Abstract
The forkhead genes are involved in patterning, morphogenesis, cell fate determination, and proliferation. Several Fox genes (Foxi1, Foxg1) are expressed in the developing otocyst of both zebrafish and mammals. We show that Foxg1 is expressed in most cell types of the inner ear of the adult mouse and that Foxg1 mutants have both morphological and histological defects in the inner ear. These mice have a shortened cochlea with multiple rows of hair cells and supporting cells. Additionally, they demonstrate striking abnormalities in cochlear and vestibular innervation, including loss of all crista neurons and numerous fibers that overshoot the organ of Corti. Closer examination shows that some anterior crista fibers exist in late embryos. Tracing these fibers shows that they do not project to the brain but, instead, to the cochlea. Finally, these mice completely lack a horizontal crista, although a horizontal canal forms but comes off the anterior ampulla. Anterior and posterior cristae, ampullae, and canals are reduced to varying degrees, particularly in combination with Fgf10 heterozygosity. Compounding Fgf10 heterozygotic effects suggest an additive effect of Fgf10 on Foxg1, possibly mediated through bone morphogenetic protein regulation. We show that sensory epithelia formation and canal development are linked in the anterior and posterior canal systems. Much of the Foxg1 phenotype can be explained by the participation of the protein binding domain in the delta/notch/hes signaling pathway. Additional Foxg1 effects may be mediated by the forkhead DNA binding domain.
Keywords: Foxg1, Fgf10, cell cycle, cell fate determination
INTRODUCTION
The mammalian forkhead family contains 43 genes and belongs to the larger family of 100 known forkhead genes in animals (Solomon et al., 2003b). The forkhead family members have the approximately 100 amino acid monomeric DNA “winged-helix” binding domain of the founding members: Drosophila forkhead (Lee and Frasch, 2004) and hepatocyte nuclear factor 3a (Tao and Lai, 1992). Forkhead genes play pivotal roles in organogenesis, including patterning and morphogenesis, through regulation of proliferation and cell fate specification (Solomon et al., 2003b). In addition, several forkhead genes are implicated in carcinogenesis through gene amplification, retroviral integration, and chromosomal translocation (Katoh and Katoh, 2004). Foxi and Foxg genes have been identified in the ear (Hatini et al., 1999; Ohyama and Groves, 2004), and Foxp2 appears important for auditory system and language development (Vargha-Khadem et al., 2005).
Work in zebrafish and mice has shown that all three Foxi genes are expressed in the otic placode (Solomon et al., 2003a; Ohyama and Groves, 2004). Foxi1 is essential for otocyst formation in zebrafish, apparently through the regulation of Pax2 and Pax8 expression (Hans et al., 2004). Mutation in the mammalian Foxi1 gene, however, results in near normal ear development. Postnatal Foxi1 null mice develop endolymphatic hydrops due to deregulation of the chloride/iodide transporter gene pendrin; and pendrin mutations are known to cause deafness in humans (Hulander et al., 1998, 2003).
Foxg1 (formerly Bf-1) expression has been analyzed up to embryonic day 12.5 using the LacZ reporter (Hatini et al., 1999). A successful Cre-expressing line, has been generated (Hebert and McConnell, 2000) and has been used to analyze conditional ear mutations (Pirvola et al., 2002; Kiernan et al., 2006). Foxg1 is also found in the developing zebrafish ear (Toresson et al., 1998) and, therefore, does seem to be conserved in its expression in vertebrates. The role of Foxg1 in eye and forebrain development has been analyzed thoroughly (Hanashima et al., 2002, 2004; Vyas et al., 2003; Martynoga et al., 2005). In the eye, Foxg1 and Foxd1 are essential for the projection of retinal ganglion cells (Herrera et al., 2004; Pratt et al., 2004), whereas other Fox genes (Foxn4) play essential roles in regulating the expression of basic helix–loop–helix (bHLH) genes associated with specific neuronal cell fate acquisition (Iwahori et al., 2004).
In this study, we use Foxg1 heterozygotic mice with the LacZ reporter substituted in the reading frame of Foxg1 to completely analyze the expression of Foxg1 beyond the previously reported early embryonic stages in the mouse inner ear (Hatini et al., 1999). We analyze the morphogenetic defects on both cochlear and vestibular outgrowth in Foxg1 null mice. Lastly, we investigate the formation of sensory epithelia, hair cells, and sensory neurons in Foxg1 null mice.
We show here that Foxg1 null mutants have both morphogenetic and histogenetic ear defects. Foxg1 mutant mice (i) have a shortened cochlea that has excessive rows of hair cells, (ii) have a vestibular system that shows a reduction or loss of canal cristae accompanied by a reduction in size or loss of canals, and (iii) have aberrations in cochlear and vestibular innervation, including lack of any innervation that connects the canal cristae to the brain.
RESULTS
Foxg1 Was Differentially Expressed in Most but Not All Inner Ear Sensory Neurons and Sensory Epithelial Cells
Using the LacZ reporter system, we describe here the extensive expression of Foxg1, not only in the embryonic ear but also in the adult ear. These data suggest that, in addition to its role in development, this transcription factor may function in adult homeostasis, comparable to recent suggestions for adult expression of Atoh1 (Matei et al., 2005a). In situ hybridization for Foxg1 at embryonic day (E) 12.5 and E18.5 showed a similar expression pattern to the LacZ data but with much lower intensity (data not shown).
Our LacZ data suggested a wide-spread and profound expression of Foxg1 in all spiral and vestibular ganglion neurons (Fig. 1F,H). In contrast, expression in hair cells was much less profound. In the utricle, the distribution of Foxg1 was in neuronal hair cells, whereas abneuronal hair cells were negative. Sections suggested that the lack of expression may coincide with changes in hair cell polarity in the striola (Fig. 1G). In the cochlea, inner hair cells were positive and outer hair cells showed only very faint LacZ expression (Fig. 1E). In contrast, virtually all supporting cells of all sensory epithelia were positive. Thus, the sensory epithelia of the canal cristae, utricle, and saccule showed a sharp delineation against the unstained nonsensory epithelia (Fig. 1). Some labeled cells were found outside the vestibular sensory epithelia such as the endolymphatic duct and parts of the canals. Labeling in the cochlea extended beyond the cells of the organ of Corti (Deiter’s, pillar and border cells) and showed expression in all cells of the cochlear duct, except for the cells of the stria vascularis (Fig. 1C,D). In summary, our data show that Foxg1 is differentially expressed in sensory and nonsensory cells of the adult inner ear.
Fig. 1.
Adult Foxg1 expression analysis using LacZ. Strong Foxg1 expression is seen in the cochlea, saccule, utricle, and canal cristae. Weaker Foxg1 expression is seen in the canals, particularly near the ampullae of the cristae; and is strongest at the inner and outer curvatures. In the utricle, an expression gradient can be seen from stronger to weaker expression in the neuronal to abneuronal portions, respectively. A: There is a faint gradient in the cochlea with the base being less stained than the apex. B: The horizontal crista sensory epithelium is outlined to show that the Foxg1 expression is limited to the supporting cells of the sensory epithelia. C: An overview of the cochlea shows very intense expression in the spiral ganglion, the inner spiral sulcus, and the organ of Corti except for the cell-free tunnel of Corti. D,E: Radial sections through the cochlea show intense staining in the Reissner’s membrane, spiral limbus, and inner spiral sulcus (below the tectorial membrane). The inner hair cells are strongly labeled, whereas outer hair cells are faintly labeled. All supporting cells are labeled, including border cells, inner and outer pillar cells, Deiter’s cells, and Hensen’s cells. The outer spiral sulcus, or Claudius’ cells are strongly labeled, but the stria vascularis is strikingly devoid of staining, as is the otic mesenchyme and the basilar membrane. F: The vestibular ganglion shows Foxg1 in all neurons, but the intensity is variable. G: The utricle of the adult shows staining in some hair cells and all supporting cells on the neuronal side with a rapid decrease of expression on the abneuronal side. A,C,D,H: All spiral ganglion neurons demonstrate Foxg1 expression to varying degrees. PC, posterior crista; HC, horizontal crista; AC, anterior crista; U, utricle; S, saccule; B, base; A, apex; SpGl, spiral ganglion; SpSl, spiral limbus; OC, organ of Corti; RM, Reissner’s membrane; SV, stria vascularis; TM, tectorial membrane; SL, spiral limbus; BM, basilar membrane; IHC, inner hair cell; OHC, outer hair cell; BC, border cell; IP, inner pillar; TC, tunnel of Corti; OP, outer pillar; DC, Deiter’s cell; HC, Hensen’s cell; HCs, hair cells; SCs, supporting cells. Scale bars = 100 μm in A–D,F–H, 10 μm in E.
Foxg1 Was Expressed in All Neurosensory Precursors
Foxg1 was expressed in the developing otic placode (Hatini et al., 1999) and at E10.5, the entire otocyst showed Foxg1 expression (Hebert and McConnell, 2000). At E11.5, Foxg1 expression, as shown by LacZ, was strong in the entire presumptive cochlea, vestibular end organs, and all sensory neurons. Additionally, strong expression was seen in the endolymphatic duct and at the base of the developing canals (Fig. 2A).
Fig. 2.
Morphogenesis of the Foxg1 null inner ear. A,C,E: Foxg1 in heterozygotic animals is expressed in the entire presumptive cochlea, the inferior and superior vestibular ganglia, the spiral ganglion, the geniculate ganglia, utricle, saccule, and all canal cristae. There is weaker expression in the developing anterior and posterior canals (but not the horizontal canal), and in the endolymphatic duct. B,D,F: In the Foxg1 null mutant, the LacZ expression pattern is basically the same as in the wild-type at E11.5. E,F: Whereas the size of the cochlea and ganglia appear to be normal, there is reduced anterior–posterior extension of the canal plates and the posterior plates that would normally fuse to form the posterior canal (E), remains unfused (F). D: The cochlea shows a delay in spiraling. Additionally, the mutant shows LacZ expression in the horizontal canal, whereas there is no Foxg1 expression in the horizontal canal of the Foxg1 heterozygote. C–F: The Foxg1 heterozygotes show a distinct horizontal crista sensory patch (C,E) that is completely absent in Foxg1 null mice (D,F). Note the more extensive reaction in Foxg1 null mice that is likely due to the double expression of LacZ under Foxg1 promoter control. AC, anterior crista; PC, posterior crista; ED, endolymphatic duct; SVG, superior vestibular ganglion; IVG, inferior vestibular ganglion; GG, geniculate ganglion; Coch, cochlea; U, utricle; S, saccule; SpGl, spiral ganglion. Scale bar = 100 μm in all images, anterior is to the left, dorsal is up.
At E13.5, Foxg1 expression labeled the developing cochlea, saccule, utricle, all three canal cristae, and the sensory neurons. The developing horizontal canal showed no Foxg1 expression. Therefore, the horizontal crista was a labeled patch against a pale background (Fig. 2C,E). The nonsensory structures of the endolymphatic duct and anterior and posterior canals also showed some expression (Fig. 2C,E). At E18.5, LacZ staining was strong in all sensory patches of the ear and in the spiral and vestibular ganglion neurons. In contrast to E13.5, the vestibular sensory epithelia showed reduced staining, particularly in the abneuronal half of the utricle (Fig. 3D). A low level of staining was also seen in the canals where it was reduced to a set of cells along the fused canal plates.
Fig. 3.
A–I: Morphological defects of the Foxg1 null at embryonic day (E) 18.5. D,H: Foxg1 heterozygotic animals have fully developed the three canals and the common crus and show Foxg1 expression in all canal cristae, utricle, saccule, and cochlea, much like the adult pattern of expression. A,C: In contrast, Foxg1 mutants, particularly on an Fgf10 heterozygotic background, have a reduced (C) or no posterior crista or posterior canal (A, not reacted for LacZ). A–C,F: All Foxg1 mutants lack a horizontal crista but do have a horizontal canal. A–C: All Foxg1 mutants have a shortened, wide cochlea (A–C) with a reduced spiral ganglion (B) that does not spiral along the cochlea. E,F,H: The anterior crista of Foxg1 mutants is somewhat smaller than that of the wild-type and is asymmetrical around the cruciate eminence. F: Note the absence of a horizontal crista in Foxg1 mutants and that the horizontal canal branches off the same ampulla as the anterior crista. F–I: The posterior crista of Foxg1 null mice is much smaller compared with the posterior crista of the wild-type. Additionally, there is no sign of a cruciate eminence. D,E,G,H,I: Note that the utricle shows less intense staining near the striolar region (D,H) and that the canal cristae have extensive staining in both hair cells and supporting cells (E,G,I). AC, anterior crista; HC, horizontal canal or horizontal crista; CC, common crus; PC, posterior crista; SpGl, spiral ganglion; U, utricle; S, saccule. Scale bars = 100 μm in A-D,F,H; 50 μm in E,G,I.
Foxg1 Mutants Lacked a Horizontal Crista and Had a Posterior Canal Morphogenetic Phenotype
The initial Foxg1 phenotype was a somewhat smaller otic vesicle at E10.5. At E11.5, the Foxg1 mutant inner ear was roughly half the width of the wild-type at this stage (Fig. 2B); the size difference resulting from a reduction in the developing canals. The difference in size of the canal area was more obvious in E12.5 and E13.5 Foxg1 null mice compared with heterozygotic littermates (Fig. 2). Due to the higher LacZ copy number there was no distinction between the canal cristae and the canals in the Foxg1 mutants. In heterozygotic animals, the central part of the canal plates fused with a separation of the common crus as previously described (Morsli et al., 1998; Chang et al., 2004). Similar fusion was seen in the anterior canal plates of the Foxg1 null mice. However, the posterior canal showed extensive LacZ expression throughout the fusion plate with limited fusion appearing at E13.5 (Fig. 2F).
In heterozygotic animals, Foxg1 expression was limited to the crista in the horizontal canal system. The mutants showed a profound expression of LacZ throughout the entire forming horizontal canal at E13.5 (Fig. 2D,F). There was, however, no obvious enlargement of a horizontal canal ampulla and no distinct staining indicative of a horizontal canal crista. Aside from a single case in which an epithelial thickening with no identifiable hair cells was observed near the base of the horizontal canal, E18.5 Foxg1 null mice showed no indication of a distinct horizontal crista or ampulla. In contrast, a horizontal canal was always present, emanating from the anterior canal ampulla (Fig. 3). These data suggest that Foxg1 null mice have a morphogenetic defect in the posterior canal and entirely lack formation of the horizontal crista and ampulla.
Foxg1 Morphogenetic Phenotype Was Enhanced in Fgf10 Heterozygotes
Previous work showed that Fgf10 null mice entirely lacked canals and showed reduced canal epithelia with a complete lack of the posterior crista, but had no obvious phenotype in the cochlea (Pauley et al., 2003; Ohuchi et al., 2005). The phenotypic similarity in the vestibular region suggested an interaction between Fgf10 and Foxg1. We crossed the Foxg1 mutant mice with the Fgf10 mutant mice. So far, we have not obtained any mice null for both Foxg1 and Fgf10. However, the mice null for Foxg1 and heterozygotic for Fgf10 showed a variable aggravation of the Foxg1 phenotype. Both Foxg1 simple nulls, as well as compound mutants, showed a wide range of posterior canal formation defects: from almost normal to near complete loss. In the most extreme case, found only in the Foxg1 null/Fgf10 heterozygote, there was no posterior canal nor could we detect any canal crista (Fig. 3A). In most cases, the size of the posterior canal was reduced and in many cases, we found incomplete separation of the posterior canal from the common crus (Fig. 3B,C).
The size variation of the posterior canal was always accompanied by a reduction of the posterior canal crista (Fig. 3F,G). Indeed, in the two ears in which we found no evidence of a posterior canal, there was also no evidence of a posterior canal crista, indicating that posterior canal formation correlates with the formation of a crista. Consistent with this assertion was the less severe reduction of the anterior canal and anterior crista in all compound mutants. In contrast to vertical canals, neither simple Foxg1 null nor compound Foxg1 null and Fgf10 heterozygotic animals had any signs of a differentiated horizontal canal crista, but always developed a horizontal canal (Fig. 3A-C). Therefore, the formation of a horizontal canal does not show a correlation with the horizontal crista formation. It is possible, however, that because an anterior crista always forms and because the horizontal canal always emerges from the anterior ampulla in Foxg1 null mice, the anterior crista can influence formation of both the anterior and the horizontal canal.
Foxg1 Null Mice Had a Shortened Cochlea With Multiple Rows of Hair Cells
The cochlea of E18.5 Foxg1 null mutants was short and formed at most one turn (Figs. 4C; 5A). In contrast, the cochlea of the Foxg1 heterozygotic animals had almost two turns. The spiral ganglion extended along the entire cochlea in the wild-type and Foxg1 heterozygotes (Figs. 1A, 3D). It was reduced in size and showed an extension of no more than two thirds the length of the short cochlea in the mutant. In extreme cases, it formed only a small ganglion near the middle of the cochlea. These data suggested a morphogenetic effect as well as an effect on neurogenesis and/or survival of sensory neurons. Because the shortened cochlea displayed alterations in hair cell organization, as previously reported for the shortened cochlea of Neurog1 null mice (Ma et al., 2000; Matei et al., 2005a), we next examined the organization of hair cells using the hair cell markers MYO VII immunocytochemistry and Atoh1 in situ hybridization (Fig. 4A-D).
Fig. 4.
Multiple rows of hair cells form in the apex of the Foxg1 mutant at embryonic day (E) 18.5. A: MYO VIIa immunostaining, a marker for hair cells, shows three rows of outer hair cells and one row of inner hair cells along the length of the cochlea in the wild-type at E18.5. B: In contrast, MYO VIIa immunostaining shows multiple rows of outer and inner hair cells near the apex in Foxg1 null mice. C,D: Likewise, in situ hybridization for Atoh1, a marker for differentiating hair cells, in the Foxg1 null cochlea shows that the organ of Corti widens to multiple rows of hair cells near the apex. E,F: The dotted line represents the approximate location of the near radial epoxy sections of a lacZ-reacted Foxg1 mutant cochlea. Up to 15 rows of poorly differentiated hair cells can be identified, partially segregated into outer and inner hair cells. The spiral artery is visible running below the organ of Corti. This artery normally runs below the tunnel of Corti and can be used as a landmark to differentiate between inner and outer hair cells and pillar cells. F: In the Foxg1 null, this landmark is of little use as the artery is tortuous and we are unable to distinguish any of the supporting cells as pillar cells and there appears to be no tunnel of Corti. Reissner’s membrane and Claudius’ cells were prominently stained. One or two rows of Hensen’s cells capped the lateral edge of the organ of Corti. F: The greater epithelial ridge showed less reaction. IHCs, inner hair cells; OHCs, outer hair cells; GER, greater epithelial ridge; CCs, Claudius’ cells; SCs, supporting cells; SPA, spiral artery; RM, Reissner’s membrane. Scale bars = 100 μm in A–D; 10 μm in E,F.
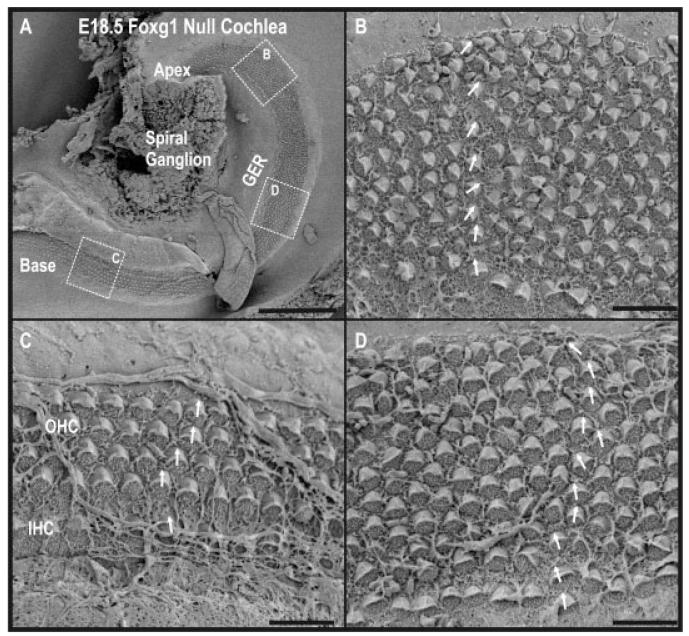
Fig. 5.
Multiple rows of hair cells are revealed by scanning electron microscopy. A: At embryonic day (E) 18.5 in the Foxg1 null, the cochlea shows almost one full turn. B–D: Hair cells throughout the cochlea show mature apical specializations including a kinocilium and stereocilia. C: Near the base, the organization of the hair cells is fairly normal. There is an additional row of outer hair cells for a total of four distinct rows, and the normal, single row of inner hair cells. Additionally, the polarity of the hair cells is normal as illustrated by the arrows. D: In the middle of this shortened cochlea, we see many more disorganized rows of hair cells and it is difficult to differentiate between inner and outer hair cells. Furthermore, the polarity of the hair cells, although generally correct, is less consistent. B: At the apex, even more and more disorganized rows of hair cells are seen. The polarity of hair cells is most profoundly disoriented in the outermost row of hair cells (top) where most cells are oriented in parallel to the long axis of the cochlea. GER, greater epithelial ridge; OHC, outer hair cells; IHC, inner hair cells. Scale bars = 100 μm in A; 10 μm in B–D.
Foxg1 null mutants showed severe disorganization of cochlear hair cells (Figs. 4, 5). Near the base, the cochlea sometimes showed a normal organization of three rows of outer hair cells and one row of inner hair cells. In the apex, however, both Atoh1 in situ hybridization and MYO VII immunocytochemistry showed multiple rows of what appeared to be both inner and outer hair cells, with no clear organization into discrete longitudinal rows (Fig. 4B,D). Thin radial sections through β-galactosidase-reacted cochleae showed a well-organized organ of Corti, which displayed a surplus of hair cells and supporting cells (Fig. 4E,F). In well-oriented radial sections, up to 14 rows of strongly LacZ-positive outer hair cells and up to four rows of more disperse and more weakly labeled inner hair cells were identified. Lack of cytological differentiation of supporting cells precluded any further identification as pillar, border, or Deiter’s cells without assistance of further molecular markers. The lateral aspect of the organ of Corti, however, showed the usual one to two rows of intensely labeled Henson’s cells followed by a single row of Claudius’ cells. The greater epithelial ridge showed a marked reduction in labeling in the presumptive spiral limbus (Fig. 4F).
Scanning electron microscopy (SEM) revealed a shortened cochlea containing hair cells with complete differentiation of the apical specializations, or hair bundles (Fig. 5). Organization of hair cell rows and polarity was disrupted. In the base (Fig. 5C), four continuous rows of outer hair cells and one row of inner hair cells were easily distinguished. Polarity of the hair cell bundles in the base appeared normal. In the middle (Fig. 5D) and apex (Fig. 5B) there were progressively more, discontinuous, rows of hair cells and distinguishing between inner and outer hair cells became increasingly difficult. Additionally, the polarity of the hair cells, particularly in the apex, was disrupted. The disruption was most profound in outermost outer hair cells, which were approximately oriented along the longitudinal axis of the cochlea (Fig. 5B arrows). The number of differentiated hair cells was estimated by counting the apical specializations seen in the SEM images. Based on this count, the number of differentiated hair cells in the Foxg1 was less than 40% of the wild-type cochlea. In summary, Foxg1 null mice have a shortened cochlea with multiple rows of fully differentiated, but somewhat disorganized, hair cells with the severity of the phenotype increasing nearer to the apex.
Innervation of the Foxg1 Mutant Ear Was Severely Disrupted
Our past research demonstrated that the injection of lipophilic tracers into the brain is a reliable technique to label the developing sensory neuron projection to the ear (Fritzsch et al., 2004, 2005a). Using this approach, we were unable to label any fibers to the posterior or anterior canal crista at any time in Foxg1 null mouse embryos. In contrast, the utricle and saccule were well innervated after dye application to the brain (Fig. 6B,C). The cochlea showed variable reduction of innervation. Closer examination showed dense innervation to the single row of inner hair cells near the base. In contrast to the three discrete rows of fibers to outer hair cells, outer hair cells of Foxg1 null mice showed a disorganized and overabundant density of fibers with occasional fibers overshooting the last row of outer hair cells (Fig. 6E,F). No distinction between inner and outer hair cell innervation was apparent in the apex, which also showed numerous fibers that extended even beyond the multiple rows of hair cells, reaching the Claudius cells of the outer spiral sulcus. However, the overshooting fibers did not enter the organ of Corti. Instead, they passed the habenula perforata and extended between the cells lining the scala tympani. Together, these data suggest that Foxg1 is essential for the development of canal cristae innervating sensory neurons and critical for neuronal path-finding in the cochlea.
Fig. 6.
Innervation defects at embryonic day (E) 18.5. A: Injection of lipophilic tracers into the brain reliably labels fibers to all six sensory epithelia of the ear (insert). A,D: In the wild-type, there is innervation to the anterior and horizontal cristae and utricle (A) and the cochlea (D). B,C: In contrast, in Foxg1 null mutants, there are no labeled fibers to the remaining two canal cristae, the anterior (B,C) and posterior crista (insert in C). In contrast, the utricle, saccule, and cochlea are well innervated after carbocyanine dye injection into the brainstem (B,C). D: The wild-type cochlea shows three rows of outer and one row of inner hair cells densely innervated, with no fibers projecting beyond the organ of Corti. F: In contrast, innervation of the apical tip of the cochlea in Foxg1 null mutants shows the multiple rows of hair cells, outlined by numerous fibers extending beyond the multiple rows of hair cells, forming loops in the outer spiral sulcus. E: Innervation of the middle turn of the Foxg1 mutant is more clearly organized and shows prominent labeling of inner hair cells but poorly organized innervation to outer hair cells and is not organized into discrete rows of fibers (compare D and E). AC, anterior crista; HC, horizontal crista; PC, posterior crista; S, saccule; SpGl, spiral ganglion; IHCs, inner hair cells; OHCs, outer hair cells; OC, organ of Corti. Scale bars = 100 μm.
Tubulin Staining Showed Delayed Onset and Disorganized Projection
We wanted to verify the absence of fibers to the anterior crista as well as the extensive overshooting of fibers to the cochlea using independent techniques. We could not label any fiber to the anterior crista of E12.5 Foxg1 null mice using the lipophilic dye technique (Fig. 7A). Overall, these data indicated a severe delay and reduction of fiber growth to the ear at this early stage, with complete loss of canal crista fibers.
Fig. 7.
Early development of innervation using tracers and acetylated tubulin staining. D: Wild-type mice have profound innervation to all vestibular sensory epithelia such as utricle, saccule, and posterior crista as early as embryonic day (E) 12.5, but show no innervation to the cochlea after cerebellar injections. A: In contrast, Foxg1 mutant littermates show only a sparse innervation to the utricle and saccule, no fibers at all to the canal cristae, but some fibers to the base of the cochlea. C,E: Of interest, labeling nerve fibers with tubulin shows, in Foxg1 mutant at E18.5, an innervation to the utricle and the anterior crista. B,E: The innervation overlaps with MYO VIIa positive hair cells (compare B and E). Note that these are fibers we were able to stain using tubulin but were unable to label using dyes injected into the brainstem. This finding suggests that theses fibers from the anterior crista do not project into the central nervous system and, thus, remain unlabeled with lipophilic tracers. Tubulin staining of the cochlea shows a more profound extension of fibers beyond the organ of Corti than lipophilic tracers do. F: Furthermore, there is a more profound innervation of the organ of Corti that also shows segregation into more densely innervated inner hair cell rows and less densely innervated outer hair cell rows. U, utricle; S, saccule; VGl, vestibular ganglion; CO, cochlea; AC, anterior crista; PC, posterior crista; OC, organ of Corti; SpGl, spiral ganglion. Scale bar = 100 μm.
All three E18.5 Foxg1 ears stained for nerve fibers with tubulin antibodies showed innervation to the cochlea, the saccule, the utricle, and the anterior crista (Fig. 7C,E). In addition to these fibers to the crista, we obtained an even more extensive projection of fibers beyond the cochlea than what we could label with dye diffusion from the brain. As noted after dye injections into the brainstem, the fibers projecting beyond the organ of Corti actually ran below the organ of Corti and the basilar membrane to extend out to the forming outer spiral limbus (Fig. 7F).
Foxg1 Null Ears Had Sensory Neurons That Did Not Project to the Brain
We next investigated the unusual innervation to the anterior crista in Foxg1 null mice by injecting dye into the sensory epithelium within the ear and studying the projection to other sensory epithelia as previously described (Tessarollo et al., 2004). Much to our surprise, by injecting dye into the cochlea, we labeled a massive projection to the anterior crista that was very similar to the projections seen with tubulin immunocytochemistry (Figs. 7,C, 8B,C). Injections of lipophilic tracers into the anterior crista revealed fibers projecting to the cochlea. Careful examination of the vestibular ganglion showed that fibers passed directly between the modiolus and the anterior vestibular nerve foramen. These fibers seem to originate from neurons in both the superior and inferior vestibular ganglion (Fig. 8).
Fig. 8.
A–F: Dye injections reveal connections between sensory epithelia in Foxg1 null mice. The differences between dye tracing after central injections and tubulin fiber labeling suggests that some sensory neurons projecting to the anterior crista do not project to the brain. To directly test this hypothesis, we injected different colored lipophilic dyes into the cochlea and anterior canal crista. E: In wild-type mice, such injections reliably label central projections and discrete populations of sensory neurons, but only some efferent fiber branches to other sensory epithelia. In contrast, dye injections into the cochlea of the Foxg1 mutant show not only fibers projecting to the brain but also labels sensory neurons in the vestibular ganglia as well as fibers entering the vestibular nerve to the anterior crista and utricle. A,B,C,F: These fibers can be traced to the utricle and anterior cristae. D,F: Injection of dye into the anterior crista with some labeling of the utricle (D) again labels vestibular neurons in both vestibular ganglia and fibers to the cochlea, including numerous fibers that extend beyond the organ of Corti (D,F). These data suggest that absence of Foxg1 alters the path-finding properties of canal sensory neurons, restricting their projection to the ear with no branches toward the brain. SVG, superior vestibular ganglion; IVG, inferior vestibular ganglion; AC, anterior crista; OC, organ of Corti; HC, horizontal canal; PC, posterior crista; U, utricle; S, saccule; C, cochlea. Scale bar = 100 μm.
In summary, sensory neuron projections show numerous aberrations, including absence of fibers that connect the anterior canal crista, the only crista with nearly normal morphology, to the brain. This crista receives at least some innervation of fibers that seems to emanate from neurons projecting to the cochlea. The massive effect on sensory neuron projection is consistent with the profound expression of Foxg1 in those neurons. The existence of “interepithelial” neurons can be compared with those of the forebrain of the Foxg1 null, in which neurons differentiate but do not project to other areas of the brain.
DISCUSSION
Our data show that Foxg1 is required for proper histogenesis of the inner ear, including formation and size determination of sensory patches, organization of hair cells, and formation and guidance of sensory neurons. Foxg1 is also required for proper morphogenesis of both the vestibular and the auditory system. We will discuss the likely molecular mechanisms that give rise to the histogenetic effects followed by a discussion of the interrelationship between histogenesis and morphogenesis.
Fewer Hair Cells Form and Entire Sensory Epithelia Are Missing in the Foxg1 Null
Other than monotreme (Ladhams and Pickles, 1996), extra rows of hair cells in mammals are known only in mutations or manipulations that affect the delta/notch/hes signaling pathway (Zine et al., 2001; Kiernan et al., 2005), the proneuronal bHLH gene Neurog1 (Ma et al., 2000; Matei et al., 2005a), the PCP pathway for convergent extension (Wang et al., 2005), or in Foxg1 null mutants (Fig. 5). Near loss and/or gain of entire sensory epithelia is only found in mutants that affect the Fgf signaling pathway (Pauley et al., 2003; Ohuchi et al., 2005), the bHLH signaling pathway (Matei et al., 2005a), and the delta/notch signaling pathway (Daudet and Lewis, 2005; Brooker et al., 2006; Kiernan et al., 2006). These similarities in phenotype suggest the possibility for interactions of these pathways at various levels (Zhong and Sternberg, 2006) with an interaction of Foxg1 with the Notch/Hes/Neurog1 pathway being the most likely.
Recent work has shown that stem cells require Hes expression for continued proliferation (Kageyama et al., 2005) and coordinated clonal expansion; and generation of neurons requires differential regulation of multiple Hes and bHLH genes in proliferating progenitors (Matter-Sadzinski et al., 2005). Alteration of delta/notch signaling can result in both up- or down-regulation of hair cell numbers (Brooker et al., 2006). Like the ear, the forebrain of the Hes1 null mice demonstrates premature differentiation of progenitor cells and a reduction of the progenitor cell population (Ishibashi et al., 1995) and halpoinsufficiency of Foxg1 causes microcephaly in man (Shoichet et al., 2005). Comparable to Hes1 null mice, reduction of size and cellular diversity in the forebrain of Foxg1 null mice comes about through reduced progenitor cell proliferation due to premature lengthening of the cell cycle (Martynoga et al., 2005). This early cell cycle lengthening causes a reduction in the progenitor pool and, combined with premature differentiation, truncates the progenitors’ proliferative capacity (Hanashima et al., 2002; Vyas et al., 2003), thus producing a much smaller forebrain containing only one type of neuron.
Existing data suggest that delta/notch signaling primarily regulates the expression of Hes1 and Hes5 by means of interaction of the activated notch fragment with RBP-J (Kuroda et al., 1999; Beres et al., 2006). HES homodimers appear to combine with GROUCHO, RUNX (Kageyama et al., 2005), and FOXG1 (Yao et al., 2001) to form an inhibitory complex that binds to the promoter region of activator type bHLH genes (Kageyama et al., 2005). Deficiency of any one of these factors would mimic, in part, the phenotype of Hes1 and Hes5 null mutants and of other impairments in the delta/notch signaling system (Zine et al., 2001; Brooker et al., 2006; Kiernan et al., 2006). Overall, these similarities suggest that all of these molecules may indeed interact in a single signaling pathway.
Neurogenin1 interacts with the HES proteins and competes for E-proteins to form heterodimers that regulate gene expression by means of E-box activation (Kageyama et al., 2005; Matter-Sadzinski et al., 2005), affects Delta expression (Ma et al., 1998), and interacts with Smad1 to block gliogenesis (Massague et al., 2005). In the Neurog1 null, the basal turn of the cochlea demonstrates the normal number of hair cells, but in the middle turn and apex, multiple rows of both inner and outer hair cells are seen with up to two rows of inners and four rows of outers (Ma et al., 2000; Matei et al., 2005a). As in the Foxg1 null, the phenotype is progressively more severe toward the apex. It has been shown that Neurog1 null (Matei et al., 2005a), Delta null (Kiernan et al., 2005), and Foxg1 null (Martynoga et al., 2005) all affect cell cycle exit and onset of differentiation. It is possible, therefore, that the similarities in phenotype in these ear mutants all relate to alteration of cell fate and cell cycle length. We currently are testing whether Neurog1 null compounds the effects of Foxg1 by breeding compound double mutants for Foxg1 and Neurog1 and by checking the cell cycle exit.
Hair Cell Polarity Is Altered in the Foxg1 Null
Among the various mutants that generate multiple rows of cochlear hair cells, only few have additional effects on hair cell polarity. The mildest phenotype with respect to additional rows, shortening of cochlear length, and disorientation is found in Neurog1 null mice (Ma et al., 2000). Our data show a more severe phenotype in shortening the cochlea, generating more rows of hair cells, and disorienting hair cell polarity in Foxg1 null mice (Figs. 5, 6). The most severe phenotype with respect to shortening the cochlea, increasing the number of hair cell rows, and disorganizing the polarity of hair cells occurs in planar cell polarity (PCP) gene mutations (Wang et al., 2005). Common to all three mutations is the more profound effect in the apex and outer rows of outer hair cells. Therefore, it is likely that all three signaling pathways interact. Exactly how accelerated cell cycle exit in Neurog1 (Fritzsch et al., 2005a) and possibly in Foxg1 (Martynoga et al., 2005) ties into the regulation of PCP regulating genes (Barald and Kelley, 2004; Lu et al., 2004; Wang et al., 2005) remains unclear. It is possible that the more profound polarity effect of Foxg1 mutants compared with Neurog1 null mice may relate to the fork-head DNA binding domain and its additional function to block Smad signaling (Seoane et al., 2004), a capacity shared with Neurog1 (Massague et al., 2005).
Neuronal Formation and Guidance Is Disrupted in the Foxg1 Null
Recent work has seen dramatic progress in the molecular understanding of fiber guidance within the ear (Fritzsch et al., 2005a,b). It has been shown that neurotrophin misexpression can cause vestibular fibers to reach the cochlea instead of vestibular epithelia (Tessarollo et al., 2004) or expand to epithelia devoid of endogenous innervation (Fritzsch et al., 2005b).
We suggest that the presence of some fibers to the anterior canal in older embryos represents an expansion of utricular fibers, and that canal cristae neurons are entirely missing in Foxg1 null mice. The complete absence of any fibers in early stages Foxg1 embryos (Fig. 7) and the presence of such fibers in later stages support this notion. Such suggestions are in line with findings in various neurotrophin mutant mice, which also have vestibular fiber rerouting as we show here for Foxg1 null mice (Fritzsch et al., 2005b).
The unusual phenotype of sensory neurons projecting to other sensory epithelia but apparently not to the brain suggests that Foxg1 affects not only the generation of sensory neurons but also their differentiation and path finding. Given the apparent involvement of Foxg1 in the bHLH signal regulation pathway (Yao et al., 2001), it is conceivable that Neurod1 signals, known to affect the pattern of innervation by means of Pou4f1 alteration (Huang et al., 2001; Kim et al., 2001), might be affected in Foxg1 null mice. We currently are breeding Foxg1 with Neurod1 heterozygotic mice to further test this possibility.
In summary, our data show that Foxg1 affects multiple levels of ear histogenesis. It is possible that most of these effects are directly linked to the emerging role of Foxg1 to interact with proteins involved in the delta/notch/hes/bHLH signaling pathway and its role in cell cycle and differentiation. If so, all other defects described here should also be investigated in mutants of these other genes in which such effects have not been analyzed (Brooker et al., 2006).
Morphogenesis and Histogenesis Are Coupled
Canal morphogenesis can be disrupted by several factors (Chang et al., 2004; Fritzsch et al., 2006). We propose that Fgf10 acts as a central node in canal morphogenesis and that prosensory crista size determines the number of sensory epithelial cells and, thereby, the amount of Fgf10 expression. Recently, genes known to affect ear growth and morphogenesis have been found to bind the promoter region of the Fgf10 gene. Among those genes are Gata3, a gene that affects ear morphogenesis (Karis et al., 2001) and several genes known to affect canal formation such as HoxA1, HoxB1, Dlx, and Nkx genes (Chang et al., 2004; Ohuchi et al., 2005). Further support for the idea that Fgf10 is a central node of canal morphogenesis is the absence of all canal formation in Fgf10 null mice (Pauley et al., 2003; Ohuchi et al., 2005). It is possible, therefore, that most of the described defects of the above mentioned genes can be attributed to deregulation of Fgf10 expression. Another category of genes appears to act downstream of Fgf10 and determines the fusion of the canal plate (Salminen et al., 2000) or the diameter of the canals (Cowan et al., 2000); and other genes known to affect canal formation seem to act in parallel to this general morphogenesis module. Importantly, loss of crista formation in Jagged1 null mice results in partial loss of canal formation (Brooker et al., 2006; Kiernan et al., 2006).
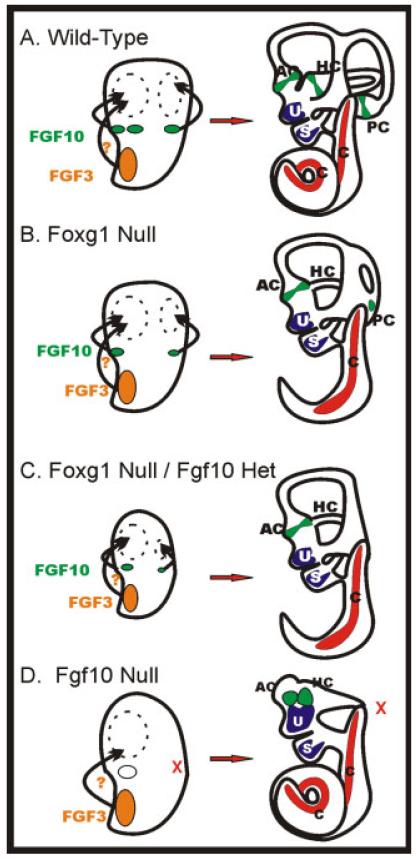
We have expanded the model proposed based on bone morphogenetic protein (BMP) signaling (Chang et al., 2004) and suggest that the level of FGF10 signaling from the prosensory cristae directs the growth of the associated canal (Fig. 9). This model is consistent with the absence of canal formation of Fgf10 null mice (Pauley et al., 2003; Ohuchi et al., 2005). In the Fgf10 null, there is some “rescue” of the anterior canal. This finding is likely mediated by Fgf3, which is expressed in the anterior portion of the developing otocyst (Fig. 9D). This Fgf3 expression may be too far away from the posterior crista to rescue its crista and canal development.
Fig. 9.
Size of the sensory epithelia precursor determines size of the developing canal. Fgf10 and perhaps Fgf3 regulate growth of the canal plates in the wild-type ear. A: These signals are critical as early as embryonic day (E) 11.5. In the Foxg1 null, no horizontal crista forms. B: The horizontal canal formation likely relies on signals from the anterior crista. Reduction in the size of the posterior crista leads to smaller posterior canals in the Foxg1 null/Fgf10 heterozygote. In extreme cases, no posterior crista or canal forms. C: Note: in no case did we obtain a posterior canal without a posterior crista. The lack of Fgf10 expression in the Fgf10 null leads to complete lack of the posterior crista and canal. D: Partial formation of the anterior and horizontal cristae may be due to Fgf3 expression in the anterior part of the otocyst. This model allows us to predict the extent of canal formation based on size of the presumptive sensory epithelia. Compiled after Pauley et al. (2003), and Wright and Mansour (2003). AC, anterior crista; HC, horizontal crista; PC, posterior crista; U, utricle; S, saccule; C, cochlea.
Foxg1, Fgf10, and Jagged1 null mice lack or have reduced prosensory canal cristae formation and all have canal formation deficits. The correlation between size of the vertical canal epithelia and morphogenesis of the associated canal suggests that loss of a vertical canal epithelium relates to loss of the appropriate canal. This suggestion was proposed previously for the Fgf10 null mice (Pauley et al., 2003; Ohuchi et al., 2005) but has remained a conjecture due to the absence of a reliable marker for the posterior crista. Given that Foxg1 is expressed in the canal epithelia early on, absence of LacZ expression in the area of the posterior crista, combined with presence of expression in other sensory epithelia such as the anterior canal crista and utricle, leads us to conclude that indeed the canal cristae for the posterior canal is missing in the most extreme phenotype of Foxg1 null mice combined with the Fgf10 heterozygote. Fgf10 in the late embryo is localized primarily in the supporting cells, and no canal defects occur in the Atoh1 null (which lacks differentiated hair cells). Therefore, the sensory epithelia primordia/supporting cells, but not the hair cells, are critical for canal development (Pauley et al., 2003; Wright and Mansour, 2003; Matei et al., 2005b). Although a direct effect of Foxg1 mutation on Fgf10 expression might be expected, the expression pattern of Fgf10, determined using in situ hybridization (Pauley et al., 2003), in the presumptive posterior crista of the Foxg1 mutant is normal (data not shown). We, therefore, assume a simple additive effect: the loss of Foxg1 leads to smaller sensory epithelia, producing less Fgf10, and this effect is compounded by reduced copy number from the Fgf10 heterozygote (Fig. 9C).
In summary, our data show that Foxg1 is at least one factor that cooperates with Fgf10 to determine size of sensory epithelia and, thereby, the signal needed to interact with BMPs for proper canal growth (Chang et al., 2004), likely through interaction with Smad signaling (Massague et al., 2005). However, these assessments hold true only for the vertical canal system. The horizontal canal system has some capacity to form independently of a horizontal crista, as demonstrated by the Foxg1 null mouse in which the horizontal canal forms but the horizontal canal crista is lacking. Combining Foxg1 with Jagged1 null mice should help to further clarify the complex interactions emerging in the delta/notch/hes/bHLH signaling system in the ear (Matei et al., 2005a; Brooker et al., 2006).
EXPERIMENTAL PROCEDURES
Embryos
The Foxg1 mouse line carrying a LacZ reporter gene in place of the Foxg1 gene (Hatini et al., 1999) was obtained and bred to homozygosity. Genotyping of adult mice was performed using polymerase chain reaction (PCR) for the LacZ reporter gene. Foxg1 homozygotic mutant embryos displayed characteristic eye and nose phenotypes and severely reduced forebrains, making phenotypic identification easy. Fgf10 mice (Pauley et al., 2003) were genotyped using PCR. Embryonic age was determined by timing of pregnancy and by staging (Theiler, 1989). For embryos, timed pregnant females were euthanized by cervical dislocation and the embryos were dissected and either immersion fixed (E9.5–E12.5) or perfusion fixed (E13.5–P0) with 4% paraformaldehyde. All procedures were approved by the Creighton IACUC committee protocol number 0630.1.
Wild-type, heterozygous, and mutant mice were collected from multiple pregnancies at E10.5, E11.5, E12.5, E13.5, and E18.5. Later time points were un-obtainable, as Foxg1 mutants die at birth. Euthanized pregnant females were used for analysis of Foxg1 expression in adults. Table 1 gives the number of heterozygotic and homozygotic mice collected at each age point as well as the numbers expected from normal Mendelian crossings. No double mutants or Fgf10 null/Foxg1 heterozygote were obtained at any time point. All Foxg1 nulls demonstrated the cochlear and horizontal crista phenotype and showed variable penetration of the posterior canal and crista phenotype.
TABLE 1. Heterozygotic and Homozygotic Mice Collected at Each Age Point and the Numbers Expected From Normal Mendelian Crossings.
| E11.5 | E12.5 | E13.5 | E18.5 | Adult | Actual | Expected Mendelian ratio | |
|---|---|---|---|---|---|---|---|
| WT | 1 | 4 | 1 | 4 | N/A | 0.06 | 0.06 or 1/16 |
| Fgf10 het | 1 | 6 | 2 | 4 | N/A | 0.08 | 0.13 or 1/8 |
| Foxg1 het | 2 | 5 | 3 | 12 | 3 | 0.13 | 0.13 or 1/8 |
| Fgf10 null | 0 | 0 | 0 | 0 | N/A | 0.00 | 0.06 or 1/16 |
| Foxg1 null | 3 | 11 | 3 | 8 | N/A | 0.15 | 0.06 or 1/16 |
| Fgf10 het / Foxg1 het | 13 | 28 | 9 | 22 | 5 | 0.44 | 0.25 or 1/4 |
| Fgf10 het / Foxg1 null | 3 | 5 | 4 | 10 | N/A | 0.13 | 0.13 or 1/8 |
| Fgf10 null / Foxg1 het | 0 | 0 | 0 | 0 | N/A | 0.00 | 0.13 or 1/8 |
| Fgf10 null / Foxg1 null | 0 | 0 | 0 | 0 | N/A | 0.00 | 0.06 or 1/16 |
E, embryonic day; WT, wild-type.
Lipophilic Dye Tracing
The 1,1′, di-octadecyl-3,3,3′,3′,-tetramethylindo-carbocyanine perchlorate (DiI) -soaked filter strips were inserted into the embryonic hindbrain to label afferent and efferent fibers to the ear, or in the ear to analyze connections between sensory epithelia and the hindbrain (Fritzsch et al., 2005a). Dissected ears were mounted flat in glycerol and imaged using a Zeiss LSM 510 confocal microscope. Image stacks were obtained and collapsed to render single plane equivalents using highest pixel intensity algorithms.
In Situ Hybridization
The expression of Atoh1 (Bermingham et al., 1999), Foxg1 (Hanashima et al., 2002), and Fgf10 (Pauley et al., 2003) was analyzed using digoxigenin (Dig) -labeled riboprobes. The whole-mount in situ hybridization followed a protocol described elsewhere (Judice et al., 2002), with minor modifications. Briefly, whole embryos (E9.5–E10.5), dissected ears (E11.5–E14.5), or dissected parts of ears (E18.5) were defatted, digested with proteinase K, washed, and hybridized overnight with the riboprobe. The tissues were treated with RNaseA to digest un-bound probe and incubated overnight with alkaline phosphatase–labeled anti-Dig Fab fragments, washed, and reacted with nitroblue phosphate/5-bromo, 4-chloro, 3-indolil phosphate (BM Purple by Roche). Reacted ears or ear parts were mounted flat in glycerol and viewed in a Nikon Eclipse 800 microscope using differential interference contrast microscopy to indicate boundaries. Images were grabbed using ImagePro software.
Immunocytochemistry
Hair cells were labeled using MYO VIIA antibodies, and nerve fibers were labeled using acetylated tubulin antibodies as previously described (Matei et al., 2005a). Antibodies for MYO VIIA were purchased from Proteus Biosciences and antibodies for acetylated tubulin were purchased from Sigma. Dilutions of primary antibodies were 1:2,000 and 1:600, respectively. Secondary antibodies were conjugated to either Alexa 543 or Alexa 648. Whole-mounted ear epithelia were viewed by using a Zeiss LSM 510 confocal microscope.
β-Galactosidase Histochemistry
Foxg1 hetero- or homozygotic embryos were hemisected (embryos E12.5 or younger) and processed for wholemount β-galactosidase histochemistry as previously described (Fritzsch et al., 2005a; Matei et al., 2005a). In older embryos, the ears were partially or completely dissected from the otic capsule to provide complete penetration of the reaction solution. Reacted ears were either whole-mounted or embedded into epoxy resin and sectioned with diamond knives at 10 μm thickness. Sections and whole-mounts were viewed with a Nikon Eclipse 800.
Scanning Electron Microscopy
Two Foxg1 null and control littermate ears were prepared for SEM as described (Ma et al., 2000). Dissected ears were postfixed in 0.5% OsO4, dehydrated in ethanol, critical point dried, mounted for examination, sputter coated, and examined in a JOEL JSM-840A operated at 5 kV. All images were imported into Corel Draw and processed into plates.
ACKNOWLEDGMENTS
We thank Drs. K. Beisel, J. Hebert, D. Ornitz, H. Zoghbi, and D. Nichols for help with probes and C. Moes for excellent histological assistance. B.F. was funded by NIH, NASA, COBRE, and by a Research Facilities Improvement Program Grant from the National Center for Research Resources, National Institutes of Health. We acknowledge the use of the confocal microscope facility of the NCCB, supported by EPSCoR EPS-0346476 (CFD 47.076).
Grant sponsor: NIH; Grant number: RO1 DC005590; Grant sponsor: NASA; Grant number: NAG 2-1611; BF; Grant sponsor: COBRE; Grant number: 1P20RR018788-01; Grant number: LB692; Grant sponsor: National Center for Research Resources, NIH, Research Facilities Improvement Program; Grant number: 1 C06 RR17417-01.
REFERENCES
- Barald KF, Kelley MW. From placode to polarization: new tunes in inner eardevelopment. Development. 2004;131:4119–4130. doi: 10.1242/dev.01339. [DOI] [PubMed] [Google Scholar]
- Beres TM, Masui T, Swift GH, Shi L, Henke RM, MacDonald RJ. PTF1 is an organ-specific and Notch-independent basic helix-loop-helix complex containing the mammalian Suppressor of Hairless (RBP-J) or its paralogue, RBP-L. Mol Cell Biol. 2006;26:117–130. doi: 10.1128/MCB.26.1.117-130.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Chang W, Brigande JV, Fekete DM, Wu DK. The development of semicircular canals in the inner ear: role of FGFs insensorycristae. Development. 2004;131:4201–4211. doi: 10.1242/dev.01292. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron. 2000;26:417–430. doi: 10.1016/s0896-6273(00)81174-5. [DOI] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola V, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Matei VA, Nichols DH, Bermingham N, Jones K, Beisel KW, Wang VY. Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev Dyn. 2005a;233:570–583. doi: 10.1002/dvdy.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Matei V, Katz DM, Xiang M, Tessarollo L. Mutant mice reveal the molecular and cellular basis for specific sensory connections to inner ear epithelia and primary nuclei of the brain. Hear Res. 2005b;206:52–63. doi: 10.1016/j.heares.2004.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Beisel KW. Cells, molecules and morphogenesis: the making of the vertebrate ear. Brain Res. 2006 doi: 10.1016/j.brainres.2006.02.078. DOI: 10.1016/j.brainres.2006.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanashima C, Shen L, Li SC, Lai E. Brain factor-1 controls the proliferation and differentiation of neocortical progenitor cells through independent mechanisms. J Neurosci. 2002;22:6526–6536. doi: 10.1523/JNEUROSCI.22-15-06526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanashima C, Li SC, Shen L, Lai E, Fishell G. Foxg1 suppresses early cortical cell fate. Science. 2004;303:56–59. doi: 10.1126/science.1090674. [DOI] [PubMed] [Google Scholar]
- Hans S, Liu D, Westerfield M. Pax8 and Pax2a function synergistically in otic specification, downstream of the Foxi1 and Dlx3b transcription factors. Development. 2004;131:5091–5102. doi: 10.1242/dev.01346. [DOI] [PubMed] [Google Scholar]
- Hatini V, Ye X, Balas G, Lai E. Dynamics of placodal lineage development revealed by targeted transgene expression. Dev Dyn. 1999;215:332–343. doi: 10.1002/(SICI)1097-0177(199908)215:4<332::AID-AJA5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Hebert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- Herrera E, Marcus R, Li S, Williams SE, Erskine L, Lai E, Mason C. Foxd1 is required for proper formation of the optic chiasm. Development. 2004;131:5727–5739. doi: 10.1242/dev.01431. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Liu W, Fritzsch B, Bianchi LM, Reichardt LF, Xiang M. Brn3a is a transcriptional regulator of soma size, target field innervation and axon path-finding of inner ear sensory neurons. Development. 2001;128:2421–2432. doi: 10.1242/dev.128.13.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulander M, Wurst W, Carlsson P, Enerback S. The winged helix transcription factor Fkh10 is required for normal development of the inner ear. Nat Genet. 1998;20:374–376. doi: 10.1038/3850. [DOI] [PubMed] [Google Scholar]
- Hulander M, Kiernan AE, Blomqvist SR, Carlsson P, Samuelsson EJ, Johansson BR, Steel KP, Enerback S. Lack of pendrin expression leads to deafness and expansion of the endolymphatic compartment in inner ears of Foxi1 null mutant mice. Development. 2003;130:2013–2025. doi: 10.1242/dev.00376. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Ang SL, Shiota K, Nakanishi S, Kageyama R, Guillemot F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- Iwahori A, Fraidenraich D, Basilico C. A conserved enhancer element that drives FGF4 gene expression in the embryonic myotomes is synergistically activated by GATA and bHLH proteins. Dev Biol. 2004;270:525–537. doi: 10.1016/j.ydbio.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Judice TN, Nelson NC, Beisel CL, Delimont DC, Fritzsch B, Beisel KW. Cochlear whole mount in situ hybridization: identification of longitudinal and radial gradients. Brain Res Brain Res Protoc. 2002;9:65–76. doi: 10.1016/s1385-299x(01)00138-6. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306:343–348. doi: 10.1016/j.yexcr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol. 2001;429:615–630. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Katoh M, Katoh M. Human FOX gene family [review] Int J Oncol. 2004;25:1495–1500. [PubMed] [Google Scholar]
- Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005;132:4353–4362. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K, Tani S, Tamura K, Minoguchi S, Kurooka H, Honjo T. Delta-induced Notch signaling mediated by RBP-J inhibits MyoD expression and myogenesis. J Biol Chem. 1999;274:7238–7244. doi: 10.1074/jbc.274.11.7238. [DOI] [PubMed] [Google Scholar]
- Ladhams A, Pickles JO. Morphology of the monotreme organ of Corti and macula lagena. J Comp Neurol. 1996;366:335–347. doi: 10.1002/(SICI)1096-9861(19960304)366:2<335::AID-CNE11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Lee HH, Frasch M. Survey of forkhead domain encoding genes in the Drosophila genome: classification and embryonicexpressionpatterns. DevDyn. 2004;229:357–366. doi: 10.1002/dvdy.10443. [DOI] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynoga B, Morrison H, Price DJ, Mason JO. Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev Biol. 2005;283:113–127. doi: 10.1016/j.ydbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005a;234:633–650. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005b;234:633–650. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter-Sadzinski L, Puzianowska-Kuznicka M, Hernandez J, Ballivet M, Matter JM. A bHLH transcriptional network regulating the specification of retinal ganglion cells. Development. 2005;132:3907–3921. doi: 10.1242/dev.01960. [DOI] [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi H, Yasue A, Ono K, Sasaoka S, Tomonari S, Takagi A, Itakura M, Moriyama K, Noji S, Nohno T. Identification of cis-element regulating expression of the mouse Fgf10 gene during inner ear development. Dev Dyn. 2005;233:177–187. doi: 10.1002/dvdy.20319. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Groves AK. Expression of mouse Foxi class genes in early craniofacial development. Dev Dyn. 2004;231:640–646. doi: 10.1002/dvdy.20160. [DOI] [PubMed] [Google Scholar]
- Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn. 2003;227:203–215. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Trokovic R, Hebert J, McConnell S, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Pratt T, Tian NM, Simpson TI, Mason JO, Price DJ. The winged helix transcription factor Foxg1 facilitates retinal ganglion cell axon crossing of the ventral midline in the mouse. Development. 2004;131:3773–3784. doi: 10.1242/dev.01246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen M, Meyer BI, Bober E, Gruss P. Netrin 1 is required for semicircular canal formation in the mouse inner ear. Development. 2000;127:13–22. doi: 10.1242/dev.127.1.13. [DOI] [PubMed] [Google Scholar]
- Seoane J, Le HV, Shen L, Anderson SA, Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–223. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- Shoichet SA, Kunde SA, Viertel P, Schell-Apacik C, von Voss H, Tommerup N, Ropers HH, Kalscheuer VM. Haploinsufficiency of novel FOXG1B variants in a patient with severe mental retardation, brain malformations and microcephaly. Hum Genet. 2005;117:536–544. doi: 10.1007/s00439-005-1310-3. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Kudoh T, Dawid IB, Fritz A. Zebrafish foxi1 mediates otic placode formation and jaw development. Development. 2003a;130:929–940. doi: 10.1242/dev.00308. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Logsdon JM, Jr, Fritz A. Expression and phylogenetic analyses of three zebrafish FoxI class genes. Dev Dyn. 2003b;228:301–307. doi: 10.1002/dvdy.10373. [DOI] [PubMed] [Google Scholar]
- Tao W, Lai E. Telencephalon-restricted expression of BF-1, a new member of the HNF-3/fork head gene family, in the developing rat brain. Neuron. 1992;8:957–966. doi: 10.1016/0896-6273(92)90210-5. [DOI] [PubMed] [Google Scholar]
- Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci. 2004;24:2575–2584. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler K. The house mouse. Springer-Verlag; New York: 1989. p. 178. [Google Scholar]
- Toresson H, Martinez-Barbera JP, Bardsley A, Caubit X, Krauss S. Conservation of BF-1 expression in amphioxus and zebrafish suggests evolutionary ancestry of anterior cell types that contribute to the vertebrate telencephalon. Dev Genes Evol. 1998;208:431–439. doi: 10.1007/s004270050200. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Copp A, Mishkin M. FOXP2 and the neuro-anatomy of speech and language. Nat Rev Neurosci. 2005;6:131–138. doi: 10.1038/nrn1605. [DOI] [PubMed] [Google Scholar]
- Vyas A, Saha B, Lai E, Tole S. Paleocortex is specified in mice in which dorsal telencephalic patterning is severelydisrupted. J Comp Neurol. 2003;466:545–553. doi: 10.1002/cne.10900. [DOI] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TJ, Mansour SL. Fgf3 and Fgf10 are required for mouse otic placode induction. Development. 2003;130:3379–3390. doi: 10.1242/dev.00555. [DOI] [PubMed] [Google Scholar]
- Yao J, Lai E, Stifani S. The wingedhelix protein brain factor 1 interacts with groucho and hes proteins to repress transcription. Mol Cell Biol. 2001;21:1962–1972. doi: 10.1128/MCB.21.6.1962-1972.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Sternberg PW. Genomewide prediction of C. elegans genetic interactions. Science. 2006;311:1481–1484. doi: 10.1126/science.1123287. [DOI] [PubMed] [Google Scholar]
- Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]