Abstract
CART peptide is known for having an inhibitory effect on cocaine- and dopamine-mediated actions after acute administration of cocaine and dopamine. In this regard, it is postulated to be a homeostatic, regulatory factor on dopaminergic activity in the nucleus accumbens (NAc). However, there is no data on the effect of CART peptide after chronic administration of cocaine, and this study addresses this. It was found that CART peptide blunted cocaine-induced locomotion (LMA) after acute administration of cocaine, as expected, but it did not affect cocaine-mediated LMA after chronic administration of cocaine. The loss of CART peptide’s inhibitory effect did not return for up to nine weeks after stopping the repeated cocaine administration. It may not be surprising that homeostatic regulatory mechanisms in the NAc are lost after repeated cocaine administration, and that this may be a mechanism in the development of addiction.
Key words/Phrases: CART peptide, CART 55-102, cocaine, acute, chronic
Introduction
The nucleus accumbens (NAc) is part of the reward pathway of the brain and is a major mediator of cocaine-induced behavioral effects including cocaine self-administration and cocaine-induced locomotor activity (LMA). Cocaine-induced behaviors are sensitive to changes in the concentration of CART55-102 peptide in the NAc [12–14]. An injection of CART 55-102 into the NAc blunts the effects of acute administration of cocaine and dopamine (DA) [13]. Conversely, depletion of endogenous CART peptide in the NAc results in a potentiation of cocaine-induced LMA [14]. There is an enhanced expression of cocaine-and-amphetamine regulated transcript (CART) in the NAc of human cocaine abusers [1, 3]. Cocaine enhances CART expression in the NAc of animals [5, 7, 9, 11]. Because of these findings, it has been postulated that CART peptide is a homeostatic regulator of DA- and cocaine-induced actions in the NAc [13, 23].
Repeated administration of cocaine can induce persistent alterations in the structure and function of the NAc (a) shortly after the last cocaine administration and (b) several weeks after withdrawal from repeated cocaine administration [2, 6, 8, 16–18, 24, 28]. For instance, chronic intermittent administration of cocaine leads to a lowering of basal dopamine levels in the NAc [22]. Also, repeated daily injections of cocaine (10 mg/kg, i.p.) for 15 days resulted in a significant increase in the number of DA D2 receptor (D2R) binding sites [8, 17] and a decrease [17] in the number of DA D1 receptor (D1R) binding sites in the NAc shortly after cessation of cocaine administration, but these changes were absent 2 weeks after halting the cocaine administration. Furthermore, chronic cocaine exposure increases the density of dendritic spines in the NAc shell shortly after chronic cocaine administration but there is a return to baseline 4 weeks after cocaine withdrawal. In the NAc core on the other hand, spines are unchanged shortly after repeated cocaine administration but are persistently decreased 4 weeks after withdrawal [6]. These findings suggest that there are dynamic changes in the NAc after cocaine administration. These modifications in the NAc presumably contribute to the addictive effect of cocaine.
Repeated intravenous administration of cocaine down regulates DA D3 receptors in the NAc [25] and DA D3 receptors regulate CART mRNA in the NAc [4, 10]. Furthermore, CART peptide attenuates the effect of combined, acute DA D1-D2 receptor activation [19], but repeated cocaine treatment causes a dysregulation of DA D1-D2 dopamine receptor signaling [2]. With these structural and functional changes in the NAc (a) shortly after chronic administration of cocaine and (b) after withdrawal from repeated administration of cocaine, it is important to determine if there are corresponding changes in the effect of CART peptide on cocaine-mediated effects. In this study, we looked at the effect of CART peptide on LMA after acute and chronic administration of cocaine and after withdrawal from chronic cocaine treatments.
Materials and Methods
A total of forty-one male Sprague Dawley rats (Charles River Inc., Wilmington, MA), weighing 386–542g during locomotor activity (LMA) experiments were used.. They were provided rat chow and water ad libitum, and maintained on a 12 hour light: dark cycle (lights on at 7am). Experiments and animal care were in accordance with the Institute of Animal Care and Use Committee of Emory University and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The rats were implanted, under isoflurane anesthesia, with bilateral stainless steel guide cannulae assembly (22 gauge; Plastics One; Roanoke, VA) using the following coordinates for the shell-core border of nucleus accumbens: A/P 1.6mm, L/M ± 1.5mm, D/V −5.7mm [21]. The experiment design was divided as follows – (a) after single cocaine injection, (b) after chronic cocaine injection, (c) 3-weeks after chronic cocaine and (d) 9-weeks after chronic cocaine.
On experiment day, rats were placed into locomotor chambers for 30 min to habituate to their surroundings before the recording of basal LMA commenced. After 30 min of basal LMA recording, rats were removed from the chambers, infused with saline (0.5 μL/side) or CART55-102 (2.5 μg in 0.5 μL of saline/side) immediately before an ip injection of equivalent volume of saline (1 mL/kg) or cocaine (10 mg/kg), and placed back into the chambers for 60 min of additional LMA recording. The selected dose of CART peptide (2.5 μg) and the selected dose of cocaine (10 mg/kg ip) were selected because of their effectiveness in previous studies [13]. The chronic cocaine (or saline) regimen proceeded every day after the above experiment and involved administration of ip cocaine (10 mg/kg) (or saline) to rats once daily. On the 14th day, like the 1st day, cocaine-mediated LMA was measured. The rats that received saline infusion into the NAc before chronic cocaine (or chronic saline) received CART55-102 after chronic cocaine (or chronic saline), and vice versa in a counterbalanced design. There were no significant differences in the body weights between the acute and chronic administration groups. For some animals, the experiment continued for up to 9 weeks after cessation of chronic cocaine administration. For these animals, each rat received both saline and CART55-102 infusions into the NAc plus ip cocaine in two experimental days separated by at least 72 hours apart (counterbalanced design) and LMA was measured.
On experiment days, intra-accumbal infusions were done using stainless steel bilateral injector cannulae (28 gauge, Plastics One), connected via polyethylene-50 (PE-50) tubing to two 25 μL microsyringes (Hamilton Co, Reno, NV). The two microsyringes were driven by pumps connected to Micro4 Microsyringe Pump Controller (World Precision Instruments, Sarasota, FL). Fluid was bilaterally injected (0.5 μL/side) for 30 seconds. The injected fluid was allowed to diffuse for an additional 30 seconds before removal of the injector cannluae. LMA was measured in 10 minute intervals using a photocell cage (Omnitech Electronics, Columbus OH) with transparent plexiglass walls (dimensions of 40 × 40 × 30cm), containing 32 photobeams located 5cm above the floor to record LMA. Each cage was placed in a stainless steel box and connected to a computer equipped with software (Digipro; Omnitech Electronics) to measure LMA.
After completion of the experiments, animals received an overdose of Ketamine (70 mg/kg) and dexmedetomidine (0.5 mg/kg), and were then perfused intracardially with saline and 4% paraformaldehyde in saline. After removal from the skull, brains were placed in 4% paraformaldehyde overnight, followed by immersion in 30% sucrose for several days and sliced (60 μm slices)using a cryostat (Leica CM1900, Leica CM3050S, Leica Microsystems, Germany). Nissl staining was performed to further confirm placement of the injector tips. A stereotaxic brain atlas was used to approximate the placement of the injector tips.
Data are all expressed as mean ± SEM, and significance occurs when p < 0.05. All analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego California USA, www.graphpad.com). LMA data were acquired in 10-minute bins and time course data and total locomotor activity data were analyzed. For analysis of the time course data, a 2-way Repeated Measures ANOVA with Bonferroni post test was used to check if there were main effects of treatment groups (between subjects), time (within subjects) and a treatment groups × time interaction. For time course data, the factors were treatment groups (4 levels: saline+saline, CART+saline, saline+cocaine, CART+cocaine) and time (9 levels). LMA data were also analyzed as total locomotor activity in 60 min– a summation of individual locomotor activity values for 60 min post-injection. For analysis of the total locomotor activity data, a 2-way ANOVA with Bonferroni post test was used. For the total LMA data, the factors were treatment groups (4 levels) and chronic cocaine (2 levels: before and after). For analysis of the total locomotor activity data before, at and after chronic cocaine administration, a 2-way ANOVA with Bonferroni post test was used. Here, the factors were treatment groups (2 levels: saline+cocaine, CART+cocaine) and chronic cocaine (4 levels: before, at, 3 weeks after, at 9 weeks after).
Results
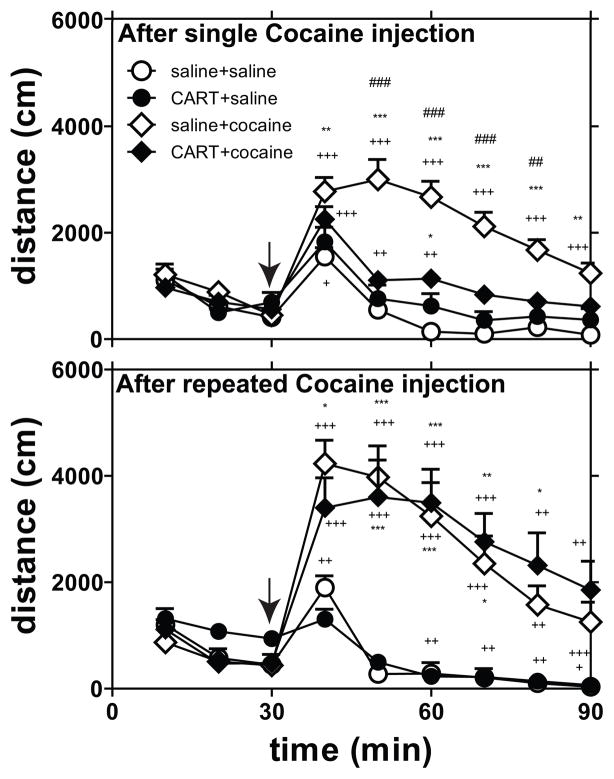
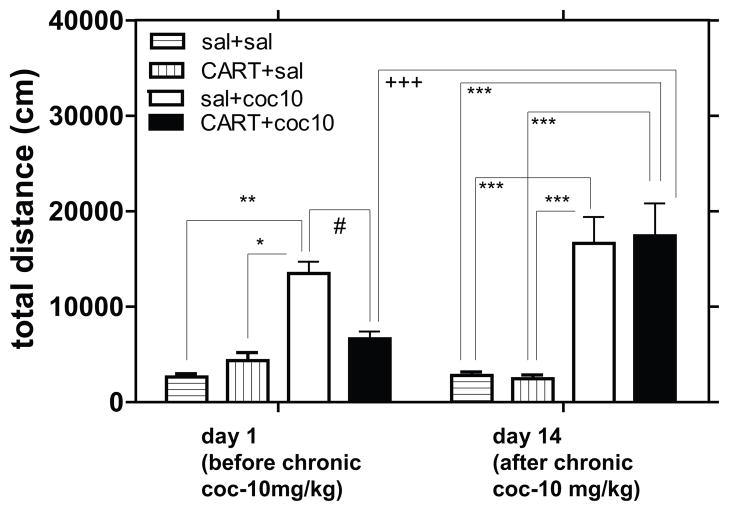
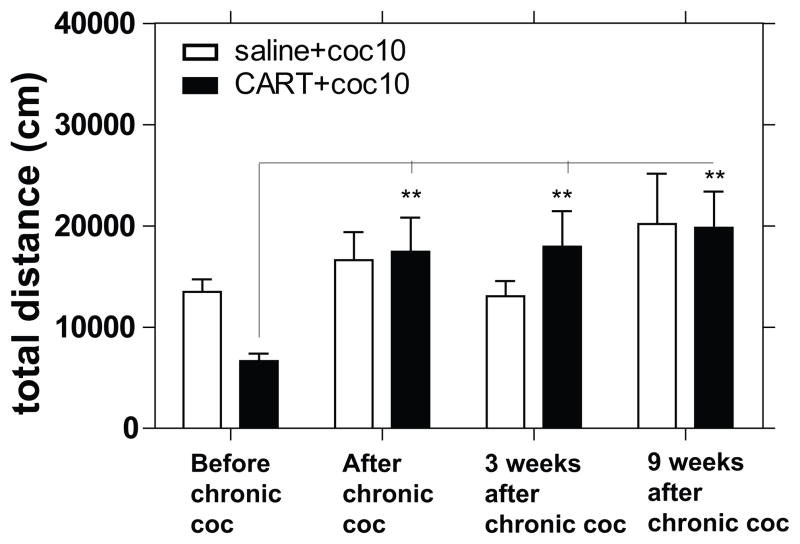
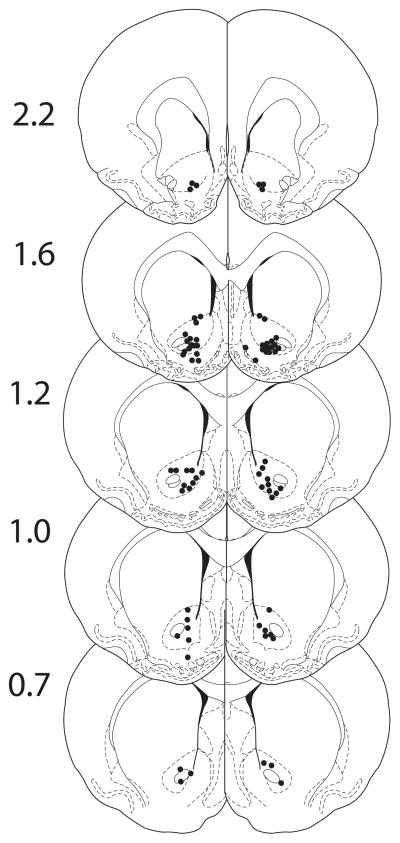
Rats received injections into the NAc of either saline alone or CART 55-102 alone, which was then immediately followed with an ip injection of either saline or cocaine, and LMA was measured. Measurements were made after one or 14 daily injections of cocaine (10mg/kg) or saline. When the individual time points of LMA were analyzed separately (Fig 1) or combined (total LMA) (Fig 2), significant differences were found. Cocaine increased total LMA as expected, and CART 55-102 reduced cocaine-induced LMA as expected (Fig 1, 2) after a single injection of cocaine (on day 1). However, on day 14, after the repeated daily cocaine administration regimen, cocaine increased LMA as expected, but CART 55-102 had no effect on cocaine-induced LMA (Fig 1, 2). Furthermore, CART 55-102 did not reduce cocaine-induced LMA at 3-or 9-weeks after withdrawal from the chronic cocaine administration (Fig 3). Thus, the loss of the inhibitory effect of CART 55-102 was long-lasting. The locations of the injections into the NAc were determined as described in Methods and as expected they were found throughout the extent of the NAc (Fig 4).
Fig 1. Time course of cocaine-induced locomotor activity.
CART55-102 (2.5 ug) or saline was administered directly into the NAc of male rats immediately before a single ip injection of saline or cocaine (coc) (10 mg/kg) (day 1) and after additional 13 days (day 14) of cocaine (10 mg/kg) administration once daily, and LMA was measured. The groups were as follows – single coc administration [saline+saline (n = 6), CART+saline (n = 5), saline+coc (n = 15), CART+coc (n = 15)] and chronic coc administration [saline+saline (n =5), CART+saline (n = 6), saline+coc (n = 13), CART+coc (n = 13)]. The rats that received CART on day 1 received saline on day 14, and vice versa. The y-axis represents total distance covered. Top Graph -- 2-way Repeated Measures ANOVA of time course data (after single cocaine injection) showed a significant treatment group effect (F 3, 37 = 18.08, ***P<0.0001), a significant time effect (F 8, 296 = 23.07, ***P<0.0001) and a significant interaction (F 24, 296 = 6.244, ***P<0.0001). Bonferroni post hoc tests showed that saline+saline was not different from CART+saline at all time points. Saline+saline was different from saline+coc at all time points. Saline+saline was different (*) from CART+coc only at the 3rd time (30 min) point after injection. Saline+coc was different (#) from CART+coc at the 2nd to 5th time points (50–80min) after injection. 1-way ANOVA of the individual treatment groups was done to determine the effect of injection by comparing the time point just before injection (time = 30 min) and all the time points post-injection. Bottom Graph -- 2-way Repeated Measures ANOVA of time course data (after chronic cocaine regimen) showed a significant treatment group effect (F 3, 33 = 5.200, **P = 0.0047), a significant time effect (F 8, 264 = 16.24, ***P<0.0001) and a significant interaction (F 24, 264 = 6.306, ***P<0.0001). Bonferroni post hoc tests showed that saline+saline was not different from CART-saline at all time points. Saline+saline was different from saline+coc up to 4 time points after cocaine injection. Saline+saline was different (*) from CART+coc from the 2nd – 5th time (50–80min) point after injection. Saline+coc was not different from CART+coc at any time point after injection. 1-way ANOVA of the individual treatment groups was done to determine the effect of injection by comparing the time point just before injection (time = 30 min) and all the time points post-injection. The p-values *p<0.05, **p<0.01, ***p<0.001 show difference when comparing all groups to saline-saline. The p-values #p<0.05, ##p<0.01, ###p<0.001 show difference when comparing between saline+coc and CART+coc groups. The p-values +p<0.05, ++p<0.01, +++p<0.001 show difference when comparing pre-injection time point (time = 30 min) to all post-injection time points for each group. The significant differences after post hoc analysis are shown on the graph.
Fig 2. The effect of CART peptide after both single and repeated injections of cocaine.
The data is a summation of the time points after cocaine (coc) administration in Fig 1. The rats that received CART on day 1 received saline on day 14, and vice versa. The y-axis represents total distance covered in 60 mins after cocaine (or saline) injection. 2-way ANOVA showed a significant treatment effect (F 3, 70 = 12.58, ***P<0.0001), an almost significant chronic coc effect (F1, 70 = 2.974, P = 0.0890) and an interaction (F 3, 70 = 3.052, *P = 0.0341). Bonferroni post hoc tests showed that CART+coc was significantly different from saline+coc on day 1 but was not different after chronic cocaine administration. Bonferroni post hoc tests also showed that the effect of CART+coc was significantly different after chronic coc administration. The p-values *p<0.05, **p<0.01, ***p<0.001 show difference when comparing all groups to saline+saline. The p-values #p<0.05, ##p<0.01, ###p<0.001 show difference when comparing between saline+coc and CART+coc groups. The p-values +p<0.05, ++p<0.01, +++p<0.001 show the difference when comparing treatments between day 1 (before chronic cocaine) and day 14 (after chronic cocaine). The significant differences after post hoc analysis are shown on the graph.
Fig 3. The effect of CART peptide after withdrawal of repeated injections of cocaine.
CART55-102 (2.5 ug) or saline was administered directly into the NAc of male rats immediately before an ip injection of saline or cocaine (coc) (10 mg/kg) acutely (day 1), after additional 13 days (day 14) of cocaine (10 mg/kg) administration once daily and at different time points after withdrawal of chronic administration of cocaine-10 mg/kg and LMA was measured. The groups were as follows (before chronic coc administration) [saline+coc (n = 15), CART+coc (n = 15)], (after chronic coc administration) [saline+coc (n = 13), CART+coc (n = 13)], (3 weeks after chronic coc administration) [saline+coc (n = 10), CART+coc (n = 10)], and (9 weeks after chronic coc administration) [saline+coc (n = 7), CART+coc (n = 8)]. The y-axis represents distance covered in 60 min after cocaine injection. The x-axis represents the conditions relative to chronic coc administration. 2-way ANOVA showed no significant treatment effect (F 1, 83 = 0.03841, P = 0.8451), a significant difference between conditions relative to time before chronic coc (F 3, 83 = 5.199, **P = 0.0024) and no interaction (F 3, 83 = 1.931, P = 0.1309). Bonferroni post hoc tests showed that the effect of CART+coc before chronic coc was significantly different from its effect after chronic coc and even after allowing rat to recover for up to 9 weeks after chronic coc. The p-values *p<0.05, **p<0.01, ***p<0.001 show difference when comparing treatment groups between all conditions relative to chronic coc. The significant differences after post hoc analysis are shown on the graph.
Fig 4. The histological analysis of the rat brains showing injection regions in the NAc.
The numbers are coordinates from Bregma in millimeters [21].
Discussion
CART peptide attenuated cocaine-induced LMA after acute administration of cocaine, but this effect was lost after repeated (14 days) cocaine administration. Also, the CART peptide inhibitory effect on cocaine-induced LMA did not return even after 9 weeks of abstinence from repeated cocaine administration. Our study confirms the effect of intra-NAc CART peptide on acute cocaine administration [13], and shows for the first time that the inhibitory effect of CART peptide is lost after repeated, non-contingent cocaine administration. There are significant differences between the design of our study and a previous study that shows that intra-NAc CART peptide suppresses locomotor sensitization 3 weeks after withdrawal from a repeated cocaine (15 mg/kg ip × 7 days) regimen [26] and there is evidence that experimental designs for the effects of repeated cocaine regimen is critical [22]. Our data implies that chronic cocaine persistently altered the CART peptide system in the NAc. Other systems in the NAc that are similarly dysregulated by chronic cocaine include the Orexin [27] and Dynorphin A [20] systems, with alterations persisting even beyond 2 months after cessation of repeated cocaine [27].
Cocaine-induced LMA is due to DA effect in the NAc and CART peptide attenuates acute DA-mediated LMA by suppressing the effect of a combined activation of DA D1R and D2R [13, 19]. After chronic cocaine administration in a similar experimental design (cocaine 10 mg/kg ip × 15 days), D1R were transiently reduced in the NAc while D2R were increased in the NAc [17]. Given that CART peptide attenuates the LMA of a combined D1R and D2R effect, the change in D1R to D2R ratio or effect in the NAc shortly after chronic administration of cocaine may be at least part of the mechanism of the effects of CART peptide (or lack of) in our study. Another possible mechanism of our results is that CART peptide is still effective but requires much higher doses to produce an effect. This could not be tested because higher doses of CART 55-102 tend to produce convulsions [15]. In any case, this would still be a cocaine-induced dysfunction of the CART peptide system in the NAc.
In conclusion, CART peptide attenuated cocaine-induced LMA after acute administration of cocaine as expected, but the inhibitory effect was lost after repeated cocaine administration and the CART peptide effect did not return even after 9 weeks of withdrawal from repeated cocaine administration. It is important to note that the studies herein are limited to LMA, and that studies with self-administration or conditioned place preference are needed as well to fully conclude that such a loss of an inhibitory system after repeated cocaine administration may be a mechanism underlying the state of addiction.
Highlights.
CART peptide inhibited cocaine-mediated locomotion after single cocaine injection.
The inhibitory effect of CART peptide is lost after chronic cocaine administration.
The effect of CART peptide was not recovered even after cocaine withdrawal.
The persistent loss of CART peptide effect suggests its role in cocaine addiction.
Acknowledgments
This project was funded by the National Center for Research Resources P51RR165 and is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132. It was also supported by DA 015040, DA 15162 and the Georgia Research Alliance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Bannon M, Kapatos G, Albertson D. Gene expression profiling in the brains of human cocaine abusers. Addict Biol. 2005;10:119–126. doi: 10.1080/13556210412331308921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaudry G, Zekki H, Rouillard C, Levesque D. Clozapine and dopamine D3 receptor antisense reduce cocaine- and amphetamine-regulated transcript expression in the rat nucleus accumbens shell. Synapse. 2004;51:233–240. doi: 10.1002/syn.10302. [DOI] [PubMed] [Google Scholar]
- 5.Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumitriu D, Laplant Q, Grossman YS, Dias C, Janssen WG, Russo SJ, Morrison JH, Nestler EJ. Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. J Neurosci. 2012;32:6957–6966. doi: 10.1523/JNEUROSCI.5718-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fagergren P, Hurd YL. Mesolimbic gender differences in peptide CART mRNA expression: effects of cocaine. Neuroreport. 1999;10:3449–3452. doi: 10.1097/00001756-199911080-00034. [DOI] [PubMed] [Google Scholar]
- 8.Goeders NE, Kuhar MJ. Chronic cocaine administration induces opposite changes in dopamine receptors in the striatum and nucleus accumbens. Alcohol Drug Res. 1987;7:207–216. [PubMed] [Google Scholar]
- 9.Hubert GW, Kuhar MJ. Cocaine administration increases the fraction of CART cells in the rat nucleus accumbens that co-immunostain for c-Fos. Neuropeptides. 2008;42:339–343. doi: 10.1016/j.npep.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter RG, Jones D, Vicentic A, Hue G, Rye D, Kuhar MJ. Regulation of CART mRNA in the rat nucleus accumbens via D3 dopamine receptors. Neuropharmacology. 2006;50:858–864. doi: 10.1016/j.neuropharm.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Hunter RG, Vicentic A, Rogge G, Kuhar MJ. The effects of cocaine on CART expression in the rat nucleus accumbens: a possible role for corticosterone. Eur J Pharmacol. 2005;517:45–50. doi: 10.1016/j.ejphar.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Jaworski JN, Hansen ST, Kuhar MJ, Mark GP. Injection of CART (cocaine- and amphetamine-regulated transcript) peptide into the nucleus accumbens reduces cocaine self-administration in rats. Behav Brain Res. 2008;191:266–271. doi: 10.1016/j.bbr.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaworski JN, Kozel MA, Philpot KB, Kuhar MJ. Intra-accumbal injection of CART (cocaine-amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. J Pharmacol Exp Ther. 2003;307:1038–1044. doi: 10.1124/jpet.103.052332. [DOI] [PubMed] [Google Scholar]
- 14.Job MO, Licata J, Hubert GW, Kuhar MJ. Intra-accumbal administration of shRNAs against CART peptides cause increases in body weight and cocaine-induced locomotor activity in rats. Brain Res. 2012;1482:47–54. doi: 10.1016/j.brainres.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keating GL, Kuhar MJ, Rye DB. High dose CART peptide induces abnormal EEG activity and behavioral seizures. Neuropeptides. 2008;42:199–204. doi: 10.1016/j.npep.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Park BH, Lee JH, Park SK, Kim JH. Cell type-specific alterations in the nucleus accumbens by repeated exposures to cocaine. Biol Psychiatry. 2011;69:1026–1034. doi: 10.1016/j.biopsych.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Kleven MS, Perry BD, Woolverton WL, Seiden LS. Effects of repeated injections of cocaine on D1 and D2 dopamine receptors in rat brain. Brain Res. 1990;532:265–270. doi: 10.1016/0006-8993(90)91768-c. [DOI] [PubMed] [Google Scholar]
- 18.Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moffett MC, Song J, Kuhar MJ. CART peptide inhibits locomotor activity induced by simultaneous stimulation of D1 and D2 receptors, but not by stimulation of individual dopamine receptors. Synapse. 2011;65:1–7. doi: 10.1002/syn.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mu P, Neumann PA, Panksepp J, Schluter OM, Dong Y. Exposure to cocaine alters dynorphin-mediated regulation of excitatory synaptic transmission in nucleus accumbens neurons. Biol Psychiatry. 2011;69:228–235. doi: 10.1016/j.biopsych.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Burlington MA: 1998. [Google Scholar]
- 22.Puig S, Noble F, Benturquia N. Short- and long-lasting behavioral and neurochemical adaptations: relationship with patterns of cocaine administration and expectation of drug effects in rats. Transl Psychiatry. 2012;2:e175. doi: 10.1038/tp.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci. 2008;9:747–758. doi: 10.1038/nrn2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, Renthal W, Graham A, Birnbaum SG, Green TA, Robison B, Lesselyong A, Perrotti LI, Bolanos CA, Kumar A, Clark MS, Neumaier JF, Neve RL, Bhakar AL, Barker PA, Nestler EJ. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace DR, Mactutus CF, Booze RM. Repeated intravenous cocaine administration: locomotor activity and dopamine D2/D3 receptors. Synapse. 1996;23:152–163. doi: 10.1002/(SICI)1098-2396(199607)23:3<152::AID-SYN4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Yoon HS, Kim S, Park HK, Kim JH. Microinjection of CART peptide 55-102 into the nucleus accumbens blocks both the expression of behavioral sensitization and ERK phosphorylation by cocaine. Neuropharmacology. 2007;53:344–351. doi: 10.1016/j.neuropharm.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Zhang GC, Mao LM, Liu XY, Wang JQ. Long-lasting up-regulation of orexin receptor type 2 protein levels in the rat nucleus accumbens after chronic cocaine administration. J Neurochem. 2007;103:400–407. doi: 10.1111/j.1471-4159.2007.04748.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Li J, Liu N, Wang B, Gu J, Zhang M, Zhou Z, Jiang Y. Signaling via dopamine D1 and D3 receptors oppositely regulates cocaine-induced structural remodeling of dendrites and spines. Neurosignals. 2012;20:15–34. doi: 10.1159/000330743. [DOI] [PubMed] [Google Scholar]