Summary
Background
Osteoblast- and osteoclast-like cells are responsible for coordinated bone maintenance, illustrated by a balanced formation and resorption. Both parameters appear to be influenced by mechanical constrains acting on each of these cell types individually. We hypothesized that the interactions between both cell types are also influenced by mechanical stimulation.
Methods
Co-cultures of osteoblast- and osteoclast-like cells were stimulated with 1,100 µstrain, 0.1 or 0.3 Hz for 1–5 min/day over 5 days. Two different setups depending on the differentiation of the osteoclast-like cells were used: i) differentiation assay for the fusion of pre-osteoclasts to osteoclasts, ii) resorption assay to determine the activity level of osteoclast-like cells.
Results
In the differentiation assay (co-culture of osteoblasts with unfused osteoclast precursor cells) the mechanical stimulation resulted in a significant decrease of collagen-1 and osteocalcin produced by osteoblast-like cells. Significantly more TRAP-iso5b was measured after stimulation for 3 min with 0.1 Hz, indicating enhanced osteoclastogenesis. In the resorption assay (co-culture of osteoblasts with fused osteoclasts) the stimulation for 3 min with 0.3 Hz significantly increased the resorption activity of osteoclasts measured by the pit formation and the collagen resorption. The same mechanical stimulation resulted in an increased collagen-1 production by the osteoblast-like cells. The ratio of RANKL/OPG was not different between the groups.
Conclusion
These findings demonstrate that already small changes in duration or frequency of mechanical stimulation had significant consequences for the behavior of osteoblast- and osteoclast-like cells in co-culture, which partially depend on the differentiation status of the osteoclast-like cells.
KeyWords: Osteoblast-like cells, Osteoclast-like cells, Co-culture, Mechanical stimulation, Differentiation
Introduction
Mechanical stimuli are important during the maintenance of bone, during fracture healing, and during defect regeneration by affecting bone formation and degradation [1, 2, 3]. In the case of defect regeneration, scaffolds are used to support the adherence and differentiation of cells necessary to form new bone and fill the gap. It is well accepted that three parameters characterize the main properties of a scaffold for bone regeneration: it can be i) osteoconductive (supports cell migration and adherence), ii) osteoinductive (contains factors that stimulate ostegenic differentiation of cells), and/or iii) osteogenic (contains cells that can form bone) [4]. Within the last decades, it became also evident that the fate of a cell is very much influenced by mechanical stimuli [5]. Therefore, scaffolds used for tissue engineering approaches should consider also the mechanical properties of the developed scaffold [6]. During the regeneration of a bony defect, new bone will be formed and the scaffolding material degraded and also the newly formed bone remodeled due to resorption. The action of the cells responsible for bone formation and resorption are tightly interlinked. Osteoclast differentiation and activation are mediated by receptor activator of nuclear factor-kB ligand (RANKL) and macrophage colony-stimulating factor (MCSF), whereas osteoprotegerin (OPG) acts as a soluble receptor antagonist for RANKL and inhibits thereby osteoclast formation. All three cytokines are synthesized by osteoblasts. Consequently, bone resorption is controlled by the quantitative OPG-RANKL synthesis from osteoblasts [7, 8]. Less is known, however, if the interaction between bone-forming osteoblasts and bone-resorbing osteoclasts is directly affected by physiological magnitudes of mechanical straining.
To investigate the effect of mechanical straining on osteoblast-like cells, in vitro studies were performed. Cyclic stretching of osteoblasts with a frequency of 1 Hz and a magnitude of 1% for various time periods showed an up-regulation of RANKL [9]. After application of cyclic tensile strain, OPG synthesis was dose-dependently increased and both sRANKL release and RANKL mRNA synthesis were reduced [10]. Tensile stretching, three-point bending, and fluid flow resulted in a change of the RANKL/OPG ratio towards OPG, which might result in the inhibition of osteoclastogenesis [11, 12, 13].
The effect of mechanical strain on osteoclast-like cells was investigated in different studies showing both stimulating and inhibiting effects [14, 15, 16, 17]. In a previous study, a device for the mechanical stimulation of cells on a bone-like structure (dentin) was evaluated. Three-point bending with approximately 1,100 µstrain was applied to osteoblast- and osteoclast-like cells separately. The stimulation induced a significant decrease of the OPG production and resulted in a significant increase of the sRANKL/OPG ratio towards sRANKL in comparison to the unstimulated osteoblast-like cells [18]. The increased RANKL/OPG ratio might stimulate the differentiation of osteoclast-like cells. Investigating the effect of three-point bending on the pure osteoclast cell culture, a stimulation-dependent decrease in the fusion and resorption activity was seen [18]. These possible contrary results demonstrate the importance of the co-culture of both cell types.
To analyze the interaction of cells with biomaterials or to determine the effects of different stimuli on the interaction between osteoblasts and osteoclasts, several co-culture systems were established [19, 20, 21, 22, 23], but there are only few studies using co-culture systems to determine the effect of mechanical stimuli on the interaction of both cell types. Kim et al. [11] stimulated murine bone marrow stromal cells (ST-2) with oscillatory fluid flow and showed in a time- and dose-dependent change in RANKL (down) and OPG (up) gene expression. Addition of osteoclast-like cells (RAW264.7) after stimulation resulted in a reduced osteoclast formation in the co-culture. In a further study, the group investigated the effect of a conditioned medium collected from osteocyte-like cells (MLO-Y4) stimulated with fluid flow on the co-culture of osteocyte-like cells or murine bone marrow stromal cells (ST-2) and osteoclast-like cells (RAW264.7) [24]. On the RNA level they saw, in contrast to the previous study, an up-regulation of both RANKL and OPG; however, on the protein level RANKL was down-regulated and OPG up-regulated.
So far, no study has investigated the direct interaction of osteoblast- and osteoclast-like cells under mechanical stimulation. The hypothesis of the present study was that the mechanical stimulation of osteoblasts and osteoclasts affects their behavior and that the effect depends on the differentiation status of the osteoclast-like cells. Osteoblasts and osteoclasts were directly co-cultured and mechanically stimulated by three-point bending with 1,100 µstrain and different durations and frequencies (3–5 min with 0.1–0.5 Hz). The cells were cultured on a bone-like structure (dentin) to allow the investigation of osteoclastic bone resorption. Parameters for osteoblast and osteoclast differentiation and osteoclastic bone resorption were analyzed.
Material and Methods
Stimulation Setup
A mechanical bending device which had been described in a previous study [18] was used. Briefly, cells were seeded and cultured on dentin discs (diameter 14 mm, thickness 0.7 mm) made out of elephant ivory (supplied by the Federal Office of Nature Conservation, Germany) followed by a 5-day period of stimulation and 1 day of rest. The dentin disc with the cells was fixed in the three-point bending device. The device fits into a 12-well plate. The force was applied through the multi-well plate cover. Using a minimum stimulus of 1,100 μm/m bending of the dentin chip the following stimuli were applied: 1 min with 0.1 Hz, 1 min with 0.3 Hz, 3 min with 0.1 Hz, 3 min with 0.3 Hz, and 5 min with 0.1 Hz. The stimulation was performed in the incubator at 37 °C and 5% CO2. The setup consisted of three stimulation devices and one unstimulated control.
Isolation and Cultivation of Human Cells
Primary human osteoblast-like cells were isolated from human trabecular bone from three different individuals. Donor bone was gained during elective orthopedic surgery (proximal tibia plateau during total knee replacement) with permission of the local ethic committee and informed consent. For isolation of the cells, bone fragments were minced into little pieces followed by digestion with collagenase type II. The isolated cells were characterized for several osteoblast-specific markers such as expression of Cbfa, osteocalcin, alkaline phosphatase activity, and the formation of mineralized matrix. The osteoblast-like cells were cultured under standard conditions (MEM-E/Ham's F-12 + 10% heat-inactivated fetal calf serum (FCS), β-glycerol phosphate, L-ascorbic acid, penicillin/streptomycin).
For osteoclast-like cells, peripheral blood was taken from three human volunteers, and cells were isolated according to previously published methods [25, 26]. Monocytes were separated from blood by density gradient centrifugation (Histopaque; Sigma, Taufkirchen, Germany) and selected by CD14+ magnetic activated cell sorting (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany).
Co-Culture
In order to evaluate the direct interaction of both cell types two different settings of co-cultivation were used: i) osteoclast differentiation: determination of fusion of mononuclear cells to osteoclast-like cells in the presence of osteoblast-like cells and ii) osteoclast resorption: determination of bone resorption activity of fused osteoclast-like cells in the presence of osteoblast-like cells.
Differentiation Assay
Osteoblast-like cells were seeded in a concentration of 3 × 104 cells per dentin chip and cultivated in 1 ml MEM-E/HAM's F-12 for 3–4 days. The cell number was determined via a noninvasive/nontoxic cell activity assay (Alamar Blue™; Biozol, Eching, Germany) and 5 × 105 monocytes per well were added. Both cell types were incubated together using MEM-E/HAM's F-12 for further 9 days followed by 5 days of three-point bending with different frequencies and durations.
Resorption Assay
In order to determine osteoclast resorption activity, isolated monocytes were seeded 5 × 105 per well on dentin chips for 14 days cultured with Alpha-Medium (Biochrom AG, Berlin, Germany) including 10% FCS. Nuclear factor-μB ligand (RANKL (20 ng/ml); Peprotech Inc, Rocky Hill, NJ, USA) and MCSF (5 ng/ml) (Sigma) were added for stimulation of fusion to multinuclear cells.
After 14 days cell number was determined using Alamar Blue Assay, and 3 × 104 osteoblast-like cells per well were added. Further cell cultivation of both cell types in MEM-E/HAM's F-12 was performed in absence of RANKL and MCSF for 3–5 days. After this pre-incubation period, the cells were mechanically stimulated by three-point bending for 5 days.
During cell cultivation, 500 μl medium was changed every 3rd day in both setups. The supernatant was stored at −20 °C for further analysis.
Assays for Determination of Cell Metabolism
Cell viability was determined using a cell activity assay (AlamarBlue) at day 1 (before stimulation) and at day 6 in accordance with the instruction of the manufacturer.
After co-cultivation the cells were fixed with formaldehyde and stained for tartrate-resistant acid phosphatases (TRAP) by adding naphtol AS-MX phosphate and fast red violet. Polynucleated (more than 3 nuclei) TRAP-positive cells were considered as osteoclast-like cells and counted using a microscope (100× magnification) equipped with a camera and an image analysis system to mark and count the cells (Microlaser microscope, P.A.L.M.; Carl Zeiss Microscope, Jena, Germany).
A pit formation assay was performed to further evaluate the activity of bone resorption by osteoclasts in co-culture. After removal of the cells with 1 mol/l NH4OH, the resorption lacunae (pits) on the dentin were stained with toluidine blue and counted (100× magnification, Microlaser microscope, P.A.L.M).
Additional quantification of osteoclasts’ resorptive activity was carried out by determination of cross-linked N-telopeptide of type I collagen (NTX-ELISA, Osteomark®; Waltham, MA, USA). The amount of TRAP-iso5b was measured by ELISA (Metra Biosystems; Mountain View, CA, USA) as a marker for osteoclast number and activity and indirect quantification of bone resorption rate. The enzyme cathepsin K as an osteoclast marker was also quantified by ELISA (Biomedica, Vienna, Austria).
Type I C-terminal collagen propeptide, produced by active osteoblasts was measured by ELISA (C1CP; Metra Biosystems). For quantification of osteoclastogenesis factors produced by osteoblasts, the amount of OPG and sRANKL-release was determined by ELISA (Immundiagnostik, Bensheim, Germany).
Total protein was measured in cell culture supernatant with the Pierce Coomassie Plus Protein Assay Reagent (Biozol), a colorimetric method for total protein quantification.
All tests were performed according to the manufacturers’ instructions.
Statistical Analysis
All experiments were performed in triplicate with cells from three different donors (total of experiments n = 9). ANOVA for independent samples was used for comparison of data and controlled with Dunnett correction for comparison against control. Statistical differences were defined at a 95% confidence level. SPSS (release 14.0; SPSS Inc. Chicago, IL, USA) software supported statistical evaluation.
Results
All data are given in percent of the mechanically unloaded control (100%). The results of the ELISAs, such as C1CP, osteocalcin, OPG, sRANKL, TRAP-iso5b and NTX, were normalized to total protein.
Differentiation Assay
No significant influences of the mechanical stimulation were seen on cell viability and total protein. The osteoclast differentiation assay showed a slight decrease of TRAP-positive multinuclear cells after stimulation for 1 min with 0.1 Hz, but this result was not significant compared to control. A slight but insignificant increase in the sRANKL/OPG ratio was seen after all mechanical stimulations (table 1).
Table 1.
Results of differentiation assay in% to control (100%), mean ± SD
| Control | 1 min 0.1 Hz | 1 min 0.3 Hz | 3 min 0.1 Hz | 3 min 0.3Hz | 5 min 0.1 Hz | |
|---|---|---|---|---|---|---|
| Cell count | 100 ± 22.49 | 133.38 ± 56.34 | 128.48 ± 45.49 | 130.94 ± 37.40 | 138.38 ± 56.87 | 117.12 ± 28.31 |
| Protein | 100 ± 14.06 | 89.78 ± 34.64 | 92.55 ± 15.42 | 98.49 ± 24.78 | 103.70 ± 26.65 | 97.13 ± 21.81 |
| TRAP-positive cells | 100 ± 9.70 | 75.72 ± 24.26 | 91.57 ± 39.99 | 87.12 ± 42.73 | 92.10 ± 41.87 | 88.96 ± 40.76 |
| sRANKL/OPG | 100 ± 49.12 | 135.30 ± 102.02 | 134.21 ± 62.10 | 128.09 ± 75.82 | 125.31 ± 84.74 | 172.78 ± 78.91 |
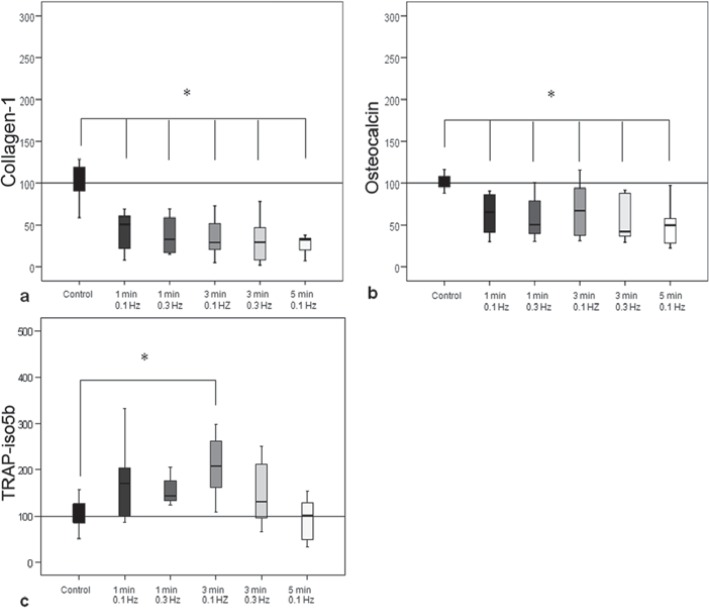
A significant decrease in the procollagen-1 production and osteocalcin synthesis was detectable in all stimulations compared to unstimulated co-culture (p < 0.027) (fig. 1a, b).
Fig. 1.
Results of the differentiation assay. a Collagen-1 synthesis by osteoblasts in co-culture with osteoclasts was significantly decreased in all groups compared to unstimulated group (control = 100%). b The osteocalcin synthesis by osteoblasts was also significantly decreased in all stimulated groups compared to unstimulated group. c Mechanical stimulation for 3 min with 0.1 Hz significantly increased the TRAP-iso5b compared to the unstimulated group (control = 100%).
Stimulation for 3 min with 0.1 Hz resulted in a significant (p = 0.008) higher amount of TRAP-iso5b produced by osteoclast-like cells compared to the unstimulated control (fig. 1c).
Resorption Assay
In the resorption assay, the cell viability and total protein were also uninfluenced by the mechanical stimulation. No significant influence on osteocalcin and catepsin K synthesis was detectable in this assay (table 2).
Table 2.
Results of resorption assay in% to control (100%), mean ± SD
| Control | 1 min 0.1 Hz | 1 min 0.3 Hz | 3 min 0.1 Hz | 3 min 0.3 Hz | 5 min 0.1 Hz | |
|---|---|---|---|---|---|---|
| Cell count | 100 ± 10.32 | 68.76 ± 24.16 | 92.15 ± 36.45 | 76.01 ± 9.96 | 84.21 ± 51.30 | 92.75 ± 36.81 |
| Protein | 100 ± 15.51 | 97.45 ± 26.39 | 101.55 ± 25.64 | 102.40 ± 34.82 | 104.66 ± 78.61 | 93.60 ± 18.26 |
| Osteocalcin | 100 +/-23.43 | 104.58 ± 35.75 | 87.56 ± 22.94 | 91.85 ± 23.68 | 118.45 ± 73.81 | 98.93 ± 37.53 |
| Cathepsin K | 100 ± 21.75 | 87.80 ± 38.96 | 117.07 ± 54.28 | 81.20 ± 30.54 | 133.47 ± 35.94 | 118.58 ± 43.16 |
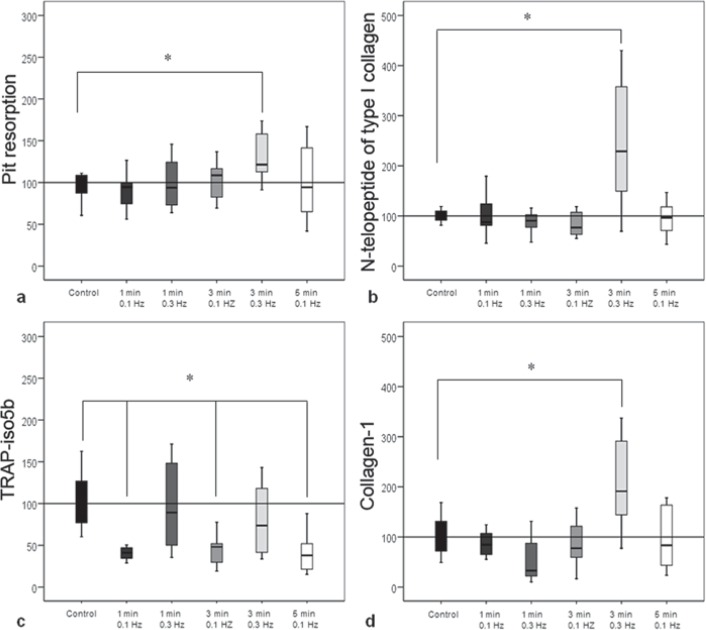
The number of resorption pits was significantly (p = 0.048) enhanced after stimulation for 3 min with 0.3 Hz (fig. 2, 3a). This increased activity was confirmed by the quantification of cross-linked N-telopeptide of type I collagen (1 min with 0.3 Hz to control, p < 0.00) (fig. 3b). The measurement of TRAP-iso5b showed a significant (p < 0.05) decrease after stimulation with 0.1 Hz at all durations (fig. 3c).
Fig. 2.
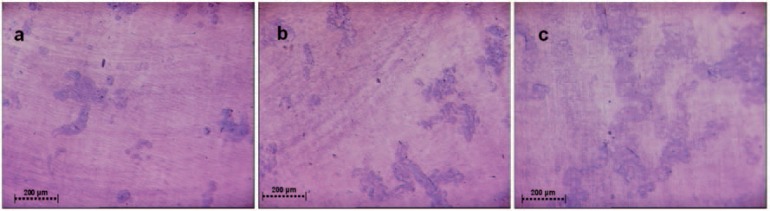
Resorption assay. a Resorption pits of the control group (no stimulation), b after stimulation for 1 min with 0.1 Hz, and c represented the stimulation group for 3 min with 0.3 Hz. In this group, resorption activity was increased.
Fig. 3.
Results of resorption assay. a The number of resorption pits was significantly increased after stimulations for 3 min with 0.3 Hz. b Quantification of resorptive activity by osteoclasts by determination of cross-linked N-telopeptide of type I collagen. A significant increase was measurable after stimulation for 3 min with 0.3 Hz. c All stimulations with 0.1 Hz resulted in significantly decreased TRAP-iso5b activity compared to unstimulated control. d The collagen-1 production by osteoblasts after co-cultivation with resorbing osteoclasts was significantly increased after stimulations for 3 min with 0.3 Hz compared to unstimulated control.
A significant (p = 0.002) increase in the synthesis of procollagen-1 by osteoblasts was seen after stimulation for 3 min with 0.3 Hz (fig. 3d).
Discussion
The hypothesis of the study was that mechanical stimulation influences the interaction of osteoblasts and osteoclasts in co-culture. To investigate this, co-cultures of osteoblast- and osteoclast-like cells were mechanically stimulated by three-point bending with different frequencies and durations. In the differentiation assay of the co-culture a significant decrease in collagen-1 and osteocalcin production by osteoblast-like cells was detected. The resorption assay of the co-culture revealed a significant increase in the pit formation, collagen degradation but also de novo collagen-1 production after stimulation for 3 min with 0.3 Hz.
Comparing the results from the co-culture to the experiments with the single osteoblast or osteoclast cultures investigating the same parameter demonstrates similarities but also differences. The decrease in collagen-1 production by osteoblast-like cells in the co-culture was also detectable in the single osteoblast culture [18]. The stimulating effect seen in the co-culture resorption assay (3 min with 0.3 Hz: increase in resorption and collagen-1 production), however, was not detected in the single osteoclast or single osteoblast culture. In contrast, a shorter and weaker stimulation (1 min with 0.3 Hz or 3 min with 0.1 Hz) resulted in a decreased osteoclastic activity [18]. Comparison of both studies, the single culture experiments and the present results from the co-culture, clearly demonstrates that the direct interaction of the cells have a high influence on the response of the cells to mechanical stimulation. From the single osteoblast-like culture one would expect an increased osteoclastic activity due to the increased sRANKL/OPG ratio after stimulation for 5 min with 0.1 Hz. The co-culture experiments resulted in an increased resorption activity, however, at a different stimulus (3 min with 0.3 Hz). In the single osteoclast culture, this stimulation had no effect on the resorption activity.
Only few studies investigating the effect of mechanical stimuli in co-culture systems of bone marrow cells or osteocyte-like cells and osteoclast-like cells are described in literature. Kim et al. [11] analyzed osteoclastogenesis of RAW264.7 cells co-cultured with murine bone marrow cells (ST-2) induced by fluid flow. In the single cell culture the fluid flow induced a significant decrease of sRANKL but an increase of OPG expression by the bone marrow cells. In the co-culture of the osteoclast-like cells and marrow cells the fluid flow significantly decreased the number of TRAP-positive cells and the resorption lacunae. The group continued the experiments and investigated the effect of fluid flow on osteocyte-like cells (MLO-Y4) [24]. Depending on the post-stimulation time, they found a down regulation of sRANKL (24 h) and up-regulation of OPG (2 h) on the protein level. The conditioned medium from the stimulated MLO cells decreased the osteoclastogenesis of RAW264.7 cells cultured with MLO cells or ST-2 bone marrow cells.
Lau et al. [27] used low-magnitude, high-frequency vibration (LMHF) to stimulate MLO-Y4 osteocytes and found a stimulation-dependent increased COX-2 expression and a significantly reduced RANKL expression. The stimulation of RAW264.7 cells with the conditioned medium significantly decreased the formation of osteoclast-like cells with more than 10 nuclei and the resorption activity. These results indicate that the marrow cells and the osteocyte-like cells respond to mechanical stimulation by increasing OPG while decreasing RANKL synthesis, resulting in an inhibition of osteoclastogenesis. The authors conclude that dynamic fluid flow at a physiological level or LMHF inhibits osteoclastogenesis by soluble factors synthesized by osteoblast-like cells and osteocytes.
The three-point bending used for mechanical stimulation in the present study revealed contrary results by an enhanced resorption activity after mechanical stimulation of the co-culture. One explanation could be the cell type used: Kim et al. [11] investigated a marrow cell line, You et al. [24] and Lau et al. [27] an osteocyte cell line (MLO-Y4), and in the present study primary osteoblast-like cells were used. It is known that the three cell types have a different phenotype and function in vivo [28]. This assumption is supported by the comparison of the effect of pulsating fluid flow on osteocytes, osteoblasts, and periosteal fibroblasts. Using the conditioned medium of the three cell types, the medium from the osteocytes inhibited osteoclastogenesis and resorption activity, whereas the medium from the osteoblast-like cells affected only with less extent the osteoclastogenesis [29]. The medium from the fibroblasts had no effect on the osteoclast-like cells. The authors concluded that the nitric oxide pathway in osteocytes is partially necessary for the communication between osteocytes and osteoclasts. In addition, a comparison of the mechanical stimulation is hardly possible. Both, the fluid flow used by Kim et al. [11] and You et al. [24] and the bending strain used in the present study are assumed to be in a physiological range, but they might have different effects on the cells. In addition, the stimulation duration differed in a great extent. In the present study the cells were stimulated over 5 days daily for 5 min. Kim et al. [11] stimulated for 1 h and analyzed the cell reaction after 0, 1.5, 24, and 72 h, whereas You et al. [24] stimulated for 2 h with 2 and 24 h rest [24]. A further important difference between the studies that might cause the different effects is the co-culturing approach. In the present study the co-culture was mechanically stimulated, whereas in the study by Kim et al. [11] only the bone marrow line was stimulated and then the RAW cells were added. This means that the osteoclast-like cells were only influenced by factors secreted from the marrow cells but not by the mechanical stimuli itself [11]. The studies from Lau et al. [27] and Tan et al. [29] used conditioned medium from the mechanically stimulated osteoblasts or osteocytes and added this to the osteoclast-like cells, which were not mechanically stimulated.
In our opinion, it is necessary to stimulate both cell types in a co-culture to investigate possible effects. Just recently a study was published investigating the effect of cultivation modifications with respect to media composition on the co-culture of human bone marrow stromal cells and human monocytes [23]. Depending on the used medium, the expression of osteoblast- or osteoclast-specific markers varied. As a marker for osteoclasts the authors used TRAP-iso5b, just like in the present study. In addition, monocytes cultured under the present conditions showed also the ability to resorb dentin as an indication for functionally active osteoclasts.
An explanation for the stimulated osteoclastic activity seen in the present study might be the relatively short stimulation (1–5 min/day). It might be speculated that this slight stimulation increases osteoclastic activity to resorb bone as physiologically seen in case of disuse or weightlessness [30] and that the stimulation used by Kim et al. [11] and You et al. [24] (2 h) may represent overuse and therefore a reduced bone resorption and increased bone formation as seen physiologically during mechanical loading [31].
The properties of the dentin, however, limited the duration and strains that could be used for stimulation [18], but the results clearly demonstrate that a short stimulation causes already changes in cell metabolism and cell activity and influences cell-cell interactions. This stimulation device and the established co-culture now allow further studies to investigate also the biological influences (e.g. the effect of growth factors) on cells under mechanical stimulation.
This study clearly shows that not only the strain influences the behavior of the cells, but also the duration and the frequency and the interaction with other cell types. For a tissue engineering approaches this means that not only the direct strain that acts on the cell determines the behavior of the cell, but also the duration and the frequency of mechanical stimulation affects the cells. In addition to these parameters, the environment including other cell types will have a dramatic effect. This very complex situation can hardly be mimicked in vitro. Cell reactor experiments can be a further approach to approximate the in vivo situation [32]. But to really test the efficacy of biomaterials or tissue engineering approaches used for defect regeneration, animal models are necessary.
Disclosure Statement
The authors declare that they have no conflict of interest.
Acknowledgment
This study was partially supported by a grant from the German Research Foundation (Deutsche Forschungsgemeinschaft; KFO 102, TP11).
References
- 1.Frost HM. Perspectives: bone's mechanical usage windows. Bone Miner. 1992;19:257–271. doi: 10.1016/0169-6009(92)90875-e. [DOI] [PubMed] [Google Scholar]
- 2.Eickhoff JA, Molczyk L, Gallagher JC, De Jong S. Influence of isotonic, isometric and isokinetic muscle strength on bone mineral density of the spine and femur in young women. Bone Miner. 1993;20:201–209. doi: 10.1016/s0169-6009(08)80001-3. [DOI] [PubMed] [Google Scholar]
- 3.Rubin CT, McLeod KJ. Promotion of bony ingrowth by frequency-specific, low-amplitude mechanical strain. Clin Orthop Relat Res. 1994;165–174 [PubMed] [Google Scholar]
- 4.Kolk A, Handschel J, Drescher W, Rothamel D, Kloss F, Blessmann M, Heiland M, Wolff KD, Smeets R. Current trends and future perspectives of bone substitute materials – from space holders to innovative biomaterials. J Craniomaxillofac Surg. 2012;40:706–718. doi: 10.1016/j.jcms.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willie B, Petersen A, Schmidt-Bleek K, Cipitria A, Mehta M, Strube P, Lienau J, Wildemann B, Fratzl P, Duda G. Designing biomimetic scaffolds for bone regeneration: why aim for a copy of mature tissue properties if nature uses a different approach? Soft Matter. 2010;6:4976–4987. [Google Scholar]
- 7.Hofbauer LC, Kuhne CA, Viereck V. The OPG/RANKL/RANK system in metabolic bone diseases. J Musculoskelet Neuronal Interact. 2004;4:268–275. [PubMed] [Google Scholar]
- 8.Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473:201–209. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Kreja L, Liedert A, Hasni S, Claes L, Ignatius A. Mechanical regulation of osteoclastic genes in human osteoblasts. Biochem Biophys Res Commun. 2008;368:582–587. doi: 10.1016/j.bbrc.2008.01.106. [DOI] [PubMed] [Google Scholar]
- 10.Kusumi A, Sakaki H, Kusumi T, Oda M, Narita K, Nakagawa H, Kubota K, Satoh H, Kimura H. Regulation of synthesis of osteoprotegerin and soluble receptor activator of nuclear factor-kappaB ligand in normal human osteoblasts via the p38 mitogen-activated protein kinase pathway by the application of cyclic tensile strain. J Bone Miner Metab. 2005;23:373–381. doi: 10.1007/s00774-005-0615-6. [DOI] [PubMed] [Google Scholar]
- 11.Kim CH, You L, Yellowley CE, Jacobs CR. Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through RANKL and OPG signaling. Bone. 2006;39:1043–1047. doi: 10.1016/j.bone.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Saunders MM, Taylor AF, Du C, Zhou Z, Pellegrini VD, Jr., Donahue HJ. Mechanical stimulation effects on functional end effectors in osteoblastic MG-63 cells. J Biomech. 2006;39:1419–1427. doi: 10.1016/j.jbiomech.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Tang L, Lin Z, Li YM. Effects of different magnitudes of mechanical strain on Osteoblasts in vitro. Biochem Biophys Res Commun. 2006;344:122–128. doi: 10.1016/j.bbrc.2006.03.123. [DOI] [PubMed] [Google Scholar]
- 14.Kurata K, Uemura T, Nemoto A, Tateishi T, Murakami T, Higaki H, Miura H, Iwamoto Y. Mechanical strain effect on bone-resorbing activity and messenger RNA expressions of marker enzymes in isolated osteoclast culture. J Bone Miner Res. 2001;16:722–730. doi: 10.1359/jbmr.2001.16.4.722. [DOI] [PubMed] [Google Scholar]
- 15.MacQuarrie RA, Fang CY, Coles C, Anderson GI. Wear-particle-induced osteoclast osteolysis: the role of particulates and mechanical strain. J Biomed Mater Res B Appl Biomater. 2004;69:104–112. doi: 10.1002/jbm.b.20031. [DOI] [PubMed] [Google Scholar]
- 16.Rubin J, Biskobing D, Fan X, Rubin C, McLeod K, Taylor WR. Pressure regulates osteoclast formation and MCSF expression in marrow culture. J Cell Physiol. 1997;170:81–87. doi: 10.1002/(SICI)1097-4652(199701)170:1<81::AID-JCP9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 17.Rubin J, Fan X, Biskobing DM, Taylor WR, Rubin CT. Osteoclastogenesis is repressed by mechanical strain in an in vitro model. J Orthop Res. 1999;17:639–645. doi: 10.1002/jor.1100170504. [DOI] [PubMed] [Google Scholar]
- 18.Kadow-Romacker A, Hoffmann JE, Duda G, Wildemann B, Schmidmaier G. Effect of mechanical stimulation on osteoblast- and osteoclast-like cells in vitro. Cells Tissues Organs. 2009;190:61–68. doi: 10.1159/000178022. [DOI] [PubMed] [Google Scholar]
- 19.Loomer PM, Ellen RP, Tenenbaum HC. Osteogenic and osteoclastic cell interaction: development of a co-culture system. Cell Tissue Res. 1998;294:99–108. doi: 10.1007/s004410051160. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Amodio S, Beertsen W, Everts V. (Pre-)osteoclasts induce retraction of osteoblasts before their fusion to osteoclasts. J Bone Miner Res. 2004;19:1722–1731. doi: 10.1359/JBMR.040509. [DOI] [PubMed] [Google Scholar]
- 21.Irie A, Takami M, Kubo H, Sekino-Suzuki N, Kasahara K, Sanai Y. Heparin enhances osteoclastic bone resorption by inhibiting osteoprotegerin activity. Bone. 2007;41:165–174. doi: 10.1016/j.bone.2007.04.190. [DOI] [PubMed] [Google Scholar]
- 22.Bernhardt A, Thieme S, Domaschke H, Springer A, Rosen-Wolff A, Gelinsky M. Crosstalk of osteoblast and osteoclast precursors on mineralized collagen–towards an in vitro model for bone remodeling. J Biomed Mater Res A. 2010;95:848–856. doi: 10.1002/jbm.a.32856. [DOI] [PubMed] [Google Scholar]
- 23.Heinemann C, Heinemann S, Worch H, Hanke T. Development of an osteoblast/osteoclast co-culture derived by human bone marrow stromal cells and human monocytes for biomaterials testing. Eur Cell Mater. 2011;21:80–93. doi: 10.22203/ecm.v021a07. [DOI] [PubMed] [Google Scholar]
- 24.You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, Kingery W, Malone AM, Kwon RY, Jacobs CR. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42:172–179. doi: 10.1016/j.bone.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Husheem M, Nyman JK, Vaaraniemi J, Vaananen HK, Hentunen TA. Characterization of circulating human osteoclast progenitors: development of in vitro resorption assay. Calcif Tissue Int. 2005;76:222–230. doi: 10.1007/s00223-004-0123-z. [DOI] [PubMed] [Google Scholar]
- 26.Sorensen MG, Henriksen K, Schaller S, Henriksen DB, Nielsen FC, Dziegiel MH, Karsdal MA. Characterization of osteoclasts derived from CD14+ monocytes isolated from peripheral blood. J Bone Miner Metab. 2007;25:36–45. doi: 10.1007/s00774-006-0725-9. [DOI] [PubMed] [Google Scholar]
- 27.Lau E, Al-Dujaili S, Guenther A, Liu D, Wang L, You L. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone. 2010;46:1508–1515. doi: 10.1016/j.bone.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235:176–190. doi: 10.1002/dvdy.20603. [DOI] [PubMed] [Google Scholar]
- 29.Tan SD, de Vries TJ, Kuijpers-Jagtman AM, Semeins CM, Everts V, Klein-Nulend J. Osteocytes subjected to fluid flow inhibit osteoclast formation and bone resorption. Bone. 2007;41:745–751. doi: 10.1016/j.bone.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res. 2006;21:605–615. doi: 10.1359/jbmr.060107. [DOI] [PubMed] [Google Scholar]
- 31.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005;36:1030–1038. doi: 10.1016/j.bone.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Petersen A, Joly P, Bergmann C, Korus G, Duda GN. The impact of substrate stiffness and mechanical loading on fibroblast-induced scaffold remodeling. Tissue Eng Part A. 2012;18:1804–1817. doi: 10.1089/ten.TEA.2011.0514. [DOI] [PubMed] [Google Scholar]