Abstract
Herein, we will review molecular aspects of vestibular ear development and present them in the context of evolutionary changes and hair cell regeneration. Several genes guide the development of anterior and posterior canals. Although some of these genes are also important for horizontal canal development, this canal strongly depends on a single gene, Otx1. Otx1 also governs the segregation of saccule and utricle. Several genes are essential for otoconia and cupula formation, but protein interactions necessary to form and maintain otoconia or a cupula are not yet understood. Nerve fiber guidance to specific vestibular endorgans is predominantly mediated by diffusible neurotrophic factors that work even in the absence of differentiated hair cells. Neurotrophins, in particular Bdnf, are the most crucial attractive factor released by hair cells. If Bdnf is misexpressed, fibers can be redirected away from hair cells. Hair cell differentiation is mediated by Atoh1. However, Atoh1 may not initiate hair cell precursor formation. Resolving the role of Atoh1 in postmitotic hair cell precursors is crucial for future attempts in hair cell regeneration. Additional analyses are needed before gene therapy can help regenerate hair cells, restore otoconia, and reconnect sensory epithelia to the brain.
Keywords: Ear, development, sensory epithelia, sensory neurons, otoconia, cupula
1. Introduction
The mammalian ear contains three sensory systems:
Angular acceleration perception is accomplished by the semicircular canals to direct specific endolymphatic movement to crista organs where hair cells convert it into electric signals. These signals are conducted by specific vestibular afferent fibers to the brainstem and the cerebellum.
Linear acceleration is detected by hair cells by movement of a gelatinous mass that contains otoconia in relationship to the endorgans where movement is converted into electric signals conducted to brainstem and cerebellum.
Sound reaches the cochlea where the basilar membrane motion and hair cell stimulation converts specific frequencies into tonotopic information that encodes place of cochlear stimulation and intensity. The spiral ganglion neurons receive this signal and then relay this information to the brainstem, but not the cerebellum.
Excluding the problem of the origin and diversification of the cochlea [55], what makes a functional vestibular system must be minimally understood at four interconnected levels requiring knowledge of both ear development and evolution.
We need to understand how the ear is patterned such that morphogenesis and histogenesis happens at the right place at the right time. For example, if a canal crista forms near the endolymphatic duct, this abnormal position would NOT permit the perception of angular acceleration mediated by endolymphatic movement as angular accelerations would only cause limited fluid movements, much like in plugged canals [115].
Hair cells have to develop at the right place with the right polarity or a mix thereof to be able to extract information from the stimuli. Having hair cells oriented 90° with respect to their normal orientation in the canal cristae would result in virtually no stimulation no matter how rapidly the head is turned.
The extracellular matrix has to be appropriately formed to permit detection of the stimulus. Having a cupula-like gelatinous structure on the utricle would render this epithelium virtually incapable to perceive linear acceleration as the cupula lacks the higher density to cause relative movements in linear acceleration. Conversely, attaching otoconia to the canal cupula causes a clinical syndrome known as cupulolithiasis as it introduces linear acceleration sensation capacity into an angular acceleration sensor.
Last, but not least, the extracted information has to be conducted in discrete and partial overlapping fashion to the hindbrain, including the cerebellum, for appropriate information processing.
In this overview we will first briefly introduce major aspects of vestibular vertebrate ear evolution to highlight how selection has shaped an unknown ancestral sensory organ into a system with three semicircular canals and crista organs to extract angular acceleration in the three cardinal planes and evolved one (macula communis), two (utricle and saccule) or three sensory organs (utricle, saccule, lagena) for perception of horizontal and vertical linear acceleration, including gravity. Next, we will analyze, in order, 1) current molecular developmental insights into patterning events and morphogenesis of canals and utricular and saccular recesses; 2) molecular biology of cupula and otoconia composition; 3) development and molecular biology of sensory neuron development, including known guiding principles for connecting these neurons specifically to the vestibular sensory organs; and 4) molecular basis of sensory epithelial development (including hair cells) as revealed by recent analysis of mutant and transgenic mice. We follow roughly the sequence of appearance of these events in ear development, with hair cell differentiation being one of the last events.
2. Vertebrate vestibular ear evolution shows changes in linear and angular acceleration sensory systems
The origin of the ear has sparked various hypotheses. The rather ubiquitous distribution of statocyts in all animal radiations suggests that bearing mechanosensory cells aggregated into statocysts are ancient structures derived from single mechanosensory cells [17,49,90]. Gene expression supports such a notion as it shows that certain genes are involved in statocyst and sensory cell development across phyla [51,73]. The chordate most closely related to vertebrates, the lancelet, has neither an ear nor organs that resemble the lateral line organs [41,75]. All craniate vertebrates have an ear that consists of two discrete sensory systems, angular and linear acceleration sensors. In cyclostome vertebrates such as lampreys and hagfish, the gravistatic receptor is a single macula covered with otoconia [79]. Evolution has apparently turned this otoconia containing organ, which resembles the invertebrate statocyst, into two or three different organs (utricle, saccule, lagena), each with a distinct orientation in space [79,119]. Such diversification in vertebrate ears implies, but does not prove, that vertebrate ear evolution is primarily the diversification of a statocyst. Interestingly, the evolution of the angular and linear acceleration system of the ear of cephalopods indicates a comparable diversification from a simple moluscan statocyst [16,94] as proposed here for the evolution of the vertebrate ear from a hypothetical statocyst of an unknown vertebrate ancestor.
In parallel to the segregation and diversification of otoconia baring organs is a diversification and morphological evolution of angular acceleration organs. Hagfish have only a single canal and Lampreys have only two vertical canals. Both taxa have only two cristae organs, an anterior and a posterior crista. This suggests that the horizontal crista evolved in jawed vertebrates together with the segregation of a single otoconia bearing organ into two [45,97]. Interestingly, cristae organs of hagfish lack cupulae. Instead, the entire ampullary lumen is surrounded by hair cells which extent their elongated kinocilium and stereocilia freely into the canals (Fig. 1). These data suggest that evolution of canal cristae in jawed vertebrates involved transformations of sensory epithelia (from an organ surrounding the entire lumen to an organ restricted to a small sensory patch), formation of a cupula to change an organ that measures only angular velocity into an organ that measures pressure of endolymph at the cupula [63], and generation of a new canal (the horizontal canal) together with the horizontal canal crista and the appropriate afferent fibers to connect this organ to the brain [45].
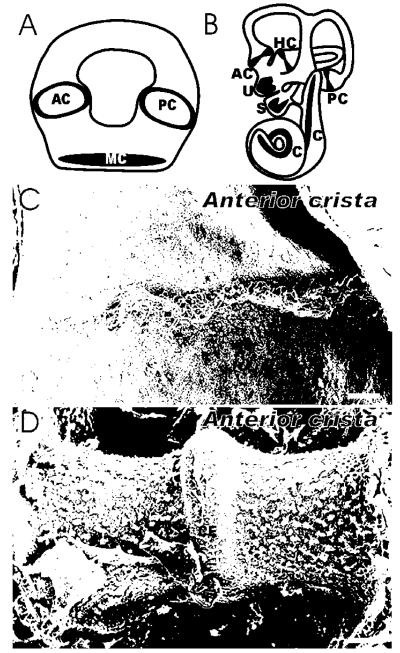
Fig. 1.
A brief survey of major evolutionary changes in the ear is presented. Hagfish, a jawless vertebrate, have a single torus that contains two canal cristae and a single common macula (A). Mammals such as mice have three canal cristae and two organs for linear acceleration perception with otoconia as well as a cochlea (B). The canal cristae show differences in their organization. Mammals have a crista that sits on a ridge separated by a cruciate eminence (CE) that is covered by a cupula (removed in D to show the hair cell bundles). In contrast, hagfish have no cupula and the sensory hair cells are arranged to form a ring around the largest diameter of ampullary enlargement (C). AC, anterior crista; C, cochlea; CE; cruciate eminence; HC, horizontal crista; MC, macula communis; PC, posterior crista; S, saccule; U, utricle. Bar indicates 100 μm. Modified after [45].
Combined with the molecular developmental conservation of mechanosensory cells and the rather ubiquitous presence of statocysts in aquatic invertebrates, the limited changes in the linear acceleration system argues that the evolutionary origin of the vertebrate ear was a statocyst like organ, with the canal system evolving independently. Hagfish have only extracellular matrix containing otoconia covering the single common macula [79,87,119]. Neither the lateral line system [13] nor the canal cristae are covered with extracellular matrix. This suggests that those covering structures evolved after the organs had evolved, possibly out of the matrix of the gravistatic organ by losing the capacity to incorporate otoconia. Molecular similarities also suggest that the cupula of cristae may have evolved out of extracellular matrix [54] of gravistatic organs in which otoconia were embedded. Developmental and evolutionary data support that the tectorial membrane of the basilar papilla (or cochlea in vertebrates) derives from gravistatic organs, suggesting that tectorial membrane formation was secondarily modified by eliminating the capacity to incorporate otoconia into the matrix [45, 54]. Development of the ear has to provide the molecular causality for such evolutionary changes (Fig. 1) and has to explain how three (instead of one) canals form, how three cristae form, and how two or more instead of one linear acceleration sensor form, including the necessary and associated changes in hair cell and sensory neurons.
3. Genes involved in patterning the ear
The ear starts as an epithelial thickening, the otic placode [147] that forms lateral to rhombomeres 5 and 6 of the hindbrain, except for lampreys and hagfish [42,102]. Placodes show specific genetic markers prior to any morphological distinction [5,104]. Precisely how large and how long lasting such a preplacodal area is remains at the moment speculative [15, 104]. Interestingly, the data across vertebrates suggest that even orthologues of genes expressed in these areas such as Foxi1 [104,134] and Fgf10 [15,103,109] may not be conserved in their function for placode induction. Such data may indicate that placodes are transient embryological adaptation and are modified to fit into the different developmental pathways of various vertebrates. Given that hair cells develop only comparatively late out of placodes, these thickenings may therefore present an embryological adaptation comparable to the placenta: necessary for development but uniquely evolved for such developmental purposes.
Like the nearby central nervous system, the otic placode undergoes invagination to form the otic cup and later the otocyst that closes off from the ectoderm. The otocyst will form between the first somite and the more rostral somitomeres. The developing otocyst will therefore be exposed to some of the same factors emanating from the notochord and spinal cord to pattern somites such as sonic hedgehog (Shh), which upregulate genes specific for muscle and tendon formation such as the basic helix-loop-helixtranscription factor (bHLH), paired box (Pax), fibroblast growth factor (Fgf) and forkhead box (Fox) genes [6,14]. In the ear, genes of these families are the bHLH genes, Neurog1, Atoh1 and Neurod1, the Pax family member, Pax2 [12,18,120], Fgf members Fgf3, Fgf10, and several Fox members [60,104]. Thus, the major players of early otic placode formation may be under molecular governance comparable to the nearby somites.
One of the first morphogenetic events of the otocyst is the growth of a plate that will form the anterior and posterior vertical canal by embryonic day 12.5 [95, 100]. Through adhesion and cell death of the central parts, the remaining ducts are formed [19,22,39]. Partially overlapping with this is the growth of another plate that will eventually form the horizontal or lateral canal (Fig. 2). At this stage, a separation will divide the superior part and the inferior part, leaving the utriculosaccular foramen [19]. In the mouse approximately 5 days after the first appearance of the placodal thickening, the vestibular portion of the ear is formed.
Fig. 2.
Morphogenetic defects of three null mutant lines are shown. Fgf10 null mutants lack formation of any canals (A,B) but form a single undifferentiated pouch that carries the reduced and malformed anterior and horizontal cristae. These mutants lack a posterior crista entirely (B). Gata3 null mutants show arrest of ear formation at the level of the otocyst with barely recognizable initiation of sensory epithelia formation (D). Interestingly, the expression of Gata3 as revealed with the β-galactosidase marker shows disorganization suggestive of alterations of expression of Gata3 (C,D). Otx1 null mutants lack formation of the horizontal canal and show a fusion of the utricular and saccular epithelium across the enlarged utriculo-saccular foramen (E,F). The loss of a horizontal canal and fusion of the utriculo-saccular epithelium in the absence of Otx1 suggests that Otx1 expression in the ear was a crucial step in vertebrate ear evolution to segregate the common macula of cyclostomes into two gravistatic organs, the utricle and saccule. AC, anterior crista; HC, horizontal crista; PC, posterior crista; S, saccule; SN, sensory neurons; U, utricle. Bar indicates 100 μm. Modified after [44,68,109].
An increasing number of genes have been identified through targeted mutations that play major roles in ear morphogenesis [23,83,103]. The genes we chose to present in some detail belong to several classes of factors that play major roles in morphogenesis: FGFs, EYA/SIX/DACH heteromeric complexes and zinc finger proteins.
FGF’s are a large family of 22 predominantly secreted ligands (except for FGF11-14) that signal to 7 specific receptors [differently spliced out of four receptor (Fgfr) genes] in a paracrine or autocrine fashion. Upon ligand binding, FGFR’s homodimerize and activate various intracellular signaling pathways that ultimately change gene expression in the targeted cell [106]. The most important FGF’s for early ear development are Fgf10, Fgf3 [152] and Fgf8 [76]. Fgf10 is expressed at the placodal stage in the mesoderm underlying the otic placode. As the placode thickens, Fgf10 expression moves into the otic ectoderm [103, 109,112,154]. Fgf3 is initially only found in the nearby rhombomeres 5 and 6 of the hindbrain. Eventually, Fgf3 is expressed in the antero-ventral aspect of the otocyst, overlapping with Fgf10 [154]. Fgf9 is only expressed in the periotic mesenchyme [114]. Expression of Fgfr1 and Fgfr2b, which is the receptor for FGF3 and FGF10 [152], is initially overlapping in the otic placode. During morphogenesis of the ear, Fgfr2b is expressed in the dorsal half of the otocyst, expanding into the cochlea caudally [112] suggesting its involvement in canal morphogenesis.
FGFs are essential for mammalian ear formation and histogenesis [27,109,113]. For example, FGF8 released from inner hair cells may determine the cell fate of pillar cells, which express FGFR3 [27,112]. Fgf10 null mutant mice (Fig. 2) have no canal formation [103,109] and show reduction and incomplete separation of canal sensory epithelia [109]. Double knockouts of both Fgf3 and Fgf10 show only limited formation of occasional micro-vesicles, if any vesicles form at all [4,153]. An ear forms in single Fgf3 null mutants but there is variable size reduction [4]. Fgf9 null mutants also have reduced canal formation somewhat similar to Fgf10 null mice [114], suggesting that mesenchymal-epithelial interactions are essential for canal morphogenesis. How FGF’s recruit other genes such as Bmp4 [20-22,53], Hmx2/3 (previously Nkx5) [11,57,149], Dlx [98,133], Gata3 [68], Otx1 [44, 101] and Sox2 [71] is not understood. The reduction or loss of semicircular canals is consistent with FGF’s established role in branching morphogenesis [109,112] also apparent in lung formation [114]. Modeling functional molecular interactions between FGF mediated FGFR intracellular signaling and other factors targeting similar promoter regions requires a much deeper knowledge of genes, their qualitative and quantitative expression patterns, and their functional interactions in ear development than is currently available [23].
Morphogenesis of the embryonic ear depends on the EYA/SIX/DACH complex [34,156,157], an evolutionary conserved gene network [59]. This developmental module or cassette is used in a variety of organs and across phyla to regulate genes that permit cell migration [136], proliferation, and cell death [157]. Null mutants show that Eya1 and Six1 are crucial for any development of the ear past the otocyst stage as only microvesicles form in Eya1/Six1 double null mutants [157,159]. Biological redundancyis suggested by the formation of the ear in altered Pax2 gene expression in spontaneous and null mutations [34,142,156, 157]. The haploid insufficiency observed in human and mouse Eya1 mutations suggest that alterations in gene dosage can affect morphogenesis [1,2,67,146,157].
Six1 affects the expression of genes known to be important in ear morphogenesis such as Bmp4, Fgf10 and Fgf3 [21,109,154], which are downregulated in Six1 null mutants. Six1 also alters the expression pattern of two genes known to be involved in ear morphogenesis, Hmx3 and Gata3 [11,68] suggesting a direct involvement of Six1 in maintenance of their correct expression pattern [157,159]. A related gene, Eya4, leads to late-onset deafness at the DFNA10 locus and endolymphatic hydrops [110,151]. Clearly, Eya1 and Six1 are the best understood genes in terms of their effect on other important genes in ear morphogenesis and still explaining their function remains tentative in the absence of any information about most of their downstream genes [159]. Further dissection of their role in ear development must be approached by using conditional mutant mouse lines to understand the contextual role these regulatory genes are playing without compromising the viability of these mutants owing to their defect of other vital organs [159].
GATA factors participate directly in several signal-transduction pathways in both invertebrates and vertebrates related to proliferation, morphogenesis and cell fate assignment [108]. Most interesting for the ear is the known interaction of GATA factors with BMP, FGF, FOX, HMX and T-box signaling. Interaction between GATA and FGF’s to neuralize ectoderm may be ancestral to chordates [10]. GATA factors also play roles in sensory organ formation in insects [108,129], possibly through the activation of bHLH factors [9].
Like some Fox genes [60,104], Gata3 is expressed at the level of the otic placode [77]. Targeted disruption of Gata3 leads to an arrest of ear development at the level of the otocyst [68] with little morphogenesis beyond a sac (Fig. 2). How GATA3 interacts with other transcription factors involved in ear morphogenesis [40, 156] is still uncertain. However, multiple binding sites for GATA factors, Otx and E-box motifs in the promoter regions of Fox genes have been described [32] and an interaction of GATA factors with FGF’s and OTX may be important for neuralization [10]. Given the early ear expression of Gata3 it is not at all surprising to see influences on transcription factors regulating morphogenesis and histogenesis of the ear [77]. Both BMP mediated and direct signaling by GATA3 [9] as well as GATA3 interaction with FOX and T-box proteins [60, 108] could effect the formation of sensory neurons. Our laboratory is currently exploring the expression of several of these genes in Gata3 null mutant mice.
Other major factors in early ear morphogenesis have been reviewed recently such as retinoids, Hox and other transcription factors [11,23,83,124] and will not be dealt with here. It suffices to say that none of the genes and their effect on ear morphogenesis is any further along in dissecting the molecular causality toward developmental defects than those three examples given above. Overall, these gene interactions seem to directly specify distinct areas to undergo morphogenesis and histogenesis, possibly through the formation of polar coordinates by which the position of these future morphogenetic and histogenetic events are specified [40]. Most interestingly for understanding the evolution of ear morphogenesis is that certain processes can be developmentally uncoupled. For example, formation of a horizontal canal and segregated utricular and saccular epithelia (Fig. 2) depend on the Otx1 gene [19], whereas formation and positioning of a horizontal crista appears to be governed by other genes [44,45].
4. Molecular composition of otoconia and cupulae
As already stated in the introduction, the ear of vertebrates is a complex structure housing three sensory systems which perceive linear acceleration (gravity), angular acceleration, and sound [79,119]. Specificity of stimulus acquisition in the ear is achieved by associating particular sensory epithelia such as utricle, saccule, canal cristae and cochlea with acellular matrix proteins inside the inner ear space that mediate unique mechanical stimuli. Otoconia provide the inertia mass to generate shearing forces for the utricle and saccule when subjected to linear acceleration such as gravity. The otoconia are typically composed of calcium carbonate crystals partially embedded in a glycoprotein matrix that is tethered by proteinaceous filaments to the underlying sensory epithelium (Fig. 3). The crystals form inside the endolymphatic space of the inner ear, which contains a fluid composed of high levels of potassium with very little calcium. Through unknown control mechanisms, the crystals are subject to disassembly under certain conditions, including age- and sex-dependent alterations [125,141]. In contrast, the cupulae of the semicircular canal cristae and the tectorial membrane of the cochlea do not contain any calcium carbonate as this would likely change the stiffness of these structures and alter their physical properties in angular acceleration and sound perception. Such data are supported by the human condition of cupulolithiasis where dislocated otoconia are associated with the posterior crista cupula and modify its physical responses such that the patients suffer from dizziness known as benign paroxysmal positional vertigo, BPPV [128,135].
Fig. 3.
The regional differences of otoconia related gene expression in the utricle and structural and protein differences in the utricular otoconia as revealed by immunocytochemistry are shown for a guinea pig. Note the differences in the size of otoconia crystals in striola (S) and extrastriolar regions (see insert). Oc90/95 is expressed in the non-sensory epithelium of the ear (red), otopetrin is expressed throughout the sensory epithelium (yellow); osteopontin is in hair cells (dark blue); otogelin is in supporting cells (cyan); α-tectorin is in supporting cells (orange); β-tectorin is in the striola (green). All proteins are found throughout the otoconia (lilac) except for otogelin (cyan) and β-tectorin (green) which are in the lower layers. No information on concentration differences of proteins within the otoconia matrix are available. Modified after [84].
In recent years the biochemical composition of otoconia, tectorial membrane and cupulae has been analyzed and several genes essential for their formation and attachment to the underlying sensory epithelia of the ear have been characterized [54,65,78,105,131,141]. These data suggest specific compositional differences between all three acellular structures of the ear. Thus far, seven major proteins of the extracellular matrices have been identified in the mammalian ear [54,141]. Additional data on another protein, otoancorin, that links the stereocilia of the hair cells to the otoconia are emerging [160].
Seven proteins have been identified to be related to otoconia formation (Fig. 3). The predominant protein in otoconia of mammals is otoconin 90/95 (OC90) [145, 150]. OC90 has conserved the structural features of the secreted phospholipase A2 (PLA2) except for the catalytic activity of the enzyme. Its highest structural similarity is to the calcium-dependent group X PLA2. The PLA2 like domains in OC90 have a higher content of anionic residues than their non-otic homologues, generating a highly acidic pH. In addition, these proteins have incorporated several chains of sulfated polysaccharides. Oc90 mRNA expression starts early in the ear of mice (around embryonic day 9.5) and expression is restricted to non-sensory areas of the ear. This unexpected expression pattern of OC90 changed the traditional view that otoconia are formed from secreted vesicles from supporting cells. In essence, Oc90 expression negatively defines sensory epithelia in developing and adult mice [141,150]. OC90 could thus present an early marker for non-sensory parts of the ear and could help elucidate changes in gene composition between non-sensory and sensory parts of the ear using quantitative and global gene expression analyses.
Another important protein that attaches the acellular matrix structures of the ear to the sensory epithelia is otogelin (otog). In Otog mutant animals, otoconia, cupulae and the tectorial membrane detach from the sensory epithelia [131] rendering the mutants unresponsive to sound and vestibular stimuli. Like OC90, otogelin is expressed early (embryonic day 10.5) and is apparently specifically expressed in the differentiating supporting cells [36]. This early expression thus suggests that some supporting cell commitment may predate proliferation of hair cells [126].
A crucial protein for otoconia formation is otopetrin [65]. If mutated, as in the tilt mutation, no otoconia will form [65,105]. In situ hybridization profiles of this gene demonstrated expression only in the otoconia bearing sensory maculae, presenting a distinguishing feature from other sensory organs of the ear. More recently, the gene responsible for another otoconia mutant mouse has been identified: head tilt mice, carry a mutation in Nox3 [74,107]. As with other mutations, it remains unclear how absence of this protein relates to loss of otoconia [132].
Unequal distribution profiles have been reported for the two proteins common to all acellular structures, α-tectorin and β-tectorin, using immunocytochemistry [54]. Interestingly, β-tectorin is only found in the inner layers of mammalian otoconia and is restricted to the striola region in birds thus indicating a possible role in the specification of striola. Despite that speculation, its function remains unclear as residual capacity to sense linear acceleration has not been tested in either Tecta or Tectb null mutant mice [54,78,144]. Expression data [118] show that in the saccule and utricle, Tecta mRNA is detected at E12.5, but Tectb mRNA is not observed until E14.5. Expression of β-tectorin mRNA ceases after P15, whereas β-tectorin mRNA expression continues within the striolar region of the utricle until at least P150. The results suggest that Tecta may play a developmental role prior to or concomitant with hair-cell differentiation, but before the appearance of hair bundles, whereas, Tectb may play a maintenance or functional role. Although Tecta is only expressed transiently during otoconia development, Tectb is continuously expressed within the striolar region of the utricle.
The last protein related to otoconia is osteopontin. Osteopontin is a non-collagenous bone matrix protein that is involved in ossification processes. mRNA has been identified in marginal cells of the stria vascularis, spiral ganglion cells, vestibular sensory hair cells and vestibular dark cells [127,137]. In contrast to most other acellular matrix proteins, the mRNA of osteopontin is expressed exclusively in vestibular hair cells [143]. In the utricle and saccule, osteopontin was detected by immunocytochemistry in the otoconia, suggesting that this protein is a component protein of mammalian otoconia.
While knowledge of the compositions of the inner ear acellular structures has thus been advanced, basic questions related to the development and maintenance of otoconia, cupula and tectorial membrane remain open. For example, how the proteins that form the otoconia assemble in the calcium poor endolymphatic fluid and how this extracellular protein assembly guides otoconia crystallization, but not calcification of the other acellular matrices is unknown. What is intriguing is that certain otoconia proteins are expressed outside the sensory epithelia [150]. In contrast, other proteins known to participate in otoconia formation, in particular otopetrin, α-tectorin and β-tectorin, are apparently strongly expressed in otoconiabearing sensory epithelia [65,118]. How all these different proteins relate to the distinct fine structure observed in acellular matrices of the ear [54,141] as well as to the different crystal structure of otoconia [58,85] and the different striolar/extrastriolar organization of otoconia [79] remains unclear.
Most important here is that the three different acellular structures of the mammalian ear, the cupulae of the cristae, the otoconia of the linear acceleration system and the tectorial membrane represent variations of a general molecular theme with a number of shared proteins being present in all three of these structures [54]. More information about the developmental expression of these proteins is needed to reveal the ontogenetic differences between the different epithelia to understand the developmental distinctions of these acellular covering structures. Likewise, a correlation is needed between the stereocilia maturation of hair cells and the development of the extracellular matrices.
5. Making sensory neurons and connecting them to the epithelia
Sensorineural development requires spatial and temporal regulation of many genes for the precise localization of the delaminating sensory neurons, ultimately upregulating the genes for sensory neuron and hair cell formation. For example, the reduction in general otocyst expression in Fgf10 might and the change in expression of Tbx1 [109,116] might alter the topology of proneuronal gene expression. Also, other proteins like GATA3, EYA1, SIX1 and FGF’s may be important for maintenance of the neurogenic capacity. Recent work on EYA1 and SIX1 show reduction and even complete loss of sensory neuron formation after an initial start [157,159]. Likewise, Gata3 null mutants [68] and sonic hedgehog (Shh) null mutants [120] show reduced formation of sensory neurons.
Loss-of-function (targeted null mutations of the respective genes) experiments have clarified some of the proneural genes crucial for inner ear primary sensory neuron development (Fig. 4). The work of Ma et al. [88] showed that inner ear primary sensory neuron formation requires the vertebrate bHLH gene, neurogenin 1 (Neurog1). As a consequence of the absence of primary sensory neuron formation in Neurog1 null mutants, the ear develops without brainstem connections as afferents do not form and neither efferents nor autonomic fibers appear to reach the ear in these animals [89]. Nevertheless, such ears develop fairly normally in their overall histology, including hair cells. This suggests that ear formation and development even of many hair cells is largely autonomous of innervation. While those hair cells that do form develop morphologically normal in the absence of innervation (except for some minor disorientation), hair cell numbers are reduced to various degrees in Neurog1 null mutant mice [96]. Most interestingly, the cochlea is shortened and the saccule is almost completely lost in these null mutants. In addition, extra rows of hair cells form in the shortened cochlea. These data suggest a significant interaction between progenitor cells that form primary neurons and progenitor cells that give rise to hair cells, supporting and other inner ear epithelial cells. The simplest explanation would be a clonal relationship of primary sensory clones with hair cell/supporting cell clones [40, 43]. However, other possible interactions can not be excluded [45] such as alteration of cell fate [96].
Fig. 4.
The effect of loss of hair cells (B,D) loss of a neurotrophin (C,E,F) or misexpression of a neurotrophin (F) on the pattern of vestibular innervation is shown. Late embryonic loss of hair cells in Pou4f3 null mice (B) and absence of differentiated hair cells in Atoh1 null mice (D) are both compatible with a targeted projection to vestibular sensory epithelia. Note, however, that the projection to the utricle is more profoundly affected (A,B,D). Loss of Bdnf results in complete loss of crista innervation and reduced innervation of the utricle (E). Some projection to cristae organs can be rescued in mutants in which sensory neuron degeneration is blocked in the absence of Bax (C). More fibers can be rescued to the canal cristae organs in Ntf3tgBDNF misexpressors (F) suggesting that a limited expression of Ntf3 is able to guide fibers to the canal cristae (C) but is not sufficient to rescue survival of the neurons (E). Note that misexpression of Bdnf under Ntf3 promoter control can also guide fibers to areas of the ear that have minor Ntf3 expression but no hair cells (arrow in F). AC, anterior crista; HC, horizontal crista; U, utricle. Bar indicates 100 μm. Modified after [50,61,139,155].
A gene that is immediately downstream of and mostly regulated by Neurog1 is Neurod1, another bHLH gene [88]. Null mutations of this gene have been analyzed and show severe reduction [72] to complete loss of sensory neurons [86]. Surviving vestibular sensory neurons may be in an unusual position and project in an aberrant pattern to only parts of the cochlea and only some vestibular sensory epithelia [47,72]. Judging from these data, Neurod1 appears to play a role in neuronal differentiation and survival, migration to appropriate areas, and target selection of peripheral neurites. Consistent with the effect on survival is the reduction and/or absence of certain neurotrophin receptor genes known to be essential for neuronal survival [72, 86]. Moreover, it is possible that the effects on survival of sensory neurons in Neurod1 null mutants are in part mediated by the reduced expression of a Pou domain factor, Pou4f1 [64]. As with Neurod1, Pou4f1 affects upregulation of certain neurotrophin receptors and thus might be only indirectly achieving its effect.
Recent molecular data suggest some candidate genes that are involved in regulating peripheral neuronal process development. Those candidate genes belong to the family of ephrin receptor and ligand complex and semaphorin ligand and receptor system known to be important in other developing systems [140]. Most simplistically, one might assume that hair cells simply attract growing neurites. If so, the molecular principle of neurite attraction should be abolished with hair cell ablation. A recent study on Pou4f3 null mutant mice that do not develop differentiated hair cells past early neonatal stages, reported growth of afferents to absent or disappearing hair cells (Fig. 4) as well as expression of neurotrophic factors in these mutants [155]. Interestingly, Atoh1 null mutant mice that never even form differentiated hair cells also display rather normal initial fiber growth (Fig. 4) but retention of fibers to the end of gestation is largely in areas of expression of the Bdnf neurotrophin [50]. Moreover, even if entire sensory epithelia formation is abrogated in specific null mutant mice, initial growth of afferents is rather targeted [109], suggesting that at least some of the pathfinding properties must reside in the interaction between growing neurites and the otocyst soluble factors.
Null mutants for the ephrin receptor B2, Ephb2, show circling behavior and altered axonal guidance of midline crossing efferents, but no data on alteration of peripheral innervation have been provided [29]. Other ephrin mutants exist but their phenotype has not yet been characterized for the ear and likely requires more detailed analysis than simple uniform fiber labeling as detailed errors might be missed using non-specific techniques. Recently, a role of one semaphorin, Sema3a, in providing a stop signal for growing afferents has been presented. Specifically, in the null mutant growing neurites do not stop at the vestibular sensory epithelia but rather continue to grow and may extend outside the ear as far as the skin above the ear [56]. While these data indicate some progress in the molecular basis of peripheral neurite guidance, it also highlights how rudimentary this insight is [46,50].
In contrast to this apparent paucity of data on molecular guidance, extensive knowledge exists on the molecular basis of sensory neuron survival [48]. Briefly, two neurotrophins (Bdnf, Ntf3) and their receptors (Ntrk2, Ntrk3) as well as the so-called low affinity receptor p75 have been identified in the developing ear using various techniques. Double null mutations of either the two neurotrophins or their two neurotrophin receptors have shown complete loss of all sensory neurons, demonstrating that those ligands and receptors are crucial for inner ear sensory neuron survival. Remaining issues center on the differential function of each neurotrophin receptor combination. Since most other vertebrates express only one neurotrophin in the ear, it also remains unclear why mammals have two. In addition, the expression patterns described show a highly dynamic change in the vestibular system [38, 111]. Data on transgenic knockin animals showed that each neurotrophin can be functionally replaced by the other neurotrophin. This suggests that the topological difference in expression matter more than any specific molecular effect [3,28]. This apparent capacity of functional replacement of each neurotrophin by the other reinvigorated the question for the functional significance of the presence of two neurotrophins as well as their differential expression.
An answer for this puzzle was recently provided using the transgenic knockin animals in which Bdnf has replaced Ntf3 (Ntf3tghBdnf). Selective tracer injection in these animals showed reorganization of the peripheral projection pattern of vestibular neurons into the basal turn of the cochlea [139] as well as rescue of projection to canal cristae in Ntf3tghBdnf mice combined with Bdnf null mutation (Fig. 4). Indeed, absence of Bdnf can result in some innervation of canal sensory epithelia (Fig. 4) in animals in which a gene important for cell death (Bax) has been eliminated [61]. These projections developed at the time the neurons are known to become susceptible to the neurotrophins for their survival suggesting that they become both neurotrophically as well as neurotropically dependent on neurotrophins at the same time. These data also suggest that the differential expression of neurotrophins in the ear plays a significant role in patterning the ear innervation. Interestingly, the central projection remained unchanged in these animals suggesting little if any influence of neurotrophins on the patterning of the central projections. Overall, these data suggest that the guidance to molecular basis processes that guide peripheral and central projection are distinct.
Most interesting are recent data on topographic segregation of afferent fibers from gravistatic organs that have hair cells with different polarities [91,92]. These data suggest that hair cells with one polarity receive an input different from those with an opposite polarity, with the striola presenting a dividing line (Fig. 5a). These data also show that no topographic segregation of afferent cells projecting to specific organs exists in the mouse ear (Fig. 5b). How such sharp boundaries form during development remains unclear.
Fig. 5.
The distribution of afferent fibers (A) and sensory neurons (B) after injections of two different lipophilic tracers into the cerebellum (red) and the superior vestibular nucleus (green) is shown in this 7 day old mouse. Note that red and green fibers are mixed to the canal cristae but are segregated along the striola in the utricle (u in A). These data imply that hair cell polarity reversal is accompanied by an almost complete segregation of fibers across the area of hair cell polarity reversal. Despite this extraordinary degree of segregation in their target organs, sensory neurons in the superior and inferior vestribular ganglion (SVG, IVG) are almost randomly mixed (B). AC, anterior crista; HC, horizontal crista; U, utricle. Bar indicates 200 μm.
In summary, certain molecular aspects of sensory neuron formation, guidance and survival have been clarified in recent years. Still, numerous open issues remain in this fast moving field and virtually no molecular data exist for the basic patterning of central projections. Interesting future work will have to sort out how the apparent relationship between sensory neurons and hair cells work at a molecular level.
6. Generating sensory epithelia and specifying position and polarity of hair cells
Hair cell development requires precise spatial and temporal regulation of many genes [45,71]. There is inadequate molecular data to specify areas of sensory patch formation but it is clear that the topology of these patches is dependent on the proper polarity acquisition of the developing otocyst [40]. A candidate for the establishment of dorso-ventral and possibly anterior posterior axis formation is Shh [120] in interaction with Gbx2 [12,83], Wnt1/3a [121] and Gli3 [23]. Longitudinal studies suggest that sensory epithelia form as a single patch that divides as the otocyst enlarges and becomes compartmentalized [38,100,109]. Null mutants strongly support the involvement of Fgf10 and Sox2 in sensory patch and hair cell formation [71,103], but the role of Bmp4 and the DELTA/NOTCH lateral inhibition process in this context is not completely clarified [6,26, 31]. Most importantly, recent data suggest formation of hair cell precursors in the absence of Atoh1, implying that Atoh1 can not activate the lateral inhibition system of delta and notch to sort out supporting cells from hair cells [50].
Unknown factors initiate the proliferation of hair cell precursor inside the growing and dividing sensory patches, such as Sox2 [71], and initiate upregulation of the retinoblastoma gene to terminate hair cell mitosis [93]. Interestingly, at least the formation of some hair cells is linked to the sensory neuron formation as Neurog1 null mutants are deficient in the number of hair cells and do never fully grow certain sensory patches, like the saccule [89]. It seems reasonable to assume that growth of epithelia depends on the proliferation of hair cell precursors, which may be set aside as early as E10.5 in the mouse [45,96]. How all these early patterning processes relate ultimately to the upregulation of genes relevant for hair cell differentiation remains unclear.
Hair cell differentiation requires a single bHLH gene, atonal homologue 1 (Atoh1), both during development [8] and during regeneration [52,66,69]. Recent data show that the gene relevant for hair cell precursor formation is still unknown [25] as undifferentiated hair cell precursors form in Atoh1 null mice [50]. Confirming and extending this findings (Fig. 6) are recent data on organ of Corti tissue culture showing that Atoh1 is not required for the initial expression of MyoVIIa, MyoVI, nicotinic aceytcholine receptor α9, fimbrin, POU4F3 and other hair cell markers [123]. This conclusion is also supported by the precocious expression of the neurotrophin Bdnf in the cochlea, prior to the upregulation of Atoh1, in what appears to be postmitotic hair cells [25,38,50]. Nevertheless, a possible involvement of Atoh1 in the selection of proliferating hair cell precursors can not be ruled out for vestibular sensory epithelia [96].
Fig. 6.
The formation of Atoh1 and Bdnf expressing cells is shown in the absence of Atoh1, a gene necessary for hair cell differentiation. Atoh1 and Bdnf genes were replaced with the LacZ reporter gene and the distribution of β-galactosidase, the enzyme generated by the LacZ gene, was revealed with a histochemical reaction that generates a blue reaction product. Note that either Atoh1-LacZ or Bdnf-LacZ reveals all hair cells of vestibular epithelia (A,C,E) in mice heterozygotic for these genes. Interestingly, in mice null for Atoh1 and that show no differentiated hair cells, expression of Atoh1-LacZ (B) and Bdnf-LacZ (D) shows a distribution of cells consistent with the formation of sensory epithelia. In addition, those sensory epithelia receive an innervation that is somewhat targeted toward those cells (E,F). Note that Bdnf expression in the utricle depends on the expression of Atoh1 (D). AC, anterior crista; HC, horizontal crista; PC, posterior crista; S, saccule; SN, sensory neurons; U, utricle. Bar indicates 100 μm. Modified after [50].
Several crucial genes for hair cell differentiation and maintenance are now characterized: Atoh1, Pou4f3, Gfi1 and Barhl1 [8,37,82,148,155]. More recent work indicates that the Pou domain factor (Pou4f3) regulates the zinc finger protein, GFI1 [62]. How those genes in turn tie into Barhl1 regulation as well as hair cell differentiation remains unclear. It appears, however, that major aspects of hair cell differentiation can be accomplished in the absence of either Pou4f3 or Gfi1 [62]. Thus Atoh1 is certainly able to promote hair cell differentiation to the extent of stereocilia formation even without those genes, but identification of hair cells appears to be even possible in the absence of Atoh1 [50] and those cells express the BDNF neurotrophin(Fig. 6).
Molecules that specify the hair cell competent area are unknown. Transfection experiments using Atoh1 gene loaded viruses have shown that cells in the greater epithelial ridge can transform into hair cells in neonates and adults [52,66,69]. How Atoh1 is initially upregulated in the cells that will become hair cells is not fully established and may vary between different sensory epithelia, the species used, as well as the technical limitations of the specific techniques employed [25,96,122]. While the role of p27 in terminal mitosis of hair cell precursors is now clear [24], other genes relevant for mitosis need to be studied are needed as well such as retinoblastoma [93]. Atoh1 drives related HLH genes (Hes1 and Hes5) that regulate the mosaic of hair cell and supporting cell formation [31,52,158]. Mutation in the Hes genes result in extra hair cell formation, supporting the notion that these genes regulate the hair cell/supporting cell mosaic and have limited effected on the overall topology and extent of hair cell progenitor formation that is regulated by as yet unknown genes.
How all those cell fate determination process are molecularly integrated into the process of cell contact formation that uses a variety of different cadherins and related molecules [70] that appear to play crucial roles in hair cell polarity formation [6,30,33,99] remains unknown. There is no direct connection with the differential distribution of actin and espin to form the stereociliary staircase and the mechanism of stereociliary bundle polarity is yet to be shown [35]. It is conceivable, but unknown, that differential distribution of espin that regulates the assembly of actin in a quantitative fashion maybe at the basis of the differential growth of stereocilia. Thus the effects described for various transmembrane and secreted signals in the establishment of hair cell polarity require a more direct link with known molecules that are differentially distributed in various hair cells.
In summary, while our molecular understanding of hair cell formation and differentiation is by far the most advanced in all ear morphogenetic and histogenetic processes, it also shows gaps that require much further work to be connected in a logical and causal sequence in order to be used in a controlled fashion to remedy one aspect of vestibular loss of function: to replace lost hair cells. Recent attempts to tie the functional maturation of hair cells [35] to the onset of transmitter release mechanisms [130] remain to be completed using appropriate mutational analysis of conditional mutants with conditional deletion of relevant genes.
At age 70 in humans the decline in vestibular and cochlear hair cell numbers may have depleted the capacity of the system to compensate and hearing and vestibular disorders become apparent. While devices to restore hearing have been designed, such as cochlear and cochlear nucleus implants, electronic devices for vestibular information delivery are in their infancy. Using the insights into the molecular basis of hair cell formation to regenerate hair cells and neurons and to connect them properly so that vestibular sensation is fully restored in the elderly would be a desired outcome of these studies summarized above.
Significant progress toward these goals show that gene therapy using Atoh1 loaded adenoviruses can transform supporting cells into hair cells [66,69]. Regenerating hair cells by forcing them to divide in retinoblastoma null mutants seem to be an alternative way that requires, however, further studies are needed to avoid the possibility of cancer formation [93]. Other avenues to achieve functional recovery are the regeneration of hair cells and sensory neurons from embryonic or adult stem cells [80,81] and isolation of proliferating ganglion cell precursors appears to be possible from the human cochlea [117]. Implanting such cells into ears depleted of either hair cells or sensory neurons or both might be a future direction to restore hearing and vestibular signal sensing. Transfering those data from the cochlea to the vestibular system is another challenge for the field [7,138]. Overall, these data support the notion that curing hair cell loss in the cochlea and the vestibular sensory organs might become a possibility although more work is needed before any of these techniques can be used for clinical trials.
7. Conclusion and outlook
Molecular progress has revealed some aspects of ear morphogenesis, sensory epithelia and extracellular matrix formation and formation and specification of sensory neuron connections. Nevertheless, these insights provide only a beginning and numerous open questions remain, requiring more sophisticated molecular analysis combined with physiological experiments in viable mutant mice that carry ear directed mutations. Progress already indicates significant clinical potential that may relate to treatments of vestibular sensation loss, by mediating hair cell and sensory neuron regeneration. Some aspects of vestibular ear evolution appear to relate to the recruitment of specific genes into the existing network of gene expression that mediates ear development. However, other aspects of vestibular ear evolution such as specific fiber projection to distinct endorgans and their central targets are less likely to depend on single genes.
Acknowledgments
This work was supported by a grant from NIH (RO1 DC005590; BF, KB) and NASA (NAG 2-1611; BF, KB), COBRE (1P20RR018788-01;YWL, BF, KB) and LB692 (BF, KB). This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number 1 C06 RR17417-01 from the National Center for Research Resources, National Institutes of Health. We acknowledge the use of the confocal microscope facility of the NCCB, supported by EPSCoR EPS-0346476 (CFD 47.076).
References
- [1].Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Levi-Acobas F, Cruaud C, Le Merrer M, Mathieu M, Konig R, Vigneron J, Weissenbach J, Petit C, Weil D. Clustering of mutations responsible for branchio-oto-renal (BOR) syndrome in the eyes absent homologous region (eyaHR) of EYA1. Hum Mol Genet. 1997;6:2247–2255. doi: 10.1093/hmg/6.13.2247. [DOI] [PubMed] [Google Scholar]
- [2].Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Weil D, Cruaud C, Sahly I, Leibovici M, Bitner-Glindzicz M, Francis M, Lacombe D, Vigneron J, Charachon R, Boven K, Bedbeder P, Van Regemorter N, Weissenbach J, Petit C. A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat Genet. 1997;15:157–164. doi: 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- [3].Agerman K, Hjerling-Leffler J, Blanchard MP, Scarfone E, Canlon B, Nosrat C, Ernfors P. BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system development. Development. 2003;130:1479–1491. doi: 10.1242/dev.00378. [DOI] [PubMed] [Google Scholar]
- [4].Alvarez Y, Alonso MT, Vendrell V, Zelarayan LC, Chamero P, Theil T, Bosl MR, Kato S, Maconochie M, Riethmacher D, Schimmang T. Requirements for FGF3 and FGF10 during inner ear formation. Development. 2003;130:6329–6338. doi: 10.1242/dev.00881. [DOI] [PubMed] [Google Scholar]
- [5].Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- [6].Barald KF, Kelley MW. From placode to polarization: new tunes in inner ear development. Development. 2004;131:4119–4130. doi: 10.1242/dev.01339. [DOI] [PubMed] [Google Scholar]
- [7].Bermingham-McDonogh O, Rubel EW. Hair cell regeneration: winging our way towards a sound future. Curr Opin Neurobiol. 2003;13:119–126. doi: 10.1016/s0959-4388(03)00018-7. [DOI] [PubMed] [Google Scholar]
- [8].Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- [9].Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- [10].Bertrand V, Hudson C, Caillol D, Popovici C, Lemaire P. Neural tissue in ascidian embryos is induced by FGF9/16/20, acting via a combination of maternal GATA and Ets transcription factors. Cell. 2003;115:615–627. doi: 10.1016/s0092-8674(03)00928-0. [DOI] [PubMed] [Google Scholar]
- [11].Bober E, Rinkwitz S, Herbrand H. Molecular basis of otic commitment and morphogenesis: a role for homeodomain-containing transcription factors and signaling molecules. Curr Top Dev Biol. 2003;57:151–175. doi: 10.1016/s0070-2153(03)57005-3. [DOI] [PubMed] [Google Scholar]
- [12].Bok J, Bronner-Fraser M, Wu DK. Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development. 2005;132:2115–2124. doi: 10.1242/dev.01796. [DOI] [PubMed] [Google Scholar]
- [13].Braun CB. Schreiner organs: a new craniate chemosensory modality in hagfishes. J Comp Neurol. 1998;392:135–163. doi: 10.1002/(sici)1096-9861(19980309)392:2<135::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [14].Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- [15].Brown ST, Martin K, Groves AK. Molecular basis of inner ear induction. Curr Top Dev Biol. 2003;57:115–149. doi: 10.1016/s0070-2153(03)57004-1. [DOI] [PubMed] [Google Scholar]
- [16].Budelmann B. Hearing in Nonarthropod Invertebrates. In: Webster DB, Fay RR, Popper AN, editors. The Evolutionary Biology of Hearing. Springer Verlag; New York, NY: 1992. pp. 141–155. [Google Scholar]
- [17].Burighel P, Lane NJ, Fabio G, Stefano T, Zaniolo G, Carnevali MD, Manni L. Novel, secondary sensory cell organ in ascidians: in search of the ancestor of the vertebrate lateral line. J Comp Neurol. 2003;461:236–249. doi: 10.1002/cne.10666. [DOI] [PubMed] [Google Scholar]
- [18].Burton Q, Cole LK, Mulheisen M, Chang W, Wu DK. The role of Pax2 in mouse inner ear development. Dev Biol. 2004;272:161–175. doi: 10.1016/j.ydbio.2004.04.024. [DOI] [PubMed] [Google Scholar]
- [19].Cantos R, Cole LK, Acampora D, Simeone A, Wu DK. Patterning of the mammalian cochlea. Proc Natl Acad Sci USA. 2000;97:11707–11713. doi: 10.1073/pnas.97.22.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chang W, Nunes FD, De Jesus-Escobar JM, Harland R, Wu DK. Ectopic noggin blocks sensory and nonsensory organ morphogenesis in the chicken inner ear. Dev Biol. 1999;216:369–381. doi: 10.1006/dbio.1999.9457. [DOI] [PubMed] [Google Scholar]
- [21].Chang W, ten Dijke P, Wu DK. BMP pathways are involved in otic capsule formation and epithelial-mesenchymal signaling in the developing chicken inner ear. Dev Biol. 2002;251:380–394. doi: 10.1006/dbio.2002.0822. [DOI] [PubMed] [Google Scholar]
- [22].Chang W, Brigande JV, Fekete DM, Wu DK. The development of semicircular canals in the inner ear: role of FGFs in sensory cristae. Development. 2004;131:4201–4211. doi: 10.1242/dev.01292. [DOI] [PubMed] [Google Scholar]
- [23].Chang W, Cole LK, Cantos R, Wu DK. Molecular genetics of vestibular organ development. In: Highstein SM, Fay RR, Popper AN, editors. The Vestibular System. Vol. 19. Springer Verlag; New York: 2004. pp. 11–56. [Google Scholar]
- [24].Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- [25].Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- [26].Cole LK, Le Roux I, Nunes F, Laufer E, Lewis J, Wu DK. Sensory organ generation in the chicken inner ear: contributions of bone morphogenetic protein 4, serrate1, and lunatic fringe. J Comp Neurol. 2000;424:509–520. doi: 10.1002/1096-9861(20000828)424:3<509::aid-cne8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- [27].Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–397. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- [28].Coppola V, Kucera J, Palko ME, Martinez-De Velasco J, Lyons WE, Fritzsch B, Tessarollo L. Dissection of NT3 functions in vivo by gene replacement strategy. Development. 2001;128:4315–4327. doi: 10.1242/dev.128.21.4315. [DOI] [PubMed] [Google Scholar]
- [29].Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron. 2000;26:417–430. doi: 10.1016/s0896-6273(00)81174-5. [DOI] [PubMed] [Google Scholar]
- [30].Dabdoub A, Donohue MJ, Brennan A, Wolf V, Montcouquiol M, Sassoon DA, Hseih JC, Rubin JS, Salinas PC, Kelley MW. Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development. 2003;130:2375–2384. doi: 10.1242/dev.00448. [DOI] [PubMed] [Google Scholar]
- [31].Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- [32].David ES, Luke NH, Livingston BT. Characterization of a gene encoding a developmentally regulated winged helix transcription factor of the sea urchin Strongylocentrotus purpuratus. Gene. 1999;236:97–105. doi: 10.1016/s0378-1119(99)00248-6. [DOI] [PubMed] [Google Scholar]
- [33].Davies A, Formstone C, Mason I, Lewis J. Planar polarity of hair cells in the chick inner ear is correlated with polarized distribution of c-flamingo-1 protein. Dev Dyn. 2005;233:998–1005. doi: 10.1002/dvdy.20376. [DOI] [PubMed] [Google Scholar]
- [34].Davis RJ, Shen W, Sandler YI, Heanue TA, Mardon G. Characterization of mouse Dach2, a homologue of Drosophila dachshund. Mech Dev. 2001;102:169–179. doi: 10.1016/s0925-4773(01)00307-0. [DOI] [PubMed] [Google Scholar]
- [35].Eatock RA, Hurley KM. Functional development of hair cells. Curr Top Dev Biol. 2003;57:389–448. doi: 10.1016/s0070-2153(03)57013-2. [DOI] [PubMed] [Google Scholar]
- [36].El-Amraoui A, Cohen-Salmon M, Petit C, Simmler MC. Spatiotemporal expression of otogelin in the developing and adult mouse inner ear. Hear Res. 2001;158:151–159. doi: 10.1016/s0378-5955(01)00312-4. [DOI] [PubMed] [Google Scholar]
- [37].Erkman L, McEvilly RJ, Luo L, Ryan AK, Hooshmand F, O’Connell SM, Keithley EM, Rapaport DH, Ryan AF, Rosenfeld MG. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996;381:603–606. doi: 10.1038/381603a0. [DOI] [PubMed] [Google Scholar]
- [38].Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fekete DM, Homburger SA, Waring MT, Riedl AE, Garcia LF. Involvement of programmed cell death in morphogenesis of the vertebrate inner ear. Development. 1997;124:2451–2461. doi: 10.1242/dev.124.12.2451. [DOI] [PubMed] [Google Scholar]
- [40].Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol. 2002;12:35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- [41].Fritzsch B. Similarities and differences in lancelet and craniate nervous systems. Isr. J. Zool. 1996;42:147–160. [Google Scholar]
- [42].Fritzsch B, Barald K, Lomax M. Early embryology of the vertebrate ear. In: Rubel EW, Popper AN, Fay RR, editors. Springer Handbook of Auditory Research. XII. Springer Verlag; New York: 1998. pp. 80–145. Development of the Auditory System. [Google Scholar]
- [43].Fritzsch B, Beisel KW. Evolution and development of the vertebrate ear. Brain Res Bull. 2001;55:711–721. doi: 10.1016/s0361-9230(01)00558-5. [DOI] [PubMed] [Google Scholar]
- [44].Fritzsch B, Signore M, Simeone A. Otx1 null mutant mice show partial segregation of sensory epithelia comparable to lamprey ears. Dev Genes Evol. 2001;211:388–396. doi: 10.1007/s004270100166. [DOI] [PubMed] [Google Scholar]
- [45].Fritzsch B, Beisel KW, Jones K, Farinas I, Maklad A, Lee J, Reichardt LF. Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol. 2002;53:143–156. doi: 10.1002/neu.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fritzsch B. Development of inner ear afferent connections: forming primary neurons and connecting them to the developing sensory epithelia. Brain Res Bull. 2003;60:423–433. doi: 10.1016/s0361-9230(03)00048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Fritzsch B, Beisel KW. Molecular conservation and novelties in vertebrate ear development. Curr Top Dev Biol. 2003;57:1–44. doi: 10.1016/s0070-2153(03)57001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fritzsch B, Coppola V, Tessarollo L, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Progress in Brain Research. 2003;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- [49].Fritzsch B, Beisel KW. Keeping sensory cells and evolving neurons to connect them to the brain: molecular conservation and novelties in vertebrate ear development. Brain Behav Evol. 2004;64:182–197. doi: 10.1159/000079746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Fritzsch B, Matei VA, Nichols DH, Bermingham N, Jones K, Beisel KW, Wang VY. Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev Dyn. 2005;233:570–583. doi: 10.1002/dvdy.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fritzsch B, Piatigorsky J. Ancestry of photic and mechanic sensation? Science. 2005;308:1113–1114. [PubMed] [Google Scholar]
- [52].Gao WQ. Hair cell development in higher vertebrates. Curr Top Dev Biol. 2003;57:293–319. doi: 10.1016/s0070-2153(03)57010-7. [DOI] [PubMed] [Google Scholar]
- [53].Gerlach LM, Hutson MR, Germiller JA, NguyenLuu D, Victor JC, Barald KF. Addition of the BMP4 antagonist, noggin, disrupts avian inner ear development. Development. 2000;127:45–54. doi: 10.1242/dev.127.1.45. [DOI] [PubMed] [Google Scholar]
- [54].Goodyear RJ, Richardson GP. Extracellular matrices associated with the apical surfaces of sensory epithelia in the inner ear: molecular and structural diversity. J Neurobiol. 2002;53:212–227. doi: 10.1002/neu.10097. [DOI] [PubMed] [Google Scholar]
- [55].Grothe B, Carr EC, Casseday JH, Fritzsch B, Köppl C. The Evolution of Central Pathways and Their Neural Processing Patterns. In: Manley GA, Popper AN, Fay RR, editors. Springer Handbook of Auditory Research: Evolution of the Vertebrate Auditory System. Spinger-Verlag; New York: 2004. pp. 289–359. [Google Scholar]
- [56].Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 Conveys Semaphorin and VEGF Signaling during Neural and Cardiovascular Development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hadrys T, Braun T, Rinkwitz-Brandt S, Arnold HH, Bober E. Nkx5-1 controls semicircular canal formation in the mouse inner ear. Development. 1998;125:33–39. doi: 10.1242/dev.125.1.33. [DOI] [PubMed] [Google Scholar]
- [58].Hallworth R, Wiederhold ML, Campbell JB, Steyger PS. Atomic force microscope observations of otoconia in the newt. Hear Res. 1995;85:115–121. doi: 10.1016/0378-5955(95)00038-6. [DOI] [PubMed] [Google Scholar]
- [59].Hanson IM. Mammalian homologues of the Drosophila eye specification genes. Semin Cell Dev Biol. 2001;12:475–484. doi: 10.1006/scdb.2001.0271. [DOI] [PubMed] [Google Scholar]
- [60].Hatini V, Ye X, Balas G, Lai E. Dynamics of placodal lineage development revealed by targeted transgene expression. Dev Dyn. 1999;215:332–343. doi: 10.1002/(SICI)1097-0177(199908)215:4<332::AID-AJA5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- [61].Hellard D, Brosenitsch T, Fritzsch B, Katz DM. Cranial sensory neuron development in the absence of brain-derived neurotrophic factor in BDNF/Bax double null. Dev Biol. 2004;275:34–43. doi: 10.1016/j.ydbio.2004.07.021. [DOI] [PubMed] [Google Scholar]
- [62].Hertzano R, Montcouquiol M, Rashi-Elkeles S, Elkon R, Yucel R, Frankel WN, Rechavi G, Moroy T, Friedman TB, Kelley MW, Avraham KB. Transcription profiling of inner ears from Pou4f3ddl/ddl identifies Gfi1 as a target of the Pou4f3 deafness gene. Hum Mol Genet. 2004;13:2143–2153. doi: 10.1093/hmg/ddh218. [DOI] [PubMed] [Google Scholar]
- [63].Highstein SM, Rabbitt RD, Holstein GR, Boyle RD. Determinants of spatial and temporal coding by semicircular canal afferents. J Neurophysiol. 2005;93:2359–2370. doi: 10.1152/jn.00533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Huang EJ, Liu W, Fritzsch B, Bianchi LM, Reichardt LF, Xiang M. Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development. 2001;128:2421–2432. doi: 10.1242/dev.128.13.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hurle B, Ignatova E, Massironi SM, Mashimo T, Rios X, Thalmann I, Thalmann R, Ornitz DM. Non-syndromic vestibular disorder with otoconial agenesis in tilted/mergulhador mice caused by mutations in otopetrin 1. Hum Mol Genet. 2003;12:777–789. doi: 10.1093/hmg/ddg087. [DOI] [PubMed] [Google Scholar]
- [66].Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- [67].Johnson KR, Cook SA, Erway LC, Matthews AN, Sanford LP, Paradies NE, Friedman RA. Inner ear and kidney anomalies caused by IAP insertion in an intron of the Eya1 gene in a mouse model of BOR syndrome. Hum Mol Genet. 1999;8:645–653. doi: 10.1093/hmg/8.4.645. [DOI] [PubMed] [Google Scholar]
- [68].Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol. 2001;429:615–630. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- [69].Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kelley MW. Cell adhesion molecules during inner ear and hair cell development, including notch and its ligands. Curr Top Dev Biol. 2003;57:321–356. doi: 10.1016/s0070-2153(03)57011-9. [DOI] [PubMed] [Google Scholar]
- [71].Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- [72].Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kozmik Z, Daube M, Frei E, Norman B, Kos L, Dishaw LJ, Noll M, Piatigorsky J. Role of Pax Genes in Eye Evolution. A Cnidarian PaxB Gene Uniting Pax2 and Pax6 Functions. Dev Cell. 2003;5:773–785. doi: 10.1016/s1534-5807(03)00325-3. [DOI] [PubMed] [Google Scholar]
- [74].Krause KH. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn J Infect Dis. 2004;57:S28–29. [PubMed] [Google Scholar]
- [75].Lacalli TC. Sensory systems in amphioxus: a window on the ancestral chordate condition. Brain Behav Evol. 2004;64:148–162. doi: 10.1159/000079744. [DOI] [PubMed] [Google Scholar]
- [76].Ladher RK, Wright TJ, Moon AM, Mansour SL, Schoenwolf GC. FGF8 initiates inner ear induction in chick and mouse. Genes Dev. 2005;19:603–613. doi: 10.1101/gad.1273605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lawoko-Kerali G, Rivolta MN, Holley M. Expression of the transcription factors GATA3 and Pax2 during development of the mammalian inner ear. J Comp Neurol. 2002;442:378–391. doi: 10.1002/cne.10088. [DOI] [PubMed] [Google Scholar]
- [78].Legan PK, Lukashkina VA, Goodyear RJ, Kossi M, Russell IJ, Richardson GP. A targeted deletion in alpha-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron. 2000;28:273–285. doi: 10.1016/s0896-6273(00)00102-1. [DOI] [PubMed] [Google Scholar]
- [79].Lewis ER, Leverenz EL, Bialek WS. The vertebrate inner ear. CRC Press; Boca Raton: 1985. p. 248. [Google Scholar]
- [80].Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- [81].Li H, Roblin G, Liu H, Heller S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc Natl Acad Sci USA. 2003;100:13495–13500. doi: 10.1073/pnas.2334503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Li S, Price SM, Cahill H, Ryugo DK, Shen MM, Xiang M. Hearing loss caused by progressive degeneration of cochlear hair cells in mice deficient for the Barhl1 homeobox gene. Development. 2002;129:3523–3532. doi: 10.1242/dev.129.14.3523. [DOI] [PubMed] [Google Scholar]
- [83].Lin Z, Cantos R, Patente M, Wu DK. Gbx2 is required for the morphogenesis of the mouse inner ear: a downstream candidate of hindbrain signaling. Development. 2005;132:2309–2318. doi: 10.1242/dev.01804. [DOI] [PubMed] [Google Scholar]
- [84].Lindeman HH. Studies on the morphology of the sensory regions o fht evestibular apparatus. Advances in Anatomy, Embryology and Cell Biology. 1969;42:7–113. [Google Scholar]
- [85].Lins U, Farina M, Kurc M, Riordan G, Thalmann R, Thalmann I, Kachar B. The otoconia of the guinea pig utricle: internal structure, surface exposure, and interactions with the filament matrix. J Struct Biol. 2000;131:67–78. doi: 10.1006/jsbi.2000.4260. [DOI] [PubMed] [Google Scholar]
- [86].Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 2000;14:2839–2854. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lysakowski A, Goldberg JM. Morphophysiology of the Vestibular Periphery. In: Highstein SM, Fay RR, Popper AN, editors. The Vestibular System. Vol. 19. Springer Verlag; New York: 2004. pp. 57–152. [Google Scholar]
- [88].Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- [89].Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Mackie GO, Singla CL. Cupular organs in two species of Corella (Tunicata: Ascidiacea) Invert. Biol. 2004;123:269–281. [Google Scholar]
- [91].Maklad A, Fritzsch B. The developmental segregation of posterior crista and saccular vestibular fibers in mice: A carbocyanine tracer study using confocal microscopy. Develop. Brain Research. 2002;135 doi: 10.1016/s0165-3806(01)00327-3. [DOI] [PubMed] [Google Scholar]
- [92].Maklad A, Fritzsch B. Development of vestibular afferent projections into the hindbrain and their central targets. Brain Res Bull. 2003;60:497–510. doi: 10.1016/s0361-9230(03)00054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Mantela J, Jiang Z, Ylikoski J, Fritzsch B, Zacksenhaus E, Pirvola U. The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development. 2005;132:2377–2388. doi: 10.1242/dev.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Markl H. The perception of gravity and of angular acceleration in invertebrates. In: Kornhuber HH, editor. Handbook of Sensory Physiology, Vol. VI/1 Vestibular System. Springer Verlag; Berlin: 1974. pp. 17–74. [Google Scholar]
- [95].Martin P, Swanson GJ. Descriptive and experimental analysis of the epithelial remodellings that control semicircular canal formation in the developing mouse inner ear. Dev Biol. 1993;159:549–558. doi: 10.1006/dbio.1993.1263. [DOI] [PubMed] [Google Scholar]
- [96].Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005;234:633–650. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Mazan S, Jaillard D, Baratte B, Janvier P. Otx1 gene-controlled morphogenesis of the horizontal semicircular canal and the origin of the gnathostome characteristics. Evol Dev. 2000;2:186–193. doi: 10.1046/j.1525-142x.2000.00062.x. [DOI] [PubMed] [Google Scholar]
- [98].Merlo GR, Paleari L, Mantero S, Zerega B, Adamska M, Rinkwitz S, Bober E, Levi G. The Dlx5 homeobox gene is essential for vestibular morphogenesis in the mouse embryo through a BMP4-mediated pathway. Dev Biol. 2002;248:157–169. doi: 10.1006/dbio.2002.0713. [DOI] [PubMed] [Google Scholar]
- [99].Montcouquiol M, Kelley MW. Planar and vertical signals control cellular differentiation and patterning in the mammalian cochlea. J Neurosci. 2003;23:9469–9478. doi: 10.1523/JNEUROSCI.23-28-09469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Morsli H, Tuorto F, Choo D, Postiglione MP, Simeone A, Wu DK. Otx1 and Otx2 activities are required for the normal development of the mouse inner ear. Development. 1999;126:2335–2343. doi: 10.1242/dev.126.11.2335. [DOI] [PubMed] [Google Scholar]
- [102].Murakami Y, Pasqualetti M, Takio Y, Hirano S, Rijli FM, Kuratani S. Segmental development of reticulospinal and branchiomotor neurons in lamprey: insights into the evolution of the vertebrate hindbrain. Development. 2004;131:983–995. doi: 10.1242/dev.00986. [DOI] [PubMed] [Google Scholar]
- [103].Ohuchi H, Yasue A, Ono K, Sasaoka S, Tomonari S, Takagi A, Itakura M, Moriyama K, Noji S, Nohno T. Identification of cis-element regulating expression of the mouse Fgf10 gene during inner ear development. Dev Dyn. 2005;233:177–187. doi: 10.1002/dvdy.20319. [DOI] [PubMed] [Google Scholar]
- [104].Ohyama T, Groves AK. Expression of mouse Foxi class genes in early craniofacial development. Dev Dyn. 2004;231:640–646. doi: 10.1002/dvdy.20160. [DOI] [PubMed] [Google Scholar]
- [105].Ornitz DM, Bohne BA, Thalmann I, Harding GW, Thalmann R. Otoconial agenesis in tilted mutant mice. Hear Res. 1998;122:60–70. doi: 10.1016/s0378-5955(98)00080-x. [DOI] [PubMed] [Google Scholar]
- [106].Ornitz DM, Marie PJ. FGF signalling pathways in enchondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16:1446–1465. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- [107].Paffenholz R, Bergstrom RA, Pasutto F, Wabnitz P, Munroe RJ, Jagla W, Heinzmann U, Marquardt A, Bareiss A, Laufs J, Russ A, Stumm G, Schimenti JC, Bergstrom DE. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004;18:486–491. doi: 10.1101/gad.1172504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Patient RK, McGhee JD. The GATA family (vertebrates and invertebrates) Curr Opin Genet Dev. 2002;12:416–422. doi: 10.1016/s0959-437x(02)00319-2. [DOI] [PubMed] [Google Scholar]
- [109].Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn. 2003;227:203–215. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Pfister M, Toth T, Thiele H, Haack B, Blin N, Zenner HP, Sziklai I, Murnberg P, Kupka S. A 4-bp Insertion in the eya-Homologous Region (eyaHR) of EYA4 Causes Hearing Impairment in a Hungarian Family Linked to DFNA10. Mol Med. 2002;8:607–611. [PMC free article] [PubMed] [Google Scholar]
- [111].Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumae U, Saarma M. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci USA. 1992;89:9915–9919. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Pirvola U, Spencer-Dene B, Xing-Qun L, Kettunen P, Thesleff I, Fritzsch B, Dickson C, Ylikoski J. FGF/FGFR-2(IIIb) signaling is essential for inner ear morphogenesis. J Neurosci. 2000;20:6125–6134. doi: 10.1523/JNEUROSCI.20-16-06125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Pirvola U, Ylikoski J, Trokovic R, Hebert J, McConnell S, Partanen J. FGFR1 Is Required for the Development of the Auditory Sensory Epithelium. Neuron. 2002;35:671. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- [114].Pirvola U, Zhang X, Mantela J, Ornitz DM, Ylikoski J. Fgf9 signaling regulates inner ear morphogenesis through epithelial-mesenchymal interactions. Dev Biol. 2004;273:350–360. doi: 10.1016/j.ydbio.2004.06.010. [DOI] [PubMed] [Google Scholar]
- [115].Rabbitt RD, Boyle R, Highstein SM. Physiology of the semicircular canals after surgical plugging. Ann N Y Acad Sci. 2001;942:274–286. doi: 10.1111/j.1749-6632.2001.tb03752.x. [DOI] [PubMed] [Google Scholar]
- [116].Raft S, Nowotschin S, Liao J, Morrow BE. Suppression of neural fate and control of inner ear morphogenesis by Tbx1. Development. 2004;131:1801–1812. doi: 10.1242/dev.01067. [DOI] [PubMed] [Google Scholar]
- [117].Rask-Andersen H, Bostrom M, Gerdin B, Kinnefors A, Nyberg G, Engstrand T, Miller JM, Lindholm D. Regeneration of human auditory nerve. In vitro/in video demonstration of neural progenitor cells in adult human and guinea pig spiral ganglion. Hear Res. 2005;203:180–191. doi: 10.1016/j.heares.2004.12.005. [DOI] [PubMed] [Google Scholar]
- [118].Rau A, Legan PK, Richardson GP. Tectorin mRNA expression is spatially and temporally restricted during mouse inner ear development. J Comp Neurol. 1999;405:271–280. [PubMed] [Google Scholar]
- [119].Retzius G. Das Gehörorgan der Wirbeltiere: II. Das Gehörorgan der Amnioten. Samson und Wallin; Stockholm: 1884. p. 345. [Google Scholar]
- [120].Riccomagno MM, Martinu L, Mulheisen M, Wu DK, Epstein DJ. Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 2002;16:2365–2378. doi: 10.1101/gad.1013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Riccomagno MM, Takada S, Epstein DJ. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005;19:1612–1623. doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Riley BB. Genes controlling the development of the zebrafish inner ear and hair cells. Curr Top Dev Biol. 2003;57:357–388. doi: 10.1016/s0070-2153(03)57012-0. [DOI] [PubMed] [Google Scholar]
- [123].Rivolta MN, Halsall A, Johnson CM, Tones MA, Holley MC. Transcript profiling of functionally related groups of genes during conditional differentiation of a Mammalian cochlear hair cell line. Genome Res. 2002;12:1091–1099. doi: 10.1101/gr.225602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Romand R, Varela-Nieto I. Development of Auditory and Vestibular Systems-3. Molecular Development of the Inner Ear. Curr Top Dev Biol. 2003;57:1–481. [Google Scholar]
- [125].Ross MD, Johnsson L-G, Peacor D. Observations on normal and degenerating human otoconia. Ann Otol. 1976;85:310–326. doi: 10.1177/000348947608500302. [DOI] [PubMed] [Google Scholar]
- [126].Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol: Suppl. 1967;220:221–244. [PubMed] [Google Scholar]
- [127].Sakagami M. Role of osteopontin in the rodent inner ear as revealed by in situ hybridization. Med Electron Microsc. 2000;33:3–10. doi: 10.1007/s007950000001. [DOI] [PubMed] [Google Scholar]
- [128].Salvinelli F, Firrisi L, Casale M, Trivelli M, D’Ascanio L, Lamanna F, Greco F, Costantino S. Benign paroxysmal positional vertigo: diagnosis and treatment. Clin Ter. 2004;155:395–400. [PubMed] [Google Scholar]
- [129].Sato M, Saigo K. Involvement of pannier and u-shaped in regulation of decapentaplegic-dependent wingless expression in developing Drosophila notum. Mech Dev. 2000;93:127–138. doi: 10.1016/s0925-4773(00)00282-3. [DOI] [PubMed] [Google Scholar]
- [130].Schimmang T, Tan J, Muller M, Zimmermann U, Rohbock K, Kopschall I, Limberger A, Minichiello L, Knipper M. Lack of Bdnf and TrkB signalling in the postnatal cochlea leads to a spatial reshaping of innervation along the tonotopic axis and hearing loss. Development. 2003;130:4741–4750. doi: 10.1242/dev.00676. [DOI] [PubMed] [Google Scholar]
- [131].Simmler MC, Cohen-Salmon M, El-Amraoui A, Guillaud L, Benichou JC, Petit C, Panthier JJ. Targeted disruption of otog results in deafness and severe imbalance. Nat Genet. 2000;24:139–143. doi: 10.1038/72793. [DOI] [PubMed] [Google Scholar]
- [132].Sollner C, Schwarz H, Geisler R, Nicolson T. Mutated otopetrin 1 affects the genesis of otoliths and the localization of Starmaker in zebrafish. Dev Genes Evol. 2004;214:582–590. doi: 10.1007/s00427-004-0440-2. [DOI] [PubMed] [Google Scholar]
- [133].Solomon KS, Fritz A. Concerted action of two dlx paralogs in sensory placode formation. Development. 2002;129:3127–3136. doi: 10.1242/dev.129.13.3127. [DOI] [PubMed] [Google Scholar]
- [134].Solomon KS, Kudoh T, Dawid IB, Fritz A. Zebrafish foxi1 mediates otic placode formation and jaw development. Development. 2003;130:929–940. doi: 10.1242/dev.00308. [DOI] [PubMed] [Google Scholar]
- [135].Squires TM, Weidman MS, Hain TC, Stone HA. A mathematical model for top-shelf vertigo: the role of sedimenting otoconia in BPPV. J Biomech. 2004;37:1137–1146. doi: 10.1016/j.jbiomech.2003.12.014. [DOI] [PubMed] [Google Scholar]
- [136].Streit A. Extensive cell movements accompany formation of the otic placode. Dev Biol. 2002;249:237–254. doi: 10.1006/dbio.2002.0739. [DOI] [PubMed] [Google Scholar]
- [137].Takemura T, Sakagami M, Nakase T, Kubo T, Kitamura Y, Nomura S. Localization of osteopontin in the otoconial organs of adult rats. Hear Res. 1994;79:99–104. doi: 10.1016/0378-5955(94)90131-7. [DOI] [PubMed] [Google Scholar]
- [138].Taylor R, Forge A. Developmental biology. Life after deaf for hair cells? Science. 2005;307:1056–1058. doi: 10.1126/science.1109680. [DOI] [PubMed] [Google Scholar]
- [139].Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci. 2004;24:2575–2584. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- [141].Thalmann R, Ignatova E, Kachar B, Ornitz DM, Thalmann I. Development and maintenance of otoconia: biochemical considerations. Ann N Y Acad Sci. 2001;942:162–178. doi: 10.1111/j.1749-6632.2001.tb03743.x. [DOI] [PubMed] [Google Scholar]
- [142].Torres M, Giraldez F. The development of the vertebrate inner ear. Mech Dev. 1998;71:5–21. doi: 10.1016/s0925-4773(97)00155-x. [DOI] [PubMed] [Google Scholar]
- [143].Uno Y, Horii A, Umemoto M, Hasegawa T, Doi K, Uno A, Takemura T, Kubo T. Effects of hypergravity on morphology and osteopontin expression in the rat otolith organs. J Vestib Res. 2000;10:283–289. [PubMed] [Google Scholar]
- [144].Verhoeven K, Van Laer L, Kirschhofer K, Legan PK, Hughes DC, Schatteman I, Verstreken M, Van Hauwe P, Coucke P, Chen A, Smith RJ, Somers T, Offeciers FE, Van de Heyning P, Richardson GP, Wachtler F, Kimberling WJ, Willems PJ, Govaerts PJ, Van Camp G. Mutations in the human alpha-tectorin gene cause autosomal dominant non-syndromic hearing impairment. Nat Genet. 1998;19:60–62. doi: 10.1038/ng0598-60. [DOI] [PubMed] [Google Scholar]
- [145].Verpy E, Leibovici M, Petit C. Characterization of otoconin-95, the major protein of murine otoconia, provides insights into the formation of these inner ear biominerals. Proc Natl Acad Sci USA. 1999;96:529–534. doi: 10.1073/pnas.96.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Vincent C, Kalatzis V, Abdelhak S, Chaib H, Compain S, Helias J, Vaneecloo FM, Petit C. BOR and BO syndromes are allelic defects of EYA1. Eur J Hum Genet. 1997;5:242–246. [PubMed] [Google Scholar]
- [147].von Kupffer C. The development of the cranial nerves of vertebrates. J Comp Neurol. 1891;1:246–264. 315–332. [Google Scholar]
- [148].Wallis D, Hamblen M, Zhou Y, Venken KJ, Schumacher A, Grimes HL, Zoghbi HY, Orkin SH, Bellen HJ. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development. 2003;130:221–232. doi: 10.1242/dev.00190. [DOI] [PubMed] [Google Scholar]
- [149].Wang W, Grimmer JF, Van De Water TR, Lufkin T. Hmx2 and Hmx3 homeobox genes direct development of the murine inner ear and hypothalamus and can be functionally replaced by Drosophila Hmx. Dev Cell. 2004;7:439–453. doi: 10.1016/j.devcel.2004.06.016. [DOI] [PubMed] [Google Scholar]
- [150].Wang Y, Kowalski PE, Thalmann I, Ornitz DM, Mager DL, Thalmann R. Otoconin-90, the mammalian otoconial matrix protein, contains two domains of homology to secretory phospholipase A2. Proc Natl Acad Sci USA. 1998;95:15345–15350. doi: 10.1073/pnas.95.26.15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Wayne S, Robertson NG, DeClau F, Chen N, Verhoeven K, Prasad S, Tranebjarg L, Morton CC, Ryan AF, Van G, Smith RJ. Mutations in the transcriptional activator EYA4 cause late-onset deafness at the DFNA10 locus. Hum Mol Genet. 2001;10:195–200. doi: 10.1093/hmg/10.3.195. [DOI] [PubMed] [Google Scholar]
- [152].Wright TJ, Hatch EP, Karabagli H, Karabagli P, Schoenwolf GC, Mansour SL. Expression of mouse fibroblast growth factor and fibroblast growth factor receptor genes during early inner ear development. Dev Dyn. 2003;228:267–272. doi: 10.1002/dvdy.10362. [DOI] [PubMed] [Google Scholar]
- [153].Wright TJ, Mansour SL. Fgf3 and Fgf10 are required for mouse otic placode induction. Development. 2003;130:3379–3390. doi: 10.1242/dev.00555. [DOI] [PubMed] [Google Scholar]
- [154].Wright TJ, Mansour SL. FGF signaling in ear development and innervation. Curr Top Dev Biol. 2003;57:225–259. doi: 10.1016/s0070-2153(03)57008-9. [DOI] [PubMed] [Google Scholar]
- [155].Xiang M, Maklad A, Pirvola U, Fritzsch B. Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation. BMC Neurosci. 2003;4:2. doi: 10.1186/1471-2202-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet. 1999;23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- [157].Zheng W, Huang L, Wei ZB, Silvius D, Tang B, Xu PX. The role of Six1 in mammalian auditory system development. Development. 2003;130:3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Zou D, Silvius D, Fritzsch B, Xu PX. Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development. 2004;131:5561–5572. doi: 10.1242/dev.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Zwaenepoel I, Mustapha M, Leibovici M, Verpy E, Goodyear R, Liu XZ, Nouaille S, Nance WE, Kanaan M, Avraham KB, Tekaia F, Loiselet J, Lathrop M, Richardson G, Petit C. Otoancorin, an inner ear protein restricted to the interface between the apical surface of sensory epithelia and their overlying acellular gels, is defective in autosomal recessive deafness DFNB22. Proc Natl Acad Sci USA. 2002;99:6240–6245. doi: 10.1073/pnas.082515999. [DOI] [PMC free article] [PubMed] [Google Scholar]