Version Changes
Updated. Changes from Version 1
We would like to thank both reviewers for their time and constructive comments on the first version of our manuscript. In response to Dr. Stefan Toegel’s review, we have included detailed information on the number of biological and technical replicates used in the revised figure legends. The revised Materials and methods section now clarifies the fact that curcumin and IL-1β were added simultaneously to the cells and explants. Curcumin was not used to “pretreat” the cells/explants prior to the addition of IL-1β. Dr. Toegel makes a very good point about the secretion of MMP-3 in the secretome of cartilage explants. However, this paper highlights the fact that curcumin is effective and non-cytotoxic at 25 micromolar. We did not include a reference protein in the western blots, as there are no proteins that can effectively be used as reference proteins in the secretome. However, we did perform a protein assay to ensure that equal quantities of protein were loaded on the polyacrylamide gels before western blotting. Finally, we did not check the Gaussian distribution of the data before applying ANOVA statistics. We are equally grateful to Dr. Oliver Grundmann for his comments about the design of our research paper. In response to Dr. Grundmann’s comments we have added some additional points to clarify the research for the readers. In response to both reviewers, we have included the number of technical and biological replicates used. At least 3 biological replicates were used in each experiment and some assays included multiple technical replicates. We have also addressed issues raised in relation to proteoglycan release assays, curcumin cytotoxicity and bioavailability.
Abstract
Objective: Curcumin (diferuloylmethane) is a phytochemical with potent anti-inflammatory and anti-oxidant properties, and has therapeutic potential for the treatment of a range of inflammatory diseases, including osteoarthritis (OA). The aim of this study was to determine whether non-toxic concentrations of curcumin can reduce interleukin-1beta (IL-1β)-stimulated inflammation and catabolism in an explant model of cartilage inflammation.
Methods: Articular cartilage explants and primary chondrocytes were obtained from equine metacarpophalangeal joints. Curcumin was added to monolayer cultured primary chondrocytes and cartilage explants in concentrations ranging from 3μM-100μM. Prostaglandin E 2 (PGE 2) and matrix metalloproteinase (MMP)-3 release into the secretome of IL-1β-stimulated explants was measured using a competitive ELISA and western blotting respectively. Proteoglycan (PG) release in the secretome was measured using the 1,9-dimethylmethylene blue (DMMB) assay. Cytotoxicity was assessed with a live/dead assay in monolayer cultures after 24 hours, 48 hours and five days, and in explants after five days.
Results: Curcumin induced chondrocyte death in primary cultures (50μM p<0.001 and 100μM p<0.001) after 24 hours. After 48 hours and five days, curcumin (≥25μM) significantly increased cell death ( p<0.001 both time points). In explants, curcumin toxicity was not observed at concentrations up to and including 25μM after five days. Curcumin (≥3μM) significantly reduced IL-1β-stimulated PG ( p<0.05) and PGE 2 release ( p<0.001) from explants, whilst curcumin (≥12μM) significantly reduced MMP-3 release ( p<0.01).
Conclusion: Non-cytotoxic concentrations of curcumin exert anti-catabolic and anti-inflammatory effects in cartilage explants.
Introduction
Osteoarthritis (OA) involves destruction of articular cartilage by a combination of mechanical injury, inflammatory mediators and proteolytic enzyme activity 1. The high cost and potential negative side effects of conventional pharmacotherapy, i.e. non-steroidal anti-inflammatory drugs (NSAIDs), has stimulated interest in natural plant products with anti-inflammatory properties, as an alternative or adjunct to conventional therapy 2. These products are being investigated for potential efficacy in a wide range of disorders with an inflammatory component, including arthritis and cancer 3, 4.
Curcumin (diferuloylmethane) is a polyphenol found in turmeric derived from the rhizomes of Curcuma longa. Curcumin is traditionally known for its powerful anti-inflammatory and anti-oxidant properties. At concentrations between 50 and 100μM it has been shown to have anti-inflammatory properties via its suppressive effects on I kappa B kinase (IKK) activity and consequently the nuclear factor-kappa B (NF-κB) signaling pathway in various cell types, including chondrocytes 5, 6. However, published work suggests that curcumin is cytotoxic to both primary chondrocytes 7 and transformed chondrocyte cell lines 8 at 50μM and above. Primary cells in their initial passages can be more phenotypically and genotypically relevant than transformed cells and are more applicable to the clinical setting 9. Therefore, the cytotoxicity observed in chondrocyte cell lines may be a consequence of the transformation induced by the SV-40 virus. The hypothesis to be tested in this study was that curcumin exerts anti-inflammatory and anti-catabolic effects on interleukin-1beta (IL-1β)-stimulated cartilage explants at non-cytotoxic concentrations. Accordingly, we evaluated the concentrations at which a commercially available curcumin formulation (sourced from Sigma-Aldrich) was cytotoxic to primary equine chondrocytes in both monolayer and explant cultures and determined whether non-toxic concentrations could reduce proteoglycan (PG) loss and inflammatory mediator production in an in vitro model of early OA.
Materials and methods
Tissues
Macroscopically normal articular cartilage samples were obtained from weight-bearing regions of the metacarpophalangeal joints of eleven horses of mixed breed, age and sex. The joint tissues were sourced from UK-based abattoirs. The joint tissues were sourced from UK-based abattoirs and veterinary practices. Animals were euthanized for non-research purposes either in accordance with Welfare of Animals (Slaughter or Killing) Regulations 1995 or the Veterinary Surgeons Act with owner consent. Approval for the use of clinical materials was obtained from the local Ethical Review Committee. Full depth cartilage from six animals was taken for explant culture and thin cartilage shavings were used for chondrocyte isolation from the remaining five animals. Cartilage samples from each animal were kept separate throughout. Cartilage shavings were aseptically harvested into low glucose Dulbecco’s modified Eagle’s medium (DMEM; HyClone, Thermo Fisher Scientific, Loughborough, UK) containing 4% penicillin/streptomycin (Sigma-Aldrich, Gillingham, UK) before washing in phosphate-buffered saline (PBS) containing 10% penicillin/streptomycin (Sigma-Aldrich, Gillingham, UK) for 20 minutes.
Chondrocyte isolation and culture
Thin cartilage slices were digested overnight in 0.1% collagenase type I (Sigma-Aldrich, Gillingham, UK) at 37°C and 5% CO 2. The resulting cell suspension was filtered and washed before undergoing first expansion in low glucose DMEM with 2% penicillin/streptomycin and 10% fetal bovine serum (FBS). Once confluency was reached, cells were passaged into 12-well plates. Only first and second passage confluent cells were used in this study.
Experimental design–monolayer cultures
Culture media was removed and replaced with treatment media (1ml/well). Control wells contained media alone (low glucose DMEM with 2% penicillin/streptomycin and 10% FBS), which formed the base for the other treatments. The NSAID carprofen (100μg/ml; Rimadyl ®, Pfizer, Sandwich, UK) was included as a positive control, due to its anti-inflammatory effects on chondrocytes 10. The nitric oxide donor, sodium nitroprusside (SNP; Sigma-Aldrich, Gillingham, UK) dissolved in DMEM (50mM) was used as a positive control for inducing cell death 11. Stock solutions of curcumin (100mM; C1386, Sigma-Aldrich, Gillingham, UK) were prepared in cell culture grade dimethyl sulfoxide (DMSO; Sigma-Aldrich, Gillingham, UK) and diluted in DMEM to 1mM. From this 1mM stock, experimental concentrations of curcumin (3μM, 6μM, 12μM, 25μM, 50μM and 100μM) were prepared in DMEM and added to the appropriate wells. A DMSO control containing a volume equivalent to that found in the highest curcumin concentration was included on each plate to ensure that any observed effects were not due to the carrier solvent. Curcumin was not used to pretreat the cells and explants prior to the addition of IL-1β. Curcumin and IL-1β were added simultaneously to the cultures. The plates were incubated at 37°C and 5% CO 2. Cytotoxicity was assessed after 24 hours, 48 hours and five days.
Cytotoxicity assays–monolayer chondrocytes
Chondrocyte viability was assessed using a commercially available live/dead assay (Invitrogen, Paisley, Scotland, UK) that utilizes calcein AM and ethidium homodimer-1 to identify live and dead cells, respectively. Media was removed and centrifuged (Eppendorf, Eppendorf rotor) at 10,000 rpm for 25 seconds at room temperature. The resulting pellet of detached cells (if any) was washed and resuspended in 20μl PBS. Adherent cells in the wells were washed in PBS before adding 20μl of the detached cell suspension to the appropriate wells and incubating in calcein AM (2μM) and ethidium homodimer-1 (4μM) in PBS (Sigma-Aldrich, Gillingham, UK) for 30 minutes at room temperature. Fluorescence was detected and captured using an inverted contrasting microscope (Leica DM IL, Leica Microsystems Ltd, Wetzlar, Germany) with Leica Application Suite imaging software (Version 2.4.0 R1, Leica Microsystems Ltd). Six random fields of view of live and dead cells were taken per well (magnification×100). Live and dead cells were counted with ImageJ Software (National Institutes of Health, Bethesda, MD) and the percentage of dead cells (expressed as a percentage of the total number of cells present) was calculated at 24 hours, 48 hours and five days for each treatment.
Cartilage explant culture
Full depth cartilage shavings were cut into 3mm discs. Three discs per well from the same animal were placed in 24-well plates containing 1ml of culture medium (serum-free low glucose DMEM supplemented with 2% penicillin/streptomycin) and allowed to equilibrate overnight at 37°C under 5% CO 2. The following day, culture media was replaced with fresh media before the experiment began.
Experimental design–cartilage explants
Cartilage from three animals was used for curcumin viability studies, and cartilage from three different animals was used for the remaining studies. All plates contained 1ml culture media, which formed the base for other treatments and acted as a control for each plate. Explant viability studies were performed by adding curcumin (12μM, 25μM and 100μM) prepared in DMEM as described above to the appropriate wells (one well per animal per treatment). SNP (50mM) was used as a positive control for cell death. The remaining 3 wells per animal per treatment were used to determine PG release from unstimulated explants in response to curcumin.
Experiments aimed at assessing the anti-catabolic and anti-inflammatory effects of curcumin were conducted by incubating explants in culture media containing recombinant equine IL-1β (R&D Systems, Abingdon, UK) (10ng/ml) and various concentrations of curcumin (3μM, 6μM, 12μM, 25μM and 50μM) prepared in IL-1β-treated media. Carprofen (100μg/ml) was prepared in IL-1β-treated media and included as a positive control. DMSO controls were performed previously and found to have no effect on PG release from both IL-1β-stimulated and unstimulated cartilage explants at volumes equivalent to that found in the highest curcumin concentration (data not shown). Plates were incubated at 37°C and 5% CO 2 for five days. After five days, explants were immediately assayed for cytotoxicity or frozen at -20°C with their corresponding supernatants for subsequent secretome assays.
Cytotoxicity assays–explant cultures
After five days, chondrocyte viability was assessed using the live/dead assay. Explants were washed in PBS then incubated with calcein AM (2μM) and ethidium homodimer-1 (8μM) in PBS for 30 minutes at room temperature. A confocal microscope (Leica TC SP2) was used to detect and measure fluorescence in 15μm z-sections though each explant (magnification×10).
DMMB assays
For evaluation of matrix PG release we used the metachromatic dye 1,9 dimethylmethylene blue (DMMB) to quantify the amount of sulfated glycosaminoglycans (GAGs) into the medium. Cartilage discs were digested in papain (Sigma-Aldrich, Gillingham, UK) for 16 hours. Papain-digested cartilage and their corresponding supernatants were assayed in 96-well plates using the DMMB (Sigma-Aldrich, Gillingham, UK) method as previously described 12. Shark chondroitin sulfate (Sigma-Aldrich, Gillingham, UK) was used as a standard (0–70μg). DMMB solution (200µl) was added to samples and standards (40μl). The plate was read (Multiskan Ascent, Thermo Labsystems, Basingstoke, UK) using Ascent Software (version 2.6, Thermo Labsystems, Basingstoke, UK). Total PG release was obtained from a spectrophotometric reading of the digested cartilage and its corresponding supernatant at 540nm. Percentage of PG release from the total PG content of the explants was calculated by dividing the supernatant value from the total PG release for each well.
Prostaglandin E 2 (PGE 2) immunoassay
A competitive immunoassay kit (R&D Systems, Abingdon, UK) was used to measure PGE 2 release according to the manufacturer’s instructions. Standards (19.6–1250pg/ml), supernatant samples and reagents were added to a 96-well plate coated in goat anti-mouse polyclonal antibody and incubated for 19 hours at 6°C. The plate was washed and developed with 200μl substrate solution per well in the dark at room temperature for 20 minutes. A stop solution was added and the plate was read immediately (Multiskan Ascent, Thermo Labsystems, Basingstoke, UK) at 450nm with wavelength correction set at 540nm using Ascent Software (version 2.6, Thermo Labsystems, Basingstoke, UK).
Western blot analysis of MMP-3 release
The protein content of explant supernatants from the PG and PGE 2 assays was quantified and aliquots containing 50µg protein were freeze-dried overnight. The resultant pellets were resuspended in 37µl sample buffer (NuPAGE Lithium dodecyl sulfate sample buffer (4×) and electrophoresed on precast 4–12% Bis-Tris 10-well gels (Invitrogen, Paisley, Scotland, UK) under denaturing and reducing conditions. Proteins were transferred to 0.45µm polyvinylidene fluoride (PVDF) membranes (GE Healthcare, Little Chalfont, UK) and blocked with 5% (w/v) non-fat milk with Tris-Buffered saline (TBS) containing 0.1% (v/v) Tween20 for 1 hour. Membranes were incubated with a goat polyclonal antibody to matrix metalloproteinase 3 (stromelysin) (MMP-3; Abcam, Cambridge, UK) diluted 1:1,000 in 5% (w/v) non-fat milk at 4°C overnight. After washing, membranes were incubated for two hours at room temperature with a secondary anti-goat antibody (1:10,000; Dako, Cambridge, UK). Membranes were washed and chemiluminescence detected using ECL+ on a Typhoon Trio+ Variable Mode Imager (both GE Healthcare, Little Chalfont, UK). Densitometric quantification of MMP-3 bands was performed using ImageJ software. Relative band intensity in comparison to controls was measured for samples from each animal.
Statistical analysis
Data were statistically analyzed using a one-way analysis of variance (ANOVA) with Tukey’s post hoc test (GraphPad InStat, version 3.05, GraphPad Software Inc., La Jolla, CA). Statistical significance was set at p<0.05. Graphs were plotted with GraphPad Prism (version 4, GraphPad Software Inc).
For chondrocyte viability quantification, results are expressed as the mean number of dead cells per field of view per treatment ± standard error of the mean (SEM). PG release percentage and PGE 2 values are reported as means of combined animals ± SEM. For MMP-3 quantification analysis, relative intensity values were reported as means of 3 combined animals ± SEM.
Results
Curcumin is cytotoxic to primary chondrocytes at 25μm and above
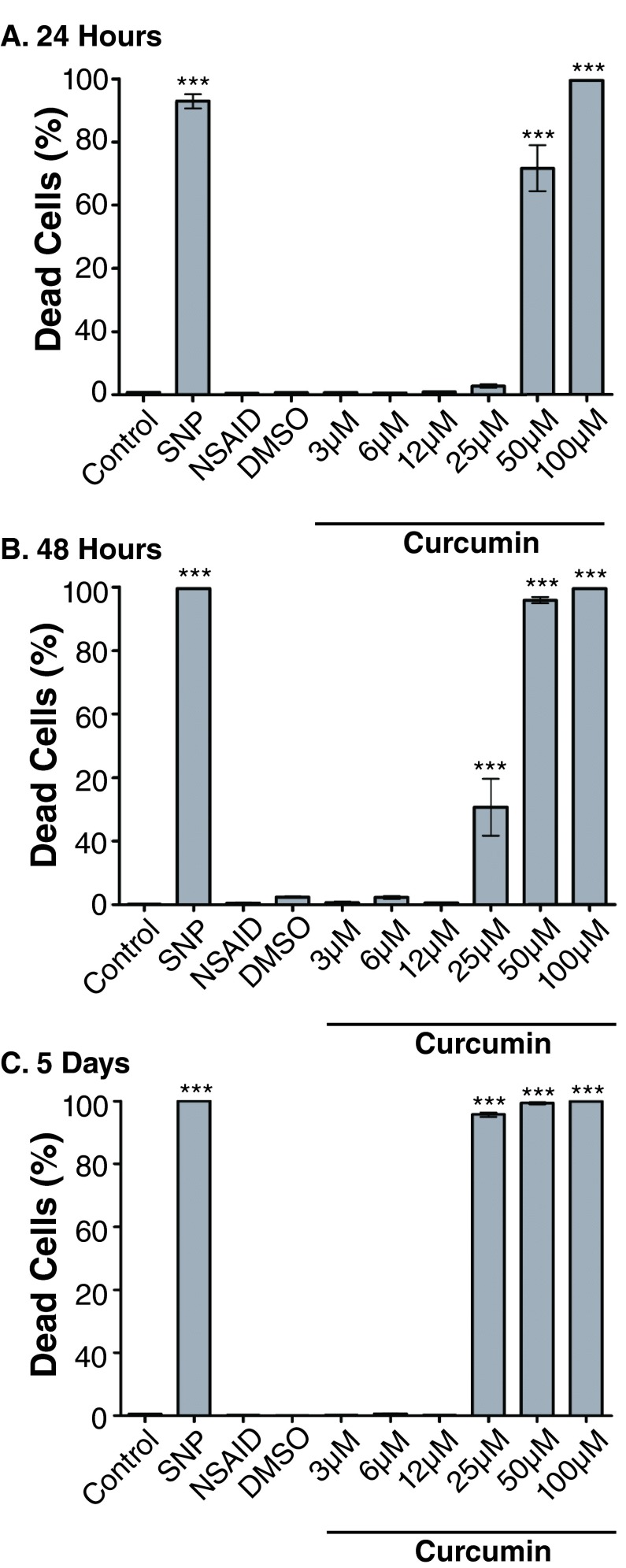
Untreated controls retained a mean cell death percentage of less than 1% at 24 hours, 48 hours and five days ( Figure 1). DMSO controls and the NSAID, carprofen, did not significantly increase cell death compared to controls at all time points. SNP effectively induced cell death ( p<0.001, all time points) with mean cell death of 92.95 ± 2.29% at 24 hours, 99.6 ± 0.17% at 48 hours and 100 ± 0.00% at five days. Curcumin significantly increased cell death compared to controls after 24 hours at 50μM (71.75 ± 7.25%, p<0.001) and 100μM (99.55 ± 0.12%, p<0.001). After 48 hours a significant increase in toxicity compared to controls was seen at 25μM (30.67 ± 8.94%, p<0.001), 50μM (95.9 ± 0.96%, p<0.001) and 100μM (99.59 ± 0.18%, p<0.001). After five days, curcumin (25μM) caused significant increases in cell death (95.71 ± 0.72%, p<0.001), 50μM (99.4 ± 0.39%, p<0.001) and 100μM (100 ± 0.00%, p<0.001).
Figure 1.
Curcumin significantly increases chondrocyte death after 24 hours ( A) at 50μM and 100μM compared to control indicated by *** ( p<0.001). After 48 hours ( B) and five days ( C), curcumin (25μM) significantly increases chondrocyte death compared to controls. The nitric oxide donor, sodium nitroprusside (SNP) (50mM) also significantly increases chondrocyte death at 24 hours, 48 hours, and five days ( p<0.001) compared to control. DMSO at concentrations found in the 100μM curcumin treatment has no effect on chondrocyte death. Results are expressed as the mean number of dead cells per field of view per treatment ± SEM. Data presented are from 5 different animals with 2 technical replicates per animal.
Curcumin is not cytotoxic to cartilage explant chondrocytes at 25μm after five days
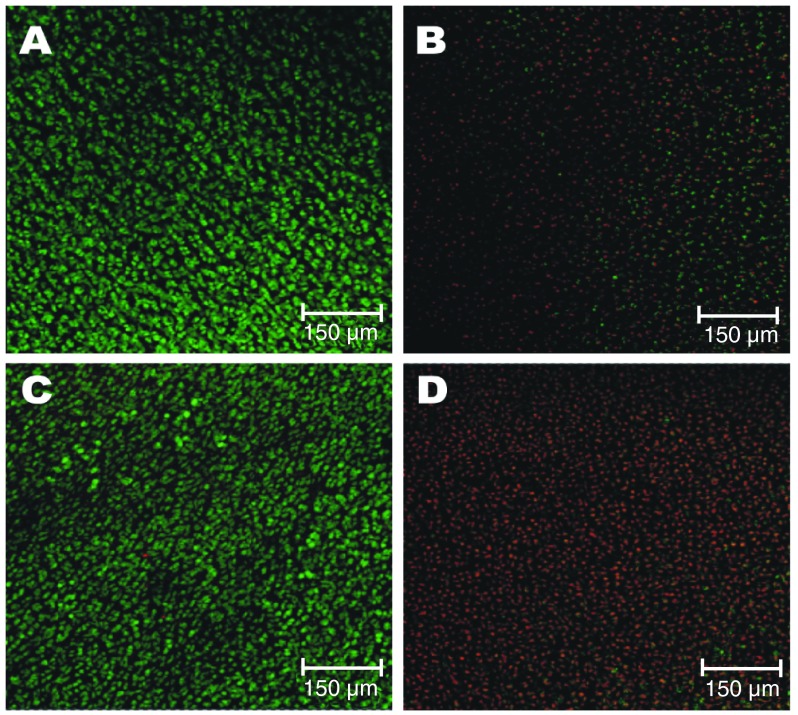
After five days in culture without serum supplementation, explants retained fully viable chondrocytes as indicated by the abundant green staining and lack of red staining in the controls ( Figure 2). SNP induced cell death in the explants as shown by the increased red staining and fewer, less vibrant green stained cells in comparison to the controls. Curcumin (25μM)-treated explants retained large numbers of green stained cells, showing no detriment to the viability of chondrocytes within cartilage explants after five days in culture. However, the large amount of red nuclei staining to the chondrocytes in the 100μM curcumin-treated explants indicates that curcumin was highly cytotoxic at this concentration.
Figure 2. Three-dimensional confocal reconstructions of z stacks through cartilage explants (15μm sections) after five days in culture.
Control ( A) consists of culture media with 2% penicillin/streptomycin. Sodium nitroprusside (SNP) (50mM) ( B) is a positive control for cell death. Treatments consist of curcumin in culture media at 25μM ( C) and 100μM ( D). Images are representative of explants in the treatments wells for each animal. Green staining indicates live metabolizing cells and red staining highlights the nuclei of dead cells. Data are from 3 different animals, with one technical replicate per animal.
Curcumin does not influence PG release from unstimulated cartilage explants
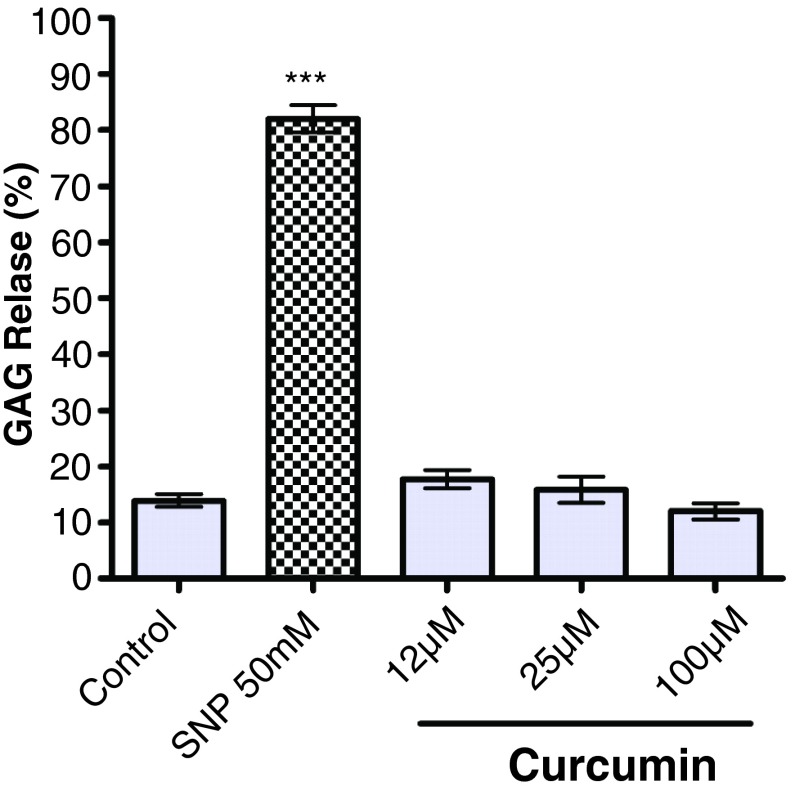
Control explants released 13.91 ± 1.13% of the total PG content of the cartilage into the media over five days (range: 159–414μg PG/ml of culture supernatant) ( Figure 3). SNP (50mM) significantly increased matrix PG release to 82.01 ± 2.43% ( p<0.001) after the same period. Curcumin did not significantly alter PG release from the explants after five days at 12μM, 25μM and 100μM.
Figure 3. Effect of curcumin on proteoglycan (PG) release from unstimulated cartilage explants.
Control column indicates cartilage discs incubated in the culture medium alone. Values are reported as the mean of three animals per treatment ± SEM. Data are from 3 different animals, with three technical replicates per animal. Significance compared to control is indicated by *** ( p<0.001). Sodium nitroprusside (SNP) (50mM) significantly increases PG release compared to control ( p<0.001). Curcumin does not alter PG release from the explants compared to control at concentrations of 12µM, 25µM or 50µM. PG loss is expressed as sulfated glycosaminoglycan (GAG) release.
Curcumin significantly suppresses IL-1β-stimulated PG and PGE 2 release in cartilage explants
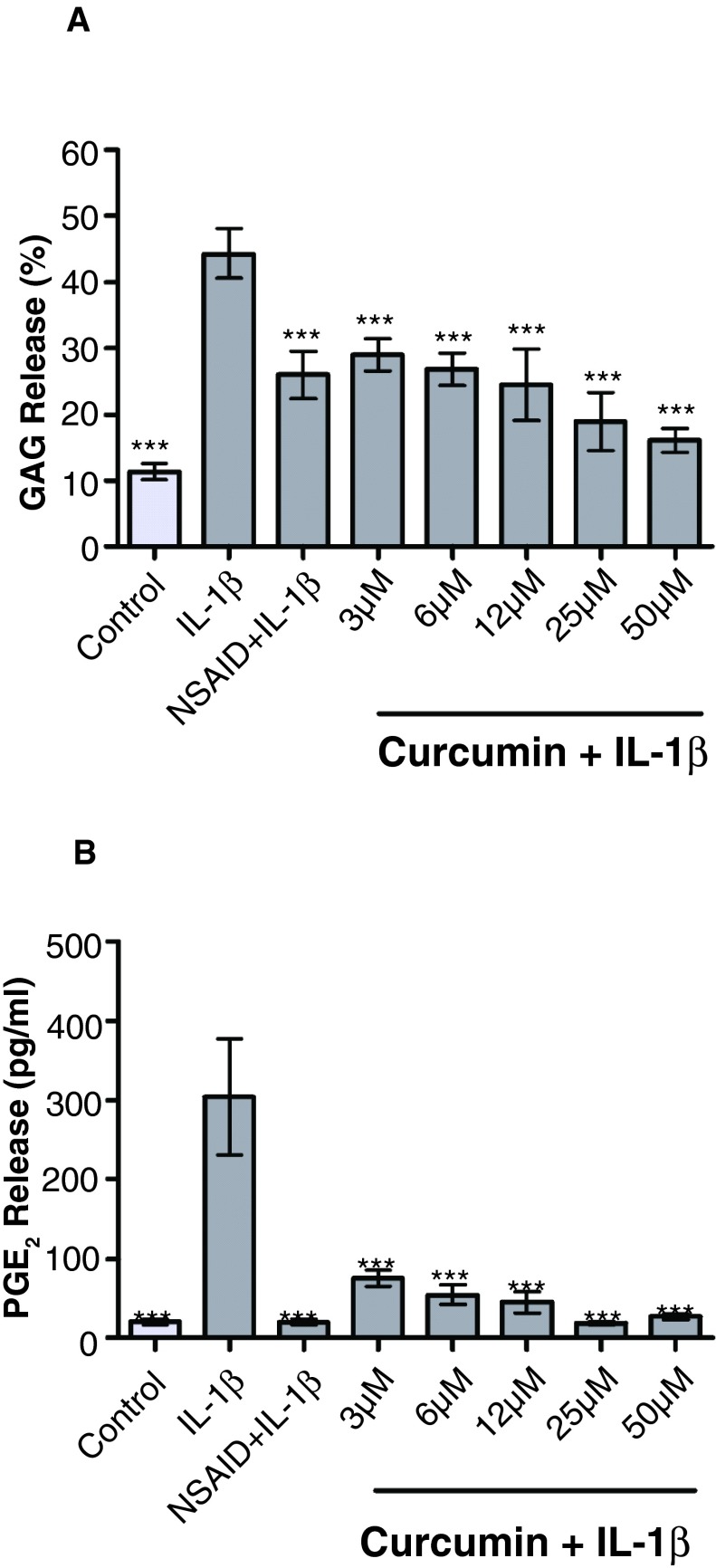
Control explants released 11.38 ± 1.26% of the total PG content of the cartilage into the media over five days (range: 86–323μg PG/ml of culture supernatant) ( Figure 4A). IL-1β (10ng/ml) significantly increased PG release to 44.31 ± 3.75% of total PG content compared to control ( p<0.001). The NSAID carprofen (100μg/ml) significantly reduced IL-1β-stimulated PG release to 25.96 ± 3.59% of total PG content ( p<0.01). Curcumin significantly decreased IL-1β-stimulated PG release in the explants to 28.98 ± 2.45% at 3µM ( p<0.05), 26.84 ± 2.49% at 6µM ( p<0.01), 24.51 ± 5.42% at 12µM ( p<0.01), 18.91 ± 4.36% at 25µM ( p<0.001) and 16.05 ± 1.82% at 50µM ( p<0.001). PGE 2 release into the media of unstimulated explants was 20.35 ± 3.67pg/ml ( Figure 4B). These levels were significantly increased ( p<0.001) to 303.3 ± 73.38pg/ml by the addition of recombinant IL-1β (10ng/ml). The NSAID significantly attenuated this effect, reducing levels to 19.72 ± 3.74pg/ml ( p<0.001). Curcumin also significantly reduced IL-1β-stimulated PGE 2 release to 75.48 ± 10.68pg/ml at 3μM ( p<0.001), 54.72 ± 12.41pg/ml at 6μM ( p<0.001), 45.65 ± 13.31pg/ml at 12μM ( p<0.001), 18.36 ± 2.38pg/ml at 25μM ( p<0.001) and 26.73 ± 3.52pg/ml at 50μM ( p<0.001).
Figure 4.
Effect of curcumin on PG release ( A) and prostaglandin E2 (PGE 2) ( B) from interleukin (IL)-1β-stimulated cartilage explants (dark grey columns) (n=3). Control (light grey column) indicates cartilage discs incubated in the culture medium alone. Values are reported as the mean of three animals per treatment ± SEM. Data are from 3 different animals, with three technical replicates per animal. Significance compared to IL-1β is indicated by * ( p<0.05), ** ( p<0.01) and *** ( p<0.001). IL-1β significantly increases proteoglycan (PG) ( p<0.001) and PGE 2 ( p<0.001) release compared to control. The non-steroidal anti-inflammatory drug (NSAID) carprofen (100µg/ml) significantly reduces IL-1β-stimulated PG ( p<0.01) and PGE 2 ( p<0.001) release. Curcumin significantly reduces IL-1β-stimulated PG release at 3µM ( p<0.05), 6µM ( p<0.01), 12µM ( p<0.01), 25µM ( p<0.001) and 50µM ( p<0.001). IL-1β-stimulated PGE 2 release is also significantly reduced at all curcumin concentrations ( p<0.001). PG loss is expressed as sulfated glycosaminoglycan (GAG) release.
Curcumin exerts dose-dependent effects on IL-1β-stimulated MMP-3 release in cartilage explants
Control explants released low levels of MMP-3, which were significantly increased by the addition of IL-1β ( p<0.001) ( Figure 5). The addition of the NSAID carprofen significantly reduced IL-1β-stimulated MMP-3 levels ( p<0.01) in all animals to near that of controls. Curcumin showed a dose-dependent significant effect on reducing the IL-1β-stimulated release at 12μM ( p<0.01), 25μM ( p<0.01) and 50μM ( p<0.001). However, the concentration at which this reduction became apparent differed between animals. For example, animal C, showed a clear reduction in MMP-3 secretion at curcumin concentrations of 12μM and above, whereas in animals A and B, the equivalent reduction was not seen until curcumin concentrations of 50μM were used. The most apparent reduction in MMP-3 secretion was always seen at 50μM with levels near to that of the controls in all animals.
Figure 5.
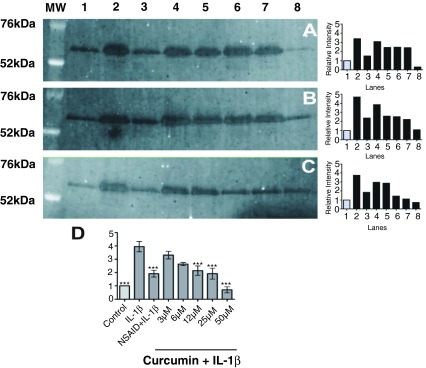
Western blot images and quantifying graphs of matrix metalloproteinase (MMP)-3 in the cartilage explant supernatants from three animals ( A, B, and C). The lane on the left of the images shows the molecular weights (MW) of standard markers (Invitrogen, Paisley, Scotland, UK) in kilodaltons (kDa). Graph D shows the relative intensity of bands from all three animals with labeled treatment axes. Values are reported as the mean of three animals per treatment ± SEM. Significance compared to interleukin (IL)-1β is indicated by ** ( p<0.01) and *** ( p<0.001). All lanes contain equal volumes of protein (50µg protein per lane). Unstimulated cartilage explants release low levels of MMP-3 as shown by the controls in lane 1. Secretion of MMP-3 from explants is significantly increased ( p<0.001) by IL-1β (10ng/ml) (Lane 2). The non-steroidal anti-inflammatory drug (NSAID) carprofen (100μg/ml) (Lane 3) significantly reduces IL-1β-stimulated MMP-3 secretion ( p<0.01) to near control levels. Curcumin at concentrations of 3μM (Lane 4) and 6μM (Lane 5) does not significantly reduce IL-1β-stimulated MMP-3 release from the explants. However, a significant reduction is observed at 12μM ( p<0.01) (Lane 6), 25μM ( p<0.01) (Lane 7) and 50μM ( p<0.001) (Lane 8) from explants in a dose dependent manner. The extent of reduction differs between animals (one technical replicate was used from each animal).
Effect of curcumin on prostaglandin E2, matrix metalloproteinase-3 and proteoglycan release in articular cartilage.
11 Data Files
Discussion
Recent research has produced conflicting results regarding the efficacy and cytotoxicity of curcumin in vitro 13– 15. This study attempted to address the issue of curcumin cytotoxicity and its anti-inflammatory effects in equine cartilage explants and monolayer chondrocyte cultures. The results of this investigation suggest that curcumin induces chondrocyte death at concentrations of 25μM and above in monolayer chondrocytes after 48 hours and five days.
The cytotoxic effects of curcumin (50μM) have been observed and documented in other cell types, notably tumor cell lines 16. Induction of apoptosis through cytochrome c release and subsequent caspase activation is thought to be a key chemopreventive effect of curcumin in cancer studies 17. In agreement with this, curcumin (50μM) has also been shown to reduce the viability of an immortalized human chondrocyte cell line after 24 hours 8. The results from this study suggest that this is also the case with primary equine chondrocytes. However, a recent study on primary human chondrocytes found that curcumin (50μM) did not reduce cell viability and successfully inhibited IL-1β-induced cytotoxicity as demonstrated by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay 18. This could be due to species-specific differences in chondrocyte susceptibility to curcumin, or equally likely, the different sources of curcumin used. This highlights the importance of assessing cytotoxicity alongside anti-inflammatory assays, with the same formulation of curcumin in each individual study.
In contrast to the monolayer cell cultures, curcumin (25μM) did not induce cytotoxicity in chondrocytes within cartilage explants after five days, although toxicity was seen at 100μM. Within cartilage, chondrocytes are embedded in a complex extracellular matrix (ECM) across which diverse diffusion gradients and fluid flow occurs 19. This could account for the differing toxicity threshold between monolayer cultures and explants. Chondrocytes are more vulnerable to cell death when they are no longer encased within a matrix, as cell adhesion and integrin attachments are important factors in promoting cell survival 20. Thus, without a protective ECM, monolayer chondrocytes may be more exposed and susceptible to external agents in the culture media than those within explants, as has been shown with bupivacaine 21. Therefore, cytotoxicity data from monolayer cultures may not reflect cytotoxic changes in 3-dimensional culture models and explant cultures.
For the purposes of addressing cytotoxicity in this study, a live/dead stain was selected to detect chondrocyte death as it can quickly provide qualitative and quantitative data on both monolayer and 3-dimensional chondrocyte cultures. The method does not distinguish between apoptosis and necrosis as the mode of cell death and although this information was not required for this study, it could be a useful addition to future studies. It should be noted here that cell death in monolayer cells can be underestimated, as dead cells can detach and be removed with the media. However, this risk was mitigated by centrifuging the discarded media so that the resulting pellet of detached cells could be resuspended and returned to the well before the live/dead stain reagents were added.
Once the uppermost observed safe level of curcumin was determined, the effect of curcumin on cartilage catabolism and inflammation was examined in explants. No significant effect of curcumin alone on matrix PG release from normal cartilage explants at both safe and toxic concentrations was observed. This suggests that curcumin does not alter the basal level of PG released from unstimulated cartilage explants in culture, even at cytotoxic concentrations. Interestingly, in these unstimulated explants, the two treatments that caused cell death had different effects on PG release: SNP (50mM) caused extensive PG loss into the media whereas curcumin (100μM) did not alter PG release compared to controls. PG loss does not directly lead to cell death in cartilage explants 22, thus the PG loss caused by SNP is unlikely to be primarily a consequence of cell death. SNP generates nitric oxide (NO), ceramide and cyanide, which aside from causing apoptosis, reduces proteoglycan and collagen synthesis by chondrocytes and increases inflammatory mediator production and MMP activity 23– 25 resulting in cartilage matrix loss. Conversely, although curcumin (100μM) induces cell death, it is likely to involve a mechanism that does not induce the release of any proteases. Curcumin is known to reduce the release of inflammatory mediators, MMPs and NO 6, 26. We have shown in this study that cytotoxic levels of curcumin reduce IL-β-stimulated PGE 2 and MMP-3 release. The reduced production of inflammatory mediators and catabolic enzymes suggests that it is unlikely to induce extensive PG loss as demonstrated in both IL-1β-treated and untreated cartilage explants in this study.
Curcumin at 3μM and above significantly reduced PG loss from IL-1β-stimulated explants. This reduction in PG loss was seen at concentrations that were non-detrimental to chondrocyte viability in explants (i.e. 25μM and under) as well as cytotoxic concentrations (100μM). Previously, we observed that curcumin (100μM) significantly reduced human IL-1β-stimulated PG release from cartilage explants, but this effect was not seen at 10μM 27. The differences in concentration efficacy between these two studies may be linked to individual variation or the source of cytokines used. Our previous study used human IL-1β on equine explants, whereas the current study used equine recombinant IL-1β. Despite a fairly conserved sequence homology between species, the use of species-specific IL-1β is more representative of the in vivo situation and thus may explain the difference in results seen between studies.
The involvement of pro-inflammatory cytokines and MMPs in OA is well documented 28, 29. MMP-3, also known as stromelysin, is produced by chondrocytes in OA cartilage tissue 30. MMP-3 gene expression is up-regulated in response to IL-1β stimulation in chondrocytes 31. Similarly, MMP-3 protein expression is increased by the addition of IL-1β to cartilage explants 32. MMP-3 was chosen as a marker of cartilage degradation in this study, as there is convincing evidence for a role for MMP-3 in cartilage destruction in OA. MMP-3 is induced by IL-1β in joint inflammation, and has the capacity to degrade collagens, proteoglycans and activate other procollagenases 33. MMP-3 and two other MMPs including MMP-1 and MMP-13 can also cleave the aggregating proteoglycan aggrecan at the interglobular domain 34– 36. Curcumin reduced MMP-3 secretion at concentrations as low as 12μM in some animals, and as high as 50μM in others, suggesting some animal-to-animal variability. However, when treated with 50μM curcumin, IL-1β-stimulated explants from all the animals used secreted MMP-3 levels that were lower than, or equivalent to, unstimulated control explants. This is in agreement with a previous study showing that curcumin (50μM) effectively reduced MMP-3 levels in IL-1β-stimulated-human chondrocyte lysates 37. However, lower curcumin concentrations have been reported as effective in cartilage from human OA patients post mortem, where curcumin reduced MMP-3 release into the media of IL-1β-stimulated chondrocytes at 15μM and in cartilage explants at 5μM 38. Many variables may account for the difference in effective curcumin concentrations, including individual variation, pre-existing joint pathology and the explant model used. It should be noted that many MMPs, including collagenases, matrilysin (MMP-7), and other stromelysins (e.g. MMP-10), are involved in osteoarthritic cartilage degradation 39– 41. Thus, the reduction in MMP-3 secretion would contribute to, but not totally account for, the reduction in PG release from IL-1β-stimulated cartilage explants.
The reported anti-inflammatory effects of curcumin have stimulated increasing interest in its potential for the treatment of inflammatory disorders 42. Curcumin is thought to exert its anti-inflammatory effects through reducing COX-1 43, COX-2 44, and microsomal PGES expression 16, thus preventing PGE 2 release. This is most likely due to its inhibitory effect on the upstream NF-κB-signaling pathway, which promotes PGE 2 production via upregulating the COX and PGES genes 45. In this study, curcumin at concentrations of 3μM and over significantly reduced PGE 2 release in response to equine IL-1β (10ng/ml). This anti-inflammatory effect is consistent with previous work in other cell culture models, such as rat peritoneal macrophages where curcumin (10µM) inhibited PGE 2 release by 45% 46, and in BV2 microglial cells where curcumin (10µM and 20µM) significantly reduced PGE 2 release in response to lipopolysaccharide (LPS) (0.5µg/ml) 47. The reduction in PGE 2 levels in response to IL-1β in our study may be attributed to the inhibitory effects of curcumin on the NF-κB pathway. Curcumin (50µM) has been shown to inhibit various steps of the NF-κB pathway, such as IL-1β-dependent phosphorylation of p65; nuclear-translocation of p65; and IκBα phosphorylation in IL-1β-stimulated human chondrocytes 6. Thus by inhibiting NF-κB, curcumin prevents the downstream inflammatory effects of COX-2 expression and PGE 2 synthesis.
Although curcumin was able to effectively attenuate the catabolic effects of IL-1β in this model, many other biochemical and biomechanical factors are capable of inducing cartilage inflammation and degeneration; these include compressive stress caused by overloading or traumatic injury of the joint 48, 49 and different cytokines such as tumor necrosis factor alpha (TNF-α) alone and in combination with oncostatin M (OSM) 50. Further studies are required to determine whether curcumin can reduce inflammation and degeneration generated by combinations of different catabolic stimuli.
The anti-inflammatory and anti-catabolic effects of curcumin shown in this study support the existing evidence that curcumin may be supportive to joint health 13– 15. However, the bioavailability of curcumin is thought to be relatively low 51 and efforts to determine whether chemically modified versions of curcumin may improve bioavailability merit further investigation 13, 52.
In summary, although this study found that curcumin at concentrations of 25μM and above is cytotoxic to monolayer chondrocytes after five days in culture, lower concentrations effectively antagonize PG and PGE 2 release in vitro and exert a potent anti-inflammatory effect on cartilage explants treated with IL-1β. Achieving micromolar concentrations of curcumin in the synovial joint, or to the pericellular matrix of chondrocytes embedded deep within the avascular articular cartilage is highly unlikely. Controversial issues associated with the metabolism and bioavailability of curcumin highlight the need for caution when extrapolating in vitro data for translational research and clinical trials. Nevertheless, our results to date suggest that a commercially available curcumin formulation at 12μM and below, has no obvious cytotoxic effects on primary chondrocytes after five days in culture. More importantly, concentrations as low as 3μM were able to effectively reduce IL-1β-stimulated cartilage degradation and inflammatory mediator production. This study supports existing evidence to suggest that curcumin may be a suitable adjunct to conventional drugs for the treatment of inflammatory and degenerative disorders such as OA. If curcumin is to be used as an anti-inflammatory supplement, further research is required to establish its bioavailability and physiologically relevant serum and synovial concentrations in vivo in humans and animals.
Acknowledgements
We would like to thank Leigh Sylvester from the School of Biosciences at the University of Nottingham for help with the confocal microscope studies.
Funding Statement
This work received major financial support from the Biotechnology and Biological Sciences Research Council through the Industrial CASE Studentship programme (Grant Number: BBS/S/M/2006/13141) and minor financial support from the WALTHAM Centre for Pet Nutrition. The BBSRC had no involvement in the study design, data collection, analysis and interpretation. The decision to submit the paper for publication was not influenced by the funding bodies.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
v2; ref status: indexed
References
- 1.Maiotti M, Monteleone G, Tarantino U, et al. : Correlation between osteoarthritic cartilage damage and levels of proteinases and proteinase inhibitors in synovial fluid from the knee joint. Arthroscopy. 2000;16(5):522–526 10.1053/jars.2000.4632 [DOI] [PubMed] [Google Scholar]
- 2.Ahmed S, Anuntiyo J, Malemud CJ, et al. : Biological basis for the use of botanicals in osteoarthritis and rheumatoid arthritis: a review. Evid Based Complement Alternat Med. 2005;2(3):301–308 10.1093/ecam/neh117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elmali N, Baysal O, Harma A, et al. : Effects of resveratrol in inflammatory arthritis. Inflammation. 2007;30(1–2):1–6 10.1007/s10753-006-9012-0 [DOI] [PubMed] [Google Scholar]
- 4.Gerhauser C, Klimo K, Heiss E, et al. : Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat Res. 2003;523–524:163–172 10.1016/S0027-5107(02)00332-9 [DOI] [PubMed] [Google Scholar]
- 5.Jobin C, Bradham CA, Russo MP, et al. : Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol. 1999;163(6):3474–3483 [PubMed] [Google Scholar]
- 6.Shakibaei M, John T, Schulze-Tanzil G, et al. : Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73(9):1434–1445 10.1016/j.bcp.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 7.Clutterbuck AL, Allaway D, Harris P, et al. : Toxic effects of curcumin (diferuloylmethane) on equine articular chondrocytes and synoviocytes in vitro. Eur Cell Mater. 2008;16(Suppl. 3):43 Reference Source [Google Scholar]
- 8.Toegel S, Wu SQ, Piana C, et al. : Comparison between chondroprotective effects of glucosamine, curcumin, and diacerein in IL-1beta-stimulated C-28/I2 chondrocytes. Osteoarthritis Cartilage. 2008;16(10):1205–1212 10.1016/j.joca.2008.01.013 [DOI] [PubMed] [Google Scholar]
- 9.Finger F, Schorle C, Zien A, et al. : Molecular phenotyping of human chondrocyte cell lines T/C-28a2, T/C-28a4, and C-28/I2. Arthritis Rheum. 2003;48(12):3395–3403 10.1002/art.11341 [DOI] [PubMed] [Google Scholar]
- 10.Dvorak LD, Cook JL, Kreeger JM, et al. : Effects of carprofen and dexamethasone on canine chondrocytes in a three-dimensional culture model of osteoarthritis. Am J Vet Res. 2002;63(10):1363–1369 10.2460/ajvr.2002.63.1363 [DOI] [PubMed] [Google Scholar]
- 11.Blanco FJ, Ochs RL, Schwarz H, et al. : Chondrocyte apoptosis induced by nitric oxide. Am J Pathol. 1995;146(1):75–85 [PMC free article] [PubMed] [Google Scholar]
- 12.Farndale RW, Sayers CA, Barrett AJ: A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248 10.3109/03008208209160269 [DOI] [PubMed] [Google Scholar]
- 13.Henrotin Y, Priem F, Mobasheri A: Curcumin: a new paradigm and therapeutic opportunity for the treatment of osteoarthritis: curcumin for osteoarthritis management. Springerplus. 2013;2(1):56 10.1186/2193-1801-2-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mobasheri A, Henrotin Y, Biesalski HK, et al. : Scientific evidence and rationale for the development of curcumin and resveratrol as nutraceutricals for joint health. Int J Mol Sci. 2012;13(4):4202–4232 10.3390/ijms13044202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henrotin Y, Clutterbuck AL, Allaway D, et al. : Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage. 2010;18(2):141–149 10.1016/j.joca.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 16.Moon Y, Glasgow WC, Eling TE: Curcumin suppresses interleukin 1beta-mediated microsomal prostaglandin E synthase 1 by altering early growth response gene 1 and other signaling pathways. J Pharmacol Exp Ther. 2005;315(2):788–795 10.1124/jpet.105.084434 [DOI] [PubMed] [Google Scholar]
- 17.Woo JH, Kim YH, Choi YJ, et al. : Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24(7):1199–1208 10.1093/carcin/bgg082 [DOI] [PubMed] [Google Scholar]
- 18.Csaki C, Mobasheri A, Shakibaei M: Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther. 2009;11(6):R165 10.1186/ar2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia AM, Frank EH, Grimshaw PE, et al. : Contributions of fluid convection and electrical migration to transport in cartilage: relevance to loading. Arch Biochem Biophys. 1996;333(2):317–325 10.1006/abbi.1996.0397 [DOI] [PubMed] [Google Scholar]
- 20.Cao L, Lee V, Adams ME, et al. : beta-Integrin-collagen interaction reduces chondrocyte apoptosis. Matrix Biol. 1999;18(4):343–355 10.1016/S0945-053X(99)00027-X [DOI] [PubMed] [Google Scholar]
- 21.Chu CR, Izzo NJ, Papas NE, et al. : In vitro exposure to 0.5% bupivacaine is cytotoxic to bovine articular chondrocytes. Arthroscopy. 2006;22(7):693–699 10.1016/j.arthro.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 22.Otsuki S, Brinson DC, Creighton L, et al. : The effect of glycosaminoglycan loss on chondrocyte viability: a study on porcine cartilage explants. Arthritis Rheum. 2008;58(4):1076–1085 10.1002/art.23381 [DOI] [PubMed] [Google Scholar]
- 23.Khatib AM, Siegfried G, Messai H, et al. : Mechanism of inhibition of endothelin-1-stimulated proteoglycan and collagen synthesis in rat articular chondrocytes. Cytokine. 2002;17(5):254–261 10.1006/cyto.2001.1001 [DOI] [PubMed] [Google Scholar]
- 24.Blanco FJ, Lotz M: IL-1-induced nitric oxide inhibits chondrocyte proliferation via PGE2. Exp Cell Res. 1995;218(1):319–325 10.1006/excr.1995.1161 [DOI] [PubMed] [Google Scholar]
- 25.Murrell GA, Jang D, Williams RJ: Nitric oxide activates metalloprotease enzymes in articular cartilage. Biochem Biophys Res Commun. 1995;206(1):15–21 10.1006/bbrc.1995.1003 [DOI] [PubMed] [Google Scholar]
- 26.Brouet I, Ohshima H: Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem Biophys Res Commun. 1995;206(2):533–540 10.1006/bbrc.1995.1076 [DOI] [PubMed] [Google Scholar]
- 27.Clutterbuck AL, Mobasheri A, Shakibaei M, et al. : Interleukin-1beta-induced extracellular matrix degradation and glycosaminoglycan release is inhibited by curcumin in an explant model of cartilage inflammation. Ann N Y Acad Sci. 2009;1171:428–435 10.1111/j.1749-6632.2009.04687.x [DOI] [PubMed] [Google Scholar]
- 28.Shinmei M, Masuda K, Kikuchi T, et al. : Production of cytokines by chondrocytes and its role in proteoglycan degradation. J Rheumatol Suppl. 1991;27:89–91 [PubMed] [Google Scholar]
- 29.Chubinskaya S, Huch K, Mikecz K, et al. : Chondrocyte matrix metalloproteinase-8: up-regulation of neutrophil collagenase by interleukin-1 beta in human cartilage from knee and ankle joints. Lab Invest. 1996;74(1):232–240 [PubMed] [Google Scholar]
- 30.Okada Y, Shinmei M, Tanaka O, et al. : Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Lab Invest. 1992;66(6):680–690 [PubMed] [Google Scholar]
- 31.Tung JT, Fenton JI, Arnold C, et al. : Recombinant equine interleukin-1beta induces putative mediators of articular cartilage degradation in equine chondrocytes. Can J Vet Res. 2002;66(1):19–25 [PMC free article] [PubMed] [Google Scholar]
- 32.Julovi SM, Yasuda T, Shimizu M, et al. : Inhibition of interleukin-1beta-stimulated production of matrix metalloproteinases by hyaluronan via CD44 in human articular cartilage. Arthritis Rheum. 2004;50(2):516–525 10.1002/art.20004 [DOI] [PubMed] [Google Scholar]
- 33.Shiomi T, Lemaitre V, D'Armiento J, et al. : Matrix metalloproteinases, a disintegrin and metalloproteinases, and a disintegrin and metalloproteinases with thrombospondin motifs in non-neoplastic diseases. Pathol Int. 2010;60(7):477–496 10.1111/j.1440-1827.2010.02547.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fosang AJ, Neame PJ, Hardingham TE, et al. : Cleavage of cartilage proteoglycan between G1 and G2 domains by stromelysins. J Biol Chem. 1991;266(24):15579–15582 [PubMed] [Google Scholar]
- 35.Fosang AJ, Last K, Knauper V, et al. : Fibroblast and neutrophil collagenases cleave at two sites in the cartilage aggrecan interglobular domain. Biochem J. 1993;295(Pt 1):273–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fosang AJ, Last K, Knauper V, et al. : Degradation of cartilage aggrecan by collagenase-3 (MMP-13). FEBS Lett. 1996;380(1–2):17–20 10.1016/0014-5793(95)01539-6 [DOI] [PubMed] [Google Scholar]
- 37.Schulze-Tanzil G, Mobasheri A, Sendzik J, et al. : Effects of curcumin (diferuloylmethane) on nuclear factor kappaB signaling in interleukin-1beta-stimulated chondrocytes. Ann N Y Acad Sci. 2004;1030:578–586 10.1196/annals.1329.067 [DOI] [PubMed] [Google Scholar]
- 38.Mathy-Hartert M, Jacquemond-Collet I, Priem F, et al. : Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm Res. 2009;58(12):899–908 10.1007/s00011-009-0063-1 [DOI] [PubMed] [Google Scholar]
- 39.Mitchell PG, Magna HA, Reeves LM, et al. : Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97(3):761–768 10.1172/JCI118475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohta S, Imai K, Yamashita K, et al. : Expression of matrix metalloproteinase 7 (matrilysin) in human osteoarthritic cartilage. Lab Invest. 1998;78(1):79–87 [PubMed] [Google Scholar]
- 41.Barksby HE, Milner JM, Patterson AM, et al. : Matrix metalloproteinase 10 promotion of collagenolysis via procollagenase activation: implications for cartilage degradation in arthritis. Arthritis Rheum. 2006;54(10):3244–3253 10.1002/art.22167 [DOI] [PubMed] [Google Scholar]
- 42.Jagetia GC, Aggarwal BB: "Spicing up" of the immune system by curcumin. J Clin Immunol. 2007;27(1):19–35 10.1007/s10875-006-9066-7 [DOI] [PubMed] [Google Scholar]
- 43.Handler N, Jaeger W, Puschacher H, et al. : Synthesis of novel curcumin analogues and their evaluation as selective cyclooxygenase-1 (COX-1) inhibitors. Chem Pharm Bull (Tokyo). 2007;55(1):64–71 10.1248/cpb.55.64 [DOI] [PubMed] [Google Scholar]
- 44.Hong J, Bose M, Ju J, et al. : Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004;25(9):1671–1679 10.1093/carcin/bgh165 [DOI] [PubMed] [Google Scholar]
- 45.Catley MC, Chivers JE, Cambridge LM, et al. : IL-1beta-dependent activation of NF-kappaB mediates PGE 2 release via the expression of cyclooxygenase-2 and microsomal prostaglandin E synthase. FEBS Lett. 2003;547(1–3):75–79 10.1016/S0014-5793(03)00672-0 [DOI] [PubMed] [Google Scholar]
- 46.Joe B, Lokesh BR: Effect of curcumin and capsaicin on arachidonic acid metabolism and lysosomal enzyme secretion by rat peritoneal macrophages. Lipids. 1997;32(11):1173–1180 10.1007/s11745-997-0151-8 [DOI] [PubMed] [Google Scholar]
- 47.Jin CY, Lee JD, Park C, et al. : Curcumin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated BV2 microglia. Acta Pharmacol Sin. 2007;28(10):1645–1651 10.1111/j.1745-7254.2007.00651.x [DOI] [PubMed] [Google Scholar]
- 48.Gosset M, Berenbaum F, Levy A, et al. : Prostaglandin E2 synthesis in cartilage explants under compression: mPGES-1 is a mechanosensitive gene. Arthritis Res Ther. 2006;8(4):R135 10.1186/ar2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patwari P, Cook MN, DiMicco MA, et al. : Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum. 2003;48(5):1292–1301 10.1002/art.10892 [DOI] [PubMed] [Google Scholar]
- 50.Hui W, Rowan AD, Richards CD, et al. : Oncostatin M in combination with tumor necrosis factor alpha induces cartilage damage and matrix metalloproteinase expression in vitro and in vivo. Arthritis Rheum. 2003;48(12):3404–3418 10.1002/art.11333 [DOI] [PubMed] [Google Scholar]
- 51.Sharma RA, McLelland HR, Hill KA, et al. : Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7(7):1894–1900 [PubMed] [Google Scholar]
- 52.Bisht S, Feldmann G, Soni S, et al. : Polymeric nanoparticle-encapsulated curcumin ("nanocurcumin"): a novel strategy for human cancer therapy. J Nanobiotechnology. 2007;5:3 10.1186/1477-3155-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]