Abstract
The bone morphogenetic protein (BMP) signaling pathway has essential functions in development, homeostasis, and in the normal and pathophysiologic remodeling of tissues. Small molecule inhibitors of the BMP receptor kinase family have been useful for probing physiologic functions of BMP signaling in vitro and in vivo, and may have roles in the treatment of BMP-mediated diseases. Here we describe the development of a selective and potent inhibitor of the BMP type I receptor kinases, LDN-212854, which in contrast to previously described BMP receptor kinase inhibitors exhibits nearly 4 orders of selectivity for BMP versus the closely related TGF-β and Activin type I receptors. In vitro, LDN-212854 exhibits some selectivity for ALK2 in preference to other BMP type I receptors, ALK1 and ALK3, which may permit the interrogation of ALK2-mediated signaling, transcriptional activity and function. LDN-212854 potently inhibits heterotopic ossification in an inducible transgenic mutant ALK2 mouse model of fibrodysplasia ossificans progressiva. These findings represent a significant step towards developing selective inhibitors targeting individual members of the highly homologous BMP type I receptor family. Such inhibitors would provide greater resolution as probes of physiologic function, and improved selectivity against therapeutic targets.
Introduction
Bone morphogenetic protein (BMP) signaling plays major roles in embryonic patterning, tissue remodeling, and maintaining physiologic homeostasis (1). It has become increasingly appreciated that disordered BMP signaling contributes to developmental and postnatal disease (2, 3). BMPs belong to the transforming growth factor beta (TGF-β) superfamily of signaling ligands that consist of more than 25 different ligands including TGF-βs, growth and differentiation factors, and Activins (1, 4). BMP ligand dimers facilitate the assembly of tetrameric receptor complexes composed of two constitutively-active type II receptor serine-threonine kinases (BMPRII, ACTRIIA, or ACTRIIB) that transphosphorylate and activate two type I receptor serine-threonine kinases (ALK1, ALK2, ALK3, or ALK6) (Figure 1a). Activated type I receptors phosphorylate BMP receptor responsive SMAD proteins 1, 5, or 8, which in association with co-SMAD4 translocate to the nucleus to regulate gene transcription (4). Activin and TGF-β ligands similarly recruit TGF-β or Activin type II receptors (TGFβR2, ACTRIIA, or ACTRIIB) with a set of type I receptors (ALK4, ALK5, or ALK7) to activate SMADs 2 and 3, which translocate with SMAD4 to the nucleus to regulate distinct transcriptional programs.
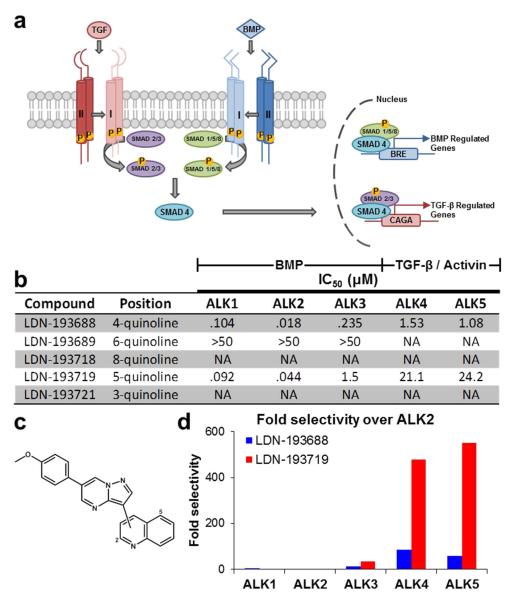
Figure 1.
Structure activity relationship of 4-methoxyphenyl pyrazolo[1,5-a]pyrimidine derivatives. a) A schematic representation of the canonical BMP and TGF-β signaling pathways. b) In vitro kinase assay IC50 measurements against BMP and TGF-β type I receptors show only the 4-quinoline (LDN-193688) and 5-quinoline (LDN-193719) derivatives are active. c) Structure of five pyrazolo[1,5-a]pyrimidine derivatives with varying quinoline attachment site. d) Fold selectivity of active inhibitors against ALK2 versus BMP and TGF-β type I receptors. Although slightly less potent against ALK2 than LDN-193688, the 5-quinoline derivative LDN-193719 has much greater selectivity for BMP vs. TGF-β type I receptors.
Specificity in BMP and TGF-β signaling is conferred in part by preferential binding of ligands with specific combinations of type I and type II receptors, although there is considerable functional redundancy among ligands and receptors (5). Biological context is provided by the spatiotemporally regulated expression of ligands and their cognate receptors in target tissues (6). The diversity of upstream ligand and receptors signals, and their pleiotropic downstream effects raises questions of how specificity is recognized and translated into biological outcome in this pathway (7). Targeting individual ligands and receptors by genetic epistasis has yielded important insights into function, but their interpretation is limited again by redundancy as well as embryonic effects. Pharmacologic strategies for modulating BMP and TGF-β signaling have emerged as a promising strategy for elucidating function and specificity in these pathways. These strategies include small molecule kinase inhibitors and recombinant protein ligand-traps (3).
A common challenge to the development of selective ATP competitive small molecule kinase inhibitors is the structural homology of highly conserved ATP binding domains (8). Structural homology is particularly high between the type I receptors of the BMP and TGF-β signaling pathways. For example, the ALK3 kinase domain possesses 66% sequence identity with that of ALK5 (9). Even greater kinase domain sequence identity is found between homologues within the BMP or TGF-β families, such as ALK1 and ALK2 (79%), ALK3 and ALK6 (86%), and ALK4 and ALK5 (90%). The high degree of structural homology between receptors poses serious challenges for the development of highly selective small molecules that can discriminate between the individual members of the TGF-β or BMP receptor families.
Highly selective inhibitors could be useful as therapeutics for diseases mediated by inappropriate signaling of an individual type I receptor, exemplified best by fibrodysplasia ossificans progressiva (FOP), an extremely rare genetic disease with a worldwide prevalence of 1 in 2 million (10). FOP manifests generally within the first decade of life with episodic soft tissue lesions that progress to ectopic endochrondral bone within skeletal muscles, ligaments and fascia, resulting in severely impaired mobility and shortened life expectancy (11). The majority of FOP cases are caused by a highly conserved missense mutation in ACVR1 encoding the BMP type I receptor ALK2 (c.617G>A; p.R206H) (10). Crystal structures of ALK2 have revealed that FOP mutations affecting the glycine-serine (GS-) rich regulatory domain disrupt stabilizing interactions with the regulatory protein FKBP12, rendering the kinase constitutively active with inappropriate downstream signaling.(12, 13) Possessing a constitutively-active intracellular kinase, ALK2R206H is unlikely to be affected by endogenous antagonists of BMP signaling such as chordin or noggin, which sequester BMP ligands, or similar ligand-traps. ALK2R206H thus represents an ideal therapeutic target for a highly selective small molecule kinase inhibitor as a treatment for FOP.
We previously described the identification of a small molecule BMP inhibitor, dorsomorphin, and the development of a highly potent derivative, LDN-193189, based on the same pyrazolo[1,5-a]pyrimidine core structure (14, 15). LDN-193189 reduced heterotopic ossification (HO) in a mouse model of FOP with an inducible constitutively active mutant ALK2Q207D (caALK2) transgene (16). LDN-193189 is a potent inhibitor of BMP signaling, but exhibits TGF-β receptor inhibition at higher concentrations. Previously well-described small molecule inhibitors of the TGF-β type I receptor kinases, such as A-83-01 and SB-505124, have both high potency and high (≥ 3 log) selectivity for TGF-β versus BMP signaling (17, 18). While TGF-β signaling inhibitors had potential utility as therapeutic agents, preclinical animal studies have associated the administration of highly potent ALK5 inhibitors with bone physeal abnormalities in immature animals, and hemorrhagic necrosis of heart valves in adult animals (19, 20). Clinically viable BMP receptor kinase inhibitors, particularly for indications requiring long term treatment, may need to minimize or eliminate off-target inhibition of the TGF-β and Activin type I receptor kinases, ALK4, ALK5, and ALK7 in light of these toxicities.
Here we describe the development of LDN-212854 a novel BMP inhibitor that exhibits substantially greater selectivity for BMP versus the TGF-β type I receptors. In addition, LDN-212854 possesses a bias towards ALK2 versus ALK1 and ALK3 compared to other inhibitors. LDN-212854 supports the concept that development of selective inhibitors of individual type I receptors may be feasible despite the high degree of homology between these receptors. LDN-212854 provides useful selectivity in vitro for resolving ALK2 versus ALK3 signaling as shown in cellular assays of hepcidin expression and osteogenic differentiation. In addition, LDN-212854 provides comparable potency in a mutant ALK2 transgenic mouse model of FOP to LDN-193189. LDN-212854 or similar compounds that selectively target individual BMP receptors can be used in vitro to ascertain individual receptor functions, and may have favorable characteristics as clinical candidates.
RESULTS and DISCUSSION
SAR of modified pyrazolo-pyrimidines
To alleviate toxicity concerns associated with TGF-β inhibition and to develop improved tools for understanding BMP signaling, we determined the structure-activity relationship (SAR) of pyrazolo[1,5-a]pyrimidine derivatives with varied quinoline attachment positions (Figure 1b-c). We previously assayed this set of derivatives using a cell-based BMP4-induced SMAD 1/5/8 phosphorylation in cell western assay (15). However, since BMP4 has been shown to preferentially bind to ALK3, this assay was likely biased towards inhibitors of ALK3 (21). To overcome these limitations we developed a highly sensitive cell-free enzymatic assay using recombinant BMP and TGF-β type I receptor kinases (ALK1, ALK2, ALK3, ALK4, and ALK5) that could detect the activity of ~ 1 nM kinase protein. The resulting SAR demonstrated that 3, 8, and 6-quinoline substituted derivatives had little activity against either BMP or TGF-β type I receptors (Figure 1b). However, the 5-quinoline derivative LDN-193719 exhibited improved BMP versus TGF-β selectivity compared to the 4-quinoline derivative LDN-193688 (550-fold versus 60-fold selectivity, Figure 1d). LDN-193719 also demonstrated greater potency against ALK2 (IC50 = 44 nM) than ALK3 (IC50 = 1.5 μM), providing a potential explanation of the weak inhibition of BMP4-induced signaling previously observed by in-cell western assay (15). To determine if increased selectivity for BMP versus TGF-β and particularly ALK2 might be generalizable to other 5-quinoline-substituted compounds, LDN-212854 was synthesized by combining the 5-quinoline moiety of LDN-193719 with the phenyl-piperazine substituent of LDN-193189. The synthetic scheme and compound characterization of LDN-212854 are provided in the Supporting Information.
Characterization of BMP Inhibitors
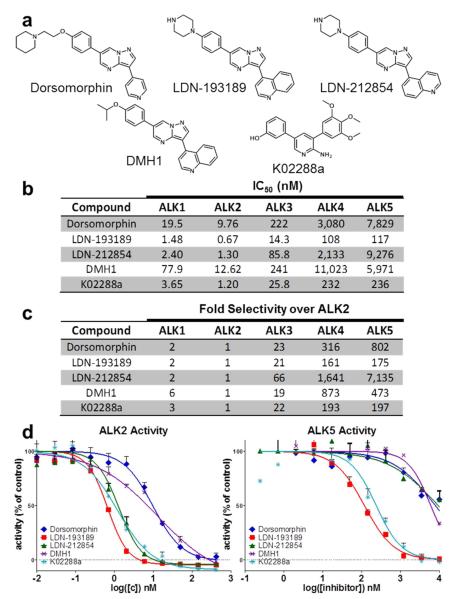
We profiled the selectivity of the known inhibitors of BMP signaling dorsomorphin, LDN-193189, DMH1, the 2-aminopyridine inhibitor K02288a, and our novel pyrazolo[1,5-a]pyrimidine 5-quinoline derivative LDN-212854 (Figure 2a) (22, 23). Based on the above SAR data, we hypothesized that the 5-quinoline derivative might improve selectivity for the BMP pathway, while maintaining potency. All inhibitors tested primarily targeted the BMP type I receptors, particularly the highly homologous receptors ALK1 and ALK2 (Figure 2b). Importantly, whereas other investigators have assayed inhibition of BMP receptor kinase activity using ≥ 100 nM enzyme (22), resulting in the lowest measurement of IC50 being bounded by half the active enzyme concentration (e.g. ~ 50 nM), we used 2.5 nM of purified kinase in this radiometric assay, permitting the measurement IC50 values substantially lower than those previously reported. For example, Vogt et al. reported an in vitro IC50 of 45 nM for LDN-193189 against ALK2, as compared to an IC50 of approximately 0.7 nM for ALK2 in our assay (24). Among compounds tested, LDN-193189 was the most potent inhibitor of ALK2, followed closely by both K02288a and LDN-212854 (both with IC50 ~ 1.2 nM). Compared to these potent compounds, dorsomorphin and DMH1 were weaker by approximately 10-fold. While LDN-193189 and K02288a inhibited the TGF-β type I receptor ALK5 with approximate IC50 measurements of 110 nM and 230 nM, respectively, whereas LDN-212854 inhibited ALK5 in the micromolar range, and thus had substantially greater selectivity for ALK2 versus ALK5 (Figure 2c–d). The selectivity for BMP versus TGF-β signaling, based on the ratio of the IC50 for the inhibition of ALK5 to ALK2, was approximately 7000-fold for LDN-212854, as compared to 800-fold, 175-fold, 470-fold, and 200-fold for dorsomorphin, LDN-193189, DMH1, and K02288, respectively (Figure S1). Thus, in a kinase inhibition assay using lower kinase concentrations than previously described, LDN-212854 demonstrated much greater selectivity for BMP versus TGF-β type I receptors than other compounds while retaining nanomolar potency.
Figure 2.
Potency and selectivity of BMP inhibitors based on in vitro kinase assay. a) Structure of previously described BMP inhibitors and LDN-212854. b) In vitro kinase assay measurements of IC50 for BMP and TGF-β type I receptors show LDN-193189 is the most potent inhibitor of ALK2, followed closely by K02288a and LDN-212854. Both DMH1 and dorsomorphin exhibited 10-fold lower potency against BMP type I receptors. c) Fold selectivity of these inhibitors against ALK2 versus closely related BMP and TGF-β type I receptors. d) Inhibition of ALK2 (BMP) and ALK5 (TGF-β) kinase activity demonstrates LDN-212854 exhibits increased selectivity for ALK2 versus ALK5. Data shown are representative of 2-3 independent experiments.
To confirm kinase assay results in cellular assays of BMP- and TGF-β-induced transcriptional activity, we used C2C12 cells stably expressing BMP responsive (BRE-Luc) and 293T cells stably expressing TGF-β responsive (CAGA-Luc) luciferase reporter transgenes (see Supporting Methods) (25, 26). Cells were transfected with adenoviruses expressing constitutively-active BMP type I receptors (caALK1, caALK2, and caALK3) and constitutively active TGF-β type I receptors (caALK4 and caALK5) in low serum conditions and in the absence of exogenous ligand, with varying concentrations of inhibitors. LDN-193189 was the most potent inhibitor of BMP signaling (IC50 ~ 11 nM for caALK2). LDN-193189 inhibited caALK5 with an IC50 ~ 213 nM, demonstrating ~20-fold selectivity for caALK2 versus caALK5 in cells (Figure 3a). Dorsomorphin was less potent than LDN-193189, and exhibited approximately 9-fold selectivity for caALK2 vs. caALK5. While the cellular assay results for these two compounds aligned closely with those obtained using the kinase assay, the activity of DMH1 and K02288a differed significantly between kinase and cell-based assays. DMH1 was a relatively weak inhibitor of caALK2 in cells (IC50 ~ 230 nM) and had greater activity against caALK5 signaling (IC50 ~ 700 nM) than might be expected based on kinase assay results. Thus, the selectivity of DMH1 for caALK2 vs. caALK5 was only 2.5-fold in cells versus 470-fold in kinase assays. These cell-based IC50 data confirmed that DMH1 is a less potent inhibitor of BMP signaling than LDN-193189 (22), however, DMH1 did not significantly improve selectivity for BMP versus TGF-β. Despite data suggesting similar potency to LDN-193189 in kinase assays, K02288a was significantly less potent in cells, inhibiting both caALK2 (IC50 ~ 225 nM) and caALK5 (IC50 ~ 700 nM) modestly and without the same degree of selectivity observed in kinase assays (~ 3-fold vs. 200-fold).
Figure 3.
Potency and selectivity of BMP inhibitors based on on BMP (BRe-Luc) and TGF-β (CAGA-Luc) transcriptional activity mediated by constitutively active ALK1-5. a) In vitro cell-based assay IC50 measurements against constitutively-active BMP (caALK1, 2, and 3) and TGF-β (ALK4 and 5) type I receptors demonstrates LDN-212854 to be more selective for BMP versus TGF-β receptors while retaining low nanomolar potency against caALK2. b–c) Inhibition of caALK1-5 by BMP inhibitors at various concentrations demonstrates that LDN-212854 preferentially inhibits caALK2 at concentrations near 100 nM, whereas other receptors are affected at higher concentrations. Data shown are calculated based on IC50 generated from at least 3 independent experiments, with data plotted as mean ± S.E.M.
In contrast, the BMP selectivity of LDN-212854 demonstrated in the kinase assay was confirmed in cells with an IC50 for caALK2 of 16 nM and an IC50 for caALK5 of approximately 2 μM, resulting in more than 130-fold selectivity for caALK2 vs. caALK5 (Figure 3a and S1). Representative inhibition curves for dorsomorphin, LDN-193189, LDN-212854, K02288a, and DMH1 against constitutively active BMP and TGF-β type I receptors also demonstrated the improved selectivity of LDN-212854 (Figure S2). At 100 nM, LDN-212854 inhibited 98% of caALK2-mediated signaling while exerting minimal effect on caALK4 or caALK5, whereas all other compounds inhibited caALK4 and caALK5 significantly at concentrations required to suppress caALK2 signaling to the same degree (Figures 3b–c and S2). In addition to having improved selectivity for caALK2 versus caALK5, LDN-212854 demonstrated a bias towards caALK2 within the BMP type I receptor family. LDN-212854 inhibited caALK2 with 6- and 10-fold more potency than caALK1 or caALK3, respectively (Figure S1).
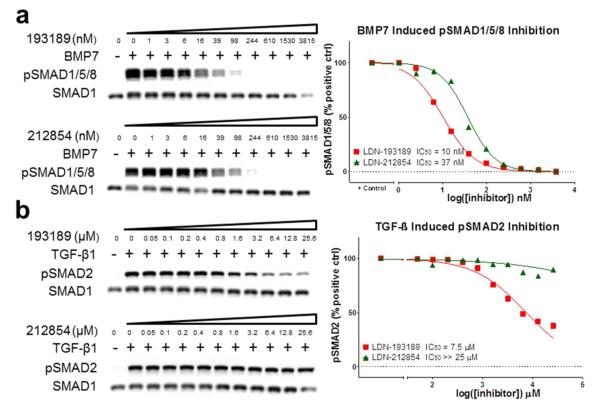
To confirm results obtained using constitutively-active type I receptors, we tested the selectivity of LDN-193189 and LDN-212854 against specific BMP and TGF-β ligands. We examined the impact of inhibitors upon BMP7-induced (20 ng mL−1, 30 min.) activation of SMAD1/5/8 using BMPR2-deficient pulmonary vascular smooth muscle cells, which we have previously found to use ALK2 almost exclusively for the transduction of BMP7 signaling (27). We used TGF-β1 ligand (5 ng mL−1, 30 min) to induce TGF-β signaling and phosphorylation of SMAD2. In this ligand-based cellular assay, LDN-212854 and LDN-193189 exhibited comparable, low nanomolar potency in blocking the phosphorylation of SMAD1/5/8 induced by BMP7 in BMPR2−/− cells (Figure 4a). Importantly, the selectivity of LDN-212854 for BMP versus TGF-β signaling was even greater than that observed using constitutively active type I receptors, with almost no inhibition of TGF-β1 signaling at the highest concentration tested (25 μM, Figure 4b), in contrast to significant effects observed above 1 μM for LDN-193189. These ligand-mediated signaling assays confirmed that LDN-212854 inhibits BMP versus TGF-β signaling with nearly 4-log selectivity, much greater than previously described BMP inhibitors and comparable to the selectivity of A-83-01 for TGF-β versus BMP signaling (17).
Figure 4.
Comparison of potency and selectivity of LDN-193189 and LDN-212854 in modulating BMP and TGF-β ligand-mediated SMAD signaling. a) Western blot analysis of BMP7 induced phosphorylation of SMAD1/5/8 in BMPR2−/− PASMCs reveals low nanomolar inhibition by both LDN-193189 and LDN-212854. b) Western blot analysis of TGF-β1 induced phosphorylation of SMAD2 in wild-type PASMC revealed significant inhibition by LDN-193189 at concentrations greater than 1 μM, and virtually no inhibition by LDN-212854 at concentrations up to 25 μM. Data shown are representative of 2 independent experiments.
LDN-212854 preferentially inhibits ALK2
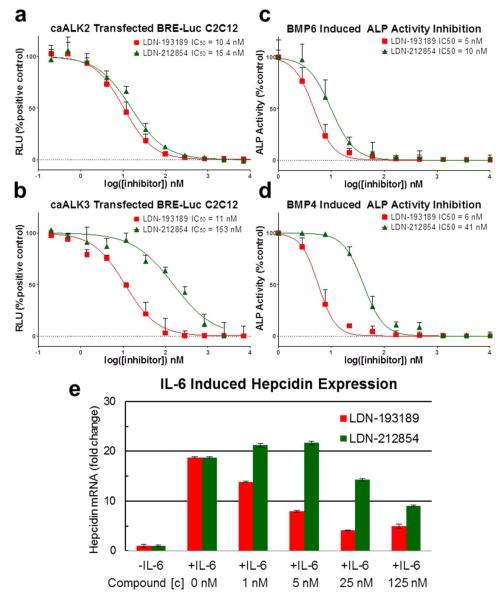
In addition to inhibiting caALK2 more potently than caALK4 and caALK5 in cell based assays, LDN-212854 also exhibited approximately one log selectivity for caALK2 versus caALK3. When C2C12 cells were transfected with caALK2, both LDN-193189 and LDN-212854 inhibited BMP transcriptional activity (BRE-Luciferase) with comparable low nanomolar efficacy (Figure 5a). When C2C12 cells were transfected with constitutively active ALK3, LDN-193189 was nearly 14-fold more potent than LDN-212854 (11 nM versus 153 nM, Figure 5b). We tested whether or not the selectivity of LDN-212854 for caALK2 versus caALK3 could be used to discriminate between the utilization of these receptors in two biological assays. First, we examined the impact of compounds on the BMP-induced osteogenic differentiation of myofibroblast C2C12 cells. When C2C12 cells were stimulated with BMP4 or BMP6 (20 ng mL−1, 6 d), osteogenic differentiation assayed by alkaline phosphatase (ALP) activity was observed. In this system, BMP6 has been shown to function primarily via ALK2, whereas BMP4 interacts primarily with ALK3 to induce ALP (21, 28). As predicted based on assays using caALK2 and caALK3, LDN-193189 inhibited both BMP6- and BMP4-induced ALP with comparable low-nanomolar potency (IC50 ~ 5 nM, Figure 5c–d). In contrast, LDN-212854 inhibited BMP6-induced ALP expression more potently than BMP4 (IC50 ~ 10 nM versus 40.5 nM). Thus, LDN-212854 exhibited greater selectivity for BMP6- versus BMP4-induced osteogenic differentiation consistent with its preference for ALK2 versus ALK3.
Figure 5.
LDN-212854 provides useful selectivity as a probe of signaling mediated by ALK2 versus ALK3, and their respective ligands. a) Representative inhibition curves of caALK2 and b) caALK3 transcriptional activity (BRE-Luc) by LDN-193189 and LDN-212854 in C2C12 cells. Both compounds potently inhibit ALK2, whereas LDN-212854 is substantially weaker against ALK3. c) Alkaline phosphatase (ALP) activity induced in C2C12 cells by BMP6, which signals primarily through ALK2, was inhibited with comparable potency by LDN-193189 and LDN-212854. d) ALP activity induced in C2C12 cells by BMP4, which signals primarily through ALK3, was more potently inhibited by LDN-193189 than LDN-212854. e) IL-6 induced hepcidin expression in HepG2 cells was inhibited potently by LDN-193189 and less potently by LDN-212854, consistent with a primarily ALK3-dependent mechanism of IL-6 induced hepcidin expression.
Next, we used LDN-212854 to probe IL-6-induced expression of hepcidin in HepG2 hepatoma cells. The in-vitro induction of hepcidin in this assay models the homeostatic pathway governing serum iron levels mediated by the BMP- and IL-6-regulated expression of hepcidin in the liver. In anemia of inflammation, IL-6 mediated signaling is thought to enhance hepcidin regulation in a BMP6-dependent fashion, inactivating iron transporter ferroportin and thereby decreasing circulating iron bioavailability for erythropoiesis (29, 30). The dose-dependent impact of LDN-193189 and LDN-212854 upon IL-6-induced (100 ng mL−1, 90 min.) expression of hepcidin mRNA was measured in cells by quantitative RT-PCR (Figure 5e). We have previously shown that ALK3 is the dominant BMP type I receptor required for IL-6-induced hepcidin expression in vitro and in vivo (31). Stimulation with IL-6 alone resulted in an 18-fold increase in hepcidin expression over control. Co-treatment with IL-6 and either LDN-193189 or LDN-212854 resulted in the inhibition of hepcidin mRNA expression (with approximate IC50 values of 5 nM and 125 nM, respectively, Figure 5e). The relatively weaker inhibition of IL-6-mediated hepcidin expression by LDN-212854 correlated with its weaker inhibition of ALK3 compared to LDN-193189 (Figure 5a-b), and supported the concept that ALK3 is the principal receptor responsible for IL-6-regulated hepcidin expression. As previously observed, maximum inhibition with either LDN-193189 or LDN-212854 did not reduce hepcidin expression to levels of controls, suggesting BMP-independent signaling mechanisms may also contribute to IL-6 induced hepcidin expression (32). Taken together these results demonstrate that LDN-212854 can provide some useful albeit limited resolution of signaling via ALK2 versus other BMP receptors in vitro. It is unlikely that LDN-212854 would discriminate the activity of ALK2 in vivo, in part due to wide ranging plasma concentrations during absorption and metabolism, but subsequent derivatives with greater selectivity might be useful in vivo probes of ALK2 function.
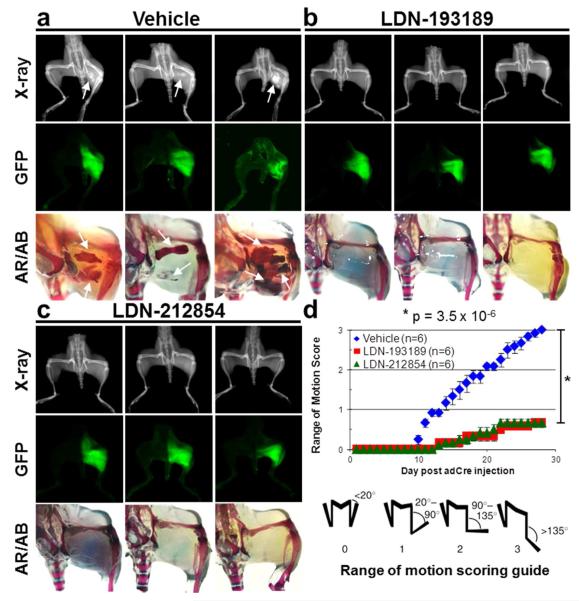
LDN-212854 inhibits ALK2Q207D-induced HO
To demonstrate the in vivo efficacy of LDN-212854, we employed a murine inducible transgenic ALK2Q207D model of heterotopic ossification (see Supporting Methods) (16). Intramuscular injection of Ad. Cre potently induces recombination of a floxed inducible ALK2Q207D and a GFP reporter, while inducing myositis and necrosis. This combination of inflammation and mutant ALK2 expression result in ectopic endochondral bone lesions reminiscent of FOP. Following retropopliteal injection of Ad. Cre (108 pfu on P7), mice were treated for 4 weeks with vehicle, LDN-193189 or LDN-212854 (6 mg kg−1, I.P., twice daily). Heterotopic bone was assayed by x-ray, and alizarin red and alcian blue staining, while GFP expression was used to confirm recombination of the ALK2Q207D transgene at the injection sites (Figure 6a-c). Bone formation and corresponding hindlimb immobility was 100% penetrant in vehicle treated mice as measured by passive range of motion scores (Figure 6d). LDN-193189 and LDN-21285 treatment prevented the formation of heterotopic bone and preserved limb range of motion with minimal or no impairment in the majority of mice. These results demonstrate that LDN-212854 can effectively neutralize ALK2 signaling in vivo. By virtue of increased BMP selectivity, 5-quinoline substituted pyrazolo[1,5a-]pyrimidine compounds such as LDN-212854 could be advantageous for the treatment of FOP by minimizing toxicity due to inhibition of TGF-β type I receptors.
Figure 6.
In vivo efficacy of LDN-212854 in a mouse model of fibrodysplasia ossificans progressiva (FOP). Mice expressing an inducible constitutively-active ALK2Q207D (CAG-Z-EGFP-caALK2) transgene were treated with a) vehicle, b) LDN-193189 or c) LDN-212854. Heterotopic ossification following injection of Ad.Cre was observed by X-ray (top panels) and staining for alizarin red and alcian blue (bottom panels). Heterotopic ossification following Ad.Cre injection was observed 100% of vehicle-treated mice, whereas ossification was essentially absent in mice treated with LDN-193189 or LDN-212854. Transgene-mediated expression of GFP (middle panel) was observed at the site of Ad.Cre injection to confirm recombination and ALK2Q207D expression. d) Passive range-of-motion was progressively impaired in vehicle-treated mice starting on day 10, whereas mobility was almost entirely preserved in mice treated with LDN-193189 and LDN-212854.
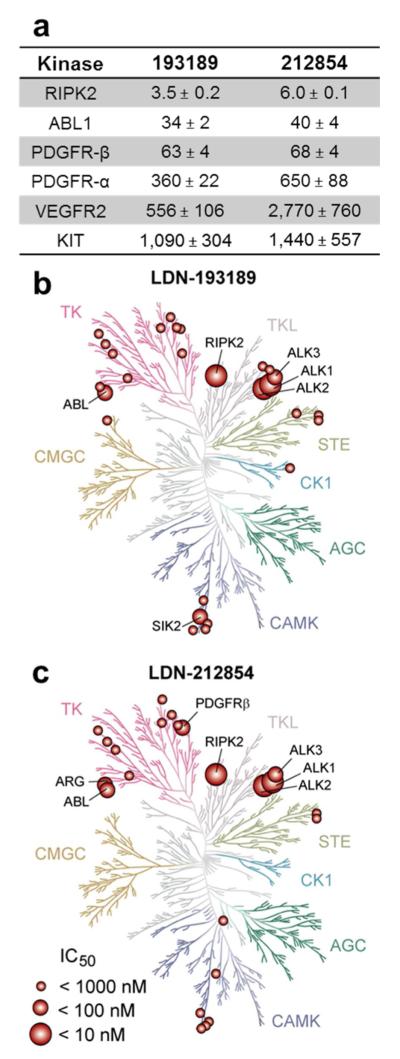
Kinase profiling
Given the highly conserved nature of the kinase domain across the human kinome, many ATP-competitive kinase inhibitors exhibit significant off-target effects (33). The off-target effects of LDN-193189 and LDN-212854 were determined against a panel of 198 kinases broadly representing the human kinome (Figure 7 and Supporting Information). Compounds were tested at 100 nM, a concentration chosen based on the high potency of these compounds against ALK2, and at 1 μM, a standard concentration for profiling off-target activities. While the 5-quinoline moiety of LDN-212854 greatly increased BMP versus TGF-β selectivity, it did not improve kinome wide selectivity. The IC50 values of LDN-193189 and LDN-212854 against several of these kinases were determined revealing significant activity against RIPK2, ABL1, and PDGFR-β (IC50 < 100 nM) whereas the IC50 values for PDGFR-α, VEGFR2 and KIT were greater than 300 nM (Figure 7a). These data confirm a recent report that RIPK2, in particular, appears to be a principal off-target liability of LDN-193189 with an IC50 comparable to that of ALK2.(24) Thus LDN-212854 and LDN-193189, when used in the low nanomolar range (≤100 nM), would effectively inhibit BMP signaling while minimizing effects against most kinases, except RIPK2. The use of LDN-193189 or LDN-212854 to inhibit BMP signaling should consider these off-target effects and particularly RIPK2, known to have important roles in modulating innate immunity and inflammation (34, 35).
Figure 7.
Kinase inhibition profile of LDN-193189 and LDN-212854. a) and b) Kinome dendrograms for LDN-193189 and LDN-212854, respectively, showing both on-target hits from our kinase assay and the top off-target hits from a screen of 198 human kinases. RIPK2 was the most potently inhibited off-target kinase followed by ABL1 and PDGFR-β, while other kinases were inhibited at much higher concentrations.
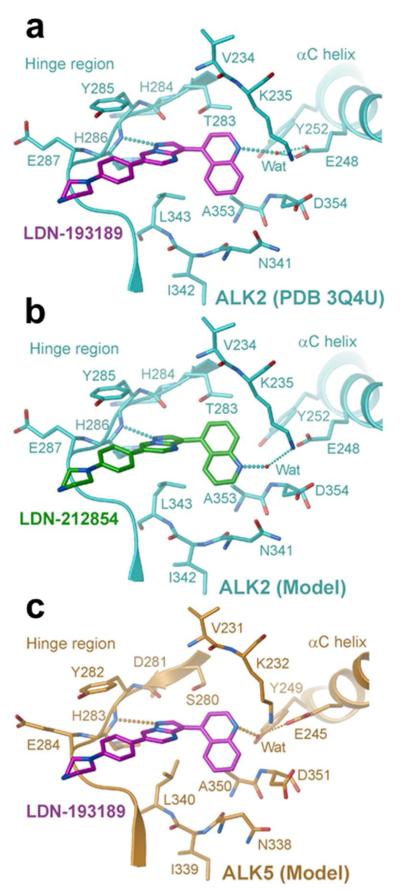
Inhibitor binding mode
To gain insight into its improved selectivity compared to LDN-193189, the binding mode of LDN-212854 to ALK2 was investigated by a molecular modeling approach, using the available co-structure of LDN-193189 as a template (36). In this complex, the pendant 4-quinoline moiety formed an important water-mediated hydrogen bond to the αC-helix residue E248 (Figure 8a). Significantly, this bond is lost upon substitution of the 5-quinoline group in LDN-212854 (Figure 8b). Instead, a new water-mediated hydrogen bond to the catalytic lysine residue K235 is predicted, as previously observed in the ALK2-K02288 structure (23). E248 and K235 are strictly conserved residues that form a necessary ion pair in all active kinases (for example, ALK5 residues E245 and K232). Therefore, sequence alone cannot explain the reduced binding of LDN-212854 to ALK5. However, inspection of the available ALK5 structures reveals a subtle shift in the orientation of the αC-helix and a conserved water molecule that is shifted to a deeper location in the ATP pocket bound between ALK5 E245 and Y249 (Figure 8c). This water molecule appears favorable for hydrogen bonding to the 4-quinoline of LDN-193189, but not to the 5-quinoline moiety of LDN-212854, potentially explaining the relatively poor inhibition of ALK5 by LDN-212854.
Figure 8.
Predicted binding mode of LDN-212854. a) ATP pocket interactions of LDN-193189 (magenta) in the ALK2 co-structure (PDB 3Q4U). A single hydrogen bond to the hinge residue H286 is made by the central pyrazolo[1,5-a] pyrimidine group. The pendant 4-quinoline moiety forms a water-mediated hydrogen bond to the αC-helix residue E248. Water is represented by a red sphere and labelled “Wat”. Hydrogen bonds are shown as a dashed line. b) Model for the binding of LDN-212854 (green) to ALK2. The pendant 5-quinoline group is predicted to form an alternative water-mediated hydrogen bond to the catalytic lysine (K235). The new water position was modeled from the ALK2-K02288 co-structure (PDB 3MTF). c) Model for the binding of LDN-193189 (magenta) to ALK5. The inhibitor was located by the superposition of ALK2 (PDB 3Q4U) and ALK5 (SB-431542 complex; PDB 3TZM) (36). ALK5 structures show a conserved water position set further back in the ATP pocket between the αC-helix residues E245 and Y249. This water can mediate hydrogen bonding to the 4-quinolone of LDN-193189, but not to the 5-quinoline moiety of LDN-212854, potentially explaining its poor binding.
Conclusion
We used highly specific and sensitive assays of BMP and TGF-β type I receptor signaling to characterize in detail several known BMP signaling inhibitors as well as a novel compound with improved selectivity for BMP versus TGF-β signaling, LDN-212854. While it does not improve kinome-wide selectivity compared to LDN-193189, LDN-212854 is notable because it preferentially targets ALK2 at substantially lower concentrations than other BMP type I receptors. Previously described inhibitors of the BMP signaling pathway based on dorsomorphin (LDN-193189, DMH1) or other scaffolds (K02288a) exhibit substantial cross-reactivity with type I receptors of the TGF-β signaling pathway, potentially limiting their utility as biological probes, as well as therapeutic agents due to the known toxicities of ALK5 inhibitors (2, 7). At concentrations of 30-100 nM LDN-212854 demonstrated nearly complete inhibition of ALK2 without significant impact on Activin and TGF-β receptors (Figure S1). LDN-212854 represents a significant step towards developing compounds that target individual receptors of the BMP signaling pathway, a goal previously thought to be limited by the high degree of structural homology between these receptors, ALK1, ALK2, ALK3 and ALK6 (9).
While we and others have demonstrated the utility of several of these compounds as biological probes in vitro and in vivo, the accurate interpretation of such experiments using small molecule kinase inhibitors requires careful consideration of their potency, selectivity, and off-target effects. Due to the increased sensitivity of our kinase assays, we could detect differences in compound potency at concentrations approaching 1 nM, a measurement that was limited in previous reports by the use of higher (≥ 100 nM) concentrations of active kinase (22, 24). The ability to resolve these important differences in activity allowed us to identify compounds such as LDN-212854 with preserved low nanomolar activity against ALK2 but decreased activity against other type I receptors. We found that compounds DMH1 and K02288 exhibited potent and selective inhibition of BMP type I receptors in kinase assays, but were less potent and selective in cell-based assays of BMP signaling. The decreased activity of these compounds in cells might be due to limited stability or membrane permeability, an interpretation that could be explored with further characterization and medicinal chemistry optimization. DMH1 was previously reported to have less activity than dorsomorphin and LDN-193189 against tyrosine kinase receptors such as VEGFR2 (22). Similarly, we found that LDN-212854 also had decreased activity against VEGFR2 (2.8 μM) than LDN-193189 (0.56 μM, Figure 7a).
The current data provide further clarity on the appropriate use of known BMP inhibitors and introduce a novel compound that is highly selective for BMP receptors with a bias towards ALK2. Given the broad developmental and homeostatic roles of receptors of the BMP receptor family, maximizing the selectivity of compounds for specific receptors of interest could be advantageous for their use in therapy, a goal that could be effectively pursued with further structural modeling and medicinal chemistry. Monoselective compounds targeting individual BMP type I receptors could yield substantial new insights into the complex physiology of this receptor pathway. The selectivity of LDN-212854 makes this compound a useful reagent for interrogating ALK2 signaling in vitro, and provides a potential template for the development of clinical compounds for treating ALK2-mediated disease such as FOP.
METHODS
Kinase Assay
Purified recombinant ALK1-5 and other kinase proteins (Invitrogen), ATP (Sigma), ATP [γ-32P] (Perkin Elmer), and dephosphorylated casein (Sigma) at final concentrations of 2.5nM, 6μM, 0.05 μCi μL−1, and 0.5 mg mL−1 respectively were aliquoted in kinase buffer (Cell Signaling) containing 0.2% bovine serum albumin supplemented with 10mM MnCL2 into 96-well plates, in combination with inhibitor compounds diluted at varying concentrations in kinase buffer (0.01nM to 10 μM) in triplicate. Positive control samples lacking inhibitor compounds, and negative controls lacking recombinant kinase were also measured in triplicate. The mixture was reacted at RT for 45 min., quenched with a final concentration of 2% phosphoric acid. The reaction mixture was transferred to 96-well P81 phosphocellulose filter plates (Millipore) and bound for 5 min. The plates were washed twenty-times with 150 μL of 1% phosphoric acid solution per well by vacuum manifold. Plates were dried at RT for 1 h, sealed, and assayed with Microscint 20 scintillation fluid (Perkin Elmer) using a Spectramax L luminometer (Molecular Devices) using the photon counting setting with an integration time of one second per well. Data was normalized to positive controls at 100% enzyme activity with negative controls being subtracted as background. GraphPad (Prism software) was used for graphing and regression analysis by sigmoidal dose-response with variable Hill coefficient.
Luciferase Reporter Assay
C2C12 myofibroblasts cells stably transfected with BMP responsive element from the Id1 promoter fused to luciferase reporter gene (BRE-Luc) were generously provided by Dr. Peter ten Dijke (Leiden University Medical Center, NL) (25). Human embryonic kidney 293T cells stably transfected with the TGF-β responsive element from the PAI-1 promoter fused to luciferase reporter gene (CAGA-Luc) were generously provided by Dr. Howard Weiner (Brigham and Women’s Hospital, Boston, MA) (26). C2C12 Bre-Luc and 293T CAGA-Luc cells were seeded at 20,000 cells in DMEM supplemented with 2% FBS per well in tissue culture treated 96-well plates (Costar® 3610; Corning). The cells were incubated for 1 h (37°C and 10% CO2) and allowed to settle and attach. Compounds of interest or DMSO were diluted in DMEM and added at final compound concentrations of 1 nM to 10 μM. Cells were then incubated for 30 min. Adenovirus expressing constitutively active BMP and TGF-β type I receptors (Ad.caALK1-5), generously provided by Dr. Akiko Hata (University of California at San Francisco), were added to achieve a multiplicity of infection (MOI) of 100. Plates were incubated overnight at 37°C. Cell viability was assayed with an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) colorimetric assay (Promega) per the manufacturer’s instructions. Media was discarded, and firefly luciferase activity was measured (Promega) according to manufacturer’s protocol. Light output was measured using a Spectramax L luminometer (Molecular Devices) with an integration time of one second per well. Data was normalized to 100% of incremental BRE-Luc activity due to adenoviruses specifying caALK1, 2, or 3, or the incremental CAGA-Luc activity due to adenoviruses specifying caALK4 or 5. Graphing and regression analysis by sigmoidal dose-response with variable Hill coefficient was performed using GraphPad Prism software.
BMP Induced ALP Activity
C2C12 myofibroblasts cells were seeded in 96-well plates (Corning) at 2,000 cells per well in DMEM supplemented with 2% FBS as previously described (37). Compounds diluted in DMEM and were added at final concentrations ranging from 1nM to 10 μM, in quadruplicate. BMP4 and BMP6 ligands diluted in DMEM were added to final concentrations of 20 ng mL−1. Positive controls were generated by omitting compounds and negative controls were generated by omitting both compounds and ligands. Cells were incubated for 6 days at 37°C and 5% CO2 and subsequently harvested using 1% Triton X-100. Extracts from each well were incubated at RT for 30 min with alkaline phosphatase (ALP) yellow (pNPP) liquid substrate for ELISA (Sigma-Aldrich) and ALP activity was measured by absorbance at 405 nM per the manufacturer’s instructions. Absorbance data was analyzed with positive controls as 100% ALP activity and negative controls being subtracted as background. Graphing and regression analysis by sigmoidal dose-response with variable Hill coefficient was performed using GraphPad Prism.
Supplementary Material
Acknowledgments
We greatly appreciate helpful discussions and advice from K. Bloch and R. Peterson. This work was supported by grants from the US National Institutes of Health DK082971-02S1 (A.H.M.), AR057374-03S1 (A.H.M.), HL079943, and AR057374 (P.B.Y.) and from the Pulmonary Hypertension Association (P.B.Y), a Leducq Foundation Transatlantic Network of Excellence Award (P.B.Y.), the Howard Hughes Medical Institute (P.B.Y.), the Harvard NeuroDiscovery Center (X.X, G.D.C.), the Partners Center for Drug Discovery (X.X., G.D.C.), and the Structural Genomics Consortium (A.N.B.). The SGC is a registered charity (number 1097737) that receives funds from the Canadian Institutes for Health Research, Genome Canada, GlaxoSmithKline, Lilly Canada, the Novartis Research Foundation, Pfizer, Takeda, AbbVie, the Canada Foundation for Innovation, the Ontario Ministry of Economic Development and Innovation, and the Wellcome Trust [092809/Z/10/Z].
Footnotes
Conflict-of-Interest Disclosure: The authors declare that they have no relevant conflicts of interest.
Supporting Information Available: This material is available free of charge at http://pubs.acs.org/journal/acbcct
Author Contributions: The experiments and compounds were conceived by A.H.M., G.D.C., and P.B.Y., and synthesized by X.X. and G.D.C. Experiments were conducted by A.H.M. and K.A.A. Molecular modeling was performed by A.N.B. The manuscript was written and revised by A.H.M., A.N.B., G.D.C and P.B.Y.
References
- 1.Waite KA, Eng C. From developmental disorder to heritable cancer: it’s all in the BMP/TGF-beta family. Nat Rev Genet. 2003;4:763–773. doi: 10.1038/nrg1178. [DOI] [PubMed] [Google Scholar]
- 2.Cai J, Pardali E, Sanchez-Duffhues G, ten Dijke P. BMP signaling in vascular diseases. FEBS Lett. 2012;586:1993–2002. doi: 10.1016/j.febslet.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Hong CC, Yu PB. Applications of small molecule BMP inhibitors in physiology and disease. Cytokine Growth Factor Rev. 2009;20:409–418. doi: 10.1016/j.cytogfr.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 5.Chen YG, Hata A, Lo RS, Wotton D, Shi Y, Pavletich N, Massague J. Determinants of specificity in TGF-beta signal transduction. Genes Dev. 1998;12:2144–2152. doi: 10.1101/gad.12.14.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramel MC, Hill CS. Spatial regulation of BMP activity. FEBS Lett. 2012;586:1929–1941. doi: 10.1016/j.febslet.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Mueller TD, Nickel J. Promiscuity and specificity in BMP receptor activation. FEBS Lett. 2012;586:1846–1859. doi: 10.1016/j.febslet.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 8.Cohen P, Alessi DR. Kinase Drug Discovery - What’s Next in the Field? ACS Chem Biol. 2013;8:96–104. doi: 10.1021/cb300610s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ten Dijke P, Ichijo H, Franzen P, Schulz P, Saras J, Toyoshima H, Heldin CH, Miyazono K. Activin receptor-like kinases: a novel subclass of cell-surface receptors with predicted serine/threonine kinase activity. Oncogene. 1993;8:2879–2887. [PubMed] [Google Scholar]
- 10.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan FS, Le Merrer M, Glaser DL, Pignolo RJ, Goldsby RE, Kitterman JA, Groppe J, Shore EM. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008;22:191–205. doi: 10.1016/j.berh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaikuad A, Alfano I, Kerr G, Sanvitale CE, Boergermann JH, Triffitt JT, von Delft F, Knapp S, Knaus P, Bullock AN. Structure of the BMP receptor ALK2 and implications for fibrodysplasia ossificans progressiva. J Biol Chem. 2012 doi: 10.1074/jbc.M112.365932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groppe JC, Shore EM, Kaplan FS. Functional modeling of the ACVR1 (R206H) mutation in FOP. Clin Orthop Relat Res. 2007;462:87–92. doi: 10.1097/BLO.0b013e318126c049. [DOI] [PubMed] [Google Scholar]
- 14.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuny GD, Yu PB, Laha JK, Xing X, Liu JF, Lai CS, Deng DY, Sachidanandan C, Bloch KD, Peterson RT. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett. 2008 doi: 10.1016/j.bmcl.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD. BMP type I receptor inhibition reduces heterotopic ossification. Nat Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tojo M, Hamashima Y, Hanyu A, Kajimoto T, Saitoh M, Miyazono K, Node M, Imamura T. The ALK-5 inhibitor A-83-01 inhibits Smad signaling and epithelial-to-mesenchymal transition by transforming growth factor-beta. Cancer Sci. 2005;96:791–800. doi: 10.1111/j.1349-7006.2005.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DaCosta Byfield S, Major C, Laping NJ, Roberts AB. SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2004;65:744–752. doi: 10.1124/mol.65.3.744. [DOI] [PubMed] [Google Scholar]
- 19.Anderton MJ, Mellor HR, Bell A, Sadler C, Pass M, Powell S, Steele SJ, Roberts RR, Heier A. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol Pathol. 2011;39:916–924. doi: 10.1177/0192623311416259. [DOI] [PubMed] [Google Scholar]
- 20.Frazier K, Thomas R, Scicchitano M, Mirabile R, Boyce R, Zimmerman D, Grygielko E, Nold J, DeGouville AC, Huet S, Laping N, Gellibert F. Inhibition of ALK5 signaling induces physeal dysplasia in rats. Toxicol Pathol. 2007;35:284–295. doi: 10.1080/01926230701198469. [DOI] [PubMed] [Google Scholar]
- 21.ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin CH, Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994;269:16985–16988. [PubMed] [Google Scholar]
- 22.Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, Hopkins CR, Lindsley CW, Hong CC. In Vivo Structure-Activity Relationship Study of Dorsomorphin Analogues Identifies Selective VEGF and BMP Inhibitors. ACS Chem Biol. 2009 doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaikuad A, Sanvitale C, Cooper C, Mahajan P, Daga N, Petrie K, Alfano I, Canning P, Krojer T, Vollmar M, Knapp S, von Delft F, Weigelt J, Arrowsmith CH, Edwards AM, Bountra C, Bullock A. RCSB PDB Protein Data Bank. Worldwide Protein Data Bank; Jun 23, 2010. Crystal structure of the ACVR1 kinase in complex with a 2-aminopyridine inhibitor. 2010. [Google Scholar]
- 24.Vogt J, Traynor R, Sapkota GP. The specificities of small molecule inhibitors of the TGFss and BMP pathways. Cell Signal. 2011;23:1831–1842. doi: 10.1016/j.cellsig.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Zilberberg L, ten Dijke P, Sakai LY, Rifkin DB. A rapid and sensitive bioassay to measure bone morphogenetic protein activity. BMC Cell Biol. 2007;8:41. doi: 10.1186/1471-2121-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oida T, Weiner HL. Murine CD4 T cells produce a new form of TGF-beta as measured by a newly developed TGF-beta bioassay. PLoS One. 2011;6:e18365. doi: 10.1371/journal.pone.0018365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu PB, Beppu H, Kawai N, Li E, Bloch KD. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J Biol Chem. 2005;280:24443–24450. doi: 10.1074/jbc.M502825200. [DOI] [PubMed] [Google Scholar]
- 28.Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci. 1999;112(Pt 20):3519–3527. doi: 10.1242/jcs.112.20.3519. [DOI] [PubMed] [Google Scholar]
- 29.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andriopoulos B, Jr., Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinbicker AU, Bartnikas TB, Lohmeyer LK, Leyton P, Mayeur C, Kao SM, Pappas AE, Peterson RT, Bloch DB, Yu PB, Fleming MD, Bloch KD. Perturbation of hepcidin expression by BMP type I receptor deletion induces iron overload in mice. Blood. 2011 doi: 10.1182/blood-2011-03-339952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinbicker AU, Sachidanandan C, Vonner AJ, Yusuf RZ, Deng DY, Lai CS, Rauwerdink KM, Winn JC, Saez B, Cook CM, Szekely BA, Roy CN, Seehra JS, Cuny GD, Scadden DT, Peterson RT, Bloch KD, Yu PB. Inhibition of bone morphogenetic protein signaling attenuates anemia associated with inflammation. Blood. 2011;117:4915–4923. doi: 10.1182/blood-2010-10-313064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1046–1051. doi: 10.1038/nbt.1990. [DOI] [PubMed] [Google Scholar]
- 34.Strober W, Watanabe T. NOD2, an intracellular innate immune sensor involved in host defense and Crohn’s disease. Mucosal immunology. 2011;4:484–495. doi: 10.1038/mi.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magalhaes JG, Lee J, Geddes K, Rubino S, Philpott DJ, Girardin SE. Essential role of Rip2 in the modulation of innate and adaptive immunity triggered by Nod1 and Nod2 ligands. European journal of immunology. 2011;41:1445–1455. doi: 10.1002/eji.201040827. [DOI] [PubMed] [Google Scholar]
- 36.Chaikuad A, Sanvitale C, Cooper CDO, Mahajan P, Daga N, Petrie K, Alfano I, Gileadi O, Fedorov O, Allerston CK, Krojer T, Vollmar M, von Delft F, Weigelt J, Arrowsmith CH, Edwards AM, Bountra C, Bullock A. RCSB PDB Protein Data Bank. Worldwide Protein Data Bank; Feb 9, 2010. Crystal structure of the ACVR1 kinase domain in complex with LDN-193189. 2011. [Google Scholar]
- 37.Yu PB, Deng DY, Beppu H, Hong CC, Lai C, Hoyng SA, Kawai N, Bloch KD. Bone Morphogenetic Protein (BMP) Type II Receptor Is Required for BMP-mediated Growth Arrest and Differentiation in Pulmonary Artery Smooth Muscle Cells. J Biol Chem. 2008;283:3877–3888. doi: 10.1074/jbc.M706797200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.