Abstract
Glial cells are being increasingly implicated in mechanisms underlying pathological pain and recent studies suggest glial gap junctions involving astrocytes may contribute. The aim of the present study was to examine the effect of a gap junction blocker carbenoxolone (CBX) on medullary dorsal horn (MDH) nociceptive neuronal properties and facial mechanical nociceptive behavior in a rat trigeminal neuropathic pain model involving partial transection of the infraorbital nerve (p-IONX). p-IONX produced facial mechanical hypersensitivity reflected in significantly reduced head withdrawal thresholds that lasted for over 3 weeks. p-IONX also produced central sensitization in MDH nociceptive neurons that was reflected in significantly increased receptive field size, reduction of mechanical activation threshold and increases in noxious stimulation-evoked responses. Intrathecal CBX treatment significantly attenuated the p-IONX-induced mechanical hypersensitivity and the MDH central sensitization parameters, compared to intrathecal vehicle treatment. These results provide the first documentation that gap junctions may be critically involved in orofacial neuropathic pain mechanisms.
Keywords: gap junction, carbenoxolone, neuropathic pain, central sensitization, infraorbital nerve, medulla
Introduction
Central sensitization reflects an increased excitability of nociceptive neurons in central nociceptive pathways following inflammation or nerve injury, and is implicated in the development and maintenance of chronic pain [19; 23; 37; 49]. In the trigeminal system, trigeminal subnucleus caudalis shares many morphological and functional similarities with the spinal dorsal horn and is often termed the medullary dorsal horn (MDH). We have previously shown that central sensitization in functionally indentified nociceptive MDH neurons occurs in acute and chronic orofacial inflammatory pain models [8–10; 12; 42; 52] as well as in chronic neuropathic pain models [4; 19; 29; 37] in which the nerve-injured animals show nociceptive behavior accompanying the MDH central sensitization.
There is increasing evidence for the involvement of glial cells, and specifically microglia and astrocytes, in the genesis and maintenance of various pathological pain states. Astrocytes and microglia are activated in response to a variety of manipulations that generate persistent nociceptive behavior in animal models of pathological pain [19; 21; 22; 24; 29; 43; 44; 48]. Neurotransmitters such as glutamate and ATP released from neurons also bind to glial receptors or pass through transporters/channels, to activate various downstream signaling systems within glial cells and induce the release of proinflammatory cytokines and other chemical mediators that act on neighboring glial cells or neurons to facilitate nociceptive signal transmission [19–22; 25; 43; 46; 47]. Glial cell inhibitors can suppress the nociceptive behavior and central sensitization occurring in animal models of pathological pain states [7; 11; 17; 19; 20; 25; 26; 37; 42; 46; 47].
The glial involvement in these pain states may involve gap junctions which are specialized intercellular transmembrane channels that connect the cytoplasm of adjacent cells, (e.g. neurons, astrocytes), allowing rapid intercellular exchange of small molecules including ions, second messengers, nutrients and metabolites [2; 40; 50; 55]. A characteristic example is the transients and oscillatory waves of cytoplasmic Ca2+ in astrocytes elicited by neuronal activity that may propagate to adjacent and/or distant astrocytes through gap junctions and hemichannels [1; 13; 36]. Ca2+ signaling and downstream cascades within the astrocytes lead to release of substances, such as glutamate, ATP and cytokines that act on adjacent and/or distant glia and neurons and may result in exacerbation of pain [3; 28; 39].
We have also recently documented that glial cells are involved in the MDH central sensitization in an orofacial acute inflammatory pain model [7; 12; 52] and that intrathecal application of a widely used gap junction blocker, carbenoxolone (CBX), blocks the development of the MDH central sensitization, suggesting that gap junctions play an important role in this acute pain model [9]. The aim of the present study was to examine the effect of CBX on MDH central sensitization and facial mechanical nociceptive behavior in a rat model of trigeminal neuropathic pain that involves partial infraorbital nerve transection (p-IONX).
Materials and Methods
Animals and neuropathic pain model
The right or the left side of the infraorbital nerve of adult male Sprague-Dawley rats (280–300 g) under isoflurane anesthesia was intraorally exposed. The infraorbital nerve supplies the maxillary upper lip as well as the anterior teeth and labial mucosa. The medial one third to half of the nerve trunk was dissected and then transected and the wound was sutured. The infraorbital nerve of sham-operated rats was identically exposed but not transected. All surgeries and procedures were approved by the University of Toronto Animal Care Committee in accordance with the regulations of the Ontario Animal Research Act (Canada). Forty-nine rats were used in this study, 25 for behavioral tests and 24 for electrophysiological recordings. In each subset of experiments, the animals were divided into four groups on the basis of whether they received p-IONX or sham treatment and whether they received CBX or the vehicle phosphate-buffered saline (PBS): p-IONX/PBS, p-IONX/CBX, sham/PBS and sham/CBX. In the behavioral experiments, the p-IONX/CBX group comprised 7 rats and each of the other three groups comprised 6 rats. In the electrophysiological experiments, each group comprised 6 rats.
Mechanosensitivity Testing
A testing tube was used to test the mechanosensitivity of the facial whisker pad, as previously described [4; 42]. One end of the tube had a hole allowing the rat’s nose, mouth and whisker pad to protrude out of the hole for testing facial mechanosensitivity. In the present study, after at least two training sessions in three days, rats adapted well to the testing tube with their nose, mouth and whisker pad protruding out of the hole in the tube. As previously described in detail [4; 42], this allowed a series of von Frey filaments (Stoelting, IL, USA) to be applied to the ipsilateral whisker pad to determine the lowest stimulus intensity required to elicit a head withdrawal response, with the cut-off intensity of 15g; the head withdrawal threshold is defined as the lowest intensity that evoked 3 or more escapes out of 5 stimulation trials. Animals were tested on 1 and 2 days before and on 1, 3, 5, 7, 10, 14, 21, and 28 days after p-IONX or sham operation.
Chemicals and Intrathecal Injection
Under 2% isoflurane anesthesia, either CBX (10 µg, Sigma) or PBS (as vehicle control) in a volume of 10 µl was intrathecally injected into the medulla with a 27-gauge dental needle connected to a 25 µl Hamilton syringe by a PE-10 tube, as previously described [42]. The CBX dose (10 µg) was based on the effective CBX doses that ranged from 0.1 µg to 25 µg, as documented in our acute orofacial pain model and other studies in adult rats [9; 33; 38]. The injections were delivered 2 hours before and then daily 1 to 6 days after the p-IONX or sham operation. On the days of mechanosensitivity testing, the intrathecal injection was delivered 2 hours before the testing.
Electrophysiological Recordings
Extracellular recordings from ipsilateral MDH nociceptive-specific (NS) and wide dynamic range (WDR) neurons were carried out 7 to 10 days after p-IONX or sham operation, the period when facial mechanical hypersensitivity following p-IONX was especially apparent (see Results). Each rat was anesthetized with intraperitoneal urethane (1 g/kg) and _-chloralose (50 mg/kg), and the trachea and left external jugular vein were cannulated. After the rat was placed in a stereotaxic apparatus, the caudal medulla was surgically exposed. The rat was artificially ventilated following immobilization by intravenous (i.v.) pancuronium bromide (0.2–0.3 ml of 2 mg/ml solution). The level of anesthesia and immobilization was maintained by a continuous i.v. infusion of a mixture of 70% urethane (0.2 g/ml) and 30% pancuronium (2 mg/ml) at a rate of 0.3–0.4 ml/h during the experimental period. A deep level of anesthesia was judged periodically by the lack of spontaneous movements and responses to paw pinching when the pancuronium-induced muscle paralysis was allowed to wear off. Heart rate, percentage expired CO2, and rectal temperature were constantly monitored and maintained at physiological levels of 333–430 beats/min, 3.5–4.2%, and 37–37.5°C, respectively.
The activity of single MDH neurons was extracellularly recorded with tungsten microelectrodes as previously described in detail [4; 12], and so will only be briefly outlined. Neuronal responses to stimulation of the orofacial region were amplified and displayed on oscilloscopes and digitized via an analog-to-digital converter. The data were collected and analyzed with Spike 2 software (Cambridge Electronic Design, UK). A wide range of mechanical (brush, pressure and pinch) and noxious thermal (radiant heat, 51–53°C) stimuli were applied to classify the MDH neurons as either wide dynamic range (WDR) or nociceptive-specific (NS) neurons. WDR neurons responded to low-threshold mechanical stimuli (brush) and increased their firing rate with increased mechanical stimulation into the noxious range, while NS neurons did not respond to low-threshold mechanical stimuli but responded to heavy pressure and pinch. The WDR and NS neurons frequently also responded to noxious heating. Neuronal properties, i.e pinch mechanoreceptive field, mechanical activation threshold, responses to 100g pinch and stimulation-response function to a series of graded mechanical stimuli by means of von Frey filament applications (6, 15, 26, 60 and 100g) of NS neurons, were characterized. Also, the tactile mechanoreceptive field as well as the pinch mechanoreceptive field, responses to 40g pinch and the stimulation-response functions of WDR neurons were determined.
Statistical Analyses
All values are presented as mean ± S.E. The differences in behavioral mechanosensitivity and neuronal stimulus-response functions were analyzed by two-way repeated measures (RM) analysis of variance (ANOVA) followed by Bonferroni post-hoc tests. The differences in other neuronal properties were analyzed by two-way ANOVA followed by Bonferroni post-hoc tests. SigmaStat for Windows Version 3.0.1 (SPSS Inc.) was used to run the analyses. The level of significance was set at P<0.05.
Results
1. Effect of intrathecal CBX on p-IONX-induced mechanical hypersensitivity
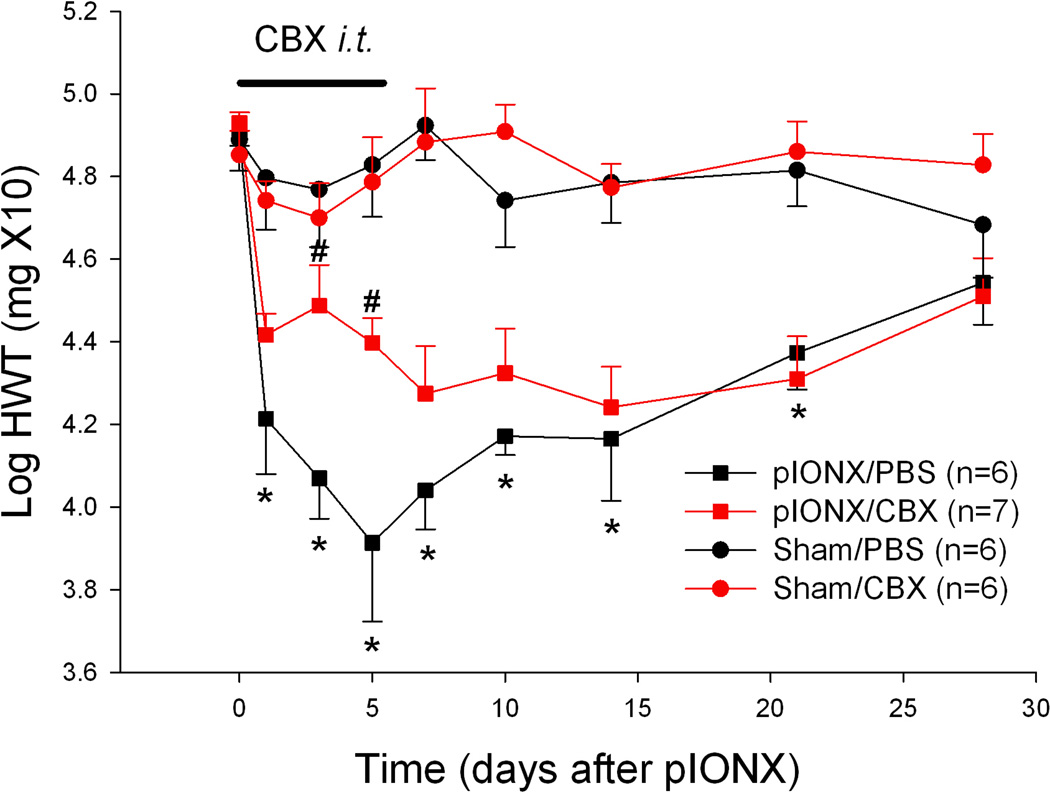
p-IONX-operated rats treated with PBS (n=6) showed facial mechanical hypersensitivity as manifested by a significantly reduced head withdrawal threshold starting one day after nerve injury. Sham-operated rats treated with PBS (n=6) did not show any significant change in withdrawal threshold after the surgery (Fig. 1). The reduced withdrawal threshold peaked around day 5, and lasted over three weeks (Fig.1), with significant main effects of time and surgery as well as their interaction, (p<0.001, two-way RM ANOVA; Table S1). To examine the possible effect of the gap junction blocker CBX in the development of the p-IONX induced mechanical hypersensitivity, CBX (or vehicle control) was intrathecally applied daily for 6 days in the p-IONX (n=7) and sham-operated (n=6) groups of rats. Intrathecal CBX significantly attenuated the withdrawal threshold reduction in the p-IONX rats, but not in the sham-operated rats, at days 3 and 5 after nerve injury (Fig.1, p<0.05 compared to PBS treatment within each time point, Bonferroni post-hoc test), with main effects of time (p<0.001), drug treatment (p=0.07), and interaction (p=0.02) between the two factors (two-way RM ANOVA; Table S1).
Fig. 1.
CBX attenuated the p-IONX-induced facial mechanical hypersensitivity. Mechanical hypersensitivity was induced after p-IONX, as shown in the comparison between p-IONX/PBS and sham/PBS animals (p<0.001 for the two factors, surgery and time, as well as the interaction between them, two-way RM ANOVA; * p<0.05 compared to baseline within the p-IONX surgery as well as compared to sham surgery within each time point, Bonferroni post-hoc test). Intrathecal CBX significantly attenuated the head withdrawal threshold (HWT) reduction in the p-IONX rats, as shown in the comparison between p-IONX/CBX and p-IONX/PBS animals (p=0.07 for the factor of drug treatment, p<0.001 for the factor of time, p=0.02 for the interaction between the two factors, two-way RM ANOVA; # p<0.05 compared to PBS treatment within each time point, Bonferroni post-hoc test).
2. Effect of intrathecal CBX on p-IONX-induced central sensitization of MDH nociceptive neurons
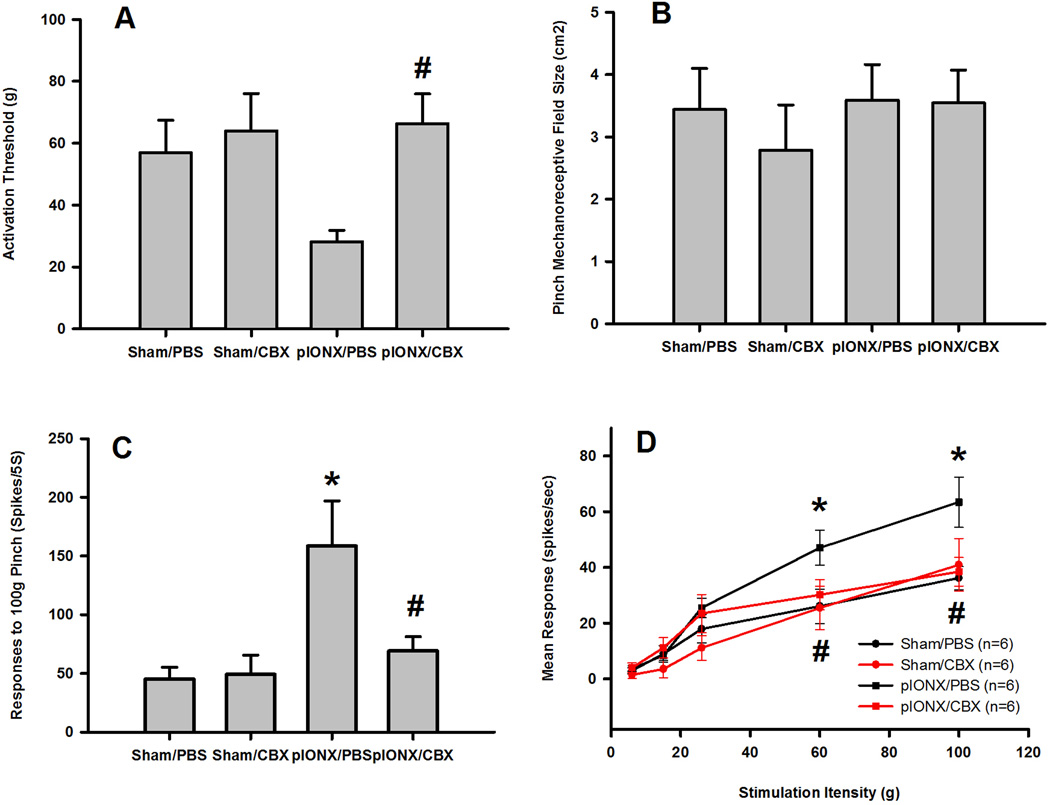
To study the neural mechanisms underlying the mechanical hypersensitivity and the blocking effect of CBX after p-IONX, the properties of MDH nociceptive neurons were examined at 7–10 days after the p-IONX operation, the period of marked mechanical hypersensitivity. Twenty-four functionally identified NS neurons in the deep lamina of MDH (657–1093 µm) were studied. p-IONX rats showed MDH central sensitization, as reflected in changes in their response properties. Compared with sham-operated rats treated with PBS, NS neurons in p-IONX rats treated with PBS showed a reduction of mechanical activation threshold (Fig. 2A, p=0.06, Bonferroni post-hoc test; Table S2), significantly increased responses to 100g pinch (Fig. 2C, P<0.05, Bonferroni post-hoc test; Table S2) and a significantly enhanced stimulation-response function (Fig. 2D, P<0.05, two-way RM ANOVA); the pinch mechanoreceptive field size of the NS neurons however remained unchanged (Fig. 2B, Table S2). There were significant main effects of drug or surgery or their interaction on NS neuronal activation threshold or responses to pinch, but no main effects on pinch mechanoreceptive field size (p<0.05, two-way ANOVA; Table S2). Intrathecal CBX but not the vehicle significantly reversed the p-IONX-induced reduction of mechanical activation threshold (Fig. 2A, p<0.05, Bonferroni post-hoc test; Table S2), increase of responses to 100g pinch (Fig. 2C, p<0.05, Bonferroni post-hoc test; Table S2) and increase of the stimulation-response function (Fig. 2D, P<0.05, two-way RM ANOVA). Intrathecal CBX had no effect in the sham-operated rats (Fig. 2A, B, C, and D; Table S2).
Fig. 2.
CBX reversed the p-IONX-induced central sensitization of MDH NS neurons. A reduction in the mechanical activation threshold (A), and significant increases in responses to pinch (C) and in the stimulation-response function (D) of NS neurons occurred in the p-IONX rats 7–10 days after the nerve injury, although the pinch mechanoreceptive field size of the NS neurons remained unchanged (B). Intrathecal CBX but not PBS attenuated all these three parameters in the p-IONX rats but had no effect in sham-operated rats. * p<0.05 compared to sham surgery, Bonferroni post-hoc test; # p<0.05 compared to PBS treatment, Bonferroni post-hoc test.
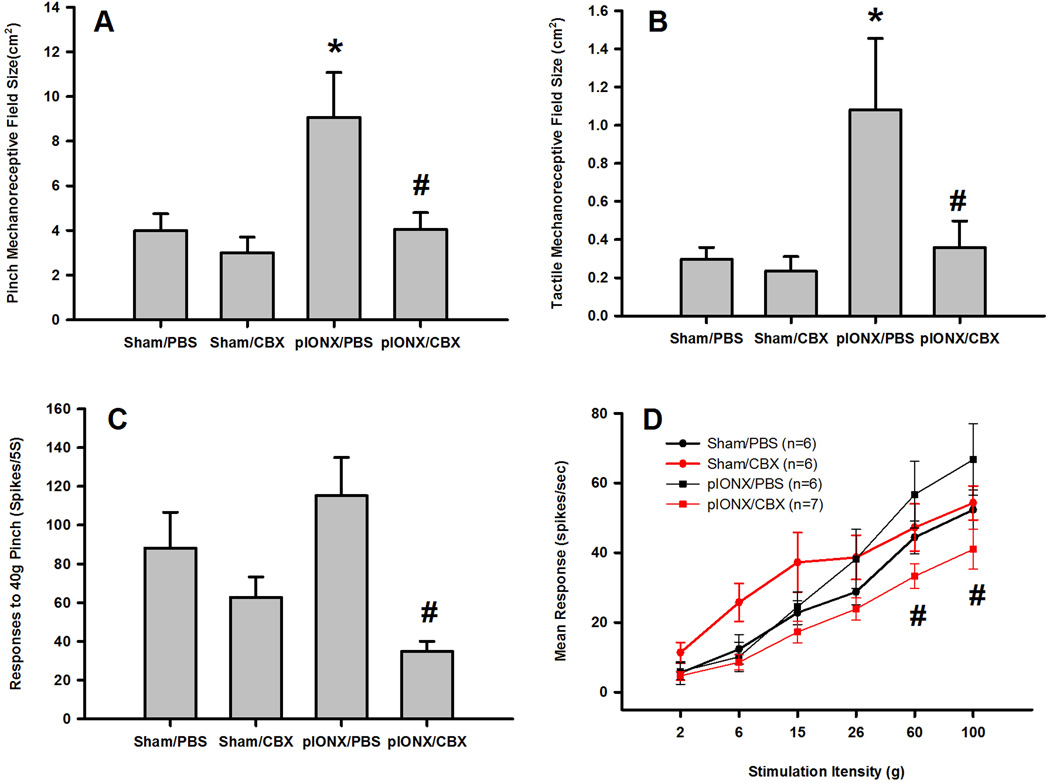
Twenty-five functionally indentified WDR neurons in the deep lamina of MDH (625–1094 µm) were also studied. p-IONX induced central sensitization of MDH WDR neurons, as shown in significantly increased pinch and tactile mechanoreceptive field sizes (Fig. 3A and B, p<0.05, Bonferroni post-hoc test; Table S3), and increase in responses to 40g pinch (Fig. 3C, Table S3) and stimulation-response function (Fig. 3D). There were significant main effects of drug and surgery on neuronal pinch mechanoreceptive field size, of drug on responses to pinch and of surgery on tactile mechanoreceptive field size (p<0.05, two-way ANOVA; Table S3). Intrathecal CBX but not the vehicle significantly reversed the p-IONX-induced increases in pinch and tactile mechanoreceptive field sizes (Fig. 3A and B, P<0.05, Bonferroni post-hoc test; Table S3), responses to 40g pinch (Fig. 3C, P<0.05, Bonferroni post-hoc test; Table S3) and stimulation-response function (Fig. 3D, P<0.05, two-way RM ANOVA) but had no effect in the sham-operated rats (Fig. 3A, B, C, and D; Table S3).
Fig. 3.
CBX reversed the p-IONX-induced central sensitization of MDH WDR neurons. p-IONX compared to sham surgery induced significant increases in tactile (A) and pinch (B) mechanoreceptive field sizes (* p<0.05 compared to sham surgery, Bonferroni post-hoc test) as well as increases in responses to pinch (C) and in the stimulation-response function (D). CBX compared to PBS significantly reduced the increases of the four neuronal properties tested (# p<0.05 compared to PBS treatment, Bonferroni post-hoc test).
Discussion
This is the first documentation that the gap junction blocker CBX attenuates the facial mechanical hypersensitivity as well as the accompanying central sensitization of functionally identified MDH nociceptive neurons induced by trigeminal nerve injury, suggesting that gap junctions play an important role in the development and/or maintenance of orofacial neuropathic pain.
Several animal models have been used to study the neural mechanisms underlying orofacial neuropathic pain [4; 16; 19; 29; 45; 53]. Mechanical and heat hypersensitivities are commonly observed after various types of trigeminal nerve injury, similar to those in sciatic or L5/L6 spinal nerve injury models. We here demonstrated in the present study that p-IONX in rats produces a prolonged mechanical hypersensitivity reflected as a lowered mechanical withdrawal threshold that begins at postoperative day 1 and is maintained for over three weeks. The extent and time course of the behavioral change induced by p-IONX in our study are consistent with those in another recent study using p-IONX in the rat [4] and with those reported in spinal neuropathic pain models [15; 30]. The behavioral hypersensitivity that has been reported in trigeminal neuropathic pain models is accompanied by significantly increased excitability of MDH nociceptive neurons indicative of central sensitization [4; 18; 19; 29; 34]. Central sensitization of functionally identified NS and WDR nociceptive neurons was also observed after p-IONX in the present study. While both groups of neurons did show several similar changes in their response properties following p-IONX, the NS and WDR neurons did differ in their receptive field features in the state of MDH central sensitization: WDR neurons showed significantly increased pinch receptive field size after p-IONX, while the pinch receptive field size of NS neurons remained unchanged after p-IONX. This indicates that some mechanisms of central sensitization may differ between NS and WDR neurons after nerve injury, consistent with other recent finding of NS and WDR neurons in the MDH following trigeminal nerve injury [4; 18]. All the examined parameters of central sensitization were blocked by CBX in this chronic nerve injury model. Since our previous study using an acute orofacial inflammatory pain model also showed that CBX blocked the development of MDH central sensitization, these findings collectively suggest the involvement of gap junctions in both acute and chronic orofacial pain processes.
Gap junctions are composed of a pair of hemichannels (connexons), and each connexon is made up of six protein subunits called connexins (Cx). In the central nervous system, neurons and glial cells express different connexins. Cx43 is the most abundant subunit expressed in astrocytes [27; 31; 32]. A limitation of the present study is that we did not make a direct assessment of Cx43, but the level of Cx43 has been shown to be elevated after sciatic nerve injury [51] or spinal cord injury [5], and Cx43/Cx30 knockout mice do not develop neuropathic pain following spinal cord injury [5]. CBX is a potent blocker of Cx43 gap junctions and hemichannels [35]. Recent studies indicate that CBX can also block other channels including P2X7 receptor-associated Pan-1 channels in spinal cord astrocytes [39]. Additionally, recent studies have demonstrated that CBX can also modulate astrocyte volume-regulated anion channels [54], inhibit the expression of IL-23 in microglia [14], modulate synaptic transmission and neuronal membrane properties [6; 41], and increase reactive oxygen species formation in neurons [56]. One other possible mechanism of CBX effect is that CBX inhibits the hemichannels and suppresses the release of glial mediators, such as proinflammatory cytokines, ATP and glutamate [38; 39].
In the present study, CBX attenuated the p-IONX-induced mechanical hypersensitivity and central sensitization of MDH nociceptive neurons associated with the p-IONX. Our previous and many other studies have implicated astrocytes in the development of neuropathic pain states [7; 20; 25; 29]. Despite the many varied potential effects of CBX, our previous studies indicating an important role of astrocytes in MDH central sensitization [7–9; 12] suggest that at least part of the observed CBX-mediated suppression of mechanical hypersensitivity and central sensitization documented in the present study is likely due to its blocking Cx43 in astrocytes, although the possibility cannot be ruled out of the contribution from Cx43 in other cell types or the other actions of CBX noted.
Astrocytes are highly interconnected by gap junctions and conduct signals in the form of intercellular Ca2+ waves. Ca2+ signaling and downstream cascades within the astrocytes lead to the release of substances such as glutamate, ATP and cytokines that act on adjacent and/or distant glia and neurons and thereby modulate neuronal activity and function within the central nervous system [1; 13; 36; 39]. An action of CBX on astrocyte gap junctions is likely a major factor underlying the present study’s findings of the effectiveness of CBX in attenuating the enhanced receptive field and response properties reflecting central sensitization of the MDH nociceptive neurons and the accompanying facial mechanical hypersensitivity following p-IONX.
In conclusion, our results indicate that gap junctions may be critically involved in orofacial neuropathic pain mechanisms and may provide a potential therapeutic target for the treatment of orofacial neuropathic pain.
Supplementary Material
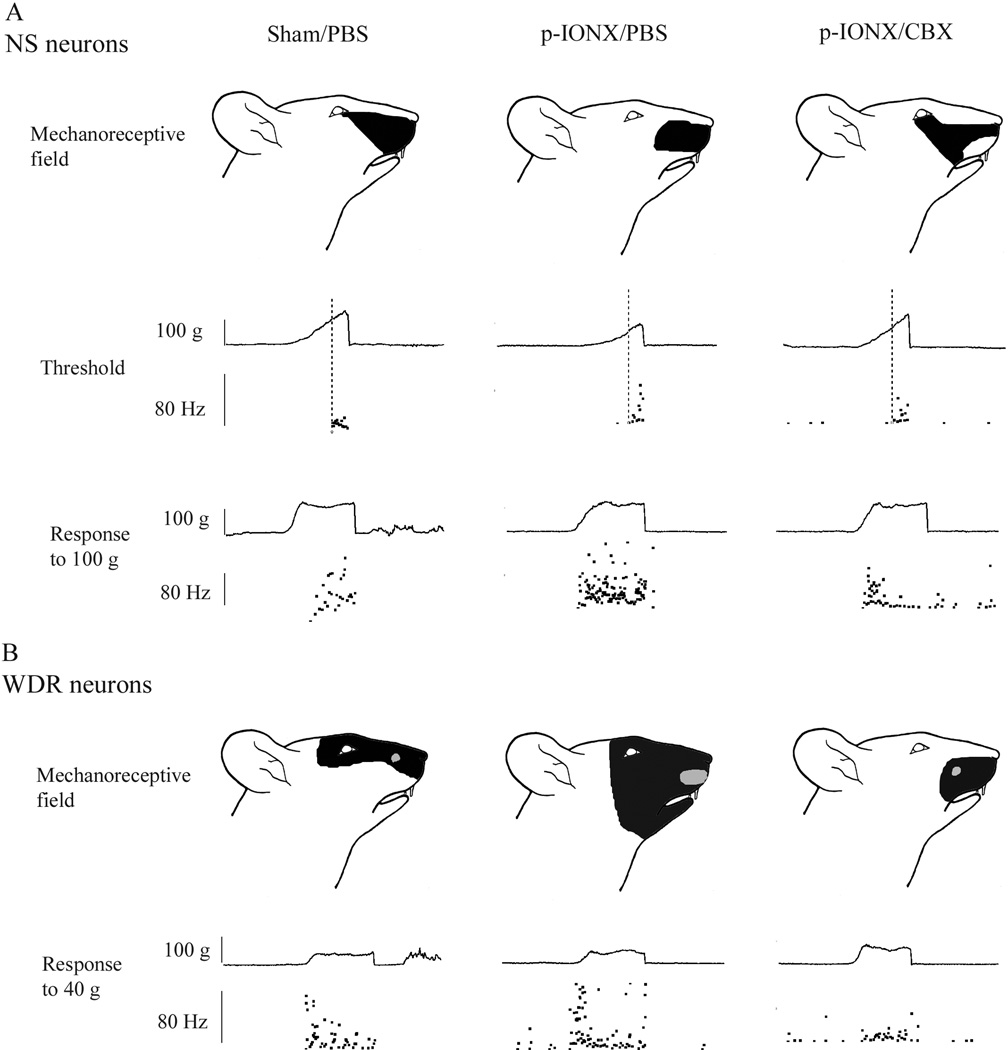
Fig. 4.
Examples showing typical mechanorecptive fields and responses of nociceptive specific (NS) and wide dynamic range (WDR) neurons in the MDH of sham animals and of p-IONX animals following PBS or CBX. In each example, the upper panel shows the mechanoreceptive field of the neuron to pinch (for NS and WDR neurons, black shading) and/or tactile touch/pressure (for WDR neurons, gray shading). The middle panel for NS neurons shows responses to graded mechanical stimuli applied to the mechanoreceptive field. The neuronal discharges, shown as dots, are displayed as an interspike instantaneous frequency plot (lower trace). The activation threshold of a given neuron is shown by the intersection of the vertical line indicating the beginning of the response and the ascending force curve (top trace). The bottom panel shows the neuronal responses to suprathreshold pressure stimuli (100 g for NS, 40 g for WDR neurons). The instantaneous frequency during the 5 s stimulation period is shown in the bottom trace and the stimulus force in the upper trace. In the middle and bottom panels, each dot represents the inverse of the preceding inter-spike interval (the instantaneous firing frequency) expressed in Hz.
Acknowledgements
This study was supported by NIH Grant DE-04786 to B.J.S and CIHR Grant MOP-82831 to J.O.D. B.J.S is the holder of a Canada Research Chair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest.
References
- 1.Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. What is the role of astrocyte calcium in neurophysiology? Neuron. 2008;59(6):932–946. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett MV, Zukin RS. Electrical coupling and neuronal synchronization in the Mammalian brain. Neuron. 2004;41(4):495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- 3.Bowser DN, Khakh BS. Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J Gen Physiol. 2007;129(6):485–491. doi: 10.1085/jgp.200709780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Y, Wang H, Chiang CY, Dostrovsky JO, Sessle BJ. Pregabalin suppresses nociceptive behavior and central sensitization in a rat trigeminal neuropathic pain model. J Pain. 2013;14(2):193–204. doi: 10.1016/j.jpain.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen MJ, Kress B, Han X, Moll K, Peng W, Ji RR, Nedergaard M. Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia. 2012;60(11):1660–1670. doi: 10.1002/glia.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chepkova AN, Sergeeva OA, Haas HL. Carbenoxolone impairs LTP and blocks NMDA receptors in murine hippocampus. Neuropharmacology. 2008;55(2):139–147. doi: 10.1016/j.neuropharm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Chiang CY, Dostrovsky JO, Iwata K, Sessle BJ. Role of glia in orofacial pain. Neuroscientist. 2011;17(3):303–320. doi: 10.1177/1073858410386801. [DOI] [PubMed] [Google Scholar]
- 8.Chiang CY, Li Z, Dostrovsky JO, Hu JW, Sessle BJ. Glutamine uptake contributes to central sensitization in the medullary dorsal horn. Neuroreport. 2008;19(11):1151–1154. doi: 10.1097/WNR.0b013e3283086781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang CY, Li Z, Dostrovsky JO, Sessle BJ. Central sensitization in medullary dorsal horn involves gap junctions and hemichannels. Neuroreport. 2010;21(3):233–237. doi: 10.1097/WNR.0b013e328336eecb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang CY, Park SJ, Kwan CL, Hu JW, Sessle BJ. NMDA receptor mechanisms contribute to neuroplasticity induced in caudalis nociceptive neurons by tooth pulp stimulation. J Neurophysiol. 1998;80(5):2621–2631. doi: 10.1152/jn.1998.80.5.2621. [DOI] [PubMed] [Google Scholar]
- 11.Chiang CY, Sessle BJ, Dostrovsky JO. Role of astrocytes in pain. Neurochem Res. 2012;37(11):2419–2431. doi: 10.1007/s11064-012-0801-6. [DOI] [PubMed] [Google Scholar]
- 12.Chiang CY, Wang J, Xie YF, Zhang S, Hu JW, Dostrovsky JO, Sessle BJ. Astroglial glutamate-glutamine shuttle is involved in central sensitization of nociceptive neurons in rat medullary dorsal horn. J Neurosci. 2007;27(34):9068–9076. doi: 10.1523/JNEUROSCI.2260-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992;8(3):429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- 14.Endong L, Shijie J, Sonobe Y, Di M, Hua L, Kawanokuchi J, Mizuno T, Suzumura A. The gap-junction inhibitor carbenoxolone suppresses the differentiation of Th17 cells through inhibition of IL-23 expression in antigen presenting cells. J Neuroimmunol. 2011;240–241:58–64. doi: 10.1016/j.jneuroim.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Hu Y, Li W, Lu L, Cai J, Xian X, Zhang M, Li Q, Li L. An anti-nociceptive role for ceftriaxone in chronic neuropathic pain in rats. Pain. 2010;148(2):284–301. doi: 10.1016/j.pain.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Imamura Y, Kawamoto H, Nakanishi O. Characterization of heat-hyperalgesia in an experimental trigeminal neuropathy in rats. Exp Brain Res. 1997;116(1):97–103. doi: 10.1007/pl00005748. [DOI] [PubMed] [Google Scholar]
- 17.Inoue K, Tsuda M. Microglia and neuropathic pain. Glia. 2009;57(14):1469–1479. doi: 10.1002/glia.20871. [DOI] [PubMed] [Google Scholar]
- 18.Iwata K, Imai T, Tsuboi Y, Tashiro A, Ogawa A, Morimoto T, Masuda Y, Tachibana Y, Hu J. Alteration of medullary dorsal horn neuronal activity following inferior alveolar nerve transection in rats. J Neurophysiol. 2001;86(6):2868–2877. doi: 10.1152/jn.2001.86.6.2868. [DOI] [PubMed] [Google Scholar]
- 19.Iwata K, Imamura Y, Honda K, Shinoda M. Physiological mechanisms of neuropathic pain: the orofacial region. Int Rev Neurobiol. 2011;97:227–250. doi: 10.1016/B978-0-12-385198-7.00009-6. [DOI] [PubMed] [Google Scholar]
- 20.Jarvis MF. The neural-glial purinergic receptor ensemble in chronic pain states. Trends Neurosci. 2010;33(1):48–57. doi: 10.1016/j.tins.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Decosterd I. Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol. 2006;2(4):259–269. doi: 10.1017/S1740925X07000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115(1–2):71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 25.McMahon SB, Malcangio M. Current challenges in glia-pain biology. Neuron. 2009;64(1):46–54. doi: 10.1016/j.neuron.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 26.Milligan ED, O'Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci. 2001;21(8):2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res Brain Res Rev. 2004;47(1–3):191–215. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Nedergaard M, Rodríguez JJ, Verkhratsky A. Glial calcium and diseases of the nervous system. Cell Calcium. 2010;47(2):140–149. doi: 10.1016/j.ceca.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Okada-Ogawa A, Suzuki I, Sessle BJ, Chiang CY, Salter MW, Dostrovsky JO, Tsuboi Y, Kondo M, Kitagawa J, Kobayashi A, Noma N, Imamura Y, Iwata K. Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. J Neurosci. 2009;29(36):11161–11171. doi: 10.1523/JNEUROSCI.3365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng HY, Chen GD, Lai CY, Hsieh MC, Lin TB. Spinal SIRP_1-SHP2 interaction regulates spinal nerve ligation-induced neuropathic pain via PSD-95-dependent NR2B activation in rats. Pain. 2012;153(5):1042–1053. doi: 10.1016/j.pain.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Rash JE, Yasumura T, Davidson KG, Furman CS, Dudek FE, Nagy JI. Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell Commun Adhes. 2001;8(4–6):315–320. doi: 10.3109/15419060109080745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J Neurosci. 2001;21(6):1983–2000. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roh DH, Yoon SY, Seo HS, Kang SY, Han HJ, Beitz AJ, Lee JH. Intrathecal injection of carbenoxolone, a gap junction decoupler, attenuates the induction of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol. 2010;224(1):123–132. doi: 10.1016/j.expneurol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Saito K, Hitomi S, Suzuki I, Masuda Y, Kitagawa J, Tsuboi Y, Kondo M, Sessle BJ, Iwata K. Modulation of trigeminal spinal subnucleus caudalis neuronal activity following regeneration of transected inferior alveolar nerve in rats. J Neurophysiol. 2008;99(5):2251–2263. doi: 10.1152/jn.00794.2007. [DOI] [PubMed] [Google Scholar]
- 35.Samoilova M, Wentlandt K, Adamchik Y, Velumian AA, Carlen PL. Connexin 43 mimetic peptides inhibit spontaneous epileptiform activity in organotypic hippocampal slice cultures. Exp Neurol. 2008;210(2):762–775. doi: 10.1016/j.expneurol.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54(7):716–725. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sessle BJ. Peripheral and central mechanisms of orofacial inflammatory pain. Int Rev Neurobiol. 2011;97:179–206. doi: 10.1016/B978-0-12-385198-7.00007-2. [DOI] [PubMed] [Google Scholar]
- 38.Spataro LE, Sloane EM, Milligan ED, Wieseler-Frank J, Schoeniger D, Jekich BM, Barrientos RM, Maier SF, Watkins LR. Spinal gap junctions: potential involvement in pain facilitation. J Pain. 2004;5(7):392–405. doi: 10.1016/j.jpain.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26(5):1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Söhl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat Rev Neurosci. 2005;6(3):191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- 41.Tovar KR, Maher BJ, Westbrook GL. Direct actions of carbenoxolone on synaptic transmission and neuronal membrane properties. J Neurophysiol. 2009;102(2):974–978. doi: 10.1152/jn.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuboi Y, Iwata K, Dostrovsky JO, Chiang CY, Sessle BJ, Hu JW. Modulation of astroglial glutamine synthetase activity affects nociceptive behaviour and central sensitization of medullary dorsal horn nociceptive neurons in a rat model of chronic pulpitis. Eur J Neurosci. 2011;34(2):292–302. doi: 10.1111/j.1460-9568.2011.07747.x. [DOI] [PubMed] [Google Scholar]
- 43.Tsuda M, Kohro Y, Yano T, Tsujikawa T, Kitano J, Tozaki-Saitoh H, Koyanagi S, Ohdo S, Ji RR, Salter MW, Inoue K. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain. 2011;134(Pt 4):1127–1139. doi: 10.1093/brain/awr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 45.Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat's infraorbital nerve. J Neurosci. 1994;14(5 Pt 1):2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watkins LR, Martin D, Ulrich P, Tracey KJ, Maier SF. Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain. 1997;71(3):225–235. doi: 10.1016/s0304-3959(97)03369-1. [DOI] [PubMed] [Google Scholar]
- 47.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24(8):450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 48.Wieseler-Frank J, Maier SF, Watkins LR. Glial activation and pathological pain. Neurochem Int. 2004;45(2–3):389–395. doi: 10.1016/j.neuint.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 49.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 50.Wu A, Green CR, Rupenthal ID, Moalem-Taylor G. Role of gap junctions in chronic pain. J Neurosci Res. 2012;90(2):337–345. doi: 10.1002/jnr.22764. [DOI] [PubMed] [Google Scholar]
- 51.Wu XF, Liu WT, Liu YP, Huang ZJ, Zhang YK, Song XJ. Reopening of ATP-sensitive potassium channels reduces neuropathic pain and regulates astroglial gap junctions in the rat spinal cord. Pain. 2011;152(11):2605–2615. doi: 10.1016/j.pain.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Xie YF, Zhang S, Chiang CY, Hu JW, Dostrovsky JO, Sessle BJ. Involvement of glia in central sensitization in trigeminal subnucleus caudalis (medullary dorsal horn) Brain Behav Immun. 2007;21(5):634–641. doi: 10.1016/j.bbi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Xu M, Aita M, Chavkin C. Partial infraorbital nerve ligation as a model of trigeminal nerve injury in the mouse: behavioral, neural, and glial reactions. J Pain. 2008;9(11):1036–1048. doi: 10.1016/j.jpain.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye ZC, Oberheim N, Kettenmann H, Ransom BR. Pharmacological "cross-inhibition" of connexin hemichannels and swelling activated anion channels. Glia. 2009;57(3):258–269. doi: 10.1002/glia.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoon SY, Robinson CR, Zhang H, Dougherty PM. Spinal astrocyte gap junctions contribute to oxaliplatin-induced mechanical hypersensitivity. J Pain. 2013;14(2):205–214. doi: 10.1016/j.jpain.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zündorf G, Kahlert S, Reiser G. Gap-junction blocker carbenoxolone differentially enhances NMDA-induced cell death in hippocampal neurons and astrocytes in co-culture. J Neurochem. 2007;102(2):508–521. doi: 10.1111/j.1471-4159.2007.04509.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.