050-3. [DOI] [PubMed] [Google Scholar]
- Gozal D. Obstructive sleep apnea in children. Minerva Pediatr. 2000;52:629–639. [PubMed] [Google Scholar]
- Gozal D, Hakim F, Kheirandish-Gozal L. Chemoreceptors, baroreceptors, and autonomic deregulation in children with obstructive sleep apnea. Respiratory physiology & neurobiology. 2013;185:177–185. doi: 10.1016/j.resp.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Khalyfa A, Capdevila OS, Kheirandish-Gozal L, Khalyfa AA, Kim J. Cognitive function in prepubertal children with obstructive sleep apnea: a modifying role for NADPH oxidase p22 subunit gene polymorphisms? Antioxid Redox Signal. 2012;16:171–177. doi: 10.1089/ars.2011.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Kheirandish-Gozal L. Obesity and excessive daytime sleepiness in prepubertal children with obstructive sleep apnea. Pediatrics. 2009;123:13–18. doi: 10.1542/peds.2008-0228. [DOI] [PubMed] [Google Scholar]
- Gozal D, Kheirandish-Gozal L. Childhood obesity and sleep: relatives, partners, or both?--a critical perspective on the evidence. Ann N Y Acad Sci. 2012;1264:135–141. doi: 10.1111/j.1749-6632.2012.06723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal E, Shah ZA, Pequignot JM, Pequignot J, Sachleben LR, Czyzyk-Krzeska MF, Li RC, Guo SZ, Gozal D. Tyrosine hydroxylase expression and activity in the rat brain: differential regulation after long-term intermittent or sustained hypoxia. J Appl Physiol. 2005;99:642–649. doi: 10.1152/japplphysiol.00880.2004. [DOI] [PubMed] [Google Scholar]
- Grace KP, Hughes SW, Horner RL. Identification of the Mechanism Mediating Genioglossus Muscle Suppression in REM Sleep. Am J Respir Crit Care Med. 2013;187:311–319. doi: 10.1164/rccm.201209-1654OC. [DOI] [PubMed] [Google Scholar]
- Gray PA, Hayes JA, Ling GY, Llona I, Tupal S, Picardo MC, Ross SE, Hirata T, Corbin JG, Eugenin J, Del Negro CA. Developmental origin of preBotzinger complex respiratory neurons. J Neurosci. 2010;30:14883–14895. doi: 10.1523/JNEUROSCI.4031-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntheroth WG. Cheyne-Stokes respiration: hypoxia plus a deep breath that interrupts hypoxic drive, initiating cyclic breathing. Med Hypotheses. 2011;77:714–716. doi: 10.1016/j.mehy.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Haupt ME, Goodman DM, Sheldon SH. Sleep related expiratory obstructive apnea in children. J Clin Sleep Med. 2012;8:673–679. doi: 10.5664/jcsm.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxhiu MA, Cherniack NS, Mitra J, van Lunteren E, Strohl KP. Nonvagal modulation of hypoglossal neural activity. Respiration. 1992;59:65–71. doi: 10.1159/000196029. [DOI] [PubMed] [Google Scholar]
- Hedner JA, Wilcox I, Laks L, Grunstein RR, Sullivan CE. A specific and potent pressor effect of hypoxia in patients with sleep apnea. Am Rev Respir Dis. 1992;146:1240–1245. doi: 10.1164/ajrccm/146.5_Pt_1.1240. [DOI] [PubMed] [Google Scholar]
- Hill AA, Garcia AJ, 3rd, Zanella S, Upadhyaya R, Ramirez JM. Graded reductions in oxygenation evoke graded reconfiguration of the isolated respiratory network. J Neurophysiol. 2011;105:625–639. doi: 10.1152/jn.00237.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL. Impact of brainstem sleep mechanisms on pharyngeal motor control. Respir Physiol. 2000;119:113–121. doi: 10.1016/s0034-5687(99)00106-1. [DOI] [PubMed] [Google Scholar]
- Horner RL. Neuromodulation of hypoglossal motoneurons during sleep. Respiratory physiology & neurobiology. 2008;164:179–196. doi: 10.1016/j.resp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Horner RL. Emerging principles and neural substrates underlying tonic sleep-state-dependent influences on respiratory motor activity. Philos Trans R Soc Lond B Biol Sci. 2009;364:2553–2564. doi: 10.1098/rstb.2009.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. The Journal of physiology. 1991;436:15–29. doi: 10.1113/jphysiol.1991.sp018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JC, St John WM, Bartlett D., Jr Respiratory-related hypoglossal nerve activity: influence of anesthetics. J Appl Physiol. 1983;55:785–792. doi: 10.1152/jappl.1983.55.3.785. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Activity of brain serotonergic neurons in the behaving animal. Pharmacol Rev. 1991;43:563–578. [PubMed] [Google Scholar]
- Jordan AS, White DP. Pharyngeal motor control and the pathogenesis of obstructive sleep apnea. Respiratory physiology & neurobiology. 2008;160:1–7. doi: 10.1016/j.resp.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass JE, Akers SM, Bartter TC, Pratter MR. Rapid-eye-movement-specific sleep-disordered breathing: a possible cause of excessive daytime sleepiness. Am J Respir Crit Care Med. 1996;154:167–169. doi: 10.1164/ajrccm.154.1.8680674. [DOI] [PubMed] [Google Scholar]
- Kim HC, Young T, Matthews CG, Weber SM, Woodward AR, Palta M. Sleep-disordered breathing and neuropsychological deficits. A population-based study. Am J Respir Crit Care Med. 1997;156:1813–1819. doi: 10.1164/ajrccm.156.6.9610026. [DOI] [PubMed] [Google Scholar]
- Kimoff RJ, Cheong TH, Olha AE, Charbonneau M, Levy RD, Cosio MG, Gottfried SB. Mechanisms of apnea termination in obstructive sleep apnea. Role of chemoreceptor and mechanoreceptor stimuli. Am J Respir Crit Care Med. 1994;149:707–714. doi: 10.1164/ajrccm.149.3.8118640. [DOI] [PubMed] [Google Scholar]
- Kline DD, Takacs KN, Ficker E, Kunze DL. Dopamine modulates synaptic transmission in the nucleus of the solitary tract. J Neurophysiol. 2002;88:2736–2744. doi: 10.1152/jn.00224.2002. [DOI] [PubMed] [Google Scholar]
- Koch H, Zanella S, Elsen GE, Smith L, Doi A, Garcia AJ, 3rd, Wei AD, Xun R, Kirsch S, Gomez CM, Hevner RF, Ramirez JM. Stable Respiratory Activity Requires Both P/Q-Type and N-Type Voltage-Gated Calcium Channels. J Neurosci. 2013;33:3633–3645. doi: 10.1523/JNEUROSCI.6390-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L, Davies RO, Pack AI. Control of Upper Airway Motoneurons During REM Sleep. News Physiol Sci. 1998;13:91–97. doi: 10.1152/physiologyonline.1998.13.2.91. [DOI] [PubMed] [Google Scholar]
- Ladewig T, Lalley PM, Keller BU. Serotonergic modulation of intracellular calcium dynamics in neonatal hypoglossal motoneurons from mouse. Brain Res. 2004;1001:1–12. doi: 10.1016/j.brainres.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Bischoff AM, Schwarzacher SW, Richter DW. 5-HT2 receptor-controlled modulation of medullary respiratory neurones in the cat. The Journal of physiology. 1995;487(Pt 3):653–661. doi: 10.1113/jphysiol.1995.sp020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DJ, Jensen CC, Mueller BA, Starr JR, Cunningham ML, Weaver EM. Pediatric sleep apnea and craniofacial anomalies: a population-based case-control study. Laryngoscope. 2010;120:2098–2105. doi: 10.1002/lary.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie P. Nothing new under the moon. Historical accounts of sleep apnea syndrome. Archives of internal medicine. 1984;144:2025–2028. doi: 10.1001/archinte.144.10.2025. [DOI] [PubMed] [Google Scholar]
- Leung CG, Mason P. Physiological properties of raphe magnus neurons during sleep and waking. J Neurophysiol. 1999;81:584–595. doi: 10.1152/jn.1999.81.2.584. [DOI] [PubMed] [Google Scholar]
- Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med. 2001;164:2147–2165. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Ramirez JM. Pattern-specific synaptic mechanisms in a multifunctional networkIEffects of alterations in synapse strength. J Neurophysiol. 2006a;95:1323–1333. doi: 10.1152/jn.00505.2004. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Ramirez JM. Pattern-specific synaptic mechanisms in a multifunctional network. II. Intrinsic modulation by metabotropic glutamate receptors. J Neurophysiol. 2006b;95:1334–1344. doi: 10.1152/jn.00506.2004. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps [see comment] Nat Neurosci. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Liu X, Sood S, Liu H, Horner RL. Opposing muscarinic and nicotinic modulation of hypoglossal motor output to genioglossus muscle in rats in vivo. The Journal of physiology. 2005;565:965–980. doi: 10.1113/jphysiol.2005.084657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P, Zhang J, Yang R, Pendlebury W. Neuronal circuitry and synaptic organization of trigeminal proprioceptive afferents mediating tongue movement and jawtongue coordination via hypoglossal premotor neurons. Eur J Neurosci. 2006;23:3269–3283. doi: 10.1111/j.1460-9568.2006.04858.x. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Fogel RB, Edwards JK, Shea SA, White DP. Local mechanisms drive genioglossus activation in obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1746–1749. doi: 10.1164/ajrccm.161.5.9907109. [DOI] [PubMed] [Google Scholar]
- Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- Mann EA, Burnett T, Cornell S, Ludlow CL. The effect of neuromuscular stimulation of the genioglossus on the hypopharyngeal airway. Laryngoscope. 2002;112:351–356. doi: 10.1097/00005537-200202000-00027. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Takeda M, Saiki C, Takahashi T, Ojima K. Effects of vagal and carotid chemoreceptor afferents on the frequency and pattern of spontaneous augmented breaths in rabbits. Lung. 1997;175:175–186. doi: 10.1007/pl00007565. [DOI] [PubMed] [Google Scholar]
- McNamara F, Wulbrand H, Thach BT. Characteristics of the infant arousal response. J Appl Physiol. 1998;85:2314–2321. doi: 10.1152/jappl.1998.85.6.2314. [DOI] [PubMed] [Google Scholar]
- Meier M, Andreas S. Mechanisms of cardiovascular co-morbidity in patients with obstructive sleep apnoea syndrome. Pneumologie. 2012;66:650–657. doi: 10.1055/s-0032-1325788. [DOI] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Liu X, Nolan P, Horner RL. Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats. The Journal of physiology. 2003a;552:975–991. doi: 10.1113/jphysiol.2003.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Nolan P, Horner RL. GABAA receptor antagonism at the hypoglossal motor nucleus increases genioglossus muscle activity in NREM but not REM sleep. The Journal of physiology. 2003b;548:569–583. doi: 10.1113/jphysiol.2002.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998;97:943–945. doi: 10.1161/01.cir.97.10.943. [DOI] [PubMed] [Google Scholar]
- O'Driscoll DM, Foster AM, Ng ML, Yang JS, Bashir F, Wong S, Nixon GM, Davey MJ, Anderson V, Walker AM, Trinder J, Horne RS. Central apnoeas have significant effects on blood pressure and heart rate in children. J Sleep Res. 2009;18:415–421. doi: 10.1111/j.1365-2869.2009.00766.x. [DOI] [PubMed] [Google Scholar]
- Oliven A, Carmi N, Coleman R, Odeh M, Silbermann M. Age-related changes in upper airway muscles morphological and oxidative properties. Exp Gerontol. 2001;36:1673–1686. doi: 10.1016/s0531-5565(01)00127-9. [DOI] [PubMed] [Google Scholar]
- Orem J, Trotter RH. Medullary respiratory neuronal activity during augmented breaths in intact unanesthetized cats. J Appl Physiol. 1993;74:761–769. doi: 10.1152/jappl.1993.74.2.761. [DOI] [PubMed] [Google Scholar]
- Otsuka R, Ono T, Ishiwata Y, Kuroda T. Respiratory-related genioglossus electromyographic activity in response to head rotation and changes in body position. Angle Orthod. 2000;70:63–69. doi: 10.1043/0003-3219(2000)070<0063:RRGEAI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Parkis MA, Bayliss DA, Berger AJ. Actions of norepinephrine on rat hypoglossal motoneurons. J Neurophysiol. 1995;74:1911–1919. doi: 10.1152/jn.1995.74.5.1911. [DOI] [PubMed] [Google Scholar]
- Pawar A, Peng YJ, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J Appl Physiol. 2008;104:1287–1294. doi: 10.1152/japplphysiol.00644.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peever JH, Shen L, Duffin J. Respiratory pre-motor control of hypoglossal motoneurons in the rat. Neuroscience. 2002;110:711–722. doi: 10.1016/s0306-4522(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Pena F, Meza-Andrade R, Paez-Zayas V, Gonzalez-Marin MC. Gasping generation in developing Swiss-Webster mice in vitro and in vivo. Neurochem Res. 2008;33:1492–1500. doi: 10.1007/s11064-008-9616-x. [DOI] [PubMed] [Google Scholar]
- Pena F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK, Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci. 2009;29:4903–4910. doi: 10.1523/JNEUROSCI.4768-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol. 2004;96:1236–1242. doi: 10.1152/japplphysiol.00820.2003. discussion 1196. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. The Journal of physiology. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000a;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. The New England journal of medicine. 2000b;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Perez-Padilla R, West P, Kryger MH. Sighs during sleep in adult humans. Sleep. 1983;6:234–243. doi: 10.1093/sleep/6.3.234. [DOI] [PubMed] [Google Scholar]
- Phillips B. Sleep-disordered breathing and cardiovascular disease. Sleep Med Rev. 2005;9:131–140. doi: 10.1016/j.smrv.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Porges WL, Hennessy EJ, Quail AW, Cottee DB, Moore PG, McIlveen SA, Parsons GH, White SW. Heart-lung interactions: the sigh and autonomic control in the bronchial and coronary circulations. Clin Exp Pharmacol Physiol. 2000;27:1022–1027. doi: 10.1046/j.1440-1681.2000.03370.x. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol. 2001;90:1986–1994. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Sensing Hypoxia: Physiology, Genetics and Epigenetics. The Journal of physiology. 2013 doi: 10.1113/jphysiol.2012.247759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhat KC, Goyal L, Bey A, Maheshwari S. Recent advances in the management of obstructive sleep apnea: The dental perspective. J Nat Sci Biol Med. 2012;3:113–117. doi: 10.4103/0976-9668.101877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praud JP, D'Allest AM, Delaperche MF, Bobin S, Gaultier C. Diaphragmatic and genioglossus electromyographic activity at the onset and at the end of obstructive apnea in children with obstructive sleep apnea syndrome. Pediatr Res. 1988;23:1–4. doi: 10.1203/00006450-198801000-00001. [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Zanella S, Koch H, Kruse SE, Lee D, Ramirez JM, Palmiter RD. Fatal breathing dysfunction in a mouse model of Leigh syndrome. J Clin Invest. 2012;122:2359–2368. doi: 10.1172/JCI62923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovacki M, Pavlovic S, Rakic A, Janelidze M, Shermulis L, Carley DW. Riluzole suppresses post-sigh, but not spontaneous apnoeas during sleep in rats. J Pharm Pharmacol. 2001;53:1555–1559. doi: 10.1211/0022357011777936. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJ, Richter DW. Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. The Journal of physiology. 1996;491(Pt 3):799–812. doi: 10.1113/jphysiol.1996.sp021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Schwarzacher SW, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Botzinger complex in vivo eliminates breathing but not gasping. The Journal of physiology. 1998;507(Pt 3):895–907. doi: 10.1111/j.1469-7793.1998.895bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Viemari JC. Determinants of inspiratory activity. Respiratory physiology & neurobiology. 2005;147:145–157. doi: 10.1016/j.resp.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Randerath WJ. Automatic positive airway pressure in titration and treatment of the obstructive sleep apnea syndrome. Pneumologie. 2007;61:228–232. doi: 10.1055/s-2007-959176. [DOI] [PubMed] [Google Scholar]
- Rees K, Spence DP, Earis JE, Calverley PM. Arousal responses from apneic events during non-rapid-eye-movement sleep. Am J Respir Crit Care Med. 1995;152:1016–1021. doi: 10.1164/ajrccm.152.3.7663777. [DOI] [PubMed] [Google Scholar]
- Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Rey S, Del Rio R, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. The Journal of physiology. 2004;560:577–586. doi: 10.1113/jphysiol.2004.072033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LB., Jr Characteristics of an inspiration-augmenting reflex in anesthetized cats. J Appl Physiol. 1962;17:683–688. doi: 10.1152/jappl.1962.17.4.683. [DOI] [PubMed] [Google Scholar]
- Robinson DM, Peebles KC, Kwok H, Adams BM, Clarke LL, Woollard GA, Funk GD. Prenatal nicotine exposure increases apnoea and reduces nicotinic potentiation of hypoglossal inspiratory output in mice. The Journal of physiology. 2002;538:957–973. doi: 10.1113/jphysiol.2001.012705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukhadze I, Fenik VB, Benincasa KE, Price A, Kubin L. Chronic intermittent hypoxia alters density of aminergic terminals and receptors in the hypoglossal motor nucleus. Am J Respir Crit Care Med. 2010;182:1321–1329. doi: 10.1164/rccm.200912-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, McKenzie DK, Gorman RB, Trinder JA, White DP, Gandevia SC. Neural drive to human genioglossus in obstructive sleep apnoea. The Journal of physiology. 2007;585:135–146. doi: 10.1113/jphysiol.2007.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboisky JP, Jordan AS, Eckert DJ, White DP, Trinder JA, Nicholas CL, Gautam S, Malhotra A. Recruitment and rate-coding strategies of the human genioglossus muscle. J Appl Physiol. 2010;109:1939–1949. doi: 10.1152/japplphysiol.00812.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboisky JP, Stashuk DW, Hamilton-Wright A, Carusona AL, Campana LM, Trinder J, Eckert DJ, Jordan AS, McSharry DG, White DP, Nandedkar S, David WS, Malhotra A. Neurogenic changes in the upper airway of patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185:322–329. doi: 10.1164/rccm.201106-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y. Reflections on the brainstem dysfunction in neurologically disabled children. Brain Dev. 2009;31:529–536. doi: 10.1016/j.braindev.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Sangkatumvong S, Khoo MC, Kato R, Detterich JA, Bush A, Keens TG, Meiselman HJ, Wood JC, Coates TD. Peripheral vasoconstriction and abnormal parasympathetic response to sighs and transient hypoxia in sickle cell disease. Am J Respir Crit Care Med. 2011;184:474–481. doi: 10.1164/rccm.201103-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saponjic J, Radulovacki M, Carley DW. Monoaminergic system lesions increase post-sigh respiratory pattern disturbance during sleep in rats. Physiol Behav. 2007;90:1–10. doi: 10.1016/j.physbeh.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- Schwenke DO, Cragg PA. Carotid bodies and the sigh reflex in the conscious and anaesthetised guinea-pig. Adv Exp Med Biol. 2000;475:801–813. doi: 10.1007/0-306-46825-5_81. [DOI] [PubMed] [Google Scholar]
- Sebe JY, Berger AJ. Inspiratory-phase short time scale synchrony in the brainstem slice is generated downstream of the pre-Botzinger complex. Neuroscience. 2008;153:1390–1401. doi: 10.1016/j.neuroscience.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaratnam SR, Parkis MA, Funk GD. Developmental modulation of mouse hypoglossal nerve inspiratory output in vitro by noradrenergic receptor agonists. Brain Res. 1998;805:104–115. doi: 10.1016/s0006-8993(98)00673-8. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Central cholinergic regulation of respiration: nicotinic receptors. Acta Pharmacol Sin. 2009;30:761–770. doi: 10.1038/aps.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SA, Horner RL, Banner NR, McKenzie E, Heaton R, Yacoub MH, Guz A. The effect of human heart-lung transplantation upon breathing at rest and during sleep. Respir Physiol. 1988;72:131–149. doi: 10.1016/0034-5687(88)90001-1. [DOI] [PubMed] [Google Scholar]
- Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–177. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- Shott SR, Cunningham MJ. Apnea and the elongated uvula. Int J Pediatr Otorhinolaryngol. 1992;24:183–189. doi: 10.1016/0165-5876(92)90145-f. [DOI] [PubMed] [Google Scholar]
- Smith ML, Niedermaier ON, Hardy SM, Decker MJ, Strohl KP. Role of hypoxemia in sleep apnea-induced sympathoexcitation. J Auton Nerv Syst. 1996;56:184–190. doi: 10.1016/0165-1838(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Soja PJ, Lopez-Rodriguez F, Morales FR, Chase MH. The postsynaptic inhibitory control of lumbar motoneurons during the atonia of active sleep: effect of strychnine on motoneuron properties. J Neurosci. 1991;11:2804–2811. doi: 10.1523/JNEUROSCI.11-09-02804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soja PJ, Morales FR, Baranyi A, Chase MH. Effect of inhibitory amino acid antagonists on IPSPs induced in lumbar motoneurons upon stimulation of the nucleus reticularis gigantocellularis during active sleep. Brain Res. 1987;423:353–358. doi: 10.1016/0006-8993(87)90862-6. [DOI] [PubMed] [Google Scholar]
- Sokoloff AJ. Localization and contractile properties of intrinsic longitudinal motor units of the rat tongue. J Neurophysiol. 2000;84:827–835. doi: 10.1152/jn.2000.84.2.827. [DOI] [PubMed] [Google Scholar]
- Sood S, Liu X, Liu H, Horner RL. Genioglossus muscle activity and serotonergic modulation of hypoglossal motor output in obese Zucker rats. J Appl Physiol. 2007;102:2240–2250. doi: 10.1152/japplphysiol.01229.2006. [DOI] [PubMed] [Google Scholar]
- Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med. 2005;172:1338–1347. doi: 10.1164/rccm.200502-258OC. [DOI] [PubMed] [Google Scholar]
- Susarla SM, Thomas RJ, Abramson ZR, Kaban LB. Biomechanics of the upper airway: Changing concepts in the pathogenesis of obstructive sleep apnea. Int J Oral Maxillofac Surg. 2010;39:1149–1159. doi: 10.1016/j.ijom.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Takemoto H. Morphological analyses of the human tongue musculature for three-dimensional modeling. J Speech Lang Hear Res. 2001;44:95–107. doi: 10.1044/1092-4388(2001/009). [DOI] [PubMed] [Google Scholar]
- Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBotzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telgkamp P, Cao YQ, Basbaum AI, Ramirez JM. Long-term deprivation of substance P in PPT-A mutant mice alters the anoxic response of the isolated respiratory network. J Neurophysiol. 2002;88:206–213. doi: 10.1152/jn.2002.88.1.206. [DOI] [PubMed] [Google Scholar]
- Thomas RJ, Terzano MG, Parrino L, Weiss JW. Obstructive sleep-disordered breathing with a dominant cyclic alternating pattern--a recognizable polysomnographic variant with practical clinical implications. Sleep. 2004;27:229–234. doi: 10.1093/sleep/27.2.229. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Lieske SP, Viemari JC, Thoby-Brisson M, Ramirez JM. Differential modulation of neural network and pacemaker activity underlying eupnea and sigh-breathing activities. J Neurophysiol. 2008;99:2114–2125. doi: 10.1152/jn.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomilehto H, Seppa J, Uusitupa M. Obesity and obstructive sleep apnea - Clinical significance of weight loss. Sleep Med Rev. 2012 doi: 10.1016/j.smrv.2012.08.002. [DOI] [PubMed] [Google Scholar]
- van Lunteren E. Muscles of the pharynx: structural and contractile properties. Ear Nose Throat J. 1993;72:27–29. 33. [PubMed] [Google Scholar]
- Vanderveken OM, Boudewyns A, Ni Q, Kashyap B, Verbraecken J, De Backer W, Van de Heyning P. Cardiovascular implications in the treatment of obstructive sleep apnea. J Cardiovasc Transl Res. 2011;4:53–60. doi: 10.1007/s12265-010-9238-y. [DOI] [PubMed] [Google Scholar]
- Verbraecken JA, De Backer WA. Upper airway mechanics. Respiration. 2009;78:121–133. doi: 10.1159/000222508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Garcia AJ, 3rd, Doi A, Ramirez JM. Activation of alpha-2 noradrenergic receptors is critical for the generation of fictive eupnea and fictive gasping inspiratory activities in mammals in vitro. Eur J Neurosci. 2011;33:2228–2237. doi: 10.1111/j.1460-9568.2011.07706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J Neurophysiol. 2006;95:2070–2082. doi: 10.1152/jn.01308.2005. [DOI] [PubMed] [Google Scholar]
- Vlemincx E, Van Diest I, Lehrer PM, Aubert AE, Van den Bergh O. Respiratory variability preceding and following sighs: a resetter hypothesis. Biol Psychol. 2010;84:82–87. doi: 10.1016/j.biopsycho.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Voituron N, Zanella S, Menuet C, Lajard AM, Dutschmann M, Hilaire G. Early abnormalities of post-sigh breathing in a mouse model of Rett syndrome. Respiratory physiology & neurobiology. 2010;170:173–182. doi: 10.1016/j.resp.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Mackiewicz M, Kubin L. Alpha(1B) receptors are the main postsynaptic mediators of adrenergic excitation in brainstem motoneurons, a single-cell RT-PCR study. J Chem Neuroanat. 2001;22:157–166. doi: 10.1016/s0891-0618(01)00124-7. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Rukhadze I, Kubin L. Hypoglossal premotor neurons of the intermediate medullary reticular region express cholinergic markers. J Appl Physiol. 2008;105:1576–1584. doi: 10.1152/japplphysiol.90670.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese-Mayer DE, Kenny AS, Bennett HL, Ramirez JM, Leurgans SE. Familial dysautonomia: frequent, prolonged and severe hypoxemia during wakefulness and sleep. Pediatr Pulmonol. 2008;43:251–260. doi: 10.1002/ppul.20764. [DOI] [PubMed] [Google Scholar]
- Wheatley JR, Mezzanotte WS, Tangel DJ, White DP. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis. 1993;148:597–605. doi: 10.1164/ajrccm/148.3.597. [DOI] [PubMed] [Google Scholar]
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, Butler JE, Saboisky JP, Gandevia SC, White DP, Trinder J. Discharge patterns of human genioglossus motor units during arousal from sleep. Sleep. 2010;33:379–387. doi: 10.1093/sleep/33.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won CH, Li KK, Guilleminault C. Surgical treatment of obstructive sleep apnea: upper airway and maxillomandibular surgery. Proc Am Thorac Soc. 2008;5:193–199. doi: 10.1513/pats.200708-121MG. [DOI] [PubMed] [Google Scholar]
- Wulbrand H, McNamara F, Thach BT. Suppression of sigma spindle electroencephalographic activity as a measure of transient arousal after spontaneous and occlusion-evoked sighs and startles. Pediatr Res. 1998;44:767–773. doi: 10.1203/00006450-199811000-00021. [DOI] [PubMed] [Google Scholar]
- Wulbrand H, McNamara F, Thach BT. The role of arousal related brainstem reflexes in causing recovery from upper airway occlusion in infants. Sleep. 2008;31:833–840. doi: 10.1093/sleep/31.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamuy J, Fung SJ, Xi M, Morales FR, Chase MH. Hypoglossal motoneurons are postsynaptically inhibited during carbachol-induced rapid eye movement sleep. Neuroscience. 1999;94:11–15. doi: 10.1016/s0306-4522(99)00355-3. [DOI] [PubMed] [Google Scholar]
- Yasaki E, Saito Y, Nakano K, Katsumori H, Hayashi K, Nishikawa T, Osawa M. Characteristics of breathing abnormality in Leigh and its overlap syndromes. Neuropediatrics. 2001;32:299–306. doi: 10.1055/s-2001-20405. [DOI] [PubMed] [Google Scholar]
- Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- Younes M, Loewen AH, Ostrowski M, Laprairie J, Maturino F, Hanly PJ. Genioglossus activity available via non-arousal mechanisms vs. that required for opening the airway in obstructive apnea patients. J Appl Physiol. 2012;112:249–258. doi: 10.1152/japplphysiol.00312.2011. [DOI] [PubMed] [Google Scholar]
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- Zhang W, Mifflin SW. Excitatory amino acid receptors within NTS mediate arterial chemoreceptor reflexes in rats. Am J Physiol. 1993;265:H770–H773. doi: 10.1152/ajpheart.1993.265.2.H770. [DOI] [PubMed] [Google Scholar]
- Zucconi M, Weber G, Castronovo V, Ferini-Strambi L, Russo F, Chiumello G, Smirne S. Sleep and upper airway obstruction in children with achondroplasia. J Pediatr. 1996;129:743–749. doi: 10.1016/s0022-3476(96)70159-2. [DOI] [PubMed] [Google Scholar]