Abstract
Common variable immunodeficiency (CVID) is a common primary immunodeficiency characterized by a failure in B-cell differentiation with defective immunoglobulin production. Affected patients are uniquely susceptible to recurrent infection with encapsulated organisms and have an increased propensity for the development of inflammatory and autoimmune manifestations. The diagnosis of CVID is commonly delayed and the underlying cause of the disorder is not understood. Replacement antibody therapy reduces the risk of serious infections. However, optimal treatment regimens for the uncommon manifestations associated with this disease, such as granulomatous lymphocytic interstitial lung disease, require further research.
Keywords: Antibody deficiency, CVID, granulomatous disease, lung disease, primary immune disease, treatment
Common variable immunodeficiency (CVID) is a heterogeneous disorder characterized by recurrent bacterial infections and impaired B-cell differentiation leading to defective immunoglobulin production. CVID is the most common clinically significant primary immunodeficiency disease. It is not a single disease, but rather a clinical syndrome that represents a family of disorders exhibiting a common phenotype. The age of onset of CVID is variable, presenting in both children and adults. The diagnosis is generally made between 20 and 40 years of age, but up 20% may present before the age of 20 years.1 Depending on the ethnicity of the population, it affects an estimated 1 in 25,000–50,000 subjects.2–4 The true incidence of CVID may be much higher because the disease is largely underappreciated and underdiagnosed, which is reflected in the common delay in diagnosis of up to 5–10 years.1,2,5–8
PRESENTATION
Nearly all patients present with recurrent upper and/or lower respiratory tract infections including bronchitis, sinusitis, otitis media, and pneumonia. Encapsulated bacteria (Haemophilus influenzae and Streptococcus pneumoniae) are the most commonly discovered pathogens.1,6,8–10 In addition, patients with CVID appear to be particularly susceptible to infections with atypical bacteria such as Mycoplasma sp. and Ureaplasma sp.8–11 Therefore, when deciding on empiric antimicrobial therapy of respiratory tract infections, agents such as macrolides or fluoroquinolones should be considered because they cover both encapsulated and atypical organisms. Pulmonary infections with Gram-negative rods should also be considered, in particular in patients with impaired cellular immunity or longstanding CVID. Opportunistic infections are rare and occur in <10% of patients.1,8,12
Unlike congenital forms of agammaglobulinemia, such as X-linked agammaglobulinemia, T-cell abnormalities are common in patients with CVID and contribute to the more variable clinical manifestations of this disease.1,8,12 A subgroup of CVID patients termed late-onset combined immune deficiency is defined by opportunistic infections and/or severe T-cell lymphopenia (CD4 < 200 cells/mm3).13 This subgroup of patients is also more likely to have a severe clinical phenotype (gastrointestinal disease, granulomatous disease, splenomegaly, and lymphomas).
Gastrointestinal tract infections with pathogens similar to those found in X-linked agammaglobulinemia (Campylobacter jejuni, Salmonella sp, and Giardia lamblia) are also common.1,8 The prevalence of hepatitis is also increased in CVID, occurring in ∼12% of patients. The prognosis due to hepatitis secondary to infection with hepatitis C is poor and may be rapidly progressive in patients with CVID.14,15 Other forms of liver disease such as nodular regenerative hyperplasia are an increasingly recognized complication of CVID.1
ETIOLOGY
The etiology of the vast majority of cases of CVID is unknown. In a small subset of patients, however, specific molecular defects have been identified. Most of these genetic abnormalities are rare with the exception of mutations in TNFRSF13B, which encodes for transmembrane activator and calcium modulator and cyclophilin ligand (TACI). Mutations in TACI occur in ∼8–10% of patients with CVID.16,17 Patients with heterozygous mutation in TACI are at high risk to develop CVID, whereas homozygous mutations of TACI always result in CVID.18 Patients with mutations in TACI are also more likely to have both autoimmune disease and splenomegaly.19–21
Other genetic defects leading to a CVID phenotype include mutations in inducible T-cell costimulator (ICOS), CD19, CD81, CD20, CD21, and B-cell activating factor of the tumor necrosis factor family receptor (BAFF-R).16,17,22–28 Some of these genetic mutations are likely to be disease causing (ICOS, CD19, CD20, and CD81) and others (BAFF-R) may require additional genetic contributions to lead to the CVID phenotype.12 Collectively, mutations in ICOS, CD19, CD81, CD20, CD21, BAFF-R, and TACI account for <10–15% of all cases of CVID.
COMPLICATIONS
Acute and chronic infections are a major cause of morbidity in patients with CVID.7,12 The occurrence of recurrent upper respiratory tract infections can result in chronic sinusitis and hearing loss. Recurrent lower respiratory tract infections (i.e., pneumonia) may lead to the development of bronchiectasis (Fig. 1), which is reported to be present in up to 20% of patients with CVID.29,30 In addition to infectious complications in the lung, parenchymal and interstitial changes may be found leading to both obstructive and restrictive defects (Table 1).
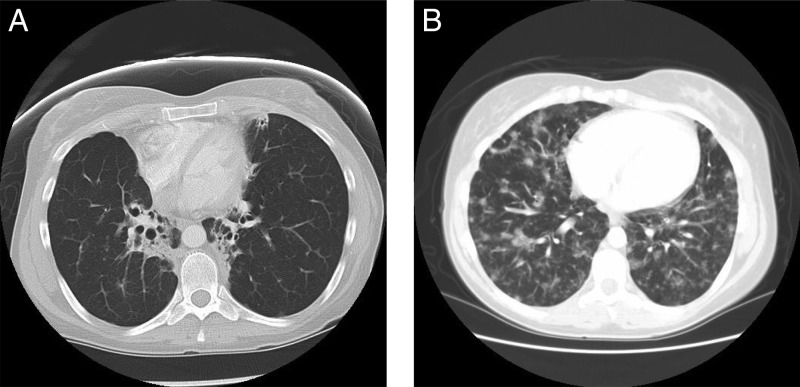
Figure 1.
(A) High-resolution computed tomography (HRCT) scans showing bronchiectasis related to CVID in the context of a normal chest radiograph (not shown). (B) HRCT scans showing granulomatous lymphocytic interstitial lung disease (GLILD) related to CVID in the context of a normal chest radiograph (not shown).
Table 1.
Common complications of CVID

CVID = common variable immunodeficiency; GLILD = granulomatous and lymphocytic interstitial lung disease.
Of particular concern, is a restrictive interstitial lung disease, which on biopsy reveals granulomatous and lymphoproliferative histopathologic patterns (lymphocytic interstitial pneumotis, follicular bronchiolitis, and lymphoid hyperplasia). The term granulomatous and lymphocytic lung disease (granulomatous lymphocytic interstitial lung disease [GLILD]) has been used to characterize this disorder30 and occurs in ∼10–25% of patients with CVID.29–35 Progressive pulmonary impairment due to GLILD appears to be an important cause of morbidity and mortality in CVID30 and shows a shortened median survival (13.7 years versus 28.8 years in those without this complication).1,12,30,36 This noncaseating granulomatous disease is systemic in nature and may be present in the lung, bone marrow, liver, and lymph nodes.37 Consequently, this disease process is often confused with sarcoidosis; but unlike sarcoid, these lesions do not remit spontaneously or resolve with steroid administration.30,31 In addition to granuloma, polyclonal lymphocytic infiltration or nonmalignant hyperplasia of the lymph nodes (cervical, mediastinal, and abdominal lymph nodes as well as the spleen) are found in at least 20% of CVID subjects.2,8,30,31,36,38,39 Lymphoid infiltrates can occur in the lungs or other organs such as the liver and kidneys. Enlarged lymph nodes usually show atypical or reactive hyperplasia, with or without preservation of germinal center boundaries.37
Patients with CVID are also at high risk to develop malignancy, which occurs in up to 15% of patients. In particular, the prevalence of non-Hodgkin's lymphomas and gastric carcinoma are increased in CVID and are a major cause of morbidity and mortality.1,38,40–42 When lymphomas appear in CVID, they are usually extranodal, B cell in origin, negative for Epstein-Barr virus, and are more common in subjects in the 4th to 7th decades of life.1,8,43
Interestingly, although patients with CVID are unable to make antibodies to foreign antigens, they show a propensity to make autoantibodies; the overall prevalence of autoimmune disease is ∼20–25%.8,44 They are susceptible to a wide range of autoimmune diseases, but autoimmune thrombocytopenia and autoimmune hemolytic anemia are the most commonly reported cytopenias occurring in 11–12% of subjects.1,2,19,45
The most common gastrointestinal manifestation of CVID is transient or persistent diarrhea, found in 21–57% of patients.44,46,47 In addition to bacterial and parasitic infections, chronic gastritis and inflammatory bowel disease are significant problems for patients with CVID.1,44 Small bowel enteropathy in CVID resembles celiac disease with short villi, crypt hyperplasia, and intraepithelial lymphocytosis.47 This small bowel enteropathy can lead to diarrhea, weight loss, and malabsorption. Large bowel enteropathy resembling Crohn's disease and ulcerative colitis has also been described in CVID,47 although it is not clear if these have the same pathogenesis as classic inflammatory bowel disease. Furthermore, a subset of individuals (8% in one cohort)7 with CVID exhibit nodular lymphoid hyperplasia on biopsy—most commonly presenting as cholestasis or portal hypertension. 41,48
Based on the European Society for Immunodeficiency CVID registry and recently reproduced in additional cohorts,49 efforts have been made to divide patients into five distinct phenotypes based on intrinsic disease-related complications: no complications, autoimmunity, polyclonal lymphocytic infiltration, enteropathy, and lymphoid malignancy.2 The outcome of individual patients appears to be dependent on the presence or absence of some of these specific complications.2 These phenotypes are not exclusive because the lymphoproliferative phenotype (GLILD, splenomegaly, and adenopathy) is frequently accompanied by autoimmune cytopenias, gastrointestinal, and hepatic disease.19,30,34,36,47,48,50,51
As treatments for infection have improved, complications such chronic lung disease, malignancy, and autoimmune disease are now the most common causes of mortality in CVID.1,2,30,31,37,42,43 In a recent analysis of 411 subjects with CVID in which 19% had died, the predominant causes of death included respiratory failure from chronic lung disease, lymphoid or other malignancy, and liver disease.1 Interestingly, not all complications were shown to be associated with reduced mortality. Risk factors for early mortality included gastrointestinal disease, liver disease, chronic lung disease, and lymphoma whereas autoimmunity and cancer other than lymphoma were not associated with early mortality.
DIAGNOSTIC EVALUATION
The diagnostic criteria for CVID include52
Decreased serum IgG level, AND decreased serum IgA or IgM
Decreased ability to make specific antibodies in response to immunizations
Exclusion of primary immunodeficiencies leading to decreased IgG
Exclusion of secondary causes of decreased serum IgG
Greater than 2 years of age
In patients with CVID, IgG levels are reduced by >2 standard deviations from the mean in all patients and <450 mg/dL in 94.2% of patients at diagnosis.2 Almost all cases of CVID have a decrease in IgA (usually <5 mg/dL) and reductions in IgM in about one-half of cases.1,2,5,6,8,52 Specific antibody responses, which are impaired in CVID, are shown by measuring specific antibodies 3–4 weeks after the administration of a protein (i.e., tetanus, diphtheria toxoid, H. influenzae B) and polysaccharide (pneumococcal vaccine) vaccines.53 Early in life CVID is not always discernible from transient hypogammaglobulinemia of infancy or congenital forms of agammaglobulinemia. Therefore, the general consensus is that this diagnosis of CVID should not be made until after a patient reaches the age of 2 years.12
Exclusion of other secondary causes of hypogammaglobulinemia is especially important in patients with isolated low IgG (Table 2).2,12,54–56 Protein loss from protein losing enteropathy or nephrotic syndrome can present as hypogammaglobulinemia and is not uncommon. Chronic oral corticosteroid use can also lead to reduced IgG levels and is a common cause of hypogammaglobulinemia in patients with severe asthma or chronic obstruction pulmonary disease. The decrease in serum IgG occurs is relatively selective with a relative sparing of the IgA and IgM and normal specific antibody production.57,58 The magnitude of the reduction of serum IgG after corticosteroid therapy is dependent on the dose and duration of steroid therapy. Although corticosteroid therapy typically does not reduce serum IgG to <400 mg/dL, reduction below this level may be seen in patients receiving high doses of corticosteroids over a long period of time. 57,58 Other common medications associated with hypogammaglobulinemia include rituximab, azathioprine, sulfasalazine, and several anticonvulsants (carbamazepine, levetiracetam, oxcarbazepine, and phenytoin).59–63
Table 2.
Causes of hypogammaglobulinemia to exclude before diagnosis of CVID

CVID = common variable immunodeficiency; XLP = X-linked lymphoproliferative disease.
The physical examination of a patient with a suspected CVID requires an in-depth focus on the involved organ systems. The chest examination often may reveal wheezing, rhonchi, or crackles in patients with CVID-associated lung disease. The clinician should observe specifically for clubbing or cyanosis and use of accessory muscles for respiration. Careful periodic examination of the lymph nodes (cervical, axillary, or inguinal) and spleen is important, because patients with CVID also frequently have adenopathy and splenomegaly, which can be quite profound.
Laboratory evaluation would include quantitative immunoglobulins (IgG, IgA, and IgM) and functional antibody testing. Flow cytometry should enumerate the total numbers of T cells, T-cell subsets (CD4 and CD8), B cells, B-cell subsets, and NK cells. B-cell subset analysis should include the percentage of total memory B cells, switched memory B cells, and CD21(lo) B cells.31,64–67
B-cell numbers are variable in CVID, and if reduced may indicate a poorer prognosis.1,8 Low numbers of switched memory B cells (CD27[+]IgM[−]IgD[−]) in the peripheral blood are frequently found in patients with a more severe phenotype of CVID (e.g., GLILD)31,68 and may portend a worse prognosis. T-cell abnormalities occur in ∼40% of patients and include anergy, T-cell lymphopenia, and poor proliferative responses to mitogens and antigens.
At initial evaluation sinus, chest, abdomen, and pelvis CT should be performed. Sinus CT is helpful in evaluation of sinus disease and in deciding if any additional antibiotic treatments may be of benefit. Abdominal CT scans are used to assess spleen size, and/or the presence of intraabdominal and retroperitoneal adenopathy. Because of the complexity of the pulmonary pathology in patients with CVID, in addition to high-resolution computed tomography (HRCT) scans of the chest, x rays, and full pulmonary function tests (including lung volumes, spirometry and diffusion capacity) should be obtained as part of the initial evaluation. HRCT scan of the chest is much more sensitive than a standard chest radiograph in detecting pulmonary parenchymal abnormalities such as interstitial lung disease or bronchiectasis.
Serologic assays that measure specific antibody are inappropriate to identify pathogens responsible for infection in patients with CVID. Diagnosis of specific pathogens in this patient population must be made by culture, polymerase chain reaction, or other direct methods of pathogen detection. Screening for human immunodeficiency virus and hepatitis B and C should be performed at the initial evaluation by polymerase chain reaction. Abnormalities in liver function tests are not uncommon and may be abnormal in as high as 43% of patients.51,69
TREATMENT
The primary treatment of CVID is antibody replacement with either i.v. or subcutaneous immunoglobulin with an initial dose of 400–600 mg/kg of gammaglobulin per month.2,70 This dose can be divided every 3–4 weeks for i.v. administration or every 1–2 weeks for subcutaneous administration. Both i.v. and subcutaneous methods have been shown to be safe and effective for replacement.12,70–73
Subsequent dosing should not be based on IgG immunoglobulin level, but rather on preventing infection. Although trough IgG levels should be drawn before initial administration and may be used to help guide therapy, recent studies have shown that individualized dose adjustments based on clinical course may be preferable to blanket goal IgG trough levels.74,75 Furthermore, higher doses are required for patients with significant pulmonary disease (i.e., bronchiectasis), sinus disease, GLILD, or splenomegaly.75 Once patients are established on immunoglobulin replacement therapy, IgG trough levels are measured every 6–12 months to ensure adequate dosing with a minimum trough level of 500 mg/gL.
There is no convincing evidence that one brand of gammaglobulin is better than others in reducing infections. However, products do differ in their preparation, and when choosing a product one must consider osmolality, pH, sodium, sugar, and IgA content. A full in-depth comparison of gammaglobulin formulations is beyond the scope of this discussion, but a summary of available brands of immunoglobulin and their characteristic can be found at the Immune Deficiency Foundation website.76
Additives in the preparations used to prevent protein aggregations77—such as amino acids, sorbitol, salt, or sucrose—may lead to adverse effects in certain clinical settings. For example, sugar content should be considered in a patient with diabetes or prediabetes. Furthermore, sucrose content has been associated with renal failure or insufficiency.78,79 Because of the large protein load, patients with renal dysfunction might want to avoid intravenous immunoglobulin (IVIg) entirely, using subcutaneous immunoglobulin instead. The major contributors to osmolality in IVIg are sodium and sugar content. Reconstitution of lyophilized preparations can also result in hyperosmolar solutions. Osmolality is an important concern because hyperosmotic states have been implicated in thrombotic complications.80
Although the mortality of CVID caused by common infectious pathogens has declined with the increased use of high-dose immunoglobulin replacement,81 patients may continue to develop obstructive, restrictive, or bronchiectatic changes.7 In fact, GLILD is refractory to gammaglobulin replacement therapy and bronchiectasis may develop despite antibody replacement therapy.29–35
For the most part, many of the complications of CVID may be treated in the same manner as immunocompetent patients with similar diseases. Oral corticosteroids, immunomodulatory dosages of IVIg (2 g/kg per month), and rituximab have been used to treat autoimmune hemolytic anemia or autoimmune thrombocytopenia in patients with CVID.19,45,82–84 Splenectomy is to be avoided, because severe infections have occurred.8,85 Treatment of bronchiectasis in patients with CVID is similar to the therapy of idiopathic bronchiectasis. Mobilization of pulmonary secretions through the use of medications such as β2-agonists along with chest physiotherapy is frequently used, although there are few trials that show the effectiveness of pulmonary hygiene.86,87
The optimal treatment for GLILD in CVID is unknown, but these patients appear to have a high mortality rate if left untreated. GLILD is resistant to corticosteroid therapy so alternative treatment regimens should be considered. Steroid sparing agents such as azathioprine, 6-mercaptopurine, cyclosporine A, mycophenolate mofetil, or methotrexate have been tried with inconsistent results.50 TNF inhibitors have also been tried and some success has been reported.88,89 However, the low prevalence of this complication in CVID patients makes controlled or open trials difficult. Recent data from a retrospective analysis of seven CVID patients with biopsy-proven GLILD aged 18–43 years suggests that combination chemotherapy with rituximab and azathioprine may be beneficial in this select patient population.68 Patients showed statistically significant improvement in HRCT score (p < 0.011) and pulmonary function testing (forced expiratory volume in 1 second, p < 0.04 and forced vital capacity, p < 0.036). These findings are encouraging but highlight the urgent need for similar prospective studies to ascertain the most effective medications, optimal timing, duration of therapy, and effect on long-term morbidity and mortality.
CONCLUSION
CVID is a complex, multifocal disease with a large array of clinical manifestations and complications. Treatment with gammaglobulin and improved antibiotic coverage have vastly improved the outlook for patients; however, as infections and infectious complications become less prominent, morbidities from disordered inflammation or immune dysregulation have become greater areas of concern. Continued studies are needed to illuminate the many causes of this disease and possibly therapeutic targets.
Footnotes
Presented at the North American Rhinology and Allergy Conference, February 4, 2013, Puerto Rico
JM Routes has received research grants from the National Institutes of Health. The remaining author has no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood 119:1650–1657, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chapel H, Lucas M, Lee M, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood 112:277–286, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Stray-Pedersen A, Abrahamsen TG, Froland SS. Primary immunodeficiency diseases in Norway. J Clin Immunol 20:477–485, 2000. [DOI] [PubMed] [Google Scholar]
- 4. Fasth A. Primary immunodeficiency disorders in Sweden: Cases among children, 1974–1979. J Clin Immunol 2:86–92, 1982. [DOI] [PubMed] [Google Scholar]
- 5. Urschel S, Kayikci L, Wintergerst U, et al. Common variable immunodeficiency disorders in children: Delayed diagnosis despite typical clinical presentation. J Pediatr 154:888894, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Oksenhendler E, Gerard L, Fieschi C, et al. Infections in 252 patients with common variable immunodeficiency. Clin Infect Dis 46:1547–1554, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Quinti I, Soresina A, Spadaro G, et al. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol 27:308–316, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: Clinical and immunological features of 248 patients. Clin Immunol 92:34–48, 1999. [DOI] [PubMed] [Google Scholar]
- 9. Gelfand EW. Unique susceptibility of patients with antibody deficiency to mycoplasma infection. Clin Infect Dis 17(suppl 1):S250–S253, 1993. [PubMed] [Google Scholar]
- 10. Roifman CM, Rao CP, Lederman HM, et al. Increased susceptibility to mycoplasma infection in patients with hypogammaglobulinemia. Am J Med 80:590–594, 1986. [DOI] [PubMed] [Google Scholar]
- 11. Hermaszewski RA, Webster AD. Primary hypogammaglobulinaemia: A survey of clinical manifestations and complications. Q J Med 86:31–42, 1993. [PubMed] [Google Scholar]
- 12. Yong PFK, Thaventhiran JED, Grimbacher B. A rose is a rose is a rose,” but CVID is not CVID: Common variable immune deficiency (CVID), what do we know in 2011? In Advances in Immunology, Chap. 2. Frederick WA. (Ed). Waltham, MA: Academic Press, 47–107, 2011. [DOI] [PubMed] [Google Scholar]
- 13. Malphettes M, Gérard L, Carmagnat M, et al. Late-onset combined immune deficiency: A subset of common variable immunodeficiency with severe T cell defect. Clin Infect Dis 49:1329–1338, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Ermis F, Akyuz F, Demir K, et al. Rapidly progressive HCV cirrhosis in a hypogammaglobulinemic patient. Intern Med 47:415–417, 2008. [DOI] [PubMed] [Google Scholar]
- 15. Fellermann K, Stange EF. Chronic hepatitis C, common variable immunodeficiency and autoimmune hemolytic anemia. Coincidence by chance or common etiology? Hepatogastroenterology 47:1422–1424, 2000. [PubMed] [Google Scholar]
- 16. Salzer U, Chapel HM, Webster AD, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet 37:820–828, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Castigli E, Wilson SA, Garibyan L, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet 37:829–834, 2005. [DOI] [PubMed] [Google Scholar]
- 18. Salzer U, Bacchelli C, Buckridge S, et al. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood 113:1967–1976, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cunningham-Rundles C. Autoimmune manifestations in common variable immunodeficiency. J Clin Immunol 28(suppl 1):S42–S45, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang L, Radigan L, Salzer U, et al. Transmembrane activator and calcium-modulating cyclophilin ligand interactor mutations in common variable immunodeficiency: Clinical and immunologic outcomes in heterozygotes. J Allergy Clin Immunol 120:1178–1185, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chinen J, Martinez-Gallo M, Gu W, et al. Transmembrane activator and CAML interactor (TACI) haploinsufficiency results in B-cell dysfunction in patients with Smith-Magenis syndrome. J Allergy Clin Immunol 127:1579–1586, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sekine H, Ferreira RC, Pan-Hammarström Q, et al. Role for Msh5 in the regulation of Ig class switch recombination. Proc Natl Acad Sci U S A 104:7193–7198, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pan-Hammarström Q, Salzer U, Du L, et al. Reexamining the role of TACI coding variants in common variable immunodeficiency and selective IgA deficiency. Nat Genet 39:429–430, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castigli E, Geha RS. TACI, isotype switching, CVID and IgAD. Immunol Res 38:102–111, 2007. [DOI] [PubMed] [Google Scholar]
- 25. Castigli E, Wilson SA, Scott S, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med 201:35–39, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salzer U, Maul-Pavicic A, Cunningham-Rundles C, et al. ICOS deficiency in patients with common variable immunodeficiency. Clin Immunol 113:234–240, 2004. [DOI] [PubMed] [Google Scholar]
- 27. Grimbacher B, Hutloff A, Schlesier M, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol 4:261–268, 2003. [DOI] [PubMed] [Google Scholar]
- 28. Orange JS, Glessner JT, Resnick E, et al. Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin Immunol 127:1360–1367, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Busse PJ, Farzan S, Cunningham-Rundles C. Pulmonary complications of common variable immunodeficiency. Ann Allergy Asthma Immunol 98:1–8, 2007. [DOI] [PubMed] [Google Scholar]
- 30. Bates CA, Ellison MC, Lynch DA, et al. Granulomatous-lymphocytic lung disease shortens survival in common variable immunodeficiency. J Allergy Clin Immunol 114:415–421, 2004. [DOI] [PubMed] [Google Scholar]
- 31. Wehr C, Kivioja T, Schmitt C, et al. The EUROclass trial: Defining subgroups in common variable immunodeficiency. Blood 111:77–85, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Arnold DF, Wiggins J, Cunningham-Rundles C, et al. Granulomatous disease: Distinguishing primary antibody disease from sarcoidosis. Clin Immunol 128:18–22, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buckley RH. Pulmonary complications of primary immunodeficiencies. Paediatr Respir Rev 5(suppl A):S225–S233, 2004. [DOI] [PubMed] [Google Scholar]
- 34. Mechanic LJ, Dikman S, Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Ann Intern Med 127:613–617, 1997. [DOI] [PubMed] [Google Scholar]
- 35. Popa V. Lymphocytic interstitial pneumonia of common variable immunodeficiency. Ann Allergy 60:203–206, 1988. [PubMed] [Google Scholar]
- 36. Morimoto Y, Routes JM. Granulomatous disease in common variable immunodeficiency. Curr Allergy Asthma Rep 5:370–375, 2005. [DOI] [PubMed] [Google Scholar]
- 37. Sander CA, Medeiros LJ, Weiss LM, et al. Lymphoproliferative lesions in patients with common variable immunodeficiency syndrome. Am J Surg Pathol 16:1170–1182, 1992. [DOI] [PubMed] [Google Scholar]
- 38. Cunningham-Rundles C, Lieberman P, Hellman G, Chaganti RS. Non-Hodgkin lymphoma in common variable immunodeficiency. Am J Hematol 37:69–74, 1991. [DOI] [PubMed] [Google Scholar]
- 39. Cunningham-Rundles C. Clinical and immunologic analyses of 103 patients with common variable immunodeficiency. J Clin Immunol 9:22–33, 1989. [DOI] [PubMed] [Google Scholar]
- 40. Salavoura K, Kolialexi A, Tsangaris G, Mavrou A. Development of cancer in patients with primary immunodeficiencies. Anticancer Res 28:1263–1269, 2008. [PubMed] [Google Scholar]
- 41. Mellemkjaer L, Hammarstrom L, Andersen V, et al. Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: A combined Danish and Swedish study. Clin Exp Immunol 130:495–500, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kinlen LJ, Webster AD, Bird AG, et al. Prospective study of cancer in patients with hypogammaglobulinaemia. Lancet 1:263–266, 1985. [DOI] [PubMed] [Google Scholar]
- 43. Cunningham-Rundles C, Siegal FP, Cunningham-Rundles S, Lieberman P. Incidence of cancer in 98 patients with common varied immunodeficiency. J Clin Immunol 7:294–299, 1987. [DOI] [PubMed] [Google Scholar]
- 44. Agarwal S, Cunningham-Rundles C. Autoimmunity in common variable immunodeficiency. Curr Allergy Asthma Rep 9:347–352, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lopes-da-Silva S, Rizzo LV. Autoimmunity in common variable immunodeficiency. J Clin Immunol 28(suppl 1):S46–S55, 2008. [DOI] [PubMed] [Google Scholar]
- 46. Washington K, Stenzel TT, Buckley RH, Gottfried MR. Gastrointestinal pathology in patients with common variable immunodeficiency and X-linked agammaglobulinemia. Am J Surg Pathol 20:1240–1252, 1996. [DOI] [PubMed] [Google Scholar]
- 47. Daniels JA, Lederman HM, Maitra A, Montgomery EA. Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): A clinicopathologic study and review. Am J Surg Pathol 31:1800–1182, 2007. [DOI] [PubMed] [Google Scholar]
- 48. Khodadad A, Aghamohammadi A, Parvaneh N, et al. Gastrointestinal manifestations in patients with common variable immunodeficiency. Dig Dis Sci 52:2977–2983, 2007. [DOI] [PubMed] [Google Scholar]
- 49. Chapel H, Lucas M, Patel S, et al. Confirmation and improvement of criteria for clinical phenotyping in common variable immunodeficiency disorders in replicate cohorts. J Allergy Clin Immunol 130:1197–1198.e9, 2012. [DOI] [PubMed] [Google Scholar]
- 50. Park JH, Levinson AI. Granulomatous-lymphocytic interstitial lung disease (GLILD) in common variable immunodeficiency (CVID). Clin Immunol 134:97–103, 2010. [DOI] [PubMed] [Google Scholar]
- 51. Malamut G, Ziol M, Suarez F, et al. Nodular regenerative hyperplasia: The main liver disease in patients with primary hypogammaglobulinemia and hepatic abnormalities. J Hepatol 48:74–82, 2008. [DOI] [PubMed] [Google Scholar]
- 52. Notarangelo L, Day N, Fleisher T. 2nd Conference of the Robert A. Good immunology society primary immune deficiencies and immune reconstitution Harvard Medical Boston, November 16th, 17th. Immunol Res 44:1–3, 2009. [DOI] [PubMed] [Google Scholar]
- 53. Orange JS, Ballow M, Stiehm ER, et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: A working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol 130(suppl):S1–S24, 2012. [DOI] [PubMed] [Google Scholar]
- 54. Chapel H, Cunningham-Rundles C. Update in understanding common variable immunodeficiency disorders (CVIDs) and the management of patients with these conditions. Br J Haematol 145:709–727, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chapel H, Geha R, Rosen F. Primary immunodeficiency diseases: An update. Clin Exp Immunol 132:9–15, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cunningham-Rundles C. Hematologic complications of primary immune deficiencies. Blood Rev 16:61–64, 2002. [DOI] [PubMed] [Google Scholar]
- 57. Lack G, Ochs HD, Gelfand EW. Humoral immunity in steroid-dependent children with asthma and hypogammaglobulinemia. J Pediatr 129:898–903, 1996. [DOI] [PubMed] [Google Scholar]
- 58. Hamilos DL, Young RM, Peter JB, et al. Hypogammaglobulinemia in asthmatic patients. Ann Allergy 68:472–481, 1992. [PubMed] [Google Scholar]
- 59. Knight AK, Cunningham-Rundles C. Oxcarbazepine-induced immunoglobulin deficiency. Clin Diagn Lab Immunol 12:560–561, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pereira LF, Sanchez JF. Reversible panhypogammaglobulinemia associated with phenytoin treatment. Scand J Infect Dis 34:785–787, 2002. [DOI] [PubMed] [Google Scholar]
- 61. Lee AH, Levinson AI, Schumacher HR., Jr Hypogammaglobulinemia and rheumatic disease. Sem Arthritis Rheum 22:252–264, 1993. [DOI] [PubMed] [Google Scholar]
- 62. Hayman G, Bansal A. Antibody deficiency associated with carbamazepine. BMJ 325:1213, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Azar AE, Ballas ZK. Reversible panhypogammaglobulinemia associated with the antiepileptic agent levetiracetam. Ann Allergy Asthma Immunol 101:108–109, 2008. [DOI] [PubMed] [Google Scholar]
- 64. Sanchez-Ramon S, Radigan L, Yu JE, et al. Memory B cells in common variable immunodeficiency: Clinical associations and sex differences. Clin Immunol 128:314–321, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ko J, Radigan L, Cunningham-Rundles C. Immune competence and switched memory B cells in common variable immunodeficiency. Clin Immunol 116:37–41, 2005. [DOI] [PubMed] [Google Scholar]
- 66. Piqueras B, Lavenu-Bombled C, Galicier L, et al. Common variable immunodeficiency patient classification based on impaired B cell memory differentiation correlates with clinical aspects. J Clin Immunol 23:385–400, 2003. [DOI] [PubMed] [Google Scholar]
- 67. Warnatz K, Denz A, Drager R, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(−)IgD(−)) in subgroups of patients with common variable immunodeficiency: A new approach to classify a heterogeneous disease. Blood 99:1544–1551, 2002. [DOI] [PubMed] [Google Scholar]
- 68. Chase NM, Verbsky JW, Hintermeyer MK, et al. Use of combination chemotherapy for treatment of granulomatous and lymphocytic interstitial lung disease (GLILD) in patients with common variable immunodeficiency (CVID). J Clin Immunol 33:30–39, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ward C, Lucas M, Piris J, et al. Abnormal liver function in common variable immunodeficiency disorders due to nodular regenerative hyperplasia. Clin Exp Immunol 153:331–337, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Orange JS, Hossny EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: A review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol 117(suppl):S525–S553, 2006. [DOI] [PubMed] [Google Scholar]
- 71. Gardulf A, Andersen V, Bjorkander J, et al. Subcutaneous immunoglobulin replacement in patients with primary antibody deficiencies: Safety and costs. Lancet 345:365–369, 1995. [DOI] [PubMed] [Google Scholar]
- 72. Berger M. Subcutaneous administration of IgG. Immunol Allergy Clin North Am 28:779–802, 2008. [DOI] [PubMed] [Google Scholar]
- 73. Chapel HM, Spickett GP, Ericson D, et al. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J Clin Immunol 20:94–100, 2000. [DOI] [PubMed] [Google Scholar]
- 74. Orange JS, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: A meta-analysis of clinical studies. Clin Immunol 137:21–30, 2010. [DOI] [PubMed] [Google Scholar]
- 75. Lucas M, Lee M, Lortan J, et al. Infection outcomes in patients with common variable immunodeficiency disorders: Relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol 125:1354–1360 e4, 2010. [DOI] [PubMed] [Google Scholar]
- 76. Immune Deficiency Foundation. 2012. Characteristics of Immune Globulin Products Currently Licensed for Use in the United States: http://primaryimmune.org/treatment-information/immunoglobulin-products.
- 77. Hooper JA. Intravenous immunoglobulins: Evolution of commercial IVIG preparations. Immunol Allergy Clin North Am 28:765–778, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shelton B, Griffin J, Goldman F. Immune globulin IV therapy: Optimizing care of patients in the oncology setting. Oncol Nurs Forum 33:911–921, 2006. [DOI] [PubMed] [Google Scholar]
- 79. Hansen-Schmidt S, Silomon J, Keller F. Osmotic nephrosis due to high-dose immunoglobulin therapy containing sucrose (but not with glycine) in a patient with immunoglobulin A nephritis. Am J Kidney Dis 28:451–453, 1996. [DOI] [PubMed] [Google Scholar]
- 80. Nydegger UE, Sturzenegger M. Adverse effects of intravenous immunoglobulin therapy. Drug Saf 21:171–185, 1999. [DOI] [PubMed] [Google Scholar]
- 81. Busse PJ, Razvi S, Cunningham-Rundles C. Efficacy of intravenous immunoglobulin in the prevention of pneumonia in patients with common variable immunodeficiency. J Allergy Clin Immunol 109:1001–1004, 2002. [DOI] [PubMed] [Google Scholar]
- 82. Kim JJ, Thrasher AJ, Jones AM, et al. Rituximab for the treatment of autoimmune cytopenias in children with immune deficiency. Br J Haematol 138:94–96, 2007. [DOI] [PubMed] [Google Scholar]
- 83. El-Shanawany TM, Williams PE, Jolles S. Response of refractory immune thrombocytopenic purpura in a patient with common variable immunodeficiency to treatment with rituximab. J Clin Pathol 60:715–716, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mahevas M, Le Page L, Salle V, et al. Efficiency of rituximab in the treatment of autoimmune thrombocytopenic purpura associated with common variable immunodeficiency. Am J Hematol 81:645–646, 2006. [DOI] [PubMed] [Google Scholar]
- 85. Sève P, Bourdillon L, Sarrot-Reynauld F, et al. Autoimmune hemolytic anemia and common variable immunodeficiency: A case-control study of 18 patients. Medicine 87:177–184, 2008. [DOI] [PubMed] [Google Scholar]
- 86. Indinnimeo L, Tancredi G, Barreto M, et al. Effects of a program of hospital-supervised chest physical therapy on lung function tests in children with chronic respiratory disease: 1-Year follow-up. Int J Immunopathol Pharmacol 20:841–845, 2007. [DOI] [PubMed] [Google Scholar]
- 87. Rosen MJ. Chronic cough due to bronchiectasis: ACCP evidence-based clinical practice guidelines. Chest 129(suppl):122S–131S, 2006. [DOI] [PubMed] [Google Scholar]
- 88. Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: A case report and review of the literature. Ann Allergy Asthma Immunol 95:293–300, 2005. [DOI] [PubMed] [Google Scholar]
- 89. Hatab AZ, Ballas ZK. Caseating granulomatous disease in common variable immunodeficiency treated with infliximab. J Allergy Clin Immunol 116:1161–1162, 2005. [DOI] [PubMed] [Google Scholar]