Summary
Increasing scientific knowledge and technical innovations in the areas of cell biology, biotechnology and medicine resulted in the development of promising therapeutic approaches for the prevention and treatment of human diseases. Advanced therapy medicinal products (ATMPs) reflect a complex and innovative class of biopharmaceuticals as these products are highly research-driven, characterised by innovative manufacturing processes and heterogeneous with regard to their origin, type and complexity. This class of ATMP integrates gene therapy medicinal products, somatic cell therapy medicinal products and tissue engineering products and are often individualized and patient-specific products. Multiple challenges arise from the nature of ATMPs, which are often developed by micro, small and medium sized enterprises, university and academia, for whom regulatory experiences are limited and regulatory requirements are challenging. Regulatory guidance such as the reflection paper on classification of ATMPs and guidelines highlighting product-specific issues support academic research groups and pharmaceutical companies to foster the development of safe and effective ATMPs. This review provides an overview on the European regulatory aspects of ATMPs and highlights specific regulatory tools such as the ATMP classification procedure, a discussion on the hospital exemption for selected ATMPs as well as borderline issues towards transplants/transfusion products.
KeyWords: ATMP, European Community, Medicinal product regulatory issues
ATMPs Are Setting New Challenges in Comparison to Small Molecule Medicinal Products
The novelty, complexity and extreme diversity of ATMPs demand new regulatory tools to allow an appropriate balancing of the risks and benefits for the patients [1]. The manufacture of ATMPs cannot be controlled as precisely as that of a chemically synthesised small-molecule product. Characterisation of the impurities poses a particular challenge. In addition, the mechanisms of action for most applications are not well established and, therefore, it is difficult to decide for cell-based therapies whether any sub-group of cells is a part of the active pharmaceutical ingredient or a potentially harmful impurity [2]. Also, cell-based therapies may work through different mechanisms as compared to conventional medicinal products, i.e., they are not metabolised but may be integrated into or rejected by the recipient [3]. Therefore, pharmacokinetic studies such as metabolism and excretion studies become less relevant, and classical carcinogenicity studies are not expected. However, the biodistribution studies especially of stem cell-based ATMPs need to be addressed in more detail to predict migration and differentiation patterns, and the persistence of cells in the patient. Animal studies as performed with small-molecule medicinal products may not be reliable because the species specificity of cells may result in markedly different interactions between the cells and their environment in animal models compared to the clinical situation. Thus, for biologicals, relevant animal models, i.e., models in which the test material is pharmacologically active so that the response leads to meaningful conclusions for the intended clinical indication, should be used wherever possible. However, for ATMPs it is often challenging to identify or generate such relevant models.
Regulatory Aspects for Marketing Authorisation in Europe
To achieve harmonised market availability within the European Union (EU), the European Commission (EC) has established a dedicated ATMP Regulation (Regulation (EC) No. 1394/2007) [4], which came into force December 30, 2008. The Regulation is supported by an amendment of the medical code (Directive 2001/83/EC), which contains updated definitions of gene therapy medicinal products (GTMPs) and cell therapy medicinal products (CTMPs). This amendment also contains specialised requirements for the marketing authorisation of ATMPs and for tissue-engineered medicinal products (TEPs), which are now defined as a class of ATMPs [5].
With implementation of the ATMP Regulation, the centralised marketing authorisation application (MAA) procedure becomes mandatory for ATMPs. To take into account the innovative character of these medicinal products, a new Committee for Advanced Therapies (CAT) was established at the European Medicines Agency (EMA) in London. The CAT comprises members with specific expertise in the area of ATMPs and scientifically evaluates the MAA preparing a draft opinion to be transmitted to the Committee for Medicinal Products for Human Use (CHMP) for adoption. The CAT gathers the expertise on this type of products in the European Community, whereas its composition ensures appropriate coverage of all scientific areas relevant to advanced therapies, i.e., gene therapy, cell therapy, tissue engineering, medical devices, pharmacovigilance and ethics. Moreover, representatives of patient associations and clinicians are part of the CAT to add the view on the medicinal products from the patient's perspective. This is particularly relevant since a lot of ATMPs are intended to treat conditions which are less in the focus of general product development.
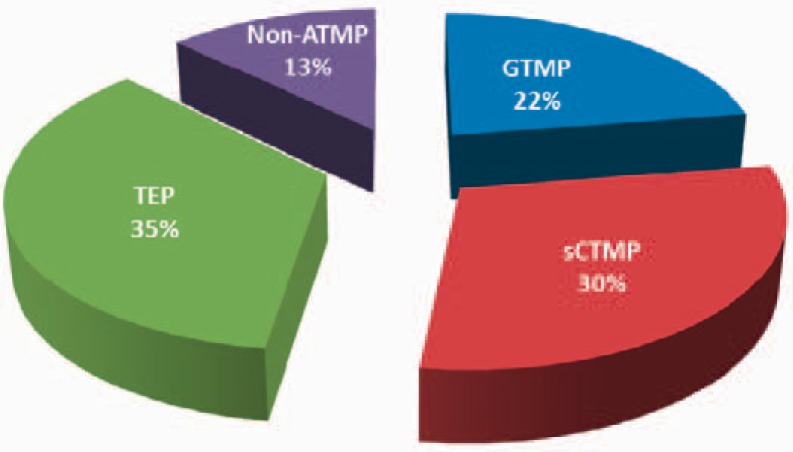
The CAT is responsible for all regulatory procedures concerning ATMP in the EU; inter alia the classification, certification and scientific evaluation of ATMP in centralized marketing authorizations. The CAT interacts with other EMA scientific committees, working parties and sections, e.g. the Pediatric Committee, the Scientific Advice Working Party and EMA's Innovation Task Force. One important task of the committee is the scientific recommendation of classification of ATMP according to Article 17 of the ATMP Regulation. Article 17 states that any applicant developing a product based on genes, cells or tissues may request a scientific recommendation on classification to establish whether the referred product falls, on scientific grounds, within the definition of an ATMP. Thus, the CAT provides a recommendation on ATMP classification after consultation with the European Commission within 60 days after receipt of the request. The outcome of all recommendations on classification is published at the EMA website where all summary reports are listed from July 2011 onwards, after deletion of all information of commercial confidential nature. The proportion of the different classes of medicinal products that had been subject to classification is visualised in figure 1.
Fig. 1.
Relation of the CAT classification recommendations from June 2009 until April 2013 in percent. The CAT provided classification recommendations for 71 medicinal products under development.
The majority of the 71 medicinal products, for which a classification was recommended, are considered as ATMPs, while only 13% were regarded as non-ATMPs. The ATMPs that were introduced to the CAT are nearly similarly distributed between the three product groups, with a majority being TEPs (35%), followed by somatic CTMPs and GTMPs (30% and 22% respectively).
While the classification of a medicinal product as a GTMP depends on the addition of a recombinant nucleic acid sequence, the classification as TEP or CTMP based on the condition of the cells being ‘engineered’, which requires the fulfillment of one of the following two conditions:
i) the cells have been subject to substantial manipulation or
ii) the cells are not intended to be used for the same essential function(s) in the recipient and the donor (non-homologous use).
This is to account for the fact that in both cases the cells, even if autologous in origin, will face a new physiological microenvironment after application, either because the cells have been changed or the environment has been changed, and their behaviour in this new environment may not be predicted from their former behaviour. A substantial manipulation is defined as one that alters biological characteristics, physiological functions or structural properties relevant for the intended regeneration, repair or replacement. One well-established example is the long-term in vitro expansion and/or in vitro differentiation of cells. In particular, the manipulations listed in Annex I of the ATMP Regulation shall not be considered as substantial manipulations [4].
The experience of the CAT with regard to the ATMP classification procedures resulted in the Reflection Paper on Classification of Advanced Therapy Medicinal Products that was published in January 2013 [6]. The paper includes a discussion on some borderline cases such as transplant/transfusion. The ATMP classification is a non-mandatory, free of charge, legally non-binding procedure that helps developers to clarify the applicable regulatory framework. It also provides clarity on the development path and scientific regulatory guidance to be followed. The ATMP classification may also be a useful tool for applicants to initiate a tailored dialogue on the product development with regulators on the European level. Due to its simple and fast process, the ATMP classification, along with other tools at the EMA (e.g. Innovation Task Force Briefing Meetings), is a first opportunity to engage with regulatory bodies. Once the candidate ATMP classification has been confirmed, the dialogue can continue with ATMP certification [7], which is exclusively provided for ATMP.
ATMP Classification – the Borderline to Transplant/Transfusion Products
Medicinal products consisting of cells or tissues are regulated via the Tissues and Cells Directive (Directive 2004/23/EC) [8], unless they are ATMP and hence fall under the ATMP Regulation. Within this area a borderline cannot always be drawn easily. One example is the recommendation of the CAT that a preparation of human pancreatic Langerhans’ islets should not be classified as an ATMP. CAT considered that, for this preparation, the described process steps do not constitute substantial manipulations for the intended use so that there is no change in the biological characteristics of the islets. In addition, the product was intended to be used for the same essential function in the recipients. This conclusion is, however, not directly applicable to any other pancreatic beta cell products which may be submitted for classification, as they may be derived from a very different and more complex process including steps that may be understood as substantial manipulations.
Some products appeared to be non-ATMP because of an only minimal manipulation or maintenance of the initial biological properties, and autologous origin has been classified by the CAT as ATMP due to their intended heterologous use. For example, autologous bone marrow-derived progenitor cells intended for treatment of patients with myocardial infarction, or even concentrates of autologous bone marrow intended for the increase of new bone formation in a critical area of atrophic non-union is considered non-homologous use and therefore the medicinal product is classified as ATMP. All ATMP classifications given by the CAT are not legally binding as the classification of medicinal products is within the responsibility of the respective National Competent Authority. However, the CAT classification procedure is one important step forward to the harmonisation of the regulatory landscape in the European community.
The Hospital Exemption for Specific ATMPs
The ATMP Regulation determines that medicinal products classified as ATMPs shall be regulated under the centralised European Marketing Authorisation procedure. Marketing authorisation is granted by the European Commission following assessment by the EMA. Article 28 of the ATMP Regulation [4] defines an exemption from the central authorisation requirement for ATMPs which are prepared on a non-routine basis and used within the same member state in a hospital in accordance with a medical prescription for an individual patient, the so-called hospital exemption. Member states are required to implement this community requirement for a hospital exemption by putting in place arrangements at the national level to meet the specific requirements set out in the ATMP Regulation.
The hospital exemption (Article 28) is applicable to all ATMPs that are
– preparation on a non-routine basis
– preparation according to specific quality standards (equivalent to those for ATMPs with a centralised marketing authorisation)
– used within the same member state
– used in a hospital
– used under the exclusive responsibility of a medical practitioner
– comply with an individual medical prescription for a custom-made product for an individual patient.
The authorisation on the basis of the hospital exemption is in the remit of the corresponding member state. Therefore, the CAT is not formally involved in the hospital exemption authorisation processes.
The Hospital Exemption for Specific ATMPs in Germany
Hospital exemption as implemented in Germany is an option for ATMPs utilised for highly innovative treatments that are fulfilling the criteria set by Article 28 of the ATMP Regulation. One example may be highly personalised patient-specific GTMPs that consist of autologous cells loaded with nucleic acids that are tumor-specific. For such a medicinal product, hospital exemption may be regarded as a suitable tool to support development and availability of these products, and thereby providing also specific treatment options for physicians and patients. Moreover, it also may be suitable to guide a particular ATMP into routine manufacturing towards a central marketing authorisation. The hospital exemption procedure in Germany, which is performed by the Paul-Ehrlich-Institut, is set up to ensure compliance with community rules for safety and efficacy, put in place appropriate standards for quality control of the manufacturing process including compliance with the good manufacturing practice requirements, review of available data/information and a discussion on the benefit/risk balance.
Conclusion
ATMPs are used in clinical settings, targeting many conditions with unmet medical needs. Numerous challenges arise from the derivation and nature of ATMP-based products. In this context, current European regulatory guidance such as the reflection paper on classification or product-specific guidelines are intended to support academic research groups and pharmaceutical companies to foster the development of innovative medicinal products. For a successful development of ATMPs an early dialogue between the scientific/clinical community and regulatory agencies at national as well as European level is of paramount importance. This way, we can expect to overcome the challenges and stimulate an informed regulatory environment.
Disclaimer
The views expressed here are personal and do not necessarily reflect the views of the Paul-Ehrlich-Institut.
Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Committee for Advanced Therapies (CAT) CAT Scientific Secretariat. Schneider CK, Salmikangas P, Jilma B, Flamion B, Todorova LR, Paphitou A, Haunerova I, Maimets T, Trouvin JH, Flory E, Tsiftsoglou A, Sarkadi B, Gudmundsson K, O'Donovan M, Migliaccio G, Ancāns J, Maciulaitis R, Robert JL, Samuel A, Ovelgönne JH, Hystad M, Fal AM, Lima BS, Moraru AS, Turcáni P, Zorec R, Ruiz S, Akerblom L, Narayanan G, Kent A, Bignami F, Dickson JG, Niederwieser D, Figuerola-Santos MA, Reischl IG, Beuneu C, Georgiev R, Vassiliou M, Pychova A, Clausen M, Methuen T, Lucas S, Schüssler-Lenz M, Kokkas V, Buzás Z, MacAleenan N, Galli MC, Lin??? A, Gulbinovic J, Berchem G, Fraczek M, Menezes-Ferreira M, Vilceanu N, Hrubisko M, Marinko P, Timón M, Cheng W, Crosbie GA, Meade N, di Paola ML, VandenDriessche T, Ljungman P, D'Apote L, Oliver-Diaz O, Büttel I, Celis P. Challenges with advanced therapy medicinal products and how to meet them. Nat Rev Drug Discov. 2010;9:195–201. doi: 10.1038/nrd3052. [DOI] [PubMed] [Google Scholar]
- 2.Salmikangas P, Flory E, Reinhardt J, Hinz T, Maciulaitis R. Regulatory requirements for clinical trial and marketing authorisation application for cell-based medicinal products (in German) Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2010;53:24–29. doi: 10.1007/s00103-009-0991-5. [DOI] [PubMed] [Google Scholar]
- 3.Reinhardt J, Flory E, Büttel I, Schröder C, Fricke S, Vucinic V, Cross M, Niederwieser D MSCs. clinical applications and European regulatory aspects. In: Hematti P, Keating A, editor. Mesenchymal Stromal Cells. Biology and Clinical Applications. New York: Springer; 2013. pp. 355–364. [Google Scholar]
- 4.Regulation (EC) 1394/2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation No 726/2004.
- 5.Sanzenbacher R, Dwenger A, Schuessler-Lenz M, Cichutek K, Flory E. European regulation tackles tissue-engineering. Nat Biotechnol. 2007;25:1089–1091. doi: 10.1038/nbt1007-1089. [DOI] [PubMed] [Google Scholar]
- 6.EMA : Reflection Paper on Classification of Advanced Therapy Medicinal Products. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/12/WC500136422.pdf
- 7.Berger A, Schule S, Flory E. The certification of advanced therapy medicinal products. A quality label for product development in small and medium-sized enterprises (in German) Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2011;54:816–821. doi: 10.1007/s00103-011-1301-6. [DOI] [PubMed] [Google Scholar]
- 8.Directive 2004/23/EC of the European Parliament and of the Council (31 March 2004) on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:102:0048:0058:EN:PDF