Abstract
Central auditory connections develop in mice before the onset of hearing, around postnatal day 7. Two previous studies have investigated the development of auditory nuclei projections and lateral lemniscal nuclear projections in embryonic rats, respectively. Here, we provide detail for the first time of the initiation and progression of projections from the inferior colliculus (IC) to the medial geniculate body (MGB) and from the MGB to the auditory cortex (AC). Overall, the developmental progression of projections follows that of terminal mitoses in various nuclei, suggesting the consistent use of a developmental timetable at a given nucleus, independent of that of other nuclei. Our data further suggest that neurons project specifically and reciprocally from the MGB to the AC as early as embryonic day 14.5. These projections develop approximately a day before the reciprocal connections between the MGB and IC and before development of projections from the auditory nuclei to the IC. The development of IC projections is prolonged and progresses from rostral to caudal areas. Brainstem nuclear projections to the IC arrive first from the lateral lemniscus nuclei then the superior olive and finally the cochlear nuclei. Overall, the auditory connection development strongly suggests that most of the overall specificity of nuclear connections is set up at least 2 weeks before the onset of sound-mediated cochlea responses in mice and, thus, is likely governed predominantly by molecular genetic clues.
Keywords: auditory system, central auditory connections, development of auditory connections, thalamocortical connections, medial geniculate body
Almost all sensory information, excluding olfaction information, reaches the forebrain cortex by means of relay stations in the thalamus. For the auditory information in mammals, this relay is the medial geniculate body (Puelles, 2001). In mammals, the ventral division of the medial geniculate body (vMGB) sends tonotopic efferent fibers to the tonotopically organized primary auditory cortex (AI; Velenovsky et al., 2003). The thalamocortical connections are reciprocal, and the AI sends corticofugal projections to the vMGB (Andersen et al., 1980; Rouiller and Welker, 1991; Hofstetter and Ehret, 1992). In contrast to the ventral division, the dorsal division of MGB (dMGB) is not tonotopically organized (Winer et al., 2001) and sends nontonotopic efferent projections toward the nontonotopic secondary auditory cortex (AII; Rouiller, 1997). Like the tonotopic system, the nontonotopic thalamocortical systems are also reciprocal; therefore, the AII send corticofugal projections to the dMGB (Rouiller, 1997). These data on various mammals suggest that the thalamocortical auditory connections likely are in the mouse both reciprocal and nonreciprocal and both tonotopical and nontonotopical projections from multiple parts of the MGB to multiple auditory cortical fields. As in humans, the left auditory cortex of the mouse appears to be 30% larger than the right (Stiebler et al., 1997; Geissler and Ehret, 2004) but may have qualitatively similar connections.
Input to the MGB predominantly comes from the ipsilateral inferior colliculus (IC). The IC, the main auditory nucleus of the midbrain, is an obligatory relay nucleus for auditory fibers from the brainstem to the MGB. It also receives thalamofugal and corticofugal fibers (Rouiller, 1997). The tonotopically organized efferent projections from the central nucleus of the IC (cIC) mainly reach vMGB, which is the origin of thalamocortical efferent fibers to the tonotopic AI. The nontonotopically organized efferent projections originate from the pericentral nucleus of the IC (pIC) and end in mammals in the dMGB (Kudo and Niimi, 1978; Kudo, 1981; Druga and Syka, 1984; Henkel and Shneiderman, 1988; Payne, 1992).
Projections to the IC originate in the cochlear nucleus complex (CN), the superior olivary complex (SOC), and the lateral lemniscus nuclei (LLn; Rouiller, 1997). The IC consists of the cIC, pericentral nucleus (pIC), and external nucleus (eIC). The cIC receives most of the efferent fibers from contralateral CN and a few fibers from the ipsilateral CN (Rouiller, 1997). Other major inputs to the cIC arise from the contralateral SOC and LLn, whereas some efferent projections come from the ipsilateral lateral superior olivary nucleus (LSO), medial superior olivary nucleus (MSO), dorsal nucleus of lateral lemniscus (dLL), and ventral nucleus of lateral lemniscus (vLL; Goldberg and Moore, 1967; Roth et al., 1978; Adams, 1979; Brunso-Bechtold et al., 1981; Kudo, 1981; Ryugo et al., 1981; Henkel and Spangler, 1983; Whitley and Henkel, 1984; Aitkin and Schuck, 1985; Maffi and Aitkin, 1987; Moore, 1988; Shneiderman et al., 1988; Schofield and Cant, 1992; Rouiller, 1997). Efferent projections from the brainstem end in the ventrolateral regions of cIC (Gabriele et al., 2000a), whereas corticocollicular fibers end in the dorso-medial region of cIC where they form highly ordered layers (Rouiller, 1997).
Little information is available on the development of auditory nuclei and their connections. “Birth dating” stuies using [3H]thymidine have shown in the rat that spiral ganglion cells form between embryonic days 13–15 (E13–E15) and are the last neurons to do so in the auditory system (Altman and Bayer, 1982). Similar studies in mice have shown proliferation of spiral neurons between E11 and E15 (Ruben, 1967), suggesting that terminal mitosis in mice occurs 1–2 days earlier than in rats (Fritzsch and Nichols, 1993). Experimental tracing studies have revealed that the first spiral neurons reach the developing cochlea in mice at E12.5. This is approximately the same time they project into the auditory nuclei (Rubel and Fritzsch, 2002; Maklad and Fritzsch, 2003a). These tracing studies have been confirmed using a specific early marker for spiral neurons, GATA3 (Karis et al., 2001). Central tonotopic projections of the spiral neurons develop in cats before the onset of hearing (Leake et al., 2002) and even in the absence of hair cell formation as in Brn3c null mutant mice (Xiang et al., 2003). These data are compatible with the idea that most pathfinding of central and peripheral processes of spiral ganglion neurons depends on molecular cues (Fritzsch, 2003; Gu et al., 2003; Tessarollo et al., 2004).
In rats, the SOC and LLn are the first auditory nuclei to start and finish neurogenesis (E9–E13). Neurons for these nuclei originate in the dorsal aspect of the medial neuroepithelium of the brainstem (Altman and Bayer, 1980), possibly in the rhombic lip (Nornes and Morita, 1979; Rubel and Fritzsch, 2002). Neurogenesis of CN occurs in the rhombic lip (His, 1888; Blake, 1900) and on the medial side of the solitary nucleus near the sulcus limitans (Willard, 1995) at E10–E15. This area may be continuous with the Math1- and Ngn1-expressing dorsal columns of the spinal cord (Maklad and Fritzsch, 2003a). How much each of these columns contributes to the auditory nuclei remains unclear.
Neurons of the IC originate in the rat from the neuroepithelium of the posterior recess of the cerebral aqueduct at E11–E17 (Altman and Bayer, 1981; Cooper and Rakic, 1981), with larger neurons being generated before smaller ones (Altman and Bayer, 1981; Oblinger and Das, 1981). Central IC nucleus neurons are formed between E11.5 and E13.5, followed by a peak production of neurons of the rostral IC at E13.5. Proliferation follows a rostral to caudal gradient within each nucleus (Cant, 1998).
In rats, MGB neurons form from the thalamic alar plate between E10 and E14 (Altman and Bayer, 1979, 1989). After their final division, MGB neurons migrate in a radial direction from the ventralmost part of the alar plate to form a lateral division of the ventral tier of prosomere 2 of the thalamus (Puelles, 2001; Puelles and Rubenstein, 2003). This part of the thalamus also contains other dorsal thalamic projection neurons. The cortical neurons form in rats from E10 to E16 from the dorsolateral wall of the cerebral vesicles (Cant, 1998). These data in rats suggest that the generation of MGB neurons (E10–E14) starts slightly later than the SOC (E9–E13), and CN begins and ends (E10–E15) before completion of the generation of IC neurons (E11–E17). On the basis of neuronal birth dates, others (Altman and Bayer, 1981) inferred that the MGB projections to the cortex might develop before the inferior colliculus fibers reach the MGB. If so, this would suggest that most of the pathfinding and the initial establishment of contacts of MGB neurons with the cortex occurs independently from any sensory input to these nuclei. More recent data on the general development of thalamic projection nuclei (Hevner et al., 2002; Lopez-Bendito and Molnar, 2003) tend to support this notion. However, no specific reference to the MGB–AC connection is provided in these studies (Hevner et al., 2002; Lopez-Bendito and Molnar, 2003), leaving the question of whether or not the MGB follows the general thalamic developmental timetable unanswered.
Aside from birth-dating studies, nothing is known about the precise timing of the embryonic development of thalamocortical auditory connections in any species (Cant, 1998). The only experimental studies investigating this issue in any auditory nuclear connections are one study on cochlear nuclei connection development (Kandler and Friauf, 1993) and one on inferior colliculus development (Gabriele et al., 2000a) in rats. These studies show that the projections from the CN to the IC develop between E15 and E17, passing SO and LL on their way to the IC. Between E18 and P5, collaterals of the CN form, and between P5 and P14, the terminal structures mature further. Contralateral LL fibers reach the IC around E19 and differentiate between P4 and P12. Some additional data exist on the late embryonic and early neonatal development of the reciprocal thalamocortical projections in cats (Payne, 1992).
Overall, the correlation between fiber outgrowth and birth date is unknown for most of the central auditory system of developing rodents and mammals. The chief goal of the present study is to specify the timing at which the afferents from the MGB reach the auditory cortex and to determine when the MGB receives afferents from the IC. By using double labeling from nearby cortical regions with lipophilic tracers having different spectral properties, we demonstrate that the MGB can be uniquely characterized, compared with adjacent thalamic areas in mice, at embryonic day 18.5. We provide data that suggest a connection to the auditory cortex as early as E14.5. We also demonstrate that the development of IC projections from the LLn, the SOC, and the CN of the brainstem develop in a rostral-to-caudal progression. Overall, these data suggest that development of central auditory projections closely follows the time line of terminal mitosis in a given auditory nucleus. These findings will help to define alterations in thalamocortical projections in congenitally deaf mice such as Brn3c null mutants (Xiang et al., 2003) or NeuroD null mutants (Liu et al., 2000; Kim et al., 2001).
MATERIALS AND METHODS
Animals
Mice used were of the CF1 strain (Jackson Laboratories). The male mouse was left in the cage for mating between 5:00 pm and 8 am the next day. Noon of the first day after fertilization was designated as E0.5. Vaginal plugs were used to indicate the onset of pregnancy. Embryos were harvested at E13.5, 14.5, 16.5, 18.5. Postnatal pups on the day of birth (E19.5) were designated postnatal day (P) 0. Pregnant females were killed by using cervical dislocation. Embryos were dissected from the uterus and perfused through the heart with 10% paraformaldehyde (PFA) in phosphate buffer (pH 7.4). The P0 pups were anesthetized with 0.1 ml of beuthanasia-D before perfusing with 10% PFA. All protocols were approved by the Creighton University IACUC committee (protocol no. 0630).
Procedure
Fixed brains were dissected the next day and further immersion fixed in 4% PFA for 1 week before proceeding. A minimum of 25 brains at each age group were used. The optimal insertion site for tracers was determined by trial and error for each stage. The insertion site was adjusted after viewing the sectioned brains. Two carbocyanine tracers (Fritzsch et al., 2002), PTIR 278 and PTIR 271 (PTI Research, Exton, PA), were applied to either the auditory cortex (AC) and/or IC using existing cortical maps of the mouse to guide the position of the insertions (Stiebler et al., 1997). We also used NeuroD LacZ mice to highlight the inferior colliculus (Fig. 1) together with the cerebellum after a β-galactosidase reaction (Kim et al., 2001). In additional sets of E18.5 embryos, inner ear applications were combined with inferior colliculus applications or double cortical insertions.
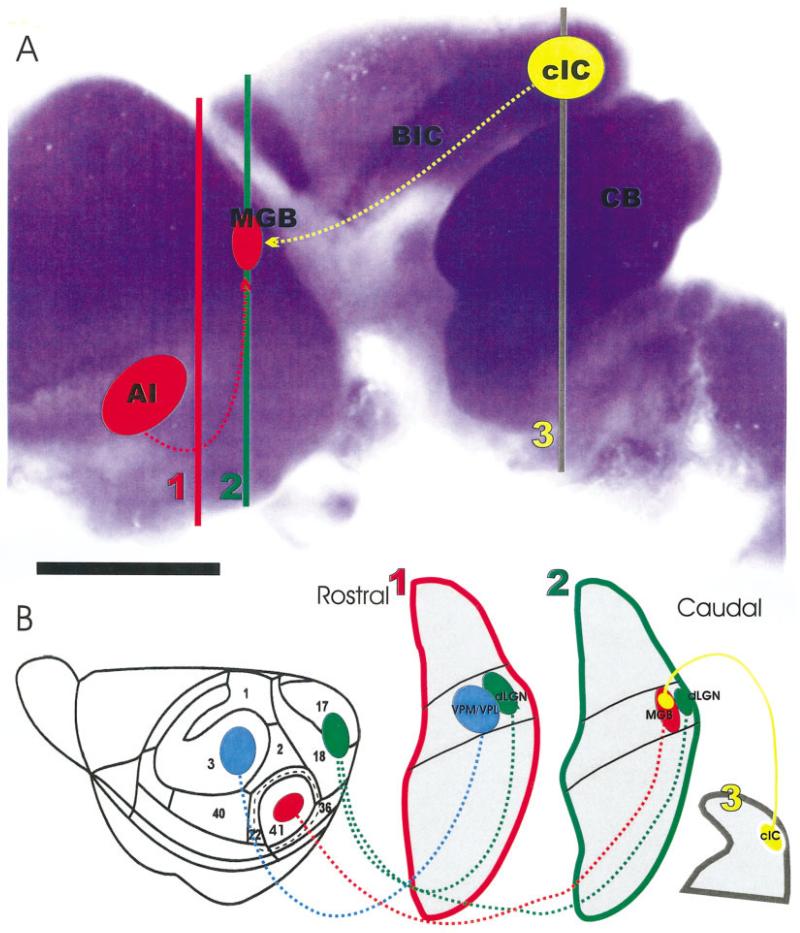
Fig. 1.
The organization of NeuroD expression in the brain, the distribution of injection sites into the presumed auditory cortex and inferior colliculus, and the nuclear and connections organization as revealed in coronal sections is shown. A: Note that the cerebellum, parts of cortex, and the inferior colliculus show strong β-galactosidase reactivity, indicating up-regulation of NeuroD in these areas. The position of the presumptive auditory cortex (AI), medial geniculate body (MGB), central nucleus of the inferior colliculus (cIC) is shown in this lateral view of an embryonic day 18 brain. B: Other injection sites in the somatosensory cortex (blue) and the visual cortex (green) as well as their corresponding thalamic relay nuclei (ventrolateral/ventromedial posterior nucleus, VPM/VPL; dorsal lateral geniculate nucleus, dLGN) are shown at the approximate section levels as indicated in A. BIC, brachium of the inferior colliculus; CB, cerebellum. Scale bar = 1 mm in A.
For insertions, crystals of lipophilic tracers were dissolved in a few drops of xylene. Filter strips were soaked in the dissolved crystals and air-dried. Pieces of filter strips were cut into small triangles (approximately 0.1 mm wide, 0.5 mm long) for insertion at the IC and AC, providing a high concentration of dye (Figs. 1, 2). Brains were left in 4% PFA, in glass vials, in a 37°C oven for the number of days needed for proper diffusion (Maklad and Fritzsch, 2002). We established the optimal time length for diffusion to determine the optimal diffusion time for each age empirically using criteria discussed below (Table 1).
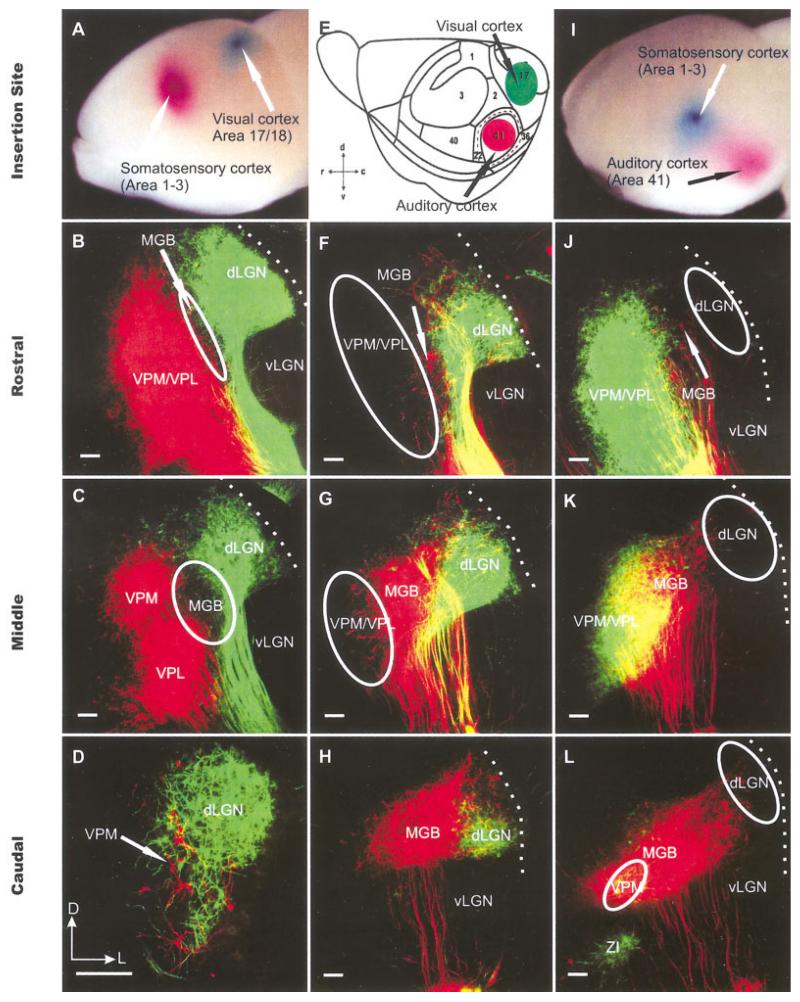
Fig. 2.
The organization of thalamocortical projection neurons as revealed in coronal sections after double dye insertions at embryonic day (E) 18.5. A,E,I: Specific sets of thalamic neurons were double labeled from auditory, visual, and somatosensory cortex with the spectrally distinct lipophilic dyes PTIR 271 (colored in green) and PTIR 278 (colored in red). This strategy allowed us to distinguish between caudomedial groups labeled from the presumptive auditory cortex, a lateral group labeled from the presumptive visual cortex, and rostromedial groups labeled from the presumptive somatosensory cortex. F-H,J-L: In agreement with the known distribution of these thalamic nuclei in adult mice, we find a caudal group labeled after presumptive auditory cortex insertions (red). F-H: More rostrally, this presumptive MGB population is dorsolateral capped by a population that is consistently labeled after presumptive visual cortex insertions. B-D,J-L: Even more rostral, we find a population only labeled after presumptive somatosensory cortex insertions, which we refer to as VPM/VPL. We can distinguish, therefore, between the different corticothalamic projections nuclei as early as E18.5. E: The cortical map follows that of Stiebler et al. (1997). dLGN; dorsal lateral geniculate nucleus; MGB, medial geniculate body; vLGN, ventral lateral geniculate nucleus; VPL, ventrolateral posterior nucleus; VPM, ventromedial posterior nucleus; ZI, zona incerta. Dotted lines indicate thalamic surface. D,E: Orientation is indicated for these and all other coronal sections of Figures 2-9: d, dorsal; c, caudal; v, ventral; r, rostral; D, dorsal; L,, lateral. Scale bars = 100 μm in B-D, F-H,J-L.
TABLE 1.
Optimal Diffusion Time for Each Age
| Age | Days | Application | Number of brains |
|---|---|---|---|
| E13.5 | 8 | AC IC | 15 |
| 7 | Rostral IC | 15 | |
| E14.5 | 10 | AC IC | 25 |
| 10 | Rostral IC | 15 | |
| E16.5 | 10 | MGB | 40 |
| 13 | AC IC | 30 | |
| E18.5 | 15 | MGB | 45 |
| 14 | AC IC | 15 | |
| P0 | 18 | AC IC | 40 |
E, embryonic day; P, postnatal day; AC, auditory cortex; IC, inferior colliculus; MGB, medial geniculate body.
Once diffusion was complete, the brains were soaked in 30% sucrose solution for five minutes to increase specific density. Added density makes orienting the brain in the 15% liquid (56°C) gelatin in a cryomold easier. Once the gelatin had solidified (approximately 10 minutes), gelatin blocks were put in 10% PFA and kept at 4°C for a minimum of 3 days. The blocks were than sectioned coronally with a Vibratome at 100-μm thickness. Sections were mounted on slides and viewed with a Bio-Rad Radiance 2000 confocal microscope mounted on top of a Nikon E800 microscope. Confocal image stacks were collected from various sections, including those with the insertion sites. Stacks of images were collapsed by using ImagePro software (Media Cybernetics, MA) to generate a single image of all labeled profiles in a given section. Diffusion was considered optimal if growth cones in the most remote projection were filled. In addition, only sections that showed no dye “bleeding” to unwanted sites, suggestive of transcellular transport (Bruce et al., 1997) were selected.
Selected insertion sites
Two sets of experiments were conducted at each age group. In one set of experiments, tracers were implanted in the AC and IC to retrogradely label cells in the MGB and to anterogradely label fibers to the MGB. In a second set of experiments, insertions were into various areas of the forebrain to label different thalamocortical projection neurons. We estimated the appropriate insertion site by projecting the existing map of cortical areas of the mouse (Stiebler et al., 1997) onto the developing forebrain (Fig. 2). Both sides of the brain were implanted with either PTIR 278 or PTIR 271 dye to achieve parallel tracing of fibers. Viewing sections through the MGB allowed us to image fibers and cells filled from the IC as distinctly different from the fibers and cells filled from the AC.
Additional experiments consisted of insertions of IC and AC on the same side to investigate the topographical location of IC projecting fibers and AC projecting neurons in the MGB. Because we used dyes with different spectral properties for the left and right side, we also could investigate in these animals the labeling of neurons in both the ipsi- and contralateral brainstem nuclei that project to the IC. In some E18.5 embryos, we also labeled the inner ear afferent and efferent projection (Karis et al., 2001; Maklad and Fritzsch, 2003b) to verify that cochlea neurons retro-gradely labeled from IC insertions were located in the cochlea projection area.
Optimization of diffusion time and insertion site
Insertion sites in the cortex and the inferior colliculus were systematically varied and the consistency of labeling in the MGB was compared (Figs. 1, 2). For example, insertions were made into three different rostrocaudal levels of the IC, one close to the anterior-inferior border of the superior colliculus, one approximately at the level of the mesencephalic flexure, and one near the caudal border, approximating the cerebellum. Diffusion times were adjusted to compensate for the longer distances to be covered. Similarly, insertions with a single dye or two dyes were systematically varied in the lateromedial aspect of the cortex, and the labeling of the MGB was compared. Optimizing insertion site and adjusting the diffusion time to the right number of days allowed us to obtain consistent results at each developmental day.
An ideal diffusion time was defined as showing single fibers with growth cones at the end of the axons or dendrites and they displayed some branches (Fig. 2). If dendritic branches were altogether missing, or axons were ending without a growth cone, an extra day of diffusion was allowed and another preparation was evaluated. There were minor differences in the number of days the dyes were allowed to diffuse from different insertion sites, because the distance needed to travel from the AC is considerably shorter than from the IC. In addition, the physical properties of the dyes used differed slightly. As a result, any application in the AC was implanted toward the end of the diffusion time. Whenever excessive labeling in other areas of the brain was noted, the diffusion time was designated as “too long.” In contrast, when only a few cells or axons were labeled without growth cones, the diffusion time was designated as “too short.” Several brains were used at each embryonic age to optimize diffusion time for each insertion site.
Statistical analysis
Microbrightfield Neurolucida software was used to trace the processes of individual neurons in stacks of confocal images that were aligned and calibrated. Only stacks of confocal images that contained clear continuation of neuronal projections were used to obtain the best tracing. The increase in dendritic span for individual AC projecting neurons and IC projecting neurons from the MGB at E14.5 and E18.5 were compared. Neuroexplorer and MS Excel were used to obtain statistical comparison of the two age groups, and MS Excel was used to plot graphs from the data obtained. Length of dendrites and size of the perikaryon of at least six neurons in the MGB, filled from insertions of the auditory cortex, were compared and histograms were generated using MS Excel. Students t test was used to verify significance of differences.
Image processing
Stacks of confocal images were imported into ImagePro software and collapsed along the Z-axis into single images. Black and white collapsed images were converted into color images by using ImagePro and, if appropriate, combined into two color images. For this presentation, we kept the color code for PTIR 271 as green and PTIR 278 as red. Images were exported as tiff files. Tiff files were assembled into plates by using CorelDraw software (Corel, Inc.). No image enhancements were used on these confocal images.
RESULTS
Establishing boundaries of the MGB in the embryonic mouse brain
The relative position of the MGB with other thalamic nuclei is, in mice, not as distinct as in cats or humans. Specifically, the MGB shows overlap with the dorsal part of the lateral geniculate nucleus (dLGN) at most of its rostrocaudal extent (Franklin and Paxinos, 1997). Over some of their overlapping area, MGB and LGN are separated by the superior thalamic radiation. Rostral continuations of the MGB contain the somatosensory thalamic relay nuclei and the ventral posterolateral and the ventral posteromedial thalamic nuclei (VPL, VPM). No criterion allowed us to distinguish those nuclei in the developing mouse brain (Figs. 1, 2), as only distinctions between large thalamic primordia such as prosomere 2 can be found in mouse embryos (Puelles and Rubenstein, 2003). We took advantage of the ability to simultaneously double label thalamic projection neurons from different cortical areas (Fig. 2) and used insertions into the auditory, visual, and somatosensory cortex to label specific sets of thalamic neurons. These approaches allowed us to distinguish between a caudomedial group of thalamocortical neurons labeled from the presumptive auditory cortex, a lateral group labeled from the presumptive visual cortex, and a rostromedial group labeled from the presumptive somatosensory cortex (Fig. 2). Consistent with the position of the superior thalamic radiation in adult mice, we found such a fiber tract almost completely separating the rostral MGB from the caudal dLGN. These data allowed us to distinguish between the different corticothalamic projection nuclei as early as E18.5 (Fig. 2) and formed the basis for our analyses in younger embryos.
Anterograde and retrograde labeling of MGB neuronal profiles after AC and IC application
E13.5
This was the earliest age at which we could successfully implant dyes into the AC and/or IC. AC insertions labeled several cells in what appeared to be the MGB (Fig. 3B). Double labeling with two different colored dyes inserted adjacent to each other showed that those neurons formed distinct groups that projected, largely nonoverlapping to the cortex, much like that in older embryos (Fig. 2). The neurons showed a single or few dendrites that were short with few side branches (Fig. 3A–D). Neurons were clearly bipolar at this developmental stage with the axon usually coming off the neuronal perikaryon opposite to the dendrite. These early thalamocortical neurons send their axons to the cortex along the meningeal surface. However, neurons that appear to develop later migrate progressively further laterally, thus generating a fiber tract in older embryos that appears to run right through the thalamus to form the superior thalamic radiation.
Fig. 3.
Organization of presumptive MGB neurons and IC fibers at embryonic day 13.5. A-D: Neurons retrogradely labeled from insertions into the presumptive auditory cortex showed a single or few dendrites that were short and showed few side branches. Neurons were clearly bipolar at this developmental stage with the axon usually coming off the neuronal perikaryon opposite to the dendrite. IC fibers projecting to the MGB could only be labeled with rostral insertions into the IC (E,F). Note that the effective uptake area of the dye is likely much smaller than the diffusion area now visible in the coronal section (F). Using the double labeling with a different colored dye insertion into the auditory cortex, we could establish the relative position of the MGB neurons and IC fibers and projecting at this stage (A,C,D). IC originating fibers showed barely discernible growth cones that occasionally were seen to approach a retrogradely labeled cortical projection neuron (A,D). IC, inferior colliculus; AC, auditory cortex; dLGN; dorsal lateral geniculate nucleus; MGB, medial geniculate body; vLGN, ventral lateral geniculate nucleus. Scale bars = 10 μ in A,F; 20 μ in B,E; 40 μ in C; 50 μ in D.
Fibers projecting to and from the IC could be labeled only with very rostral insertions into the IC, almost at the level of the brachium of the inferior colliculus (BIC; Fig. 1). Those insertions into the IC (Fig. 3F) labeled numerous cell populations in the thalamus (Fig. 3E). By using the double labeling with two different colored dyes inserted into the cortex, we could establish the approximate location of the MGB at this stage (Fig. 3B,C). Comparing the retrogradely filled cortical projection neurons with the anterogradely filled fibers and cells after IC insertion showed a few neurons labeled in the ventral MGB and single fibers projecting into the dorsal MGB (Fig. 2B–D). These fibers showed barely discernible growth cones that occasionally were seen to approach a retrogradely labeled cortical projection neuron (Fig. 3A,D). This proximity suggests that axonal growth cones of IC neurons may already interact with the growing dendrites of the MGB that project to the auditory cortex at this early stage in development, although visualization of such possible interactions was rare. Neurons retrogradely labeled from the IC were found sparingly and interspersed with cortical projection neurons (Fig. 3C). These data suggest that colliculofugal and colliculopetal projections originate almost simultaneously from partially overlapping areas of the MGB.
E14.5
At this stage, the location of MGB is characterized by an obvious bump, adjacent to the hippocampal fold in the diencephalic region with the dorsal half (the future dLGN) belonging to prosomere 2 (P2) and the ventral half (the future vLGN) belonging to prosomere 3 (Puelles, 2001; Puelles and Rubenstein, 2003). Occasionally, we obtained retrograde labeling of P3 neurons, but the boundary of P2 and P3 was distinct with respect to a massive projection of P2 to the cortex, as in earlier stages (Fig. 3B and data not shown). The location of the MGB was also validated with double labeling of the AC and IC. AC insertions revealed a bundle of fibers that ended near MGB neurons, suggesting that the AC insertion was in the primary auditory cortex region, known to be reciprocally connected to the MGB (Rouiller, 1997). The AC projecting neurons had few dendritic branches with axons reaching the auditory cortex.
As thalamocortical projections showed only quantitative changes, we concentrated at this developmental stage on the IC connections with the brainstem and the thalamus. Rostral IC insertions labeled a few neurons primarily in the reticular formation ventral to P2 that possessed dendritic branches. Caudal IC insertions labeled even fewer neurons with fewer and shorter dendrites in the MGB. Only rostral IC insertions yielded a few fibers that terminated predominantly in the MGB. Simultaneous insertion of AC with a different dye showed that the few IC axons mingled with the dendrites of MGB neurons projecting to the AC.
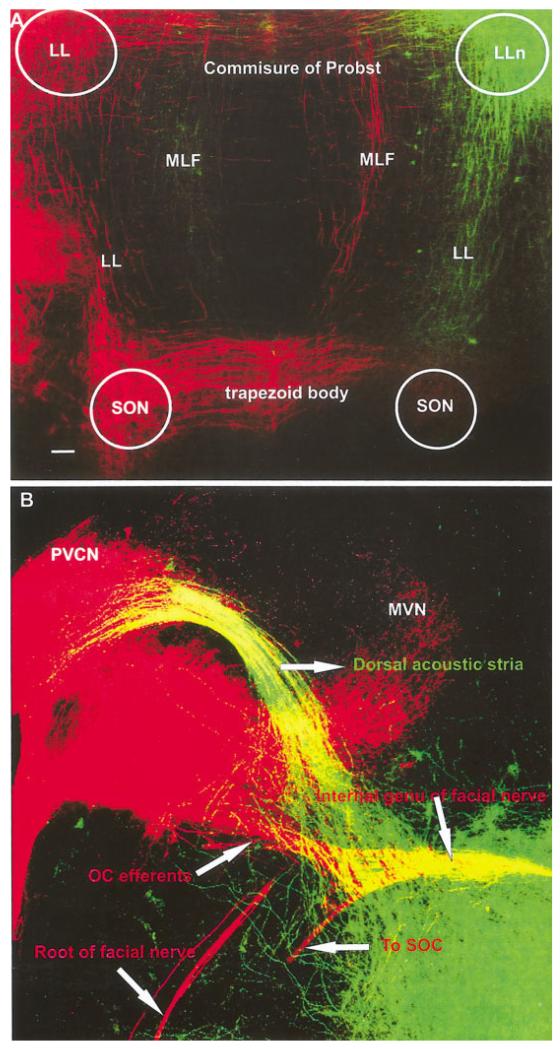
In addition to the thalamic fibers and neurons labeled after IC insertions, fibers, and neurons in the brainstem were also labeled. We found that lateral lemniscus (LL) fibers projected prominently to the SO region ipsilaterally. We also found that LL fibers crossed the commissure of Probst in rhombomere 1 to reach the contralateral LLn (Fig. 3A,B). These fibers originate from dLLn neurons (Fig. 4B-D) and from several other sources. Fibers labeled from the IC projected along the medial longitudinal fascicle toward the contralateral brainstem reticular formation (Fig. 4A,B). In addition, IC fibers crossed to invade the cIC as well as filling their cells of origin (Fig. 4A,C,D). These contralaterally projecting IC neurons were most frequently found in the dorsal IC (Fig. 4D). These neurons were small with few dendrites and side branches. We could not label any fibers projecting beyond the superior olive, which showed only few neurons projecting to the ipsilateral lateral lemniscus (Fig. 4B). No labeling of cochlear nuclei was obtained at this stage of development.
Fig. 4.
IC insertions and labeled fibers and neurons in the brainstem at embryonic day 14.5. A,B: Insertions into the IC-labeled ipsilateral LL fibers that extended toward to the SO region where few neurons were retrogradely filled. We also found that LL fibers crossed the commissure of Probst to reach the contralateral LL. A-D: These fibers did not only extend to dorsal LL (dLL) neurons (B-D) but also projected along the medial longitudinal fascicle toward the contralateral brainstem reticular formation (A,B). A,C,D: In addition, contralateral central nucleus of the IC (cIC) fibers crossed to invade the cIC as well as filling their cells of origin. D: These contralateral cIC neurons were most frequently found dorsal in central nucleus of the IC. B: We could not label any fibers projecting beyond the superior olive, which showed only few neurons projecting to the ipsilateral lateral lemniscus. IC, inferior colliculus; LL, lateral lemniscus; MLF, medial longitudinal fascicle; SO, superior olive. Scale bars = 40 μ in A; 10 μm in B,C; 20 μ in D.
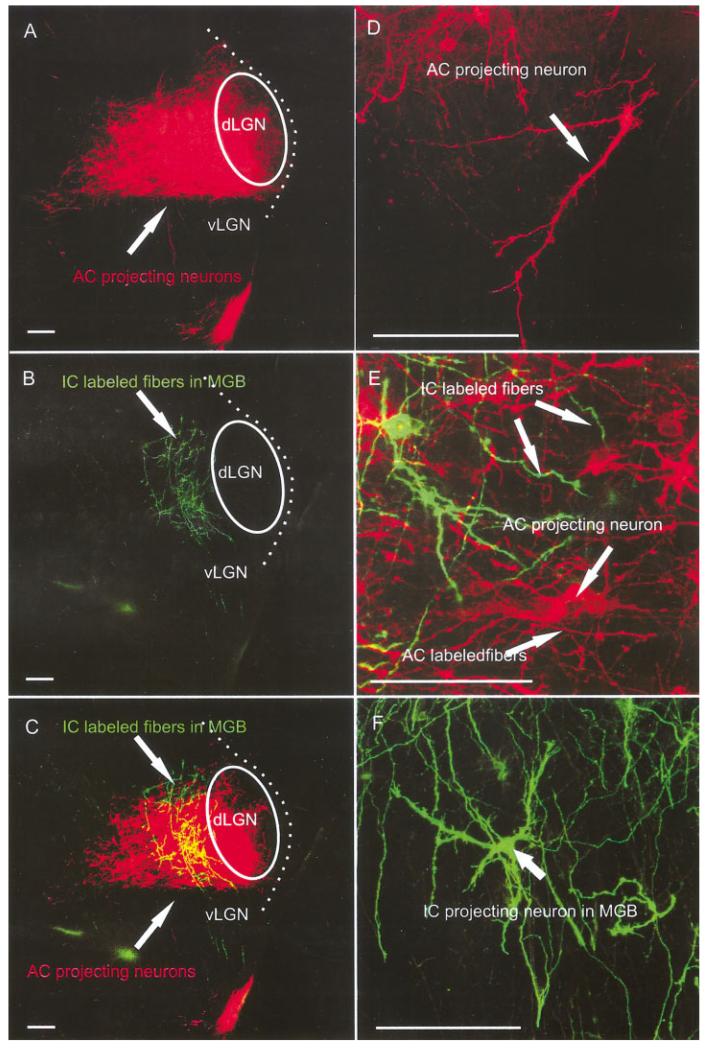
E16.5
AC insertions labeled numerous AC projecting neurons from the MGB (Fig. 4A,B). Single MGB neurons, retrogradely filled from the AC, showed a multipolar appearance with numerous dendrites and a prominent cell body and axon (Fig. 5B,C). Insertion into the rostral IC showed the expansion of fiber bundles originating in the IC in the MGB. Occasional cell bodies with a few dendrites were labeled, interspersed among the thalamocortical neurons (Fig. 5D). Rostral IC insertions showed more densely clustered fibers than caudal IC insertions. Some IC projection fibers in the MGB ended in growth cones, which apparently approached the dendrites of the AC-projecting neurons (Fig. 5A–C). The predominance in number and branching of AC projecting neurons compared with the few IC-derived fibers suggests that the thalamocortical projections develop earlier than colliculothalamic projections. Massive rostral IC insertions labeled many more fibers and IC projecting neurons in the area of the MGB (Fig. 5D) and also some neurons in the superior olives (SO) and cochlear nuclei (CN; data not shown).
Fig. 5.
A-D: AC and IC insertions show labeled fibers and neurons in the thalamus at embryonic day 16.5. Single MGB neurons retrogradely filled from the AC were densely packed in what appeared to be the MGB. B,C: Individual neurons were multipolar with numerous dendrites and a prominent cell body and axon. Simultaneous insertion of rostral IC with a different dye showed the expansion of IC projection fiber bundles. A,C: Some IC projection fibers in the MGB were simple fibers ending in growth cones, which apparently approached the dendritic growth cones on the AC projecting neurons. Many more AC projecting neurons than IC fibers were labeled, suggesting that the thalamocortical projections was more developed than colliculothalamic projections and may be established with limited interaction with the growth cone of IC-derived fibers only. D: Massive rostral IC insertions labeled many more fibers and also IC projecting neurons in the area of the MGB. AC, auditory cortex; IC, inferior colliculus; MGB, medial geniculate body. Scale bars = 10 μ in A; 20 μ in B; 60 μ in C; 40μ in D.
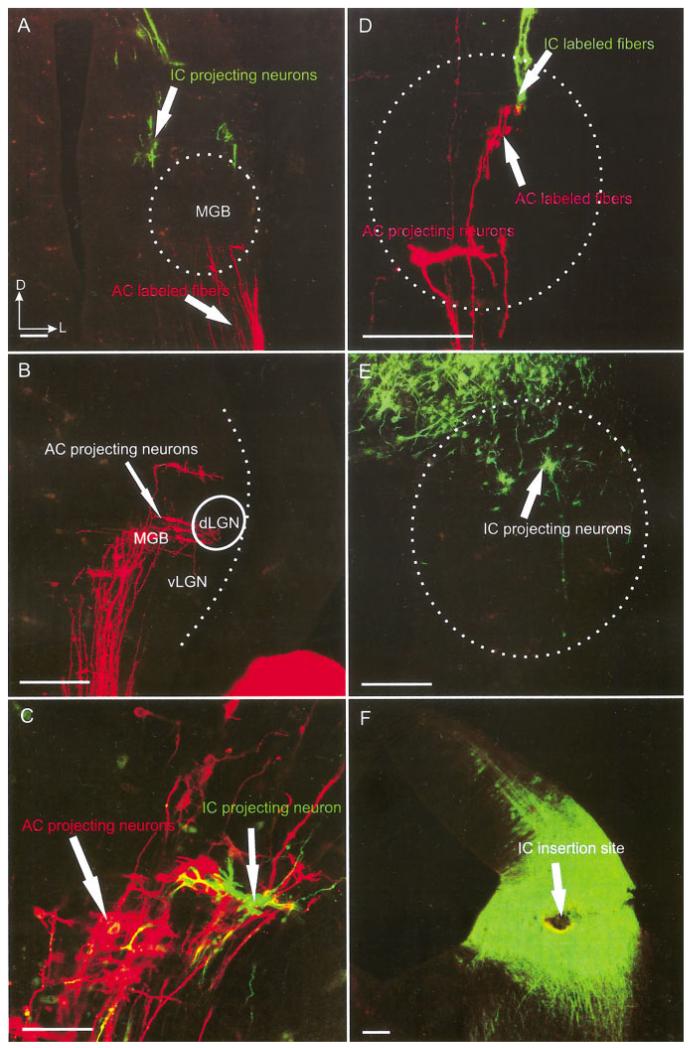
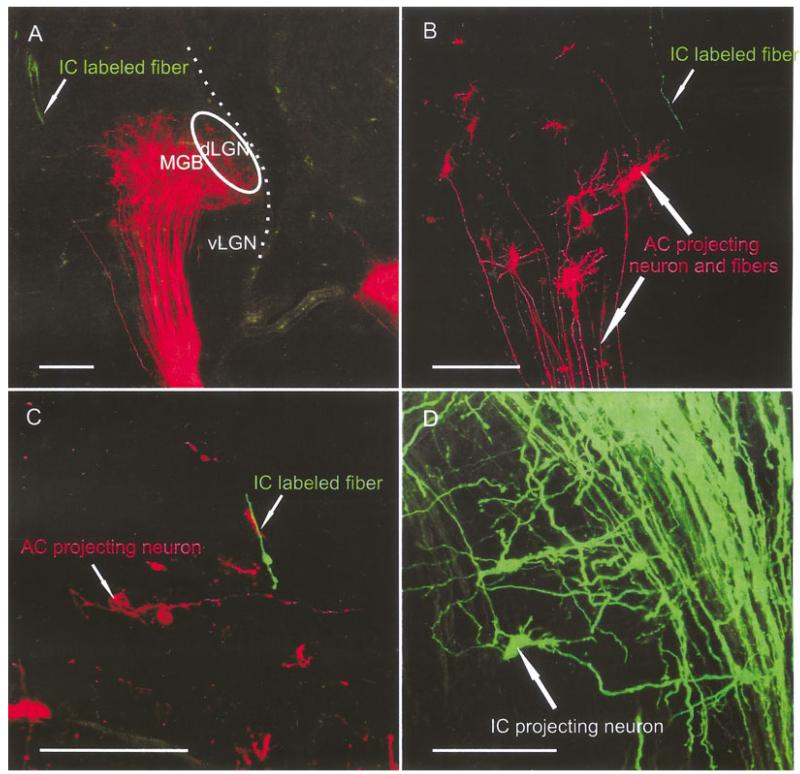
E18.5
At E18.5, AC insertions showed more prominent projections and the dendritic branching in the MGB was extensive (Fig. 6). Restricted insertions into small areas of the auditory cortex, with little labeling of fibers of passage to other cortical areas, showed labeling of what appeared to be subnuclei in the MGB (Figs. 6, 7A). Simultaneous insertion of IC and AC showed that AC projecting neurons in the MGB receive numerous fibers originating from the IC (Fig. 7A–C). The IC fibers terminated in growth cones that interdigitated with the similarly abundant dendritic arbors of cortical projection neurons in a highly complex manner. The IC projection seemed to gravitate toward the more ventral aspect of the MGB, an area that might develop into the tonotopically organized vMGB later. Smaller insertions labeled isolated IC and AC neurons that showed multiple, long dendrites with many side branches and growth cones (Fig. 7D–F). At this stage, cortical projection neuron dendritic growth cones were less conspicuous, while IC growth cones were more prominent and possibly more abundant. This difference may indicate that dendritic growth began to slow down while the IC fibers were still immature.
Fig. 6.
Insertion site into the auditory cortex and the retrogradely filled neurons in the MGB at embryonic day 18.5. Note that only a small part of the MGB was labeled after this fairly small insertion into the auditory cortex, suggesting an already restricted projection to small cortical areas at this stage of development. This insertion had very few fibers of passage labeled to other cortical areas. MGB, medial geniculate body; dLGN; dorsal lateral geniculate nucleus; vLGN, ventral lateral geniculate nucleus. Scale bar = 10 μm.
Fig. 7.
AC and IC insertions reveal more extensive cell and fiber overlap at E18.5. A-C: Caudal IC insertions labeled fibers overlapping with MGB neurons labeled after auditory cortex insertions. D-F: Higher magnifications showed the more elaborate dendritic trees of the MGB neurons (D), IC projecting neurons (F), and a prominent overlap of dendrites and axons of retrogradely filled neurons as well as anterogradely filled fibers (E). AC, auditory cortex; IC, inferior colliculus; dLGN; dorsal lateral geniculate nucleus; MGB, medial geniculate body; vLGN, ventral lateral geniculate nucleus. Scale bars = 10 μ in A-C; 60 μ in D-F.
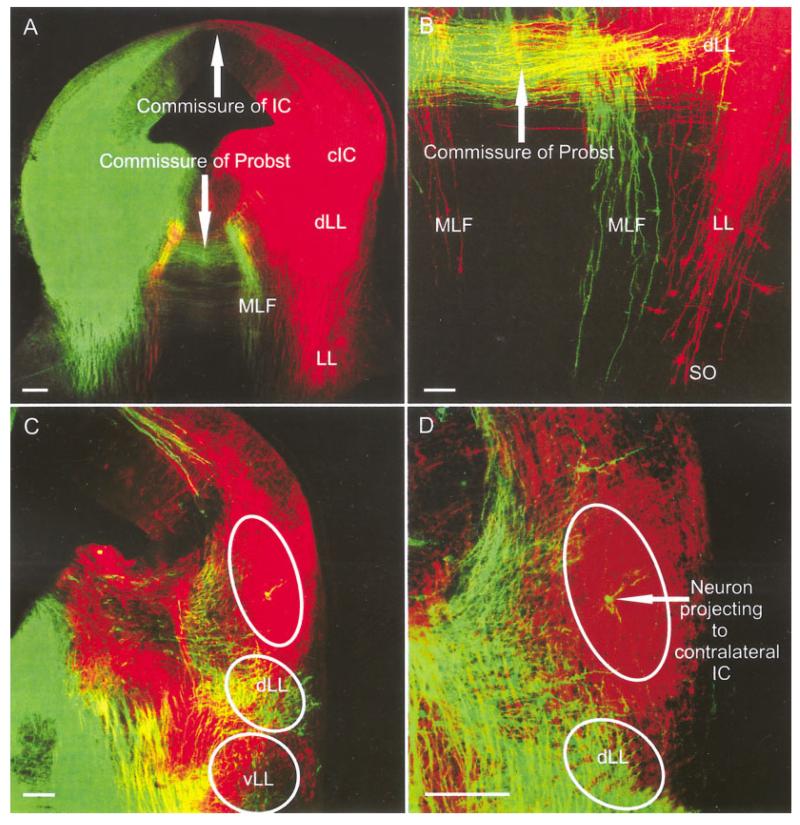
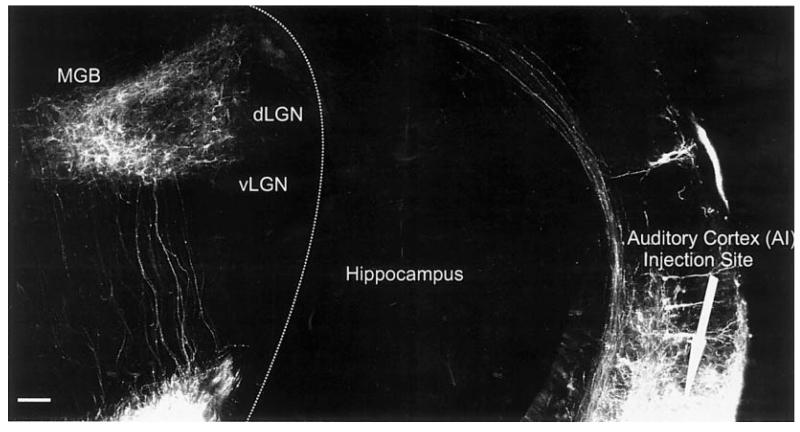
Double labeling of the left and right IC showed the passage of fibers from dLL and cIC to the contralateral side, ultimately forming the intercollicular commissure (data not shown) and the commissure of Probst (Fig. 8A). Most of these fibers ended in the ipsilateral LL (Fig. 8A). Many lemniscal fibers could be traced beyond the LLn and reached the ipsilateral SO where they ended in numerous retrogradely labeled cells. Many lemniscal fibers decussated rostral to the trapezoid body to reach the contralateral SO (Fig. 8A). Some fibers could be traced to the contralateral side and were found to form the dorsal acoustic stria that could be traced to the contralateral dorsal cochlear nucleus (DCN) and posteroventral cochlear nucleus (PVCN) at this stage (Fig. 8B). Double labeling of the dorsal acoustic stria, and its origin in the dorsal and ventral cochlear nuclei together with afferent and efferent fibers and cells filled from the ear, showed that the dorsal acoustic stria in mice is tightly bundled near the auditory nuclei. However, as the fibers pass through the vestibular nuclei they disperse around the area where the inner ear efferent fibers diverge from the facial branchial motor fibers (Fig. 8B), only to coalesce into a tight bundle again when crossing to the contralateral side of the brainstem.
Fig. 8.
Projections to and from the IC as revealed with different colored lipophilic dyes at embryonic day 18.5. A: Bilateral IC insertions labeled the commissure of Probst, fibers and cells in the ipsilateral and contralateral SO, as well as a massive decussation of all acoustic striae in the trapezoid body. The medial longitudinal fascicle (MLF) ran in parallel to the lateral lemniscus (LL), carrying fibers to the reticular formation. B: The dorsal and intermediate acoustic striae continue to the posterior part of the posterior ventral cochlear nucleus (PVCN). Comparison with the vestibular and cochlear inner ear afferent and efferents (labeled after dye insertion into the ear and the facial nerve; red) with the brainstem fibers labeled from the IC (green) showed that these acoustic striae disperse near the area where the efferent bundle segregates from the facial branchial motoneurons fibers heading toward the facial root. IC, inferior colliculus; SO, superior olive; SON, SO nucleus; LLn, lateral lemniscus nucleus; MVN, medial vestibular nucleus. Scale bar = 10 μ.
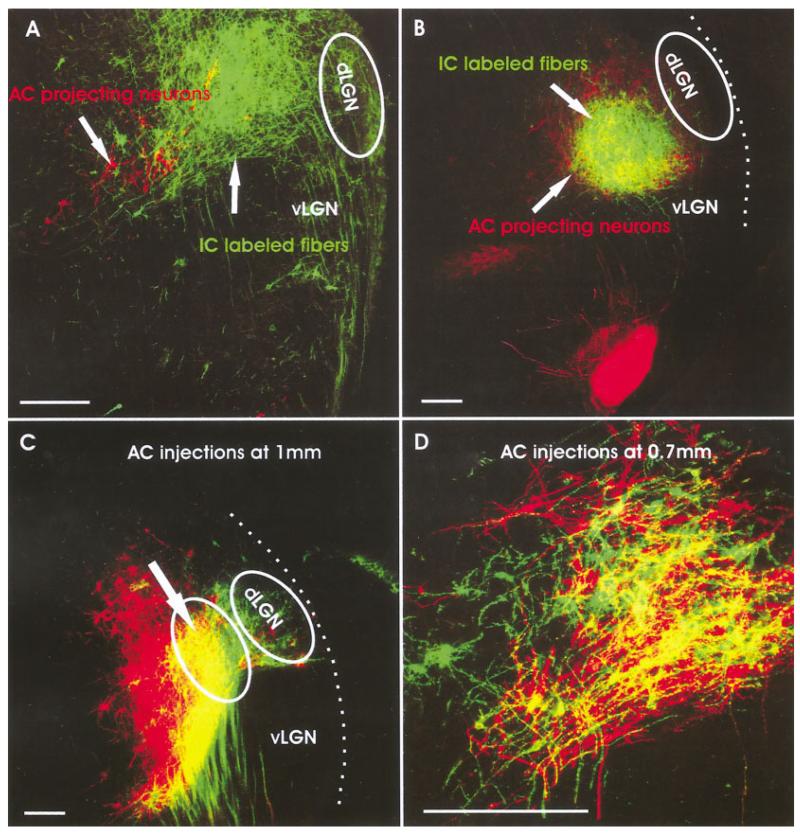
P0
At this stage of development, we concentrated on the degree of overlap after various adjacent AC insertions to understand the degree of segregation present in the MGB with respect to auditory projections as the IC projections almost completely overlapped with the retrogradely filled AC projection neurons in the MGB (Fig. 8A,B). Insertions were between 0.7 and 1.5 mm apart along the dorsoventral aspect of the forebrain that appeared to reflect the boundaries between AI and AII as depicted for adult mice (Stiebler et al., 1997). Our data showed that insertions of 1 mm distance or more resulted in almost complete segregation of labeled cell populations (Fig. 9C). Nevertheless, individual neurons were interspersed among the second labeled population and showed strong similarities in overall dendritic branching (Fig. 9C). Insertions that were 0.75 mm or less apart showed almost complete overlap of cells (Fig. 9D). Specific clustering of cells in these later insertions suggested limited segregation (Fig. 9D). Comparable data do not exist in older animals, and it remains unclear how our morphological data at this stage compare with the electrophysiological data obtained in adult mice (Stiebler et al., 1997).
Fig. 9.
The overlap of AC projecting neurons and IC fibers as well as the segregation of neurons projecting to distant sites of the auditory cortex is shown for newborn mice. A,B: Insertions into the IC reveal massive projections to the MGB that partially or completely overlap with MGB neurons retrogradely filled from the AC. C: Distantly spaced insertions with two different dyes show largely segregated neuronal populations with limited overlap of some cortical projecting neurons. D: Insertions spaced just less than 0.75 mm apart showed substantial overlap of neurons. AC, auditory cortex; IC, inferior colliculus; dLGN; dorsal lateral geniculate nucleus; MGB, medial geniculate body; vLGN, ventral lateral geniculate nucleus. Scale bars = 10 μ.
Statistical analysis of AC and IC neuron development
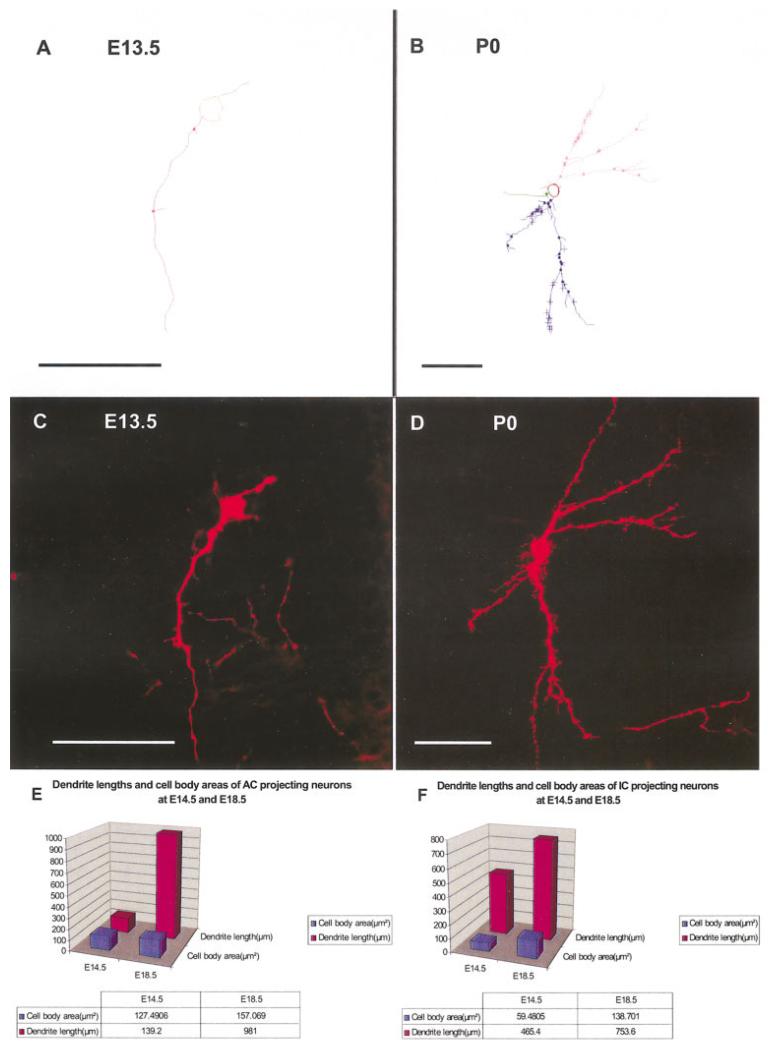
At E14.5 and E18.5, we analyzed a total of five retrogradely filled thalamocortical and thalamocollicular neurons in the area of the MGB. Single neurons were scanned with the confocal microscope, and image stacks were analyzed tracing the perikaryon and the dendrites using Neurolucida (Fig. 10A–D). The primary data were evaluated by generating means of the perikaryal areas and dendritic length. The data showed a significant increase in size over time (P > 0.05). However, growth of the dendrites was much more profound than area increase of the perikaryon and differences between cortical and collicular projection neurons were seen (Fig. 10E,F).
Fig. 10.
Dendritic analysis suggests differential growth of cortical and collicular projection neurons. A-D: We used confocal image stacks and Neurolucida software to analyze the branching pattern of individual neurons that could be imaged entirely. These cells showed increased complexity and more elaborate dendritic trees over time. E,F: We quantified the total area and length of all dendrites in at least three individual neurons of two stages, and the comparison of the means showed a tendency for the growth of the total length of the dendrites to be more profound in the cortical projecting neurons (E) than in the collicular projection neurons (F). E, embryonic day; P, postnatal day. Scale bars = 50 μm.
DISCUSSION
Timing of fiber growth with respect to the onset of auditory function
Maturation of the auditory brainstem response (ABR) indicates that the mouse peripheral hearing system establishes sound pressure hearing only after the first postnatal week (Ehret, 1976, 1997; Romand, 1992; Walsh and Romand, 1992; Cant, 1998). In contrast, all major connections of spiral sensory neurons with hair cells and the cochlear nuclei (Xiang et al., 2003) as well as all connections between cochlear nuclei and other nuclei of the central auditory pathway are established before birth, in the apparent absence of any patterned sensory stimulation (Cant, 1998; Rubel and Fritzsch, 2002). Except for embryonic chicken (Jones et al., 2001), no data exist that show spontaneous activity in the embryonic auditory system. Our data confirm and extend the findings on peripheral and central maturation of the cochlea and the brainstem (Kandler and Friauf, 1993; Cant, 1998; Gabriele et al., 2000a; Leake et al., 2002; Rubel and Fritzsch, 2002) to all other auditory nuclei of the brain of the mouse and show that the basic circuitry of the mammalian auditory system may develop in the absence of sound pressure initiated acoustic stimulation. Targeted projection to specific areas of developing nuclei is likely not dependent on acoustic input for initial development. Therefore, two essential elements in the establishment of circuitry, pathfinding and apparent rough target selection by axons, do not depend on physiologically relevant stimulation of the auditory system. However, development of more refined afferent organization in a given auditory nucleus may depend on proper afferent activity in the neonate (Gabriele et al., 2000b; Leake et al., 2002; Rubel and Fritzsch, 2002) as well as on the presence of specific numbers of afferents (Fritzsch et al., 1997).
These suggestions are in agreement with findings on the establishment of thalamocortical connections in general in wild-type and mutant mice. For example, tracing studies in the somatosensory system of mice show that cortical pioneer fibers reach the thalamus around embryonic day 12.5, much like what we found in our study for the auditory system (Lopez-Bendito and Molnar, 2003). Of interest, analyses of some mutant data have already established basic molecular mechanisms underlying corticothalamic connection formation. Tbr1 is a gene that apparently affects cortical but not thalamic differentiation (Hevner et al., 2002). In the Tbr1 null mutant mice, cortical axons enter the subpallium at the same time as in wild-type control animals, but most stop growing without entering the diencephalon (Hevner et al., 2002). Likewise, thalamic axons extend in Tbr1 null mice into the external capsule without entering the cortex. In another mouse, the Gbx2 null mutants that affects the thalamus, but not the cortex, thalamic axons are reduced in number and grow no further than the subpallium (Hevner et al., 2002). In yet another mutant, Pax6 null mice, both cortical and thalamic axons apparently fail to reach their target (Hevner et al., 2002). It has been concluded that neither corticothalamic nor thalamocortical fibers can reach and invade their specific targets independently. Apparently, either fiber can reach the subpallium but require molecularly intact reciprocal projections for further guidance. Therefore, disturbance of either cortical or thalamic development or both causes a reciprocal reduction of thalamocortical and corticothalamic connections. Thus, even when the primary defect of a mutation is either in the thalamus or the cortex, it will result in pathfinding errors of the reciprocal projection in the internal capsule, because cortical and thalamic axons associate with each other in the internal capsule. Although no data on these issues are available specifically for auditory thalamocortical connections, the similarities in the timetable of development suggest similarities in developmental mechanisms. Indeed, in all projections to and from the thalamus, there appears to be an almost coincidental appearance of fibers and retrogradely filled neurons in a given area, suggesting that the above outlined principle may apply to colliculothalamic projections as well.
Much like efferent fibers to the ear seem to grow along afferent fibers to reach their target (Fritzsch, 1999; Fritzsch et al., 1998), corticothalamic and thalamocortical fibers may grow preferentially along each other (Hevner et al., 2002), suggesting some molecular recognition cues that could possibly be specific for a given corticothalamic system. Beyond that, our data presented here show that this reciprocity is only needed for the connections between specific thalamic and cortical areas but not necessarily for targets within that area. Similar data in the inner ear also suggest that afferents may be necessary to guide efferents to the cochlea but not necessarily within the cochlea (Fritzsch, 2003; Tessarollo et al., 2004). Our insertions of different colored dyes placed adjacently in the same cortical area show limited reciprocity of thalamofugal and thalamopetal cells and fibers as well as some overlap of thalamocortical neurons in tightly spaced insertions. More data are needed to establish the finer details of the apparent reciprocity of corticothalamic interconnections within and between corticothalamic areas, in particular in mutants with selective ablation of corticothalamic areas (Krubitzer and Kahn, 2003). Whether or not the outliers in the present study will be corrected for later on or are in mice also the basis of the recently described multisensory zone (Brett-Green et al., 2003) requires further investigation.
Auditory system maturation compared with other sensory systems
Overall, the auditory system follows a developmental timetable comparable to that of other sensory systems, despite that overall maturation of the auditory periphery is delayed compared with other sensory systems such as the somatosensory system. Whereas recent data show that topographically distinct connections between the cochlea and cochlear nuclei can develop grossly normal even in the absence of hair cells (Xiang et al., 2003), the specific role of proper stimulation or the potential existence of spontaneous activity in the embryonic auditory system of mammals (Jones et al., 2001) requires further exploration beyond the already existing data (Gabriele et al., 2000b; Leake et al., 2002). Most importantly, it needs to be established how neonatal plasticity after the removal of a specific set of hair cells using high energy sound bursts can affect the cortical tonotopic representation. Such data need to be compared with cortical reorganization obtained after removal of one or more whiskers (Welker and Van der Loos, 1986; Senft and Woolsey, 1991) or to entire cortical sensory map changes after specific sensory inputs are lost (Bronchti et al., 1992; Weeks et al., 2000; Krubitzer and Kahn, 2003). Clearly, activation-dependent effects exist for the auditory cortex as much as for other cortical areas (Rauschecker, 1999, 2002) and the auditory brainstem (Rubel and Fritzsch, 2002). For example, while 85% of cochlear spiral ganglion cells are lost in NT-3 null mutants, there appears to be an enlargement of the remaining fiber projection to the cochlear nuclei. This finding suggests that reduced competition may expand the cochleotopic representation, possibly concomitant to the expansion of the peripheral target area (Fritzsch et al., 1997). Unfortunately, the cortical representation and degree of development of all connections have not been analyzed in mature Brn3c null mutants in which cochlear hair cells never mature but die off in late embryos (Xiang et al., 2003) or in mature NT-3 null mutants that die as neonates (Fritzsch et al., 1997). Such data could for the first time provide needed evidence to establish the role of spontaneous activity in the central auditory system for the development, maintenance, and fine tuning of connections, including the tonotopic projection into the cochlear nuclei. Comparing such data with the effects of binocular deprivation on the role of visual cortical connections (Bronchti et al., 1992; Weeks et al., 2000) as well as the many neonatal ablation studies in the auditory system (Gabriele et al., 2000b; Rubel and Fritzsch, 2002) could provide further mechanistic insights. Clearly, the degree of discreet and partially segregated thalamocortical projections as well as the degree of differentiation found in individual newborn thalamocortical neurons will allow us to evaluate any effect of such mutations on the thalamic development as many ear specific mutations are viable at least until birth and appropriate conditional mutants will allow studying neonates.
The cochlear nucleus efferents follow three pathways: (1) by means of the ventral acoustic stria or trapezoid body which contains axons from anteroventral cochlear nucleus and PVCN (Rouiller, 1997); (2) by means of the intermediate acoustic stria or stria of Held originate from PVCN to end in periolivary nuclei, vLL, and cIC (Rouiller, 1997); (3) by means of the dorsal acoustic stria or stria of Monakow, which carries axons from the DCN, which bypass SOC to reach the contralateral cIC and contralateral dLL and vLL (Rouiller, 1997). We show here, for the first time, an area of distinct pathfinding of the dorsal and intermediate acoustic stria near the area of segregation of inner ear efferent fibers from facial branchial motoneuron fibers (Karis et al., 2001). Once candidate molecules for the pathfinding of these fibers have been identified, it will be interesting to see how they are distributed in this area of the brainstem that belongs to rhombomere 4. Mutations known to affect rhombomere 4 (Muller et al., 2003) need to be analyzed to understand the effect on potential alterations of the pathfinding properties of these projections and whether they exist at all in such mutants.
Some ascending fibers from CN to IC are composed of very thin fibers that travel along with other LL fibers to cross the midline at the level of trapezoid body. Ascending fibers to the IC also come from vLL, dLL, and SOC (Rouiller, 1997). The SOC receives bilateral ascending fibers from the ventral cochlea nucleus (vCN) and descending fibers from ipsilateral IC. The SOC projects its fibers to IC, LL, CN, and the cochlea (Helfert and Aschoff, 1997). The dLL project bilaterally to the IC and its contralateral fibers cross the brainstem caudal to the aqueduct (Gabriele et al., 2000a). The dLL receives the majority of input fibers from the SO and the contralateral CN, and some from the contralateral dLL by means of the commissure of Probst (Gabriele et al., 2000a). In addition to all those fibers, we found fibers crossing in the commissure of Probst to extend along the medial longitudinal fascicle to the contralateral side. Those fibers seem to originate from the reticular formation medial to the SOC complex. However, their origin and distribution in the IC requires further investigation.
Overall development
Neurons in the MGB could be labeled from the AC as early as E13.5. These neurons displayed a specific distribution in the dorsal thalamus that was comparable to that found in later stages. By E13.5, MGB neurons could be labeled from the IC and fibers from the IC started to invade the MGB. Simultaneous labeling of IC fibers and MGB neurons from the AC with different colored dyes showed an increasing complexity of fiber and dendrite interaction between E13.5 and P0. At E14.5, neurons had few, short dendrites. However, as development progressed beyond E16.5 to P0, the neurons developed more complex dendrites and axons developed side branches with growth cones. Most importantly is the specific and partially overlapping of IC afferents with MGB neurons filled from the auditory cortex (Fig. 7). At P0, MGB neurons projected largely segregated but partially overlapping to areas of the auditory cortex. Large overlap was found in closely spaced insertions up to P0 (less than 1 mm apart), suggesting that the terminal arbors of MGB neurons extend beyond the boundaries of their designated terminal fields in the AC. At P0, there are still growth cones on the tips of axons, suggesting that the maturation process is ongoing. More data on neonatal mice are needed to show how this embryonic organization corresponds to that of functionally mature animals.
Overall, our data on temporal progression of development in mice conform closely to the data on rats, assuming a 1- to 2-day delay in maturation of rats. For example, in rats, axonal outgrowth from the CN reaches the IC around E18 (Kandler and Friauf, 1993) and we can retrogradely fill CN neurons as early as E16.5 in mice. Contralateral dLL neurons reach the IC in rats at E15 (Gabriele et al., 2000a), and we can label such cells in mice at E14.5. Thus, in the brainstem, our data on mice closely compare with those of two previous studies and fits closely to peripheral ear developmental data, which also indicate that the mouse is approximately 2 days ahead of rats in development (Fritzsch and Nichols, 1993).
Overall, these data suggest that the MGB projection is topographically specific from the beginning and that only local “fine tuning” might occur in neonates before or after the onset of hearing, suggesting that the development of auditory pathway connection and perhaps even its tonotopic organization is independent of any acoustic stimulus (Cant, 1998). The grossly normal formation of these connections is apparently specified by genes at the onset. Spontaneous activity, shown to play major roles in other systems such as the visual system (Rauschecker, 2002), seems to be of little importance here as no spontaneous activity has been reported thus far in such young embryonic mice (Jones et al., 2001). We are now using some of the mouse models with genetic alteration specifically of the periphery such as loss of hair cells or alteration of peripheral and central sensory neuron projections (Fritzsch et al., 1997; Xiang et al., 2003) to investigate how the central organization will be affected by these changes.
ACKNOWLEDGMENTS
We thank S. Pauley for help with the English. We also thank Dr. Brian Gray of PTI Research for the generous donation of the lipophilic tracer dyes used in this study, and Drs. Betsy Ohlsson-Wilhelm and Kathy Muirhead of SciGro, Inc., for review of the article.
Grant sponsor: National Institute on Deafness and Other Communication Disorders; Grant number: 1RO1 DC005590.
LITERATURE CITED
- Adams JC. Ascending projections to the inferior colliculus. J Comp Neurol. 1979;183:519–538. doi: 10.1002/cne.901830305. [DOI] [PubMed] [Google Scholar]
- Aitkin L, Schuck D. Low frequency neurons in the lateral central nucleus of the cat inferior colliculus receive their input predominantly from the medial superior olive. Hear Res. 1985;17:87–93. doi: 10.1016/0378-5955(85)90134-0. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the diencephalon in the rat. V. Thymidine-radiographic observations on internuclear and intranuclear gradients in the thalamus. J Comp Neurol. 1979;188:473–499. doi: 10.1002/cne.901880309. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the brain stem in the rat. III. Thymidine-radiographic study of the time of origin of neurons of the vestibular and auditory nuclei of the upper medulla. J Comp Neurol. 1980;194:877–904. doi: 10.1002/cne.901940410. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Time of origin of neurons of the rat inferior colliculus and the relations between cytogenesis and tonotopic order in the auditory pathway. Exp Brain Res. 1981;42:411–423. doi: 10.1007/BF00237506. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the cranial nerve ganglia and related nuclei in the rat. Adv Anat Embryol Cell Biol. 1982;74:1–90. doi: 10.1007/978-3-642-68479-1. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the rat thalamus: V. The posterior lobule of the thalamic neuroepithelium and the time and site of origin and settling pattern of neurons of the medial geniculate body. J Comp Neurol. 1989;284:567–580. doi: 10.1002/cne.902840406. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Knight PL, Merzenich MM. The thalamocortical and corticothalamic connections of AI, AII, and the anterior auditory field (AAF) in the cat: evidence for two largely segregated systems of connections. J Comp Neurol. 1980;194:663–701. doi: 10.1002/cne.901940312. [DOI] [PubMed] [Google Scholar]
- Blake JA. The roof and lateral recesses of the fourth ventricle, considered morphologically and embryologically. J Comp Neurol. 1900;10:79–108. [Google Scholar]
- Brett-Green B, Fifkova E, Larue DT, Winer JA, Barth DS. A multisensory zone in rat parietotemporal cortex: intra- and extracellular physiology and thalamocortical connections. J Comp Neurol. 2003;460:223–237. doi: 10.1002/cne.10637. [DOI] [PubMed] [Google Scholar]
- Bronchti G, Schonenberger N, Welker E, Van der Loos H. Barrelfield expansion after neonatal eye removal in mice. Neuroreport. 1992;3:489–492. doi: 10.1097/00001756-199206000-00008. [DOI] [PubMed] [Google Scholar]
- Bruce LL, Christensen MA, Fritzsch B. Electron microscopic differentiation of directly and transneuronally transported DiI and applications for studies of synaptogenesis. J Neurosci Methods. 1997;73:107–112. doi: 10.1016/s0165-0270(96)02218-2. [DOI] [PubMed] [Google Scholar]
- Brunso-Bechtold JK, Thompson GC, Masterton RB. HRP study of the organization of auditory afferents ascending to central nucleus of inferior colliculus in cat. J Comp Neurol. 1981;197:705–722. doi: 10.1002/cne.901970410. [DOI] [PubMed] [Google Scholar]
- Cant NB. Structural development of the mammalian central auditory pathways. In: Rubel ED, Popper AN, Fay RR, editors. Development of the auditory system. Springer; New York: 1998. [Google Scholar]
- Cooper ML, Rakic P. Neurogenetic gradients in the superior and inferior colliculi of the rhesus monkey. J Comp Neurol. 1981;202:309–334. doi: 10.1002/cne.902020303. [DOI] [PubMed] [Google Scholar]
- Druga R, Syka J. Ascending and descending projections to the inferior colliculus in the rat. Physiol Bohemoslov. 1984;33:31–42. [PubMed] [Google Scholar]
- Ehret G. Critical bands and filter characteristics in the ear of the housemouse (Mus musculus) Biol Cybern. 1976;24:35–42. doi: 10.1007/BF00365592. [DOI] [PubMed] [Google Scholar]
- Ehret G. The auditory cortex. J Comp Physiol [A] 1997;181:547–557. doi: 10.1007/s003590050139. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. Academic Press; San Diego: 1997. The mouse brain in stereotaxic coordinates. [Google Scholar]
- Fritzsch B. Ontogenetic and evolutionary evidence for the motoneuron nature of vestibular and cochlear efferents. In: Berlin CI, editor. The efferent auditory system: basic science and clinical applications. Singular Publishing; San Diego: 1999. pp. 31–59. [Google Scholar]
- Fritzsch B. Development of inner ear afferent connections: forming primary neurons and connecting them to the developing sensory epithelia. Brain Res Bull. 2003;60:423–433. doi: 10.1016/s0361-9230(03)00048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Nichols DH. DiI reveals a prenatal arrival of efferents at the differentiating otocyst of mice. Hear Res. 1993;65:51–60. doi: 10.1016/0378-5955(93)90200-k. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Farinas I, Reichardt LF. Lack of neurotrophin 3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion. J Neurosci. 1997;17:6213–6225. doi: 10.1523/JNEUROSCI.17-16-06213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Barbacid M, Silos-Santiago I. The combined effects of trkB and trkC mutations on the innervation of the inner ear. Int J Dev Neurosci. 1998;16:493–505. doi: 10.1016/s0736-5748(98)00043-4. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Muirhead KA, Gray B, Maklad A. Diffusion and imaging properties of three new lipophilic tracers, PKH2, PKH26 and PTIR271, and their use for triple labeling of neuronal profiles. Soc Neurosci. 2002 doi: 10.1016/j.brainresbull.2005.05.016. Abstract 519.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriele ML, Brunso-Bechtold JK, Henkel CK. Development of afferent patterns in the inferior colliculus of the rat: projection from the dorsal nucleus of the lateral lemniscus. J Comp Neurol. 2000a;416:368–382. doi: 10.1002/(sici)1096-9861(20000117)416:3<368::aid-cne8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Gabriele ML, Brunso-Bechtold JK, Henkel CK. Plasticity in the development of afferent patterns in the inferior colliculus of the rat after unilateral cochlear ablation. J Neurosci. 2000b;20:6939–6949. doi: 10.1523/JNEUROSCI.20-18-06939.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler DB, Ehret G. Auditory perception vs. recognition: representation of complex communication sounds in the mouse auditory cortical fields. Eur J Neurosci. 2004;19:1027–1040. doi: 10.1111/j.1460-9568.2004.03205.x. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Moore RY. Ascending projections of the lateral lemniscus in the cat and monkey. J Comp Neurol. 1967;129:143–156. [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolod-kin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfert RH, Aschoff A. Superior olivary complex and nuclei of the lateral lemniscus. In: Ehret G, Romand R, editors. The central auditory system. Oxford University Press; New York: 1997. pp. 193–258. [Google Scholar]
- Henkel CK, Shneiderman A. Nucleus sagulum: projections of a lateral tegmental area to the inferior colliculus in the cat. J Comp Neurol. 1988;271:577–588. doi: 10.1002/cne.902710408. [DOI] [PubMed] [Google Scholar]
- Henkel CK, Spangler KM. Organization of the efferent projections of the medial superior olivary nucleus in the cat as revealed by HRP and autoradiographic tracing methods. J Comp Neurol. 1983;221:416–428. doi: 10.1002/cne.902210405. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Miyashita-Lin E, Rubenstein JL. Cortical and thalamic axon pathfinding defects in Tbr1, Gbx2, and Pax6 mutant mice: evidence that cortical and thalamic axons interact and guide each other. J Comp Neurol. 2002;447:8–17. doi: 10.1002/cne.10219. [DOI] [PubMed] [Google Scholar]
- His W. Zur Geschichte des Gehirns sowie der zentralen und peripherischen Nervenbahnen. Abh Math-Phys K1 Saechs Akad Wiss. 1888;24:341–392. [Google Scholar]
- Hofstetter KM, Ehret G. The auditory cortex of the mouse: connections of the ultrasonic field. J Comp Neurol. 1992;323:370–386. doi: 10.1002/cne.903230306. [DOI] [PubMed] [Google Scholar]
- Jones TA, Jones SM, Paggett KC. Primordial rhythmic bursting in embryonic cochlear ganglion cells. J Neurosci. 2001;21:8129–8135. doi: 10.1523/JNEUROSCI.21-20-08129.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Friauf E. Pre- and postnatal development of efferent connections of the cochlear nucleus in the rat. J Comp Neurol. 1993;328:161–184. doi: 10.1002/cne.903280202. [DOI] [PubMed] [Google Scholar]
- Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol. 2001;429:615–630. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L, Kahn DM. Nature versus nurture revisited: an old idea with a new twist. Prog Neurobiol. 2003;70:33–52. doi: 10.1016/s0301-0082(03)00088-1. [DOI] [PubMed] [Google Scholar]
- Kudo M. Projections of the nuclei of the lateral lemniscus in the cat: an autoradiographic study. Brain Res. 1981;221:57–69. doi: 10.1016/0006-8993(81)91063-5. [DOI] [PubMed] [Google Scholar]
- Kudo M, Niimi K. Ascending projections of the inferior colliculus onto the medial geniculate body in the cat studied by anterograde and retrograde tracing. Brain Res. 1978;155:113–117. doi: 10.1016/0006-8993(78)90310-4. [DOI] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT. Postnatal refinement of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol. 2002;448:6–27. doi: 10.1002/cne.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ. Essential role of BETA2/ NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 2000;14:2839–2854. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bendito G, Molnar Z. Thalamocortical development: how are we going to get there? Nat Rev Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- Maffi CL, Aitkin LM. Differential neural projections to regions of the inferior colliculus of the cat responsive to high frequency sounds. Hear Res. 1987;26:211–219. doi: 10.1016/0378-5955(87)90113-4. [DOI] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. The developmental segregation of posterior crista and saccular vestibular fibers in mice: a carbocyanine tracer study using confocal microscopy. Dev Brain Res. 2002;135:1–17. doi: 10.1016/s0165-3806(01)00327-3. [DOI] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Development of vestibular afferent projections into the hindbrain and their central targets. Brain Res Bull. 2003a;60:497–510. doi: 10.1016/s0361-9230(03)00054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Partial segregation of posterior crista and saccular fibers to the nodulus and uvula of the cerebellum in mice, and its development. Brain Res Dev Brain Res. 2003b;140:223–236. doi: 10.1016/s0165-3806(02)00609-0. [DOI] [PubMed] [Google Scholar]
- Moore DR. Auditory brainstem of the ferret: sources of projections to the inferior colliculus. J Comp Neurol. 1988;269:342–354. doi: 10.1002/cne.902690303. [DOI] [PubMed] [Google Scholar]
- Muller M, Jabs N, Lork DE, Fritzsch B, Sander M. Nkx6.1 controls migration and axon pathfinding of cranial branchio-motoneurons. Development. 2003;130:5815–5826. doi: 10.1242/dev.00815. [DOI] [PubMed] [Google Scholar]
- Nornes HO, Morita M. Time of origin of the neurons in the caudal brain stem of rat. Dev Neurosci. 1979;2:101–114. [Google Scholar]
- Oblinger MM, Das GD. Neurogenesis in the brain stem of the rabbit: an autoradiographic study. J Comp Neurol. 1981;197:45–62. doi: 10.1002/cne.901970105. [DOI] [PubMed] [Google Scholar]
- Payne BR. Development of the auditory cortex. In: Romand R, editor. Development of the auditory and vestibular systems 2. Elsevier; Amsterdam: 1992. pp. 357–390. [Google Scholar]
- Puelles L. Thoughts on the development, structure and evolution of the mammalian and avian telencephalic pallium. Philos Trans R Soc Lond B Biol Sci. 2001;356:1583–1598. doi: 10.1098/rstb.2001.0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Auditory cortical plasticity: a comparison with other sensory systems. Trends Neurosci. 1999;22:74–80. doi: 10.1016/s0166-2236(98)01303-4. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Cortical map plasticity in animals and humans. Prog Brain Res. 2002;138:73–88. doi: 10.1016/S0079-6123(02)38072-5. [DOI] [PubMed] [Google Scholar]
- Romand R. Development of the auditory and vestibular systems 2. Elsevier; Amsterdam: 1992. p. 609. [Google Scholar]
- Roth GL, Aitkin LM, Andersen RA, Merzenich MM. Some features of the spatial organization of the central nucleus of the inferior colliculus of the cat. J Comp Neurol. 1978;182:661–680. doi: 10.1002/cne.901820407. [DOI] [PubMed] [Google Scholar]
- Rouiller EM. Functional organization of the auditory pathways. In: Ehret G, Romand R, editors. The central auditory system. Oxford University Press; New York: 1997. pp. 3–96. [Google Scholar]
- Rouiller EM, Welker E. Morphology of corticothalamic terminals arising from the auditory cortex of the rat: a Phaseolus vulgaris-leucoagglutinin (PHA-L) tracing study. Hear Res. 1991;56:179–190. doi: 10.1016/0378-5955(91)90168-9. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioauto-graphic study of terminal mitoses. Acta Otolaryngol Suppl. 1967;220:221–244. [PubMed] [Google Scholar]
- Ryugo DK, Willard FH, Fekete DM. Differential afferent projections to the inferior colliculus from the cochlear nucleus in the albino mouse. Brain Res. 1981;210:342–349. doi: 10.1016/0006-8993(81)90907-0. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Cant NB. Organization of the superior olivary complex in the guinea pig: II. Patterns of projection from the periolivary nuclei to the inferior colliculus. J Comp Neurol. 1992;317:438–455. doi: 10.1002/cne.903170409. [DOI] [PubMed] [Google Scholar]
- Senft SL, Woolsey TA. Growth of thalamic afferents into mouse barrel cortex. Cereb Cortex. 1991;1:308–335. doi: 10.1093/cercor/1.4.308. [DOI] [PubMed] [Google Scholar]
- Shneiderman A, Oliver DL, Henkel CK. Connections of the dorsal nucleus of the lateral lemniscus: an inhibitory parallel pathway in the ascending auditory system? J Comp Neurol. 1988;276:188–208. doi: 10.1002/cne.902760204. [DOI] [PubMed] [Google Scholar]
- Stiebler I, Neulist R, Fichtel I, Ehret G. The auditory cortex of the house mouse: left-right differences, tonotopic organization and quantitative analysis of frequency representation. J Comp Physiol [A] 1997;181:559–571. doi: 10.1007/s003590050140. [DOI] [PubMed] [Google Scholar]
- Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci. 2004;24:2575–2584. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velenovsky DS, Cetas JS, Price RO, Sinex DG, McMullen NT. Functional subregions in primary auditory cortex defined by thalamocortical terminal arbors: an electrophysiological and anterograde labeling study. J Neurosci. 2003;23:308–316. doi: 10.1523/JNEUROSCI.23-01-00308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh EJ, Romand R. Functional development of the cochlea and cochlear nerve. In: Romand R, editor. Development of auditory and vestibular systems 2. Elsevier; Amsterdam: 1992. pp. 161–220. [Google Scholar]
- Weeks R, Horwitz B, Aziz-Sultan A, Tian B, Wessinger CM, Cohen LG, Hallett M, Rauschecker JP. A positron emission tomographic study of auditory localization in the congenitally blind. J Neurosci. 2000;20:2664–2672. doi: 10.1523/JNEUROSCI.20-07-02664.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker E, Van der Loos H. Quantitative correlation between barrel-field size and the sensory innervation of the whiskerpad: a comparative study in six strains of mice bred for different patterns of mystacial vibrissae. J Neurosci. 1986;6:3355–3373. doi: 10.1523/JNEUROSCI.06-11-03355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley JM, Henkel CK. Topographical organization of the inferior collicular projection and other connections of the ventral nucleus of the lateral lemniscus in the cat. J Comp Neurol. 1984;229:257–270. doi: 10.1002/cne.902290210. [DOI] [PubMed] [Google Scholar]
- Willard FH. Development of the mammalian auditory hindbrain. In: Malhotra S, editor. Advances in neural science. JAI Press Inc.; Greenwich CT: 1995. pp. 205–234. [Google Scholar]
- Winer JA, Diehl JJ, Larue DT. Projections of auditory cortex to the medial geniculate body of the cat. J Comp Neurol. 2001;430:27–55. [PubMed] [Google Scholar]
- Xiang M, Maklad A, Pirvola U, Fritzsch B. Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation. BMC Neurosci. 2003;4:2. doi: 10.1186/1471-2202-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]