Abstract
Increasing evidence indicates the functional expression of ionotropic γ-aminobutyric acid receptor (GABAA-R) in astrocytes. However, it remains controversial in regard to the intracellular Cl− concentration ([Cl−]i) and the functional role of anion-selective GABAA-R in astrocytes. In gramicidin perforated-patch recordings from rat hippocampal CA1 astrocytes, GABA and GABAA-R specific agonist THIP depolarized astrocyte membrane potential (Vm), and the THIP induced currents reversed at the voltages between −75.3 to −78.3 mV, corresponding to a [Cl−]i of 3.1 – 3.9 mM that favors a passive distribution of Cl− anions across astrocyte membrane. Further analysis showed that GABAA-R induced Vm depolarization is ascribed to HCO3− efflux, while a passively distributed Cl− mediates no net flux or influx of Cl-that leads to an unchanged or hyperpolarized Vm. In addition to a rapidly activated GABAA-R current component, GABA and THIP also induced a delayed inward current (DIC) in 63% of astrocytes. The DIC became manifest after agonist withdrawal and enhanced in amplitude with increasing agonist application duration or concentrations. Astrocytic two-pore domain K+ channels (K2Ps), especially TWIK-1, appeared to underlie the DIC, because 1) acidic intracellular pH, as a result of HCO3− efflux, inhibited TWIK-1; 2) the DIC remained in the Cs+ recording solutions that inhibited conventional K+ channels and 3) the DIC was completely inhibited by 1 mM quinine but not by blockers for other cation/anion channels. Altogether, HCO3− efflux through activated GABAA-R depolarizes astrocyte Vm and induces a delayed inhibition of K2Ps K+ channels via intracellular acidification.
Keywords: Astrocytes, GABAA receptors, bicarbonate, TWIK-1, patch clamp, hippocampus
Introduction
Ionotropic γ-aminobutyric acid receptors (GABAA-Rs) are anion-selective channels activated by inhibitory neurotransmitter GABA. The functional GABAA-Rs are pentamer assembling from 19 subunits (α1-6, β1-3, γ1-3, δ, ε, θ, π and ρ1-3) into different subtypes (Olsen and Sieghart 2008). The expression of ionotropic GABAA-R in astrocytes has been indicated by immunocytochemistry (Bureau et al. 1995) and functional studies, including GABAA-R currents measured from astrocytes in situ (Bekar et al. 1999; MacVicar et al. 1989; Meier et al. 2008; Steinhauser et al. 1994) and in acutely isolated preparation of rat hippocampus (Fraser et al. 1995; Zhou and Kimelberg 2001). Activation of astrocytic GABAA-R has been shown to elevate intracellular Ca2+ concentration (Bernstein et al. 1996; Fraser et al. 1995; Meier et al. 2008) and modulate outward K+ channel activity (Bekar and Walz 1999; Bekar and Walz 2002). GABAA-R activation is also critical for the proliferation of astrocyte-like GFAP-expressing progenitors in postnatal subventricular zone (Liu et al. 2005).
Developmentally regulated intracellular chloride concentration ([Cl−]i) from high to low underlies the functional transition of neuronal GABAA-R from excitatory to inhibitory (Ben-Ari 2002). However, it remains controversial in regard to the [Cl−]i in astrocytes; while cultured astrocytes contained a high [Cl−]i of around 30-40 mM (Bekar and Walz 2002; Kimelberg 1981), glial cells identified electrophysiologically from guinea-pig olfactory cortex showed a low [Cl−]i of 3.2 mM (Ballanyi et al. 1987). A high [Cl−]i mainly results from an inward transport of Cl− anions via Na+-K+-2Cl− cotransporter (NKCC). Interestingly, the amount of mRNA of Na+-K+-2Cl− cotransporter in cultured astrocytes and astrocytes isolated from postnatal day one (P1) mouse was 2.7-fold higher than that of the astrocytes isolated from P17 mouse, while the mRNAs of K+-Cl− cotransporter KCC1 and KCC2, which function to extrude Cl− from cells, developmentally increased 1.5- and 1.4-fold in isolated astrocytes from P1 to P17 mouse, respectively (Cahoy et al. 2008). This leaves the question of the actual [Cl−]i in neonatal and mature astrocytes to be further investigated.
Should a low [Cl−]i content exist in mature astrocytes, whether GABAA-R activation has a discernible impact on astrocyte membrane potential is questionable, because the Cl− equilibrium potential (ECl) established by a passive distribution of Cl− anions equals to the K+ equilibrium potential (EK) and the membrane potential. Therefore, the precise influence of GABAA-R on astrocyte membrane potential and its downstream physiological function need to be determined.
To approach these questions, conventional and gramicidin perforated patch clamp recordings were used to study astrocytic GABAA-R in acute rat hippocampal slices. We revealed a passively distributed [Cl−]i in hippocampal mature astrocytes, and showed that GABAA-R mediated HCO3− efflux depolarized astrocyte membrane potential and inhibited two-pore domain K+ channels (K2Ps) in a delayed fashion that was likely due to intracellular acidification.
Materials and Methods
Hippocampal slice preparation
Rat hippocampal slices were prepared from postnatal day (P) 21 to 29 Sprague-Dawley rats. The procedures were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee, The Ohio State University and Wadsworth Center, New York State Department of Health. Rats were anesthetized with 8% chloral hydrate in 0.9% NaCl followed by decapitation. The brain was rapidly removed from skull and placed in ice-cold oxygenated (95% O2 /5% CO2) slice preparation solution containing (in mM): 125 NaCl, 3.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 0.1 CaCl2, 3 MgCl2 and 10 glucose. Coronal hippocampal slices at 300 μm thickness were cut at 4°C with a Pelco 1500 Vibratome (Ted Pella, Inc., Redding, CA) and transferred to a nylon slice holder basket immersed in artificial cerebral spinal fluid (aCSF) containing (in mM): 125 NaCl, 3.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2 and 10 glucose (osmolality, 295 ± 5 mOsm) at room temperature (20-22°C). Slices were kept in aCSF with continuous oxygenation for at least 60 min before recording.
Electrophysiology
Individual slice was transferred into a recording chamber (RC-22, Warner Instruments, Holliston, MA) with constant perfusion of oxygenated aCSF at 2.5 ml/min rate. Astrocytes in the CA1 stratum radiatum were identified by differential interference contrast (DIC) video microscopy (Olympus BX51), and a cooling LED camera (WAT-902H Ultimate) to display the slice image on a monitor using a 4× or 40× water-immersion objectives. Recording electrodes were fabricated from borosilicate capillaries (1.5/0.86 mm outer/inner diameter, Warner Instruments, Holliston, MA) using a Flaming/Brown Micropipette Puller (Model P-87, Sutter Instrument). The electrodes had the open tip resistance of 2.5-5.0 MΩ when filled with the electrode solution contained (in mM): 140 KCl, 0.5 CaCl2, 1 MgCl2, 5 EDTA, 10 HEPES, 3 Mg2+-ATP and 0.3 2Na+-GTP that was titrated with KOH to pH 7.25-7.27. The final osmolality was 280.0 ± 5.0 mOsm.
Whole-cell membrane current or membrane potential was amplified by MultiClamp 700A or 700B amplifiers and the data acquisition was controlled by PClamp 9.2 or 10.2 software (Molecular Devices, Sunnyvale, CA). DIGIDATA 1322A interface was used to convert digital-analog signals between amplifier and computer. Membrane capacitance (CM), access resistances (Ra), membrane resistance (Rm) and input resistance (Rin) were measured by using the “Membrane test” protocol built into the pClampex. The voltage error in voltage clamp recording was calculated from Ra /(Ra+Rm)*100%. The membrane potential (Vm) was read either in “I = 0” mode or measured directly in current clamp mode with no holding currents. Astrocytes with a resting membrane potential more positive than −75 mV were discarded. All the experiments were conducted at room temperature of 20-22°C.
Gramicidin stock solution was made by adding 5 mg gramicidin into 1 ml dimethylsulfoxide (DMSO). Working solution was made by adding 3.6 μl stock solution into 1 ml electrode solution (18 μg gramicidin/ml) and used for two hours. The electrode was first dipped in gramicidin-free electrode solution for ~10 seconds, and followed by backfilling with 2 μl gramicidin-free and 8 μl gramicidin-containing electrode solutions sequentially(final gramicidin concentration: 14.5 μg/ml). A +10 mV/10 ms voltage step was delivered every 20 s from the holding potential of −70 mV to monitor the perforation process. From the step induced current (I10 mV), the input resistant (Rin) was calculated from Rin = 10 mV/ I10 mV (Sup. Fig. 1). A successful perforation process typically took ~30 min to reach a stable and acceptable Rin of ~ 50 MΩ. Change in membrane conductance during recording was monitored by a periodically delivered voltage ramp command; the membrane was first stepped from −70 mV down to −160 mV and followed by a voltage ramp from −160 mV to +20 mV at the rate of 180 mV/sec. The net GABAA-R currents induced by THIP were resolved by subtraction of voltage ramp induced currents in control from THIP.
For focal application, a drug-containing electrode with < 1 μm opening diameter was advanced to the recorded cell 20-30 μm away from soma (Fig. 3B). For GABA EC50 analysis, two drug application electrodes were used (inset in Fig. 5A); one electrode contained 1 mM GABA and the second contained a lower concentration of GABA. Two doses of GABA were applied sequentially from low to high and the current induced by low concentration GABA was normalized to the one induced by 1 mM GABA. Thereafter, the second electrode was replaced with another concentration of GABA for the next test. Typically 3 - 5 GABA concentrations could be tested from each recorded astrocyte (Fig. 5A).
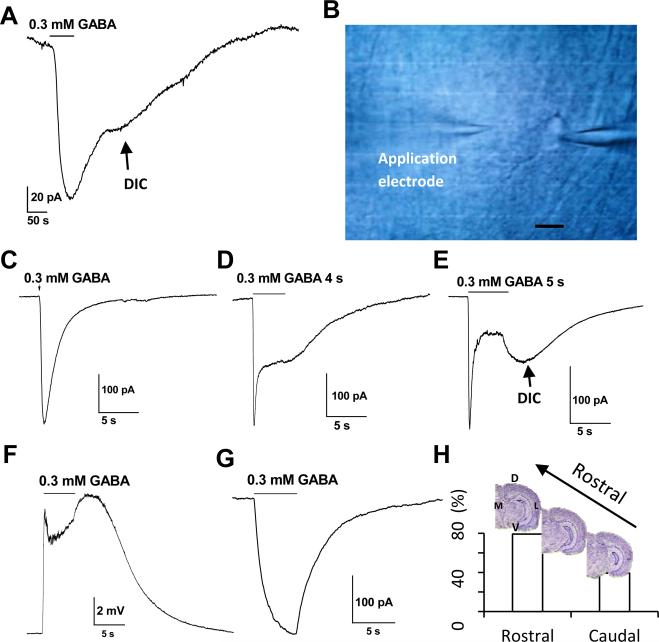
Figure 3. GABAA-R activation induced delayed DIC.
A. Bath application of 0.3 mM GABA for 1 min induced a rapid initial receptor current that was followed by a delayed inward current (DIC) after GABA withdrawal. B. Shown in the image was the placement of the second electrode on the top-left serving for focal drug application to the recorded cell on the right electrode. Scale bar is 10 μm. C-E. Focal application of 0.3 mM GABA at variable durations; the induction of DIC depended on the duration of the GABA exposure. F. A 5-second application of 0.3 mM GABA induced two phases of Vm depolarization. The second phase, or delayed Vm depolarization (DVD), peaked 2 s after the withdrawal of GABA, which was consistent with the peak time of DIC in the same cell (E). G. A representative recording without GABA induced DIC. H. Frequency of the DIC appearance in hippocampal slices prepared from rostral (79.2%, 38/48 cells), vs. caudal side (39.3%, 11/28 cells). The difference between the frequencies in these subregions is statistically significant at P < 0.01. M: middle, D: dossal, L: lateral, V: ventral.
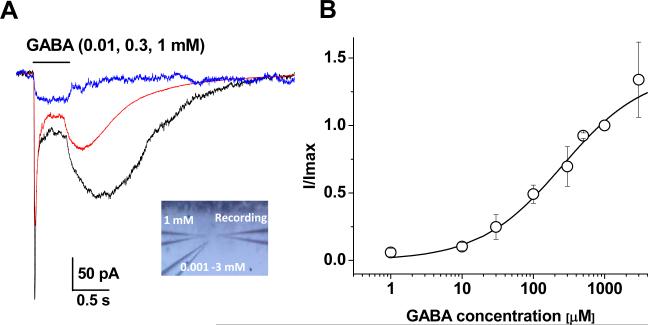
Figure 5. Dependence of DIC on GABA concentration.
A. Representative GABAA-R currents induced by 0.01 mM, 0.3 mM and 1 mM GABA from one astrocyte. The GABA induced DIC became evident in 0.3 mM GABA and further enhanced in 1 mM GABA. The inset shows three-electrode configuration for dose-response experiments. The right electrode was for recording and the two left electrodes were used for GABA application. B. Dose-response relation of GABA induced initial GABAA-R currents. GABA-induced currents at various concentrations were normalized to 1 mM in the same cell. Each data point represents the mean of 3 to 4 cells. Error bars are SEMs. The smooth line gives the best fit according to the Hill equation (see Materials and Methods), yielding an EC50 of 0.3 mM and nH of 0.75.
Solutions and reagents
TTX, D-AP5, NBQX (sodium salt), THIP, bicuculline, thapsigargin, SR95531, GABA, gramicidin, nipecotic acid and meclofenamic acid (MFA) were purchased from Tocris Bioscience (Ellisville, Missouri, USA). Other chemicals were purchase from Sigma (St. Louis, MO, USA). They were dissolved in either DMSO or H2O as stock solutions and stored in −20 °C freezer. 0.5 μM tetrodotoxin (TTX), 10 μM AP-5 and 30 μM NBQX were included in bath solution to inhibit neuronal synaptic transmission (Xie et al. 2008).
In some experiments, the intracellular Cl− was reduced or totally replaced by gluconate, or K+ both in bath and electrode solutions was substituted with equimolar Cs+ for inhibition of conventional K+ channels. The 0 mM [Na+]e focal application solution was achieved by equimolar replacement of NaCl with Choline-Cl and NaHCO3 with Choline-HCO3.
Data Analysis
(1) Intracellular Cl concentration
Intracellular Cl− concentration was resolved from the reversal potential of GABAA-R currents from the equation bellow:
| (1) |
Where T is temperature (295.15 K, corresponding to 22°C), R and F are universal gas constant and Faraday constant, respectively. [Cl−]i and [Cl−]e are intra- and extracellular Cl− concentrations respectively. The intracellular HCO3− ([HCO3−]i) used in this calculation was 16 mM and the relative permeability of HCO3− to Cl− (PHCO3/PCl) was 0.2 (Kaila and Voipio 1987; Staley et al. 1995).
(2) Correction of voltage error in the reversal potential of GABAA-R currents
Voltage error in astrocyte voltage clamp recording was corrected to resolve the actual reversal potential of GABAA-R currents (EGABA-A).
| (2) |
Where the Vr is the reversal potential of ramp voltage command induced currents in control aCSF solution or in the presence of GABAA-R specific agonist THIP (Fig. 2C). VTHIP is the measured reversal potential resulting from the subtraction of voltage ramp induced currents between control and THIP (Fig. 2C).
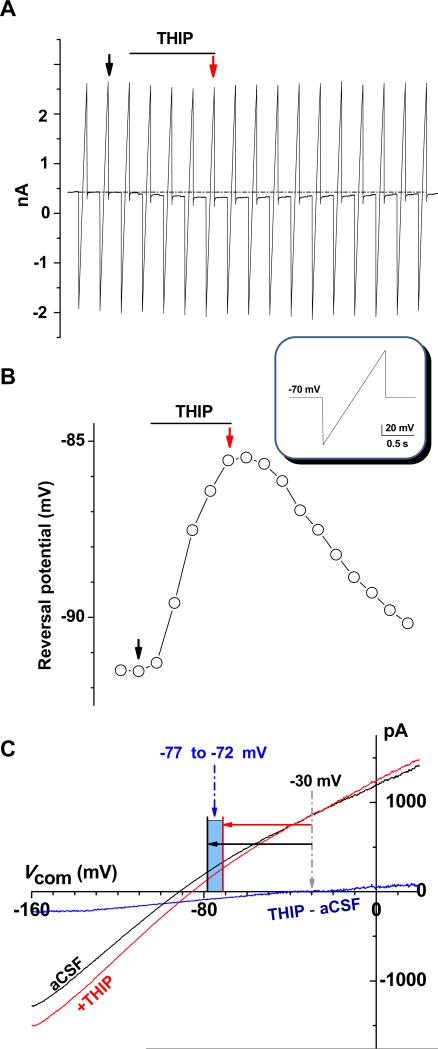
Figure 2. Astrocytic GABAA-R currents.
A. Gramicidin perforated patch recording from an astrocyte; the cell was held at −70 mV and a 1 second long voltage ramp from −160 mV to +20 mV was delivered every 20 second to induce currents (inset below A). Addition of 100 μM THIP shifted the holding currents downwardly (dotted line). B. Chronological display of reversal potentials of ramp induced current traces shown in A. The black and red arrows in A and B pointed to the ramp induced current traces and their corresponding reversal potentials in aCSF control and THIP application, respectively. C. Black and red traces in A are plotted against the applied voltages. THIP induced GABAA-R currents (blue) were resolved by off-line digital subtraction of red trace from the black trace that yielded an apparent EGABAA-R of −30 mV. As the voltage ramp induced currents carried a 75% of voltage error, the error correction yielded the actual EGABAA-R in the range of −77 mV to −72 mV for this recording.
(3) Astrocyte membrane potential (Vm) established by transmembrane K+ ions
| (3) |
This assumption is made upon a close approximation of EK with Vm in perforated patch recording (see Results).
(4) Anion driving force of GABAA-R
In perforated patch recording, astrocyte Vm closely matched EK (see Results). Because passive Cl− distribution means ECl =EK= Vm, therefore, astrocyte [Cl−]i in passive distribution can be calculated from the following equation:
| (4) |
Thus, the equation (1) can be altered to:
| (5) |
Based on (3) and (5), the total anion driving force via GABAA-R is:
| (6) |
Because [Cl−]e [K+]e is present in both numerator and denominator in the logarithm, the sign of anion driving force is determined by:
| (7) |
This relationship indicates that the direction of anion flux is determined by HCO3− and K+, but not by Cl−.
(5) The GABA dose-response curve was fitted by the Hill equation below
| (8) |
Data are presented as means ± SEM, unless indicated otherwise. Chi square test and Student's t test were performed to determine the statistical significance of the differences between the two groups. The level of significance was set at P < 0.05.
Results
Activation of GABAA-R depolarizes astrocyte membrane potential under intact [Cl−]i
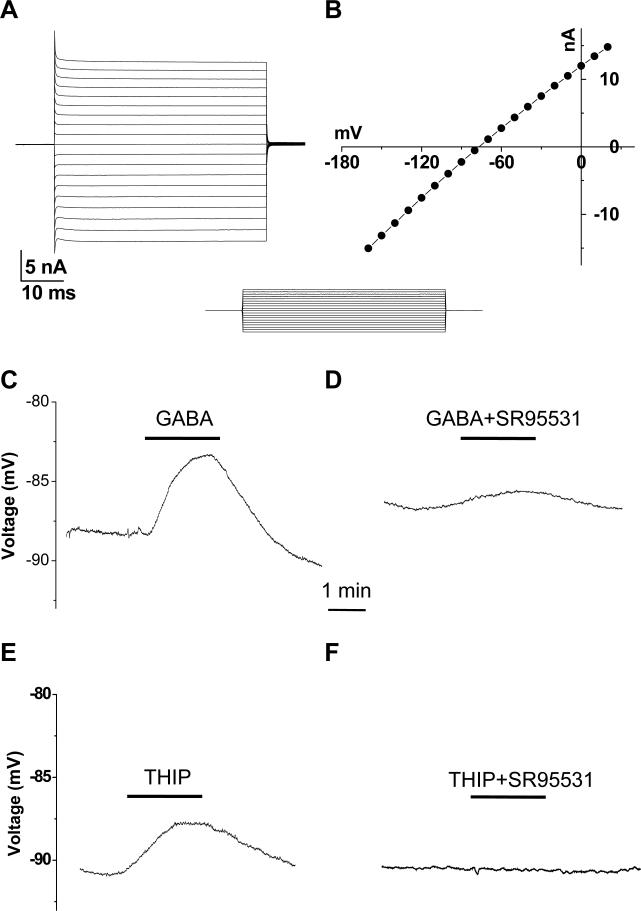
Astrocytes showing a linear voltage-current (I-V) relationship and low membrane resistance (Rm) have been reported since 1990's (Berger et al. 1991; D'Ambrosio et al. 1998; Jabs et al. 1997; Kang et al. 1998; Steinhauser et al. 1992). Mature astrocytes in rat hippocampal CA1 stratum radiatum identically show the same electrophysiological characteristics after P21 (Zhou et al. 2006), therefore, these criteria were used to identify mature astrocytes in the present study. GABA has been shown to induce GABAA-R currents in hippocampal astrocytes (Fraser et al. 1995; Meier et al. 2008; Steinhauser et al. 1994; Zhou and Kimelberg 2001). To determine the role of GABAA-R on membrane potential (Vm) and [Cl−]i in intact astrocytes, gramicidin perforated patch recording (PPR) was used (see Methods and Sup. Fig. 1 for details). Stably established PPR recordings had a membrane potential (Vm), Ra and Rm of −88.0 ± 0.4 mV, 42 ± 7.8 MΩ and 13.8 ± 2.0 MΩ, respectively (n = 8).
In current clamp recording, 0.3 mM GABA and a specific GABAA-R agonist 0.1 mM THIP depolarized astrocytic Vm by 2.4 ± 0.4 mV (n = 6) and 3.8 ± 0.3 mV (n = 5), respectively (Fig. 1 C, E), and the depolarization was almost inhibited by 0.01 mM SR95531, a specific GABAA-R antagonist (Fig.1 D, F), indicating that under the condition of intact [Cl−]i, GABAA-R activation led to Vm depolarization in hippocampal astrocytes.
Figure 1. GABAA receptor activation induced astrocyte Vm depolarization in gramicidin.
A. A mature astrocyte characterized by its passive membrane conductance that was induced by the voltage commands shown in the inset below the recording; the cell was held at −80 mV at resting and stepped to command voltages of −160 to 20 mV in 10 mV increment. B. The current-to-voltage (I-V) relationship of this the same cell. C, E. A 2-min application of 0.3 mM GABA or 0.1 mM THIP induced Vm depolarization in astrocytes that could be substantially (D) or completely (F) inhibited by 0.01 mM SR95531.
To determine [Cl−]i of astrocytes, a voltage ramp command was applied to the established PPRs (Fig. 2A), first in aCSF solution, then in addition of 0.1 mM THIP (Fig. 2A). The net GABAA-R currents were resolved by subtracting currents induced in control aCSF from the currents in the presence of THIP (Fig. 2C) that resulted in a GABAA-R reversal potential (EGABA-A) of −38.5 ± 7.1 mV (n = 5). As the reversal potentials were resolved from current subtraction that carried 75% of voltage error, the error in each recording was corrected as exemplified in Fig. 2C. The corrected EGABA-A values were −78.3 ± 2.2 mV to −75.3 ± 2.3 mV (n = 5), corresponding to a total anion driving force (Vm - EGABA-A) of −5.4 mV to −9.7 mV. The [Cl−]i was calculated according to the Equ. 1. The resulted [Cl−]i, 3.1 ± 0.6 to 3.9 ± 0.7 mM (n = 5), was comparable to a passively distributed [Cl−]i of 3.4 mM as calculated from the Equ. 4.
Bicarbonate efflux underlies GABAA-R induced astrocyte Vm depolarization
To explore the mechanism underlying a low [Cl−]i mediated Vm depolarization, we first calculated the Cl− driving force (CDF) below.
Where the Vm is the membrane potential of PPRs, and ECl is the Cl− equilibrium potential calculated based on [Cl−]e=134.5 mM in bath and [Cl−]i = 3.1 – 3.9 mM in PPRs. A positive CDF value indicates Cl− influx that should not underlie GABAA-R activation induced Vm depolarization.
GABAA-R is also permeable to HCO3− with a relative permeability of PHCO3/PCl = 0.2(Kaila and Voipio 1987; Staley et al. 1995). According to the used [HCO3−]e of 25 mM in bath and a [HCO3−]i of 16 mM (Staley et al. 1995), the EHCO3- was −12.2 mV and the Bicarbonate Driving Force (BDF) was:
An outward BDF indicates efflux of [HCO3−]i via activated GABAA-R that must be responsible for the observed Vm depolarization in astrocytes.
Characteristics of GABAA-R currents in astrocytes
To further characterize astrocyte GABAA-R currents, symmetric Cl− gradient was used in the recording solutions. The Vm and Rin were −78.4 ± 0.2 mV(n = 84) and 18.4 ± 1.3 MΩ (n = 89) (Sup. Fig. 2). Astrocytes were held at their resting potentials for bath application of 0.3 mM GABA for 1 min, which induced an inward current of −162 ± 43 pA (n = 10) (Fig. 3 A). Noticeably, withdrawal of GABA was followed by a delayed inward current (DIC) in 70% of astrocytes (n = 7/10).
To segregate DIC better from the initial GABAA-R currents, focal GABA application was used to synchronize receptor activation (Fig. 3B). A short pulse (< 1s) focal application of 0.3 mM GABA increased peak receptor current amplitude considerably compared to bath GABA application, i.e., −321 ± 56 pA (n = 36), so was a faster time-to-peak kinetic of receptor activation (Fig. 3C). However, a short pulse focal GABA application was insufficient to induce DIC in the vast majority of cells (98%) (n = 91/93).
The induction of DIC was clearly depended on the time of GABA application. A longer 5-second GABA induced a salient DIC in 63.4% of cells (59/93) (Fig. 3C-E), and this application time was adopted in the following experiments unless indicated otherwise.
While the initial GABA receptor current reached the peak rapidly within 0.2-1 s (n = 50), the DICs became evident only after GABA/THIP withdrawal. In current clamp recording, focal GABA application also induced a delayed Vm depolarization (DVD). Next, the time-to-peak of DIC and DVD was measured from the same astrocytes for comparison. The values were matched closely one another, i.e., 6.6 ± 0.5 s for DIC vs. 6.8 ± 0.3 s for DVD (n = 4; P = 0.75, Fig. 3E-F), which confirmed that the DIC and DVD represent the same event subsequent to astrocytic GABAA-R activation. Contrary to the DIC that was induced at a clamped voltage of −80 mV (Fig. 3E), DVD appeared at the resting membrane potential without voltage control (Fig. 3F). We next varied the voltages from −110 mV to −60 mV and the results clearly showed that DVD was independent of the applied voltages (Fig. 7 A), suggesting that inhibition of voltage-independent K+ channels, such as two-pore domain K+ channels (K2Ps), could be accountable for DVD/DIC.
Figure 7. HCO3− efflux was requird for the induction of DVD.
A. Representative recordings from an astrocyte, where DVD appeared when the Vm was changed from resting (A1) to −105 mV (A2) and −59 mV (A3). B. Representative Vm recordings from another astrocyte, where depolarization of Vm from resting (B1) to −13 mV (B2) abolished DVD.
The amplitude of DICs varied considerably between astrocytes; three representative DICs with different current amplitudes, compared to their preceding receptor currents, are shown in Fig. 4A-C. It took 1-2 min for the induced DIC to recover fully and DIC could be re-evoked multiple times from the same cell.
Figure 4. Dependence of DIC on GABAA-R activation.
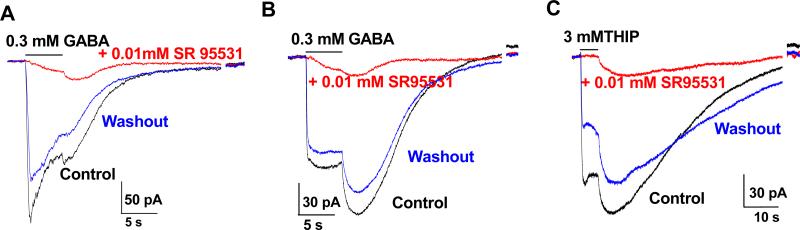
A, B, C. The amplitude of the DIC induced by either 0.3 mM GABA or 0.1 mM THIP varied from cell to cell (black traces), but depended on the preceding gating of GABAA-R; the DICs were substantially abolished in the presence of 0.01 mM SR95531 for GABAA-R inhibition.
The DIC appeared more frequently in slices prepared from the rostral than that of the caudal side of hippocampus (79.2%, n = 38/48 vs. 39.3%, n = 11/28, P < 0.01; Fig. 3H). No significant difference in DIC frequency was found between recordings obtained from superficial (or close to pyramidal layer) and deep half of stratum radiatum slices (58.3 %, n = 14 / 24, vs. 57.1% n = 16 / 28). Interestingly, a higher percentage of DIC in rostral slices coincided with a higher level of TWIK-1 K+ channel mRNA expression in this region (Talley et al. 2001).
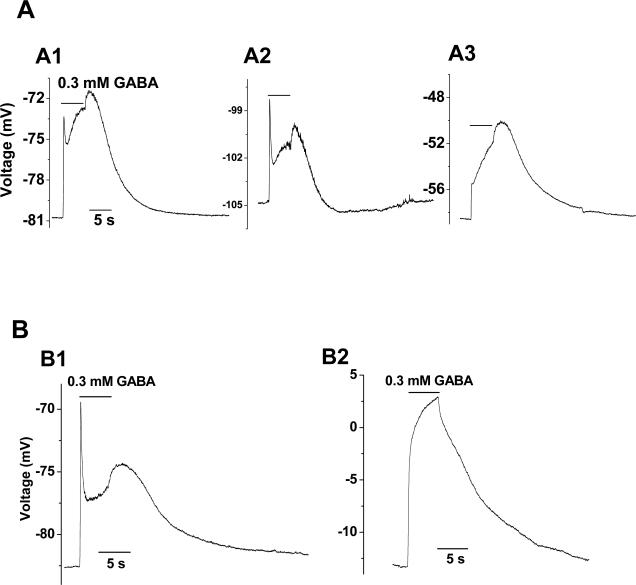
NG2 glia also express functional GABAA-Rs and could be identified based on their voltage-gated K+ and Na+ conductance expression and lack of intercellular coupling (Xie et al. 2007). However, DIC was never inducible from NG2 glia (n = 16; Sup. Fig. 3A), nor the interneurons (n = 5; Sup. Fig. 3A), suggesting an astrocytic specific of DIC.
Although neuronal GABAA-R activation has been shown to induce K+ transient in stratum pyramidale resulting from outward transport of K+ via neuronal K+-Cl− cotransporter KCC2 (Grover et al. 1993; Viitanen et al. 2010), we found that the induction of neuronal K+ transient required a much excessive exposure of pyramidal neurons to GABA (Sup. Fig. 4). Thus, DIC and K+ transient are separate events subsequent to astrocytic and neuronal GABAA-R activation, respectively.
A preceding GABAA-R activation appeared to be required for the induction of DIC, because DIC was largely inhibited in the presence of SR95531 (Fig. 4, n = 5).
The amplitude of DIC also depended on applied GABA concentration (Fig. 5A), and an apparent threshold of GABA concentration for DIC induction was around 0.1 mM. We here also examined the dose-response relationship between GABA concentration and the amplitude of the initial peak receptor currents (see Methods for details). The resultant EC50 and Hill coefficient were 0.3 mM and 0.75, respectively (Fig. 5B). This EC50 value indicated a rather low affinity of astrocytic GABAA-R to GABA compared to the recombinant neuronal subtypes of GABAA-R (Esmaeili et al. 2009). The EC50 was also 6-fold higher than the one examined from the freshly isolated hippocampal astrocytes (Fraser et al. 1995).
GABAA-R mediated inhibition of Cs− permeable K2P likely underlies DIC
Activation of astrocytic GABAA-R has been shown to transiently elevate intracellular Ca2+ (Fraser et al. 1995; Meier et al. 2008). A potential contribution of Ca2+ to the DIC was tested by inclusion of Ca2+ channel blockers, 0.1 mM Ni2+, 0.1 mM Cd+ and 0.15 mM verapamil, in the bath solution. Ca2+ release from intracellular stores was inhibited by 0.1 mM ruthenium red. In a separate experiment, the Ca2+ stores were depleted by bath application of 5 μM thapsigargin. These conditions together did not inhibit or modify DIC (Sup. Fig. 5A-D), and the DIC was not affected by Ca2+ -free bath solution either (data not shown).
Pharmacological concentration of GABA could over-activate Na+ dependent electrogenic GABA transporters. However, GABA uptake inhibitor, 0.1 mM nipecotic acid, induced only a small current (−11.0 ± 4.5 pA, n = 4; Sup. Fig. 6A), and did not affect DIC (Sup. Fig. 6B-C).
GABAA-R activation induced DIC could also be ascribed to activation of some unknown type of anion channel. However, 0.1 mM NPPB, a broad spectrum anion channel inhibitor (Zhang et al. 2011), did not affect DIC( Sup. Fig. 5A). A potential involvement of anion channel to DIC was explored further by reducing or total replacement of KCl with K+-gluconate in electrode solution. However, 5 mM or 0 mM [Cl−]i did not affect DIC (Sup. Fig.5E).
We also explored a possible contribution of [Na+]e to the DIC. As shown in Sup. Fig. 5F, when the DIC was induced, focal application of 0 mM [Na+]e did produce a partial reduction of DIC. However, 0 mM [Na+]e alone also induced a outward current with a comparable size in the same cell. Therefore, it was unlikely that 0 mM [Na+]e has a significant contribution to the DIC. Na+ channels blockers 0.5 μM TTX and 0.05 mM amiloride also did not affect the appearance of the DIC (data not shown). A potential impact of gap junction coupling on the induction of DIC was tested by bath application of gap junction inhibitor, 0.1 mM meclofenamic acid (MFA)(Xu et al. 2010). DIC was not affected by MFA (data not shown).
HCO3− efflux via activated GABAA-R has been shown to acidify intracellular pH (Kaila and Voipio 1987) that is inhibitory for TWIK-1 K+ channels (Lesage et al. 1996) expressed abundantly in astrocytes (Zhou et al. 2009). We hypothesized that HCO3− efflux induced intracellular acidification and TWIK-1 inhibition underlies DIC.
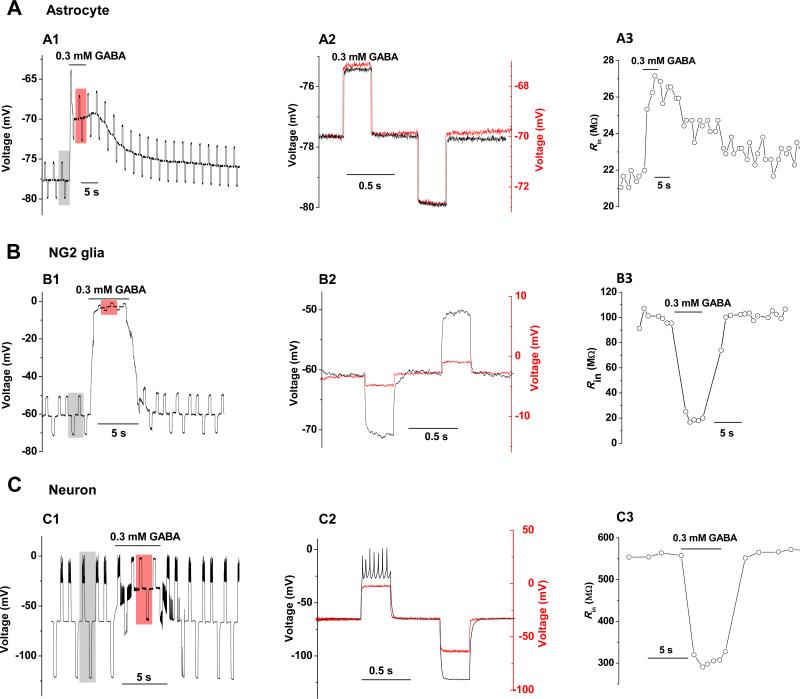
Activation of GABAA receptor is known to decrease membrane resistance (Rm). However, GABA induced a progressive increase in input resistance (Rin) in astrocytes that amounted to 5.6 ± 0.8 MΩ (n = 4, Fig. 6A). The results indicated that astrocytic GABAA-R activation is associated with a strong suppression of background open channels and the latter overwhelmed the receptor activation mediated Rm decrease. This effect was astrocytic specific, because GABAA-R activation decreased Rm markedly in NG2 glia (Fig. 6B), pyramidal neurons and interneuron (Fig. 6C) that accounted to 76 ± 12 MΩ (n = 2), 107 ± 17 MΩ (n = 4) and 300 MΩ (n = 1), respectively.
Figure 6. Activation of astrocytic GABAA-R is associated with a rapid increase in input resistance.
Membrane input resistance (Rin) was measured by a pair of ± 0.1 nA/ 300 ms current pulse delivered periodically to a recorded astrocyte (A), NG2 glia (B) and SR interneuron (C. To compare GABA effect on Rin, two representative recording segments from control (shaded grey square) and GABA application (shaded red square) are superimposed in A2, B2 and C2, where increase in Rin in astrocytes and decrease in Rin in a NG2 glia and an interneuron are shown. A3, B3, C3. Chronological display of Rin changes from control to GABA application and washout for an astrocyte, NG2 glia and interneuron, respectively.
To test the dependence of DVD on the HCO3− efflux, we altered the HCO3− driving force from manipulation of Vm. At the holding Vm more negative than the resting Vm to drive HCO3− efflux, the DVD was readily inducible (Fig.7A), while the DVD was abolished at a holding Vm near the EHCO3 of −12 mV (n = 5) (Fig. 7B). These results indicated that HCO3− efflux and the following intracellular acidification should be required for DVD induction.
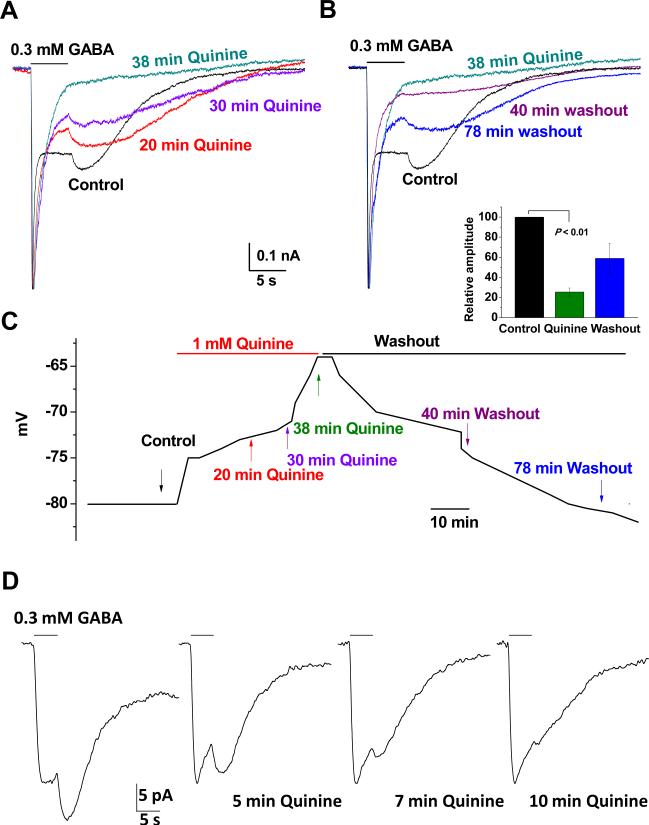
We next sought to determine if TWIK-1 blocker quinine could inhibit DIC. Quinine is a commonly used TWIK-1 channel inhibitor (Lesage et al. 1996; Ma et al. 2011; Zhou et al. 2009). Bath application of 1 mM quinine resulted in a progressive Vm depolarization (Fig. 8C). Following to Vm depolarization, quinine inhibited DIC in a time-dependent fashion (Fig. 8 A-B). The inhibitory effect of quinine on DIC was quantatively calculated by using the ratio of the amplitude of DIC (IDIC) over its preceding GABAA-R currents (IGABA-R), i.e., IDIC/IGABA-R. In the end of 40 min of quinine treatment, the IDIC/IGABA-R ratio reduced to 25.5 ± 4.0% of the control level (n = 3, P < 0.01; Fig. 8 A-B and inset below B). Note that the remaining 25.5% of IDIC/IGABA-R (green traces) was indistinguishable from the nondesensitizing GABAA-R currents, or the quinine produced actual inhibition should be considerably greater than 74.5%. The results indicated that quinine sensitive K+ channels, most likely K2Ps, were required for the induction of DIC. The quinine produced inhibition was reversible and repeatable in the same recording (data not shown).
Figure 8. Quinine inhibition of GABA induced DIC.
A. Time-dependent inhibition of GABA induced DIC by bath application of 1 mM quinine. 0.3 mM GABA induced receptor currents and DIC at different times by focal application. After control recording, 1 mM quinine was bath applied that inhibited the DIC in a time-dependent fashion. B. Continued recording from the cell in A. After washout, the DIC appeared 78 min later with 57% recovery in current amplitude. Quinine inhibition (inset) was calculated and presented as IDIC/IGABA_R that decreased to 25.5% of the control level in the end of 40 min of quinine application. C. Change in Vm during quinine application and washout for the cell shown in A and B, where quinine produced Vm depolarization, coincided with the quinine mediated DIC inhibition. D. DIC remained in Cs+ a recording solution that was inhibited in a time-dependent fashion. The holding level of the recording traces in A, B and D were normalized to the same level for comparison of DIC amplitude.
Astrocytic K2Ps are highly permeable to Cs+, a commonly used cation substitute for inhibition of conventional K+ channels (Hille 2001). Had K2P inhibition truly underlied the DIC, an intact DIC would remain in the recording solutions without K+ and quinine should still be effective. In the recordings, where the K+ ions were totally replaced by Cs+ in the solutions, GABA and THIP still induced DIC and the currents were effectively inhibited by 1 mM quinine (n = 4, P < 0.05) (Fig. 8D).
Collectively, the evidences indicated strongly that inhibition of Cs+ permeable K2Ps, especially TWIK-1, most likely was accountable for the GABAA-R induced DIC in astrocytes.
The DIC in our experimental condition require prolonged exposure of nonphysiological concentration of GABA, therefore it is necessary to determine to what extent this could occur in situ. In dual interneuron recording in CA1 striatum radiatum, over stimulation of neuron with positive current injection induced neither astrocytic GABAA-R currents nor DIC (Sup. Fig. 7). The functional implication might be that synchronized GABA release from multiple GABAergic neurons are required to activate astrocytic GABAA-R and induce the following DIC.
Discussion
The [Cl−]i follows a passive distribution in mature hippocampal astrocytes
In the present study, we have revealed, for the first time, a rather low [Cl−]i of 3.1 to 3.9 mM in mature rat hippocampal astrocytes that favors a passive distribution of Cl− anion. Such a low [Cl−]i differs strikingly from the high [Cl−]i of 30 to 40 mM high in cultured astrocytes (Bekar and Walz 2002; Kimelberg 1981). In an early study by Ballanyi and colleagues, a low [Cl−]i of 3.2 mM was reported from glial cells of guinea-pig olfactory cortex measured with Cl− sensitive microelectrode (Ballanyi et al. 1987). In the same study, the glial cells were identified to have a membrane potential more negative than −75 mV and lack of synaptic potentials, therefore, they most likely represented astrocytes, although some oligodendrocytes might also be included.
High intracellular Cl− content is mainly ascribed to active accumulation of Cl− from Na+-K+-2Cl− cotransporter. According to a transcriptome study, the high [Cl−]i in cultured astrocytes may well be resulted from a 2.7-fold higher Na+-K+-2Cl− cotransporter expression compared to astrocytes isolated from young mature P17 mouse (Cahoy et al. 2008). Interestingly, the same study also revealed a 2.7 fold higher Na+-K+-2Cl− cotransporter expression in astrocytes isolated from neonatal P1 mouse compared to P17 mouse, implying a similarity between cultured astrocytes and immature astrocytes. It would be interesting to know in the future whether an age-dependent down regulation in Na+-K+-2Cl− cotransporter expression would correlate to a decreasing [Cl−]i during astrocyte maturation (Cahoy et al. 2008).
Similar to the mature neurons (Ben-Ari 2002; Owens et al. 1996), our results revealed a low [Cl−]i in mature hippocampal astrocytes. In contrast, other mitotically active cell types in the brain, such as NG2 glia and subventricular zoon neuronal progenitors maintain a high [Cl−]i (Lin and Bergles 2004; Wang et al. 2003), so is the cultured astrocytes and glioma (Sontheimer 2008; Walz 2002). Increasing evidence indicates that cultured astrocytes are similar to reactive astrocytes in various pathological states, and reactive astrocytes appear to have a up-regulated K+ uptake ability that is coupled with Cl− influx, suggesting a high Cl− content in reactive astrocytes (MacVicar et al. 1989; Walz and Wuttke 1999). These data together favors a notion that a low [Cl−] is a characteristic of differentiated cells in the central nervous system.
Bicarbonate efflux via activated GABAA-R induces Vm depolarization in astrocytes
We showed in the perforated patch recordings that GABAA-R activation mediated a Vm depolarization. As noted that passive Cl− distribution means tied values of ECl and EK (- 93 mV) with a zero driving force for Cl- flux. The fact that Vm in perforated patch recordings (−88 mV) is very close to ECl and EK indicates a negligible driving force (Vm -ECl) for Cl− flux during astrocytic GABAA-R activation.
In cultured astrocytes, bicarbonate efflux via activating GABAA-R induced astrocyte Vm depolarization (Kaila et al. 1991). The same mechanism also underlies the Vm depolarization of astrocytes in slices. We here extent the analysis further to show that the only impact of astrocytic GABAA-R activation on Vm is depolarization. Because [Cl−] becomes irrelevant to the Vm depolarization, thus, the direction of HCO3− flux follows to a relation of [HCO3−]i[K+]i - [HCO3−]e[K+]e (Equ. 7). The physiological [HCO3−] varies between 24 mM to 26 mM in most of extracellular fluids and 10mM to 15 mM inside cells (Litvin et al. 2003; Tresguerres et al. 2010), and the [K+]e fluctuates between 4 mM to 12 mM (Heinemann and Lux 1977; Walz 2000), thus, the [HCO3−]i[K+]i-[HCO3−]e[K+]e, should always be a negative value that drives HCO3− efflux and Vm depolarization. In summary, HCO3− efflux and Vm depolarization should be the direct functional consequences of astrocytic GABAA-R activation, a role that has been previously demonstrated from muscle fibers (Kaila and Voipio 1987). The HCO3− efflux also implies a role of astrocytic GABAA-R in pH regulation.
A critical factor that determines the amount of HCO3− efflux is Vm. However, the Vm measured from conventional whole-cell (−78 mV) and perforated patch (−88 mV) recordings varied by 10 mV (P < 0.01) and the latter was more close to the EK (−93 mV) in our experimental conditions (Fig. 2B). At least two factors could underlie the difference between the two recording modes. First, the distribution of Vm (Sup. Fig. 2B) showed that some of the Vm values did exceed the −80 mV to close to the Vm of perforated patch recordings, thus, variation in gigaohm seal quality in whole-cell recording may partially contribute to the difference. Second, perforated patch recording does not involve extensive dialysis of cytoplasmic content that may also underlie the observed difference.
Activation of astrocytic GABAA-R induces delayed inhibition of astrocytic K2P
Our data showed that GABAA-R activation is followed by a delayed inhibition of astrocytic K2P K+ channels. The present study showed that the DIC remained intact when intracellular and extracellular [Ca2+], [Na+] and [Cl−] were altered and DIC was insensitive to a variety of cation/anion channel inhibitors. The involvement of K2P in DIC has been indicated, as DIC was intact in Cs+ based recording solutions, a condition that inhibits conventional K+ channels, such as Kir4.1, but remains the function of Cs+ permeable K2Ps in astrocytes. The involvement of Cs+ sensitive K2P in DIC was further supported by inhibition of DIC by quinine (Lesage et al. 1996; Ma et al. 2011; Zhou et al. 2009). The involvement of TWIK-1 in DIC has been proposed because 1) GABAA-R activation leads to intracellular acidification in cultured astrocytes (Kaila et al. 1991); 2) intracellular acidification is shown to inhibit TWIK-1 K+ channels (Lesage et al. 1996) and 3) TWIK-1 is highly expressed in astrocytes and sensitive to quinine with a EC50 of 85 μM (Zhou et al. 2009). These together Previously, GABAA-R activation has been shown to alter [Cl−]i that in turn modulate astrocyte outwardly rectifying K+ channels (Bekar and Walz 2002). In addition to that, the present study show that GABAA-R activation could modify other K+ channels expressed in mature hippocampal astrocytes via bicarbonate efflux and intracellular acidification.
The affinity of astrocytic GABAA-R to GABA
By using focal GABA application to astrocytes in situ, we revealed a GABA EC50 value of 300 μM, which was 6-fold higher than a EC50 value (50 μM) analyzed from astrocytes freshly isolated from rat hippocampus of similar ages (Fraser et al. 1995). The freshly isolated astrocytes offer the advantages of being able to clearly determine the properties of astrocytes without having to control the indirect effect from the surrounding neurons or other glial cells. The isolated astrocytes also have significantly improved Rm for a better voltage clamping quality (Kimelberg et al. 2000). However, the poor voltage clamping quality in slice recording should mask the current amplitude induced by high concentration of GABA more severely than the ones induced by the lower concentrations. In other words, the EC50 in our study should be further underestimated. The actual reasons underlying the different EC50 values between the two studies are unclear.
Nevertheless, astrocytic GABAA-R showed a much lower affinity to endogenous ligand GABA compared to neurons that have a EC50 in the range of 0.2 – 0.4 μM (Wu et al. 2007). In view of the low ambient GABA in the range of 0.1 – 0.4 μM (Attwell et al. 1993), it is unlikely that neuronal GABA release could induce phasic GABAA-R activation. Therefore, astrocytic GABAA-R may mainly function to sense ambient GABA and this has been shown to play role in maintaining the morphology of astrocytes in developing and adult brain (Velez-Fort et al. 2012).
Supplementary Material
Supplementary Figure 1. Gramicidin perforated-patch recording from a mature astrocyte. A. Fluorescent image of a recording electrode containing 0.1% Lucifer yellow (LY) and gramicidin during perforated-patch recording. LY remained in the electrode throughout the recording. B. Fluorescent image of LY during conventional whole-cell recording, where LY diffused into the cytoplasm of the recorded cell and adjacent astrocytic syncytium. C. Time course of gramicidin perforated patch formation. After forming of gigaohm cell-attach mode, cell was held at −70 mV and a +Δ10 mV/10 ms voltage step was delivered every 20 second to monitor the input resistance (Rin) change. In this recording, a time-dependent increase in step induced currents (C) and a progressive decrease in Rin (D) occurred concomitantly that stabilized at around 25 min of gramicidin perforation.
Supplementary Figure 2. Distribution of astrocyte whole-cell membrane input resistance and membrane potential. Distribution of membrane input resistance (Rin) (n = 89) and whole-cell resting membrane potential (Vm) (n = 84) as indicated. Data were calculated from conventional whole-cell recording with K+ based solutions.
Supplementary Figure 3. Absence of DIC in NG2 glia and neurons. A. 0.3 mM GABA did not induce DIC in a NG2 glia (n = 16). The whole-cell current profile of the same NG2 glia is also shown below A. B. 0.3 mM GABA application induced a larger inward current from an interneuron with no detectable DIC and washout of GABA was followed by a transient increase in spontaneous spikes. Both cells were recorded from CA1 stratum radiatum region.
Supplementary Figure 4. No detectable K+ transient from pyramidal neurons under our experimental conditions. A. Configuration of recording and drug application electrodes for this experiment. A1. (1) is the recording electrode, (2) and (3) are two application electrodes with different opening diameter/ [GABA] of < 1 μm / 0.3 mM and 2.3 μm / 1 mM, respectively. A2. A recorded pyramidal neuron is shown in high magnification; the arrow points to the cell soma located at stratum pyramidale (SP) and the arrowheads point to the apical dendrites that extended into the stratum radiatum (SR). Insert below shows the current profile of the recorded pyramidal neuron. B. Black and red traces are currents induced by focal application of GABA from electrode (2) and (3), respectively. The application duration was identical at 5 s. The current induced by GABA from electrode (2) turned off rapidly, but the current induced by electrode (3) possessed a prolonged inward component that took more than 80 s to recover fully, suggesting induction of K+ transient under this condition. C. Vm responses of the same neuron to 0.3 mM GABA via electrode (2); first with a short pulse (black trace), then for 5 s (red). No prolonged Vm change was detectable from both conditions. D. The same neuron was applied GABA from electrode (3), first with a pulse (black), then for 5 s (red). And the latter induced a prolonged Vm depolarization that took more than 3 min to recover to the basal Vm. Replications of same GABA applications in voltage clamp were shown in the inset of D. The results showed that a substantial K+ transient could be induced with more excessive application of GABA to SP.
Supplementary Figure 5. DIC are not mediated by [Ca2+]i [Ca2+]e, [Mg2+]e, [Na+]e or [Cl]i. A. Application of a mixture of inhibitors for Ca2+ channels and intracellular Ca2+ store release did not inhibit GABA induced DIC. In this experiment, a broad spectrum Cl− channel inhibitor NPPB was also included. B-D. DIC was not affected by bath application of either 5 μM thapsigargin alone (C) (n =2) or 5 μM thapsigargin plus 0 mM Ca2+( D) (n = 3). E. DIC could be induced in K+-gluconate electrode solution containing 0 mM [Cl−]i. F. DIC were independent of [Na+]e. After induction of DIC (black trace), the DIC was modified by 0 mM [Na+]e (red trace) applied through the 2nd focal application electrode. However, 0 mM [Na+]e alone also induced a similar size of outward current (blue) in the same cell.
Supplementary Figure 6. Effect of nipecotic acid on GABA induced astrocytic DIC. A. 0.1 mM nipecotic acid alone induced −17 pA inward current in an astrocyte. B-C. In the presence of 0.1 mM nipecotic acid, the 0.3 mM GABA induced DIC was not affected in two astrocytes. D. 0.1 mM nipecotic acid induced −5.8 pA currents in an interneuron located at stratum radiatum.
Supplementary Figure 7. Neither GABAA-R currents nor DIC could be detected in astrocyte in response to stimulation of single adjacent GABAnergic interneuron. Dual patch recordings from an astrocyte (upper panel) and an interneuron (lower panel) in the CA1 stratum radiatum region spaced 30 μm apart. 200 pA currents injection resulted in intensive firings in the interneuron (C). Although the enhanced firing should trigger enhanced GABA release to the adjacent astrocyte (A), neither GABAA-R nor DIC were detected. In B, negative currents of −200 pA were injected to the same recorded neuron that did not induce Vm change in the adjacent astrocyte (B).
Acknowledgements
This study was supported by grants from the National Science Foundation (IOS0641828 to MZ), National Institute of Neurological Disorders and Stroke (RO1NS062784 to MZ) and a Startup fund from The Ohio State University School of Medicine (to MZ). We thank Dr. Wei Wang for her critical comment on the manuscript, and Dr. John A. Payne, Department of Physiology and Membrane Biology, University of California, for discussion of the Cs+ effect on neuronal KCC2 cotransporter.
Reference
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11(3):401–7. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Grafe P, ten Bruggencate G. Ion activities and potassium uptake mechanisms of glial cells in guinea-pig olfactory cortex slices. J Physiol. 1987;382:159–74. doi: 10.1113/jphysiol.1987.sp016361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekar LK, Jabs R, Walz W. GABAA receptor agonists modulate K+ currents in adult hippocampal glial cells in situ. Glia. 1999;26(2):129–38. [PubMed] [Google Scholar]
- Bekar LK, Walz W. Evidence for chloride ions as intracellular messenger substances in astrocytes. J Neurophysiol. 1999;82(1):248–54. doi: 10.1152/jn.1999.82.1.248. [DOI] [PubMed] [Google Scholar]
- Bekar LK, Walz W. Intracellular chloride modulates A-type potassium currents in astrocytes. Glia. 2002;39(3):207–16. doi: 10.1002/glia.10096. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3(9):728–39. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Berger T, Schnitzer J, Kettenmann H. Developmental changes in the membrane current pattern, K+ buffer capacity, and morphology of glial cells in the corpus callosum slice. J Neurosci. 1991;11(10):3008–24. doi: 10.1523/JNEUROSCI.11-10-03008.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein M, Lyons SA, Moller T, Kettenmann H. Receptor-mediated calcium signalling in glial cells from mouse corpus callosum slices. J Neurosci Res. 1996;46(2):152–63. doi: 10.1002/(SICI)1097-4547(19961015)46:2<152::AID-JNR3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Bureau M, Laschet J, Bureau-Heeren M, Hennuy B, Minet A, Wins P, Grisar T. Astroglial cells express large amounts of GABAA receptor proteins in mature brain. J Neurochem. 1995;65(5):2006–15. doi: 10.1046/j.1471-4159.1995.65052006.x. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28(1):264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio R, Wenzel J, Schwartzkroin PA, McKhann GM, 2nd, Janigro D. Functional specialization and topographic segregation of hippocampal astrocytes. J Neurosci. 1998;18(12):4425–38. doi: 10.1523/JNEUROSCI.18-12-04425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili A, Lynch JW, Sah P. GABAA receptors containing gamma1 subunits contribute to inhibitory transmission in the central amygdala. J Neurophysiol. 2009;101(1):341–9. doi: 10.1152/jn.90991.2008. [DOI] [PubMed] [Google Scholar]
- Fraser DD, Duffy S, Angelides KJ, Perez-Velazquez JL, Kettenmann H, MacVicar BA. GABAA/benzodiazepine receptors in acutely isolated hippocampal astrocytes. J Neurosci. 1995;15(4):2720–32. doi: 10.1523/JNEUROSCI.15-04-02720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover LM, Lambert NA, Schwartzkroin PA, Teyler TJ. Role of HCO3- ions in depolarizing GABAA receptor-mediated responses in pyramidal cells of rat hippocampus. J Neurophysiol. 1993;69(5):1541–55. doi: 10.1152/jn.1993.69.5.1541. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Lux HD. Ceiling of stimulus induced rises in extracellular potassium concentration in the cerebral cortex of cat. Brain Res. 1977;120(2):231–49. doi: 10.1016/0006-8993(77)90903-9. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable cells. Sinauer; Sunderland, MA: 2001. [Google Scholar]
- Jabs R, Paterson IA, Walz W. Qualitative analysis of membrane currents in glial cells from normal and gliotic tissue in situ: down-regulation of Na+ current and lack of P2 purinergic responses. Neuroscience. 1997;81(3):847–60. doi: 10.1016/s0306-4522(97)00207-8. [DOI] [PubMed] [Google Scholar]
- Johnson D, Wu SM-S. Foundations of cellular neurophysiology: The MIT Press. 1995:19. [Google Scholar]
- Kaila K, Panula P, Karhunen T, Heinonen E. Fall in intracellular pH mediated by GABAA receptors in cultured rat astrocytes. Neurosci Lett. 1991;126(1):9–12. doi: 10.1016/0304-3940(91)90358-z. [DOI] [PubMed] [Google Scholar]
- Kaila K, Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987;330(6144):163–5. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1(8):683–92. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Active accumulation and exchange transport of chloride in astroglial cells in culture. Biochim Biophys Acta. 1981;646(1):179–84. doi: 10.1016/0005-2736(81)90285-6. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Cai Z, Schools G, Zhou M. Acutely isolated astrocytes as models to probe astrocyte functions. Neurochem Int. 2000;36(4-5):359–67. doi: 10.1016/s0197-0186(99)00144-8. [DOI] [PubMed] [Google Scholar]
- Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. Embo J. 1996;15(5):1004–11. [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2004;7(1):24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem. 2003;278(18):15922–6. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8(9):1179–87. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zhang X, Chen H. TWIK-1 two-pore domain potassium channels change ion selectivity and conduct inward leak sodium currents in hypokalemia. Sci Signal. 2011;4(176):ra37. doi: 10.1126/scisignal.2001726. [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Tse FW, Crichton SA, Kettenmann H. GABA-activated Cl- channels in astrocytes of hippocampal slices. J Neurosci. 1989;9(10):3577–83. doi: 10.1523/JNEUROSCI.09-10-03577.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier SD, Kafitz KW, Rose CR. Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia. 2008;56(10):1127–37. doi: 10.1002/glia.20684. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60(3):243–60. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16(20):6414–23. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H. An unexpected role for ion channels in brain tumor metastasis. Exp Biol Med (Maywood) 2008;233(7):779–91. doi: 10.3181/0711-MR-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269(5226):977–81. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- Steinhauser C, Berger T, Frotscher M, Kettenmann H. Heterogeneity in the Membrane Current Pattern of Identified Glial Cells in the Hippocampal Slice. Eur J Neurosci. 1992;4(6):472–484. doi: 10.1111/j.1460-9568.1992.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Steinhauser C, Jabs R, Kettenmann H. Properties of GABA and glutamate responses in identified glial cells of the mouse hippocampal slice. Hippocampus. 1994;4(1):19–35. doi: 10.1002/hipo.450040105. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21(19):7491–505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresguerres M, Buck J, Levin LR. Physiological carbon dioxide, bicarbonate, and pH sensing. Pflugers Arch. 2010;460(6):953–64. doi: 10.1007/s00424-010-0865-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez-Fort M, Audinat E, Angulo MC. Central Role of GABA in Neuron-Glia Interactions. Neuroscientist. 2012 doi: 10.1177/1073858411403317. [DOI] [PubMed] [Google Scholar]
- Viitanen T, Ruusuvuori E, Kaila K, Voipio J. The K+-Cl cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J Physiol. 2010;588(Pt 9):1527–40. doi: 10.1113/jphysiol.2009.181826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem Int. 2000;36(4-5):291–300. doi: 10.1016/s0197-0186(99)00137-0. [DOI] [PubMed] [Google Scholar]
- Walz W. Chloride/anion channels in glial cell membranes. Glia. 2002;40(1):1–10. doi: 10.1002/glia.10125. [DOI] [PubMed] [Google Scholar]
- Walz W, Wuttke WA. Independent mechanisms of potassium clearance by astrocytes in gliotic tissue. J Neurosci Res. 1999;56(6):595–603. doi: 10.1002/(SICI)1097-4547(19990615)56:6<595::AID-JNR5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Wang DD, Krueger DD, Bordey A. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J Physiol. 2003;550(Pt 3):785–800. doi: 10.1113/jphysiol.2003.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Diez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron. 2007;56(5):851–65. doi: 10.1016/j.neuron.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Lynch DT, Schools GP, Feustel PJ, Kimelberg HK, Zhou M. Sodium channel currents in rat hippocampal NG2 glia: characterization and contribution to resting membrane potential. Neuroscience. 2007;150(4):853–62. doi: 10.1016/j.neuroscience.2007.09.057. [DOI] [PubMed] [Google Scholar]
- Xie M, Wang W, Kimelberg HK, Zhou M. Oxygen and glucose deprivation-induced changes in astrocyte membrane potential and their underlying mechanisms in acute rat hippocampal slices. J Cereb Blood Flow Metab. 2008;28(3):456–67. doi: 10.1038/sj.jcbfm.9600545. [DOI] [PubMed] [Google Scholar]
- Xu G, Wang W, Kimelberg HK, Zhou M. Electrical coupling of astrocytes in rat hippocampal slices under physiological and simulated ischemic conditions. Glia. 2010;58(4):481–93. doi: 10.1002/glia.20939. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cao HJ, Kimelberg HK, Zhou M. Volume regulated anion channel currents of rat hippocampal neurons and their contribution to oxygen-and-glucose deprivation induced neuronal death. PLoS One. 2011;6(2):e16803. doi: 10.1371/journal.pone.0016803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Kimelberg HK. Freshly isolated hippocampal CA1 astrocytes comprise two populations differing in glutamate transporter and AMPA receptor expression. J Neurosci. 2001;21(20):7901–8. doi: 10.1523/JNEUROSCI.21-20-07901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Schools GP, Kimelberg HK. Development of GLAST(+) astrocytes and NG2(+) glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. J Neurophysiol. 2006;95(1):134–43. doi: 10.1152/jn.00570.2005. [DOI] [PubMed] [Google Scholar]
- Zhou M, Xu G, Xie M, Zhang X, Schools GP, Ma L, Kimelberg HK, Chen H. TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J Neurosci. 2009;29(26):8551–64. doi: 10.1523/JNEUROSCI.5784-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Gramicidin perforated-patch recording from a mature astrocyte. A. Fluorescent image of a recording electrode containing 0.1% Lucifer yellow (LY) and gramicidin during perforated-patch recording. LY remained in the electrode throughout the recording. B. Fluorescent image of LY during conventional whole-cell recording, where LY diffused into the cytoplasm of the recorded cell and adjacent astrocytic syncytium. C. Time course of gramicidin perforated patch formation. After forming of gigaohm cell-attach mode, cell was held at −70 mV and a +Δ10 mV/10 ms voltage step was delivered every 20 second to monitor the input resistance (Rin) change. In this recording, a time-dependent increase in step induced currents (C) and a progressive decrease in Rin (D) occurred concomitantly that stabilized at around 25 min of gramicidin perforation.
Supplementary Figure 2. Distribution of astrocyte whole-cell membrane input resistance and membrane potential. Distribution of membrane input resistance (Rin) (n = 89) and whole-cell resting membrane potential (Vm) (n = 84) as indicated. Data were calculated from conventional whole-cell recording with K+ based solutions.
Supplementary Figure 3. Absence of DIC in NG2 glia and neurons. A. 0.3 mM GABA did not induce DIC in a NG2 glia (n = 16). The whole-cell current profile of the same NG2 glia is also shown below A. B. 0.3 mM GABA application induced a larger inward current from an interneuron with no detectable DIC and washout of GABA was followed by a transient increase in spontaneous spikes. Both cells were recorded from CA1 stratum radiatum region.
Supplementary Figure 4. No detectable K+ transient from pyramidal neurons under our experimental conditions. A. Configuration of recording and drug application electrodes for this experiment. A1. (1) is the recording electrode, (2) and (3) are two application electrodes with different opening diameter/ [GABA] of < 1 μm / 0.3 mM and 2.3 μm / 1 mM, respectively. A2. A recorded pyramidal neuron is shown in high magnification; the arrow points to the cell soma located at stratum pyramidale (SP) and the arrowheads point to the apical dendrites that extended into the stratum radiatum (SR). Insert below shows the current profile of the recorded pyramidal neuron. B. Black and red traces are currents induced by focal application of GABA from electrode (2) and (3), respectively. The application duration was identical at 5 s. The current induced by GABA from electrode (2) turned off rapidly, but the current induced by electrode (3) possessed a prolonged inward component that took more than 80 s to recover fully, suggesting induction of K+ transient under this condition. C. Vm responses of the same neuron to 0.3 mM GABA via electrode (2); first with a short pulse (black trace), then for 5 s (red). No prolonged Vm change was detectable from both conditions. D. The same neuron was applied GABA from electrode (3), first with a pulse (black), then for 5 s (red). And the latter induced a prolonged Vm depolarization that took more than 3 min to recover to the basal Vm. Replications of same GABA applications in voltage clamp were shown in the inset of D. The results showed that a substantial K+ transient could be induced with more excessive application of GABA to SP.
Supplementary Figure 5. DIC are not mediated by [Ca2+]i [Ca2+]e, [Mg2+]e, [Na+]e or [Cl]i. A. Application of a mixture of inhibitors for Ca2+ channels and intracellular Ca2+ store release did not inhibit GABA induced DIC. In this experiment, a broad spectrum Cl− channel inhibitor NPPB was also included. B-D. DIC was not affected by bath application of either 5 μM thapsigargin alone (C) (n =2) or 5 μM thapsigargin plus 0 mM Ca2+( D) (n = 3). E. DIC could be induced in K+-gluconate electrode solution containing 0 mM [Cl−]i. F. DIC were independent of [Na+]e. After induction of DIC (black trace), the DIC was modified by 0 mM [Na+]e (red trace) applied through the 2nd focal application electrode. However, 0 mM [Na+]e alone also induced a similar size of outward current (blue) in the same cell.
Supplementary Figure 6. Effect of nipecotic acid on GABA induced astrocytic DIC. A. 0.1 mM nipecotic acid alone induced −17 pA inward current in an astrocyte. B-C. In the presence of 0.1 mM nipecotic acid, the 0.3 mM GABA induced DIC was not affected in two astrocytes. D. 0.1 mM nipecotic acid induced −5.8 pA currents in an interneuron located at stratum radiatum.
Supplementary Figure 7. Neither GABAA-R currents nor DIC could be detected in astrocyte in response to stimulation of single adjacent GABAnergic interneuron. Dual patch recordings from an astrocyte (upper panel) and an interneuron (lower panel) in the CA1 stratum radiatum region spaced 30 μm apart. 200 pA currents injection resulted in intensive firings in the interneuron (C). Although the enhanced firing should trigger enhanced GABA release to the adjacent astrocyte (A), neither GABAA-R nor DIC were detected. In B, negative currents of −200 pA were injected to the same recorded neuron that did not induce Vm change in the adjacent astrocyte (B).