Abstract
Background:
Sinonasal papillomas are benign epithelial neoplasms arising from Schneiderian mucosa. The three subtypes, exophytic, oncocytic, and inverted (inverted papilloma [IP]), should be distinguished from one another histopathologically. This study (1) highlights the histopathological and clinical differences between the Schneiderian papilloma subtypes and (2) identifies clinical features that potentially predict papilloma subtypes.
Methods:
A retrospective review was performed of patients with Schneiderian papillomas over an 11-year period.
Results:
Seventy patients with sinonasal papillomas who underwent sinus surgery were identified. There were 50 (71%) male and 20 (29%) female subjects diagnosed at an average age of 53 years (range, 13–80 years). Exophytic (n = 25), oncocytic (n = 9), and IP (n = 37) were identified. IP was associated with transformation into squamous cell carcinoma in three (8%) cases and dysplasia in three (8%) cases. Neither oncocytic nor exophytic subtypes were associated with dysplasia or malignancy. On multivariate analysis of potential predictors of papilloma subtype, history of chronic rhinosinusitis (CRS) and location of papilloma were significantly associated with papilloma subtype. Using classification and regression tree model, papilloma subtypes can be predicted based on presence or absence of CRS and papilloma location with nominal 82.4% accuracy.
Conclusion:
The inverted and exophytic type are the most common sinonasal papillomas, with the inverted type having an 8% rate of malignant transformation in this study. In contrast, the oncocytic type was not associated with dysplasia or malignancy in our series despite reports in the literature indicating malignant potential. History of CRS and papilloma location can provide clues to the histological subtype, which is important for surgical planning and patient counseling.
Keywords: Cylindrical cell, exophytic, inverted, oncocytic, Schneiderian papillomas, sinonasal papillomas
Schneiderian papillomas are papillomas arising in the sinonasal tract, which is lined with Schneiderian epithelium, ectodermally derived respiratory mucosa.1,2 This distinctive epithelium can give rise to three histologically unique types of papillomas: exophytic (fungiform, septal, and squamous), inverted (inverting), and oncocytic (cylindrical cell and columnar) papillomas.
In 1854, Ward first documented the occurrence of a papilloma in the sinonasal cavity.3 Reingertz, in 1935, was the first to histologically describe the appearance of inverted papillomas (IPs).4 Since then, several different names have been used to characterize these three distinct types of papillomas. In 1991, the World Health Organization classified sinonasal papillomas into three distinct histopathological subtypes: exophytic, inverted, and oncocytic.5
The purpose of this study is to clarify the etiology, histopathology, clinical behavior, malignant potential, and management of these different types of sinonasal papillomas based on a review of patients with Schneiderian papillomas over an 11-year period at a single academic medical center.
MATERIALS AND METHODS
Pathology specimens were reviewed from sinus surgeries performed at University of California–Los Angeles (UCLA) Medical Center between January 2000 and July 2011. Seventy patients were diagnosed with Schneiderian papillomas over this 11-year period. All specimens were reviewed by the senior head and neck pathologist (S.B.). A retrospective patient chart review was then conducted to determine type of sinonasal papilloma, age, sex, tobacco and alcohol exposure, history of allergic rhinitis, history of chronic rhinosinusitis (CRS), number of prior sinus surgeries, location, associated findings, and malignancies.
Statistical Analysis
Age, sex, tobacco and alcohol exposure, allergic rhinitis, CRS, prior sinus surgeries, and location were evaluated for their potential to predict the subtype of papilloma. All potential predictors were categorical except for age. Tobacco exposure was classified as nonsmoker, prior smoker, or current smoker. Alcohol use was classified as none, social drinker (<1 drink/wk), regular drinker (1–4 drinks/wk), or heavy drinker (≥4 drinks/wk). CRS was defined by the guidelines delineated by the 2007 Academy of Otolaryngology–Head and Neck Surgery's panel on adult rhinosinusitis.6 Prior sinus surgery was classified as none, one prior surgery, or two or more prior surgeries. Sites of tumor involvement were obtained by review of the operative report and intake history and included nasal cavity; frontal, maxillary, ethmoid, or sphenoid sinuses; or nasopharyngeal soft palate. The nasal cavity included the following: nasal septum, lateral nasal wall, vestibule, inferior, middle, or superior turbinates.
Statistical Methods
In univariate analysis, categorical predictors were evaluated individually for a significant association with papilloma subtype using the chi-square test. To evaluate the quantitative variable of age, the mean age at presentation was calculated for each papilloma subtype and then compared using one-way analysis of variance and corresponding pairwise t-test comparisons under the one-way analysis of variance model. The eight predictors were then evaluated simultaneously using a multivariate classification and regression tree (CART) model, which is a recursive binary partitioning model. The p values for the binary partitions were computed using Fisher's exact text. Statistical analysis was conducted using SAS Version 9.3 (SAS, Inc., Cary, NC) and Answer Tree 3.0 (SPSS, Inc., Chicago IL).
Our retrospective study was approved by the UCLA Institutional Review Board, project title IRB11-002254, and the requirement for informed consent was waived. Our study was Health Insurance Portability and Accountability Act compliant.
RESULTS
Between January 2000 and July 2011, 70 patients with Schneiderian papillomas were identified. Patient demographics are summarized in Table 1. Of these 70 patients, 50 (71%) were male and 20 (29%) were female subjects. The average age at diagnosis was 53 years (range, 13–80 years).
Table 1.
Demographics
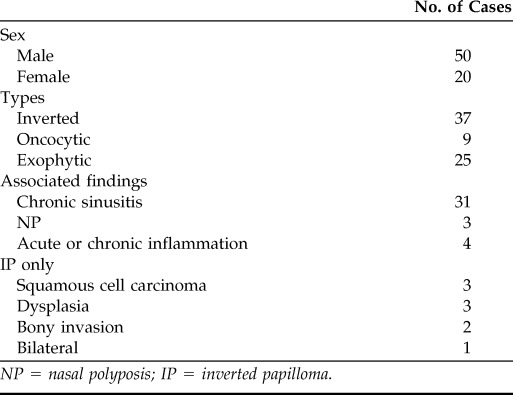
NP = nasal polyposis; IP = inverted papilloma.
Inverted (n = 37; Fig. 1), oncocytic (n = 9; Fig. 2), and exophytic types (n = 25; Fig. 3) were identified (one patient had a papilloma with features of both IP and exophytic papilloma), providing a total of 71 observations. The differences among the different types are summarized in Table 2. IPs were associated with transformation into squamous cell carcinoma in three (8%) cases, dysplasia in three (8%) cases, and bony invasion in two (6%) cases (Table 1). Neither oncocytic nor exophytic subtypes were associated with dysplasia or malignancy.
Figure 1.
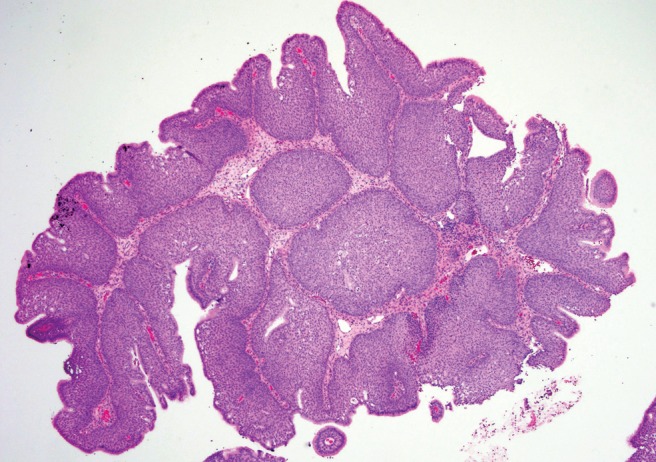
Low-power magnification, hematoxylin and eosin stain illustrating inverted papilloma (IPs) revealing markedly thick inverted growth of nonkeratinizing transitional cells.
Figure 2.
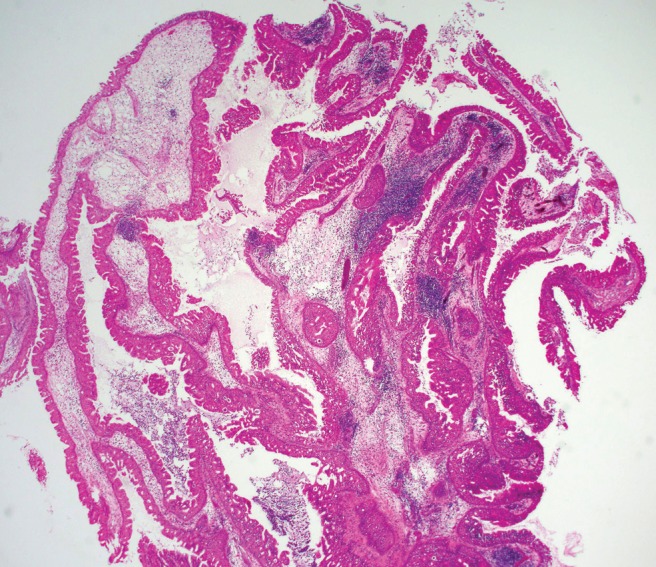
Low-power magnification, hematoxylin and eosin stain showing oncocytic Schneiderian papilloma exhibiting both exophytic and endophytic patterns with several layers of pseudostratified columnar (cylindrical) cells containing uniform small dark round nuclei and eosinophilic cytoplasm.
Figure 3.
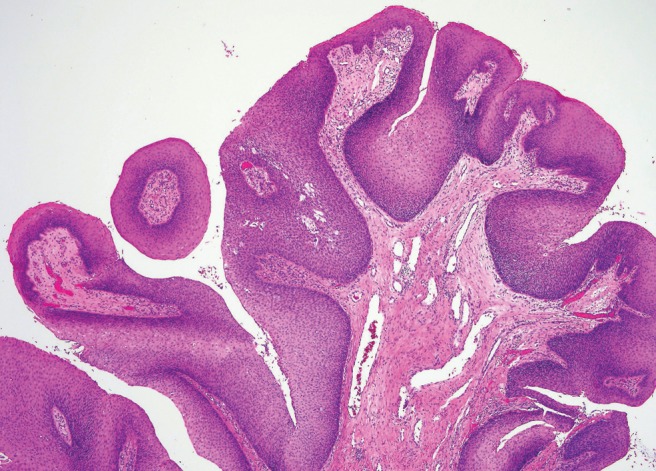
Low-power magnification, hematoxylin and eosin stain depicting exophytic papilloma with branching, exophytic proliferations, and a fibrovascular core, lined by well-differentiated stratified squamous epithelium.
Table 2.
Potential predictors of papilloma subtype
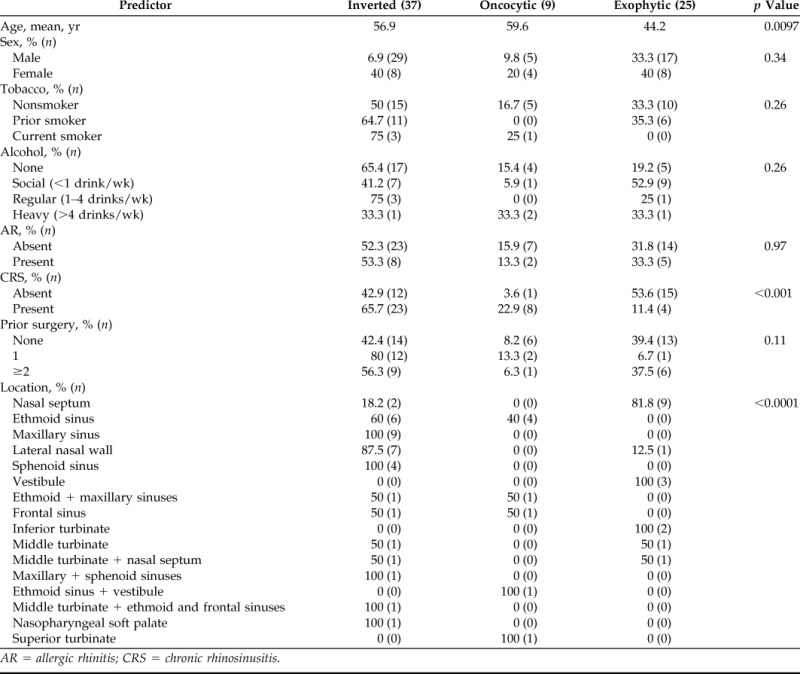
AR = allergic rhinitis; CRS = chronic rhinosinusitis.
In univariate analysis, history of CRS (p < 0.001)), location (p < 0.0001), and age (p = 0.0097) were significantly associated with subtype of papilloma. Thirty-five of the tumors (50%) were associated with CRS as diagnosed on history and physical exam findings on presentation. Three (4%) of the papillomas with CRS also were associated with nasal polyposis (NP). Given the small number of patients with CRS with NP, these were combined with cases of CRS without NP, forming a single group of patients with CRS. Fifty-three percent (15/28) of patients without a history of CRS had an exophytic papilloma on final pathology. For patients with CRS, 66% (23/35) of the papillomas proved to be the inverted subtype (Table 2).
Papillomas arose from 11 possible locations (Table 2). Those papillomas where the specific site of attachment could not be determined from the medical record were excluded (n = 11: 1 oncocytic, 8 exophytic, and 2 inverted). The majority of papillomas were found within the nasal cavity (29/60). The ethmoid (10/60) and maxillary sinuses (9/60) represented commonly involved sites of the paranasal sinuses. All 25 cases of exophytic papillomas were confined to the nasal cavity, with the majority originating from the nasal septum (9/25). One hundred percent (9/9) of papillomas confined to the maxillary sinus were the inverted subtype. Another common subsite within the nasal cavity, the lateral nasal wall, harbored papillomas of the inverted subtype 87.5% (7/8). Within the ethmoid sinuses, papillomas were oncocytic in 40% of cases (4/10) and inverted in 60% of cases (6/10). Papillomas that were seen arising from two or more subsites were given their own classification.
The mean age at presentation of the patients with the oncocytic, exophytic, and inverted subtype were 59.6, 44.2, and 56.9 years, respectively. Patients with the exophytic subtype were younger, presenting in their fourth decade of life (p = 0.0097) and the remaining two subtypes were more likely to occur in older patients.
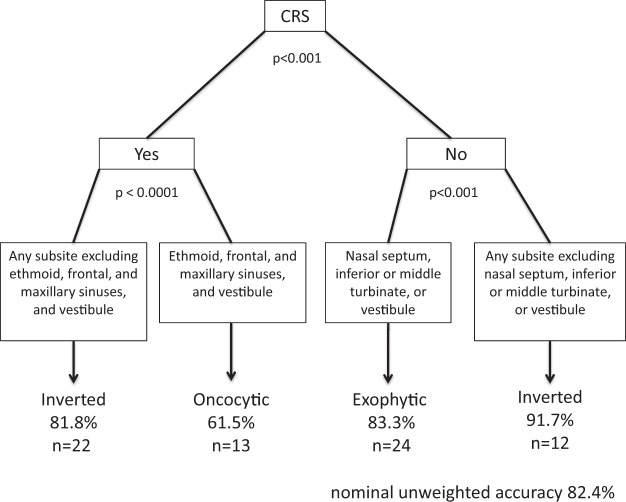
A history of CRS and tumor location were simultaneously significant together on multivariate analysis (Fig. 4). Once these two factors were controlled, the remaining patient characteristics of tobacco use, alcohol use, and age were not significant on multivariate analysis.
Figure 4.
Classification and regression tree (CART) model for multivariate analysis assessing significance of chronic rhinosinusitis (CRS) status and papilloma location in predicting papilloma subtype.
Finally, we used a CART method to predict papilloma type by tumor location. The classification tree initially partitions cases based on the presence or absence of CRS and subsequently divides patients according to location of the papilloma. The resultant predictive algorithm is presented in Fig. 4. (1) Patients with CRS and papillomas arising from any sinonasal site excluding ethmoid, frontal, and maxillary sinuses and vestibule were shown to be the inverted subtype, as they were in 81.8% (p < 0.0001). (2) Patients with CRS whose papilloma is localized to the ethmoid, frontal, or maxillary sinuses or vestibule are predicted to be oncocytic, as 61.5% of these cases were oncocytic (p < 0.0001). (3) Patients without CRS whose papilloma stems from either the nasal septum, the inferior or the middle turbinate and vestibule are predicted to be exophytic, as they were in 83.3% of these cases (p < 0.001). (4) Patients without CRS whose papilloma originates from any sinonasal subsite excluding the nasal septum, inferior or middle turbinate, and vestibule are predicted to be inverted, as they were in 91.7% of these cases (p < 0.001). Using this CART model, papilloma subtype can be predicted based on these two risk factors with a nominal unweighted accuracy of 82.4%.
DISCUSSION
Sinonasal papillomas account for 0.5–4% of all nasal tumors.7 IP and exophytic papillomas are the most commonly diagnosed subtypes, each accounting for almost one-half of all sinonasal papillomas, and oncocytic papillomas are the rarest type, found only in 3–5% of all papillomas.2,8 This trend persisted in this study, with IP and exophytic papillomas comprising 87%, and the oncocyltic (OSP) subtype was seen in only 13% of cases. A 3:1–8:1 male/female predominance exists with exophytic papillomas and IP, whereas OSP have shown no sex predilection.2 However, in this patient population gender did not show a significant relationship to any papilloma subtype. The majority of sinonasal papillomas occurs between the fourth to sixth decades of life, which is corroborated in our study, with younger patients at an average age of 44 years being more likely to have an exophytic papilloma, and patients one decade older were more likely to show one of the remaining two types.1,9–11
Exophytic papillomas almost exclusively arise from the nasal septum with rare cases arising from the vestibule or middle turbinate, which was supported with our data.12 In contrast, IP and OSP generally originate from the lateral nasal wall, ethmoid sinus, maxillary sinus, and, less commonly, from the frontal and sphenoid sinuses or nasal septum.8,9 In this series, the association between papilloma subtype and location proved to be statistically significant on univariate and multivariate analysis. IPs tended to arise from the maxillary, ethmoid, and sphenoid sinuses and the lateral nasal wall. Exophytic papillomas were most common within the nasal cavity on the nasal septum and within the vestibule, and OSP exhibited a predilection for the ethmoid sinuses.
Although there are no pathognomonic findings on radiographic imaging distinguishing each sinonasal papilloma type from inflammatory polyps or chronic sinusitis, certain findings can suggest its presence. CT findings highly suggestive of papilloma include a contiguous nasal cavity and sinus mass with heterogenous contrast enhancement and unilateral sinus opacification.8–10 Thinning or bowing of adjacent bone can be seen with progressive enlargement of this tumor, most commonly at the medial wall of the maxillary sinus and the lamina papyracea. When bony erosion or destruction is seen, associated malignancy must be considered. Focal hyperostosis and osteitis within the opacified sinus has been shown to predict the site of origin of IP on both retrospective and prospective analysis; however, this finding is generally not seen with the oncocytic and exophytic types.7,9,13 When this was studied for predictive value in prospective analysis, focal osteitis on preoperative CT of the paranasal sinuses predicted the correct site of origin of the IP in 95% of cases.13 The mechanism by which IP causes this bony reaction may possibly be secondary to the secretion of bone morphogenic peptide tumor cells.14 Although focal osteitis would have been another useful factor to investigate for its ability to discern papilloma subtypes, the majority of our cases in our retrospective review did not have scans available to include in this review. MRI can be useful in defining the extent of the tumor and differentiating the tumor mass from inspissated mucus and for detecting intracranial and intraorbital extension of tumor.
The exact pathogenesis of papillomas has not yet been delineated. The human papilloma virus (HPV) types 6, 11, 16, and 18 have been implicated as a leading factor in the development of IP and exophytic papillomas, although HPV does not seem to be related to the formation of OSP.1,9–11 HPV-6 and -11 were positive in the two patients that were subtyped with exophytic papillomas. However, the mere presence of HPV may not be enough to support the viral etiology of these papillomas.15 Chronic inflammation and CRS have also been hypothesized as a precursor for the development of sinonasal papillomas.11,12,15 In our series, 62% of cases were associated with CRS; however, the exact nature of this relationship between papillomas and CRS may be simply associative rather than causative.
In this study, a variety of patient and surgical characteristics were analyzed to identify risk factors of each papilloma subtype. On univariate analysis of a series of 70 patients, age, history of CRS, and originating subsite were found to be statistically significant predictors of papilloma subtype. This significant predictive value was upheld for CRS history and originating subsite on multivariate analysis, providing not only clues on how to predict papilloma subtype but a proposed diagnostic algorithm (Fig. 4). If information regarding CRS history and site of papilloma origin within the paranasal sinuses are known, the papilloma subtype can potentially be predicted before surgery. This would carry great clinical efficacy for the rhinologist planning excision. Although final pathological analysis is the gold standard by which to classify papillomas, having the ability to possibly deduce the papilloma subtype would enable better surgical planning and preoperative counseling.
Presence and absence of CRS were found to be a significant risk factor in both univariate and multivariate analysis for oncocytic and IP subtypes in specific locations of the paranasal sinuses (Fig. 4). Other potential risk factors, including alcohol use, tobacco use, and history of prior sinus surgery, failed to show a significant association with any subtype of papilloma. It should be noted that a sample size of only nine oncocytic patients limits the ability to detect or confirm univariate and multivariate associations. Some associations not seen, such as the lack of association with alcohol or tobacco, may be an artifact of this limited sample size.
Transformation of sinonasal papillomas into malignancy has been described in IP and oncocytic papillomas, but not in exophytic papillomas.10 Five to 15% of cases of IP can be associated with malignancy and 4–17% of cases of oncocytic papillomas have been associated with malignancy.2,16,17 Malignant transformation can occur at the time of diagnosis or develop later at the site of previous resection.9 In this series, all cases of dysplasia or malignancy were seen with IP, and of these, all were diagnosed at the initial resection. The predominant malignancy associated with IP and oncocytic papillomas is squamous cell carcinoma, although verrucous carcinoma and adenocarcinoma have also been reported with IP. In contrast, mucoepidermoid carcinoma, epidermoid carcinoma, and sinonasal undifferentiated carcinomas have been seen with oncocytic papilloma.8,9,16 The etiology of carcinoma development is controversial, with theories attributing malignant transformation to HPV-16 and -18. Some suggest that infection with HPV may occur early in the process of tumorigenesis with several other insults required to transform benign IP to dysplasia or malignancy.9 Another proposed theory suggests that differential expression of certain cell cycle proteins may significantly contribute to this transformation process.18 Recurrences or atypical features do not seem to be related to the carcinomatous transformation.15
The histopathological examination is needed to differentiate each of these papillomas. On gross analysis, IP usually appears large, firm, and gray in color with a multinodular, polypoid, uneven surface.9 Histologically, IP shows markedly thick inverted or endophytic growth of nonkeratinizing transitional cells (Fig. 1). The thick epithelium undergoes squamous maturation and inverts into the stroma with a distinct basement membrane that separates the epithelium from the underlying connective tissue stroma. Surface keratinization and a granular cell layer are uncommon. Numerous intraepithelial microcysts containing cell debris, macrophages, and mucin are present.
In contrast, oncocytic papillomas appear as soft, fleshy pink papillary tissue. On histological evaluation, these papillomas exhibit both exophytic and endophytic patterns and have several layers of pseudostratified columnar (cylindrical) cells with uniform small dark round nuclei, eosinophilic cytoplasm, and minimal epidermoid component (Fig. 2).8,19 Similar to IP, numerous intraepithelial mucus-containing microcysts can be seen within the epithelium; however, the diffuse oncocytic nature of the epithelium distinguishes OSP from the other sinonasal papillomas.8,20 OSP must be differentiated from well-differentiated adenocarcinoma by the presence of stratified epithelium, intact basement membranes, and the absence of nuclear pleomorphism, mitotic activity, and bony destruction.
Exophytic papillomas grossly appear as gray–tan, exophytic, “mushroom-shaped” verrucous papillary proliferations classically arising from the anterior nasal septum attached to the underlying mucosa by a narrow stalk.7,10,19 Histologically, exophytic papillomas have a cellular composition like IP with branching, exophytic proliferations with a fibrovascular core lined by well-differentiated stratified squamous epithelium (Fig. 3). Unlike IP, keratin formation can be present in the surface epithelium, seen as hyper- and parakeratosis, and chronic inflammatory cells are uncommon. Koilocytosis may be seen in the superficial cells.
Treatment of all sinonasal papillomas includes complete surgical excision; however, in practice, feasibility of achieving this depends on the extent of disease, available technology, and the comfort and skill of the surgeon. Although external approaches were once exclusively used, endoscopic resection has been gaining popularity because of decreased morbidity without compromising recurrence rates. Ultimately, the type of approach depends on the site of tumor attachment and extension.9,20–24 The site of tumor attachment is crucial to identify to ensure complete resection, which involves complete removal of the affected mucosa and mucoperiosteum, because incomplete or limited resections are thought to be the leading cause of recurrence.25 Additionally, complete resection allows pathological analysis to rule out a synchronous malignant transformation.25 Microscopic foci of residual papilloma can be concealed within the bone at the attachment site. Therefore, drilling the bone at the site of attachment can further reduce the risk of recurrence.26 These practices typically apply to IP and oncocytic types, which appear to behave similarly in terms of local destruction and malignant transformation, sparing the exophytic type.17
Radiation therapy is generally not recommended for the treatment of sinonasal papillomas unless associated with malignancy, although some reports suggest the use of radiation therapy for locally advanced papillomas or papillomas with multiple recurrences.8,22 In the case of an IP, another goal of surgery is the creation of cavity that allows for easy endoscopic surveillance.
Recurrence after surgical resection varies widely in the literature and usually suggests incomplete resection.1,23 Five to 60% of IP cases can recur, and 25–35% of OSP cases can recur within 5 years of resection.8,9,16,17 Although much less common, recurrences can occur with exophytic papillomas in up to 22%.2,10 Several factors have been shown to increase the risk of recurrence for IP including tobacco exposure, increased tumor size, increased hyperkeratosis, squamous epithelial hyperplasia, increased number of mitoses, bilaterality, and the lack of inflammatory polyps.1,9,27–29 In addition, IP originating from the frontal sinus tend to have multiple recurrences, likely because of technical difficulties operating in this location.9,28 Most recurrences usually occur within the first 3 years, although recurrences 10 years postoperatively have been reported.9,23 Long-term follow-up is recommended given that recurrences can occur after prolonged periods of time.
CONCLUSION
Sinonasal papillomas are benign epithelial neoplasms arising from Schneiderian mucosa. Although the incidence of IP is much higher compared with the other papilloma subtypes, oncocytic and exophytic papillomas must be recognized and differentiated. Age, history of CRS, and papilloma location were found to be statistically significant predictors for papilloma subtype.
ACKNOWLEDGMENTS
Jeffrey Gornbein, Dr.P.H., from the UCLA Department of Biomathematics contributed to the review of the statistical analysis and presentation of the data.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Batsakis JG, Suarez P. Schneiderian papillomas and carcinomas: A review. Adv Anat Pathol 8:53–64, 2001. [DOI] [PubMed] [Google Scholar]
- 2. Cheng T-Y, Ueng S-H, Chen Y-L, et al. Oncocytic Schneiderian papilloma found in a recurrent chronic paranasal sinusitis. Chang Gung Med J 29:336–341, 2006. [PubMed] [Google Scholar]
- 3. Ward N. A mirror of the practice of medicine and surgery in the hospitals of London. London Hosp Lancet 2:480–482, 1854. [Google Scholar]
- 4. Ringertz N. Pathology of malignant tumors arising in the nasal and paranasal cavities and maxilla. Acta Otolaryngol (Stockh) 27(suppl):31–42, 1938. [Google Scholar]
- 5. Shanmugaratnam K, Sobin LH. The World Health Organization histological classification of tumours of the upper respiratory tract and ear. A commentary on the second edition. Cancer 71:2689–2697, 1993. [DOI] [PubMed] [Google Scholar]
- 6. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: Adult sinusitis. Otolaryngol Head Neck Surg 137(suppl):S1–S31, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Bawa R, Allen GC, Ramadan HH. Cylindrical cell papilloma of the nasal septum. Ear Nose Throat J 74:179–181, 1995. [PubMed] [Google Scholar]
- 8. Barnes L, Bedetti C. Oncocytic Schneiderian papilloma: A reappraisal of cylindrical cell papilloma of the sinonasal tract. Hum Pathol 15:344–351, 1984. [DOI] [PubMed] [Google Scholar]
- 9. Anari S, Carrie S. Sinonasal inverted papilloma: Narrative review. J Laryngol Otol 124:705–715, 2010. [DOI] [PubMed] [Google Scholar]
- 10. Perez-Ordoñez B. Hamartomas, papillomas and adenocarcinomas of the sinonasal tract and nasopharynx. J Clin Pathol 62:1085–1095, 2009. [DOI] [PubMed] [Google Scholar]
- 11. Wood JW, Casiano RR. Inverted papillomas and benign nonneoplastic lesions of the nasal cavity. Am J Rhinol Allergy 26:157–163, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Orlandi RR, Rubin A, Terrell JE, et al. Sinus inflammation associated with contralateral inverted papilloma. Am J Rhinol 16:91–95, 2002. [PubMed] [Google Scholar]
- 13. Bhalla RK, Wright ED. Predicting the site of attachment of sinonasal inverted papilloma. Rhinology 47:345–348, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Okamoto T, Kodama S, Nomi N, et al. Expression of bone morphogenic protein in sinonasal inverted papilloma with new bone formation. Allergy Rhinol (Providence) 2:16–20, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Melroy CT, Senior BA. Benign sinonasal neoplasms: A focus on inverting papilloma. Otolaryngol Clin North Am 39:601–617, x, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Chrysovergis A, Paschalidis J, Michaels L, Bibas A. Nasopharyngeal cylindrical cell papilloma. J Laryngol Otol 125:86–88, 2011. [DOI] [PubMed] [Google Scholar]
- 17. Kaufman MR, Brandwein MS, Lawson W. Sinonasal papillomas: Clinicopathologic review of 40 patients with inverted and oncocytic Schneiderian papillomas. Laryngoscope 112:1372–1377, 2002. [DOI] [PubMed] [Google Scholar]
- 18. Kim S, Lee OY, Choi J, et al. Pattern of expression of cell cycle-related proteins in malignant transformation of sinonasal inverted papilloma. Am J Rhinol Allergy 25:75–81, 2011. [DOI] [PubMed] [Google Scholar]
- 19. Hyams VJ. Papillomas of the nasal cavity and paranasal sinuses: A clinicopathological study of 315 cases. Ann Otol Rhinol Laryngol 80:192–206, 1971. [DOI] [PubMed] [Google Scholar]
- 20. Kapadia SB, Barnes L, Pelzman K, et al. Carcinoma ex oncocytic Schneiderian (cylindrical cell) papilloma. Am J Otolaryngol 14:332–338, 1993. [DOI] [PubMed] [Google Scholar]
- 21. Busquets JM, Hwang PH. Endoscopic resection of sinonasal inverted papilloma: A meta-analysis. Otolaryngol Head Neck Surg 134:476–482, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Lawson W, Ho BT, Shaari CM, Biller HF. Inverted papilloma: A report of 112 cases. Laryngoscope 105:282–288, 1995. [DOI] [PubMed] [Google Scholar]
- 23. Mirza S, Bradley PJ, Acharya A, et al. Sinonasal inverted papillomas: Recurrence, and synchronous and metachronous malignancy. J Laryngol Otol 121:857–864, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Vrabec DP. The inverted Schneiderian papilloma: A 25-year study. Laryngoscope 104:582–605, 1994. [DOI] [PubMed] [Google Scholar]
- 25. Lund VJ, Stammberger H, Nicolai P, et al. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol Suppl 22:1–143, 2010. [PubMed] [Google Scholar]
- 26. Chiu AG, Jackman AH, Antunes MB, et al. Radiographic and histologic analysis of the bone underlying inverted papillomas. Laryngoscope 116:1617–1620, 2006. [DOI] [PubMed] [Google Scholar]
- 27. Wormald PJ, Ooi E, van Hasselt CA, Nair S. Endoscopic removal of sinonasal inverted papilloma including endoscopic medial maxillectomy. Laryngoscope 113:867–873, 2003. [DOI] [PubMed] [Google Scholar]
- 28. Katori H, Nozawa A, Tsukuda M. Histopathological parameters of recurrence and malignant transformation in sinonasal inverted papilloma. Acta Otolaryngol 126:214–218, 2006. [DOI] [PubMed] [Google Scholar]
- 29. Moon IJ, Lee DY, Suh MW, et al. Cigarette smoking increases risk of recurrence for sinonasal inverted papilloma. Am J Rhinol Allergy 24:325–329, 2010. [DOI] [PubMed] [Google Scholar]