Abstract
We report the case of a patient treated with dabrafenib and trametinib (mitogen-activated protein kinase pathway inhibitors) for stage 3b cutaneous melanoma who developed bilateral uveitis. Although there have been reports of ocular side effects with this class of drugs, uveitis has not been previously reported to the best of our knowledge. This case indicates the wide range of side effects that can be seen with the newer targeted biological therapies.
Key words: Dabrafenib, Trametinib, Uveitis, Melanoma
Introduction
Molecularly targeted therapies are potentially more specific with fewer toxic side effects than many traditional chemotherapeutic agents used in the treatment of cancer [1]. Nevertheless, they can be associated with a variety of toxicities. We report a case of a patient treated with dabrafenib and trametinib [mitogen-activated protein kinase (MAP kinase) pathway inhibitors] for stage 3b cutaneous melanoma who developed bilateral uveitis while on therapy. To the best of our knowledge, although there have been reports of ocular side effects with this class of drugs, uveitis has not been previously reported [2].
Case Report
A 64-year-old male underwent adjuvant treatment with oral dabrafenib 150 mg b.d. and oral trametinib 2 mg o.d. for a cutaneous nodular ulcerating melanoma under his left breast, as part of the COMBI-AD clinical trial (a trial of dabrafenib and trametinib after surgery to remove melanoma). He previously underwent surgical resection of the tumour (Breslow thickness 2.15 mm, BRAF gene positive, stage 3b) and also had positive sentinel node biopsies. Ocular examinations form part of the protocol of this trial, owing to previous associations between these agents and retinal vein occlusion and central serous chorioretinopathy [3].
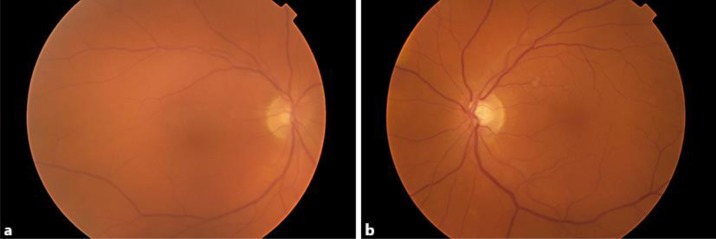
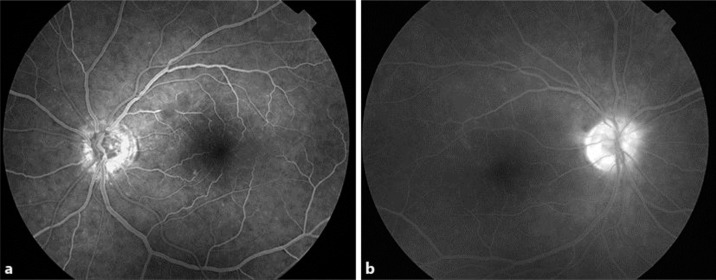
At his initial screening visit, ocular examination was unremarkable, apart from an area of peripheral retinal degeneration in his right eye. Two weeks after starting the trial medications, he experienced pyrexia and malaise. His medications were temporarily stopped and he was started on ibuprofen; an ocular examination at this stage also proved normal. One week later, the decision was made to stop the trial medications permanently. However, he developed floaters in the left eye after a few days, and an ocular examination revealed vitritis in both eyes (2+ vitreous cells, 0.5+ vitreous haze), with vitreous snowballs in the left eye anterior to the superotemporal retinal arcade (fig. 1). His visual acuity remained unaffected at 6/6. Fluorescein angiography indicated early patchy choroidal hyperfluorescence in both eyes together with late optic disc leakage (fig. 2).
Fig. 1.
Fundus photographs demonstrating 0.5+ vitreous haze in both eyes associated with vitreous snowballs anterior to the superotemporal retinal arcade in the left eye. All findings resolved spontaneously without treatment.
Fig. 2.
Fluorescein angiography demonstrating early patchy choroidal hyperfluorescence in a, and late optic disc leakage in b.
Endogenous endophthalmitis was considered unlikely owing to the absence of focal chorioretinal involvement, the lack of significant immunosuppression and the absence of any predisposing invasive procedures within the recent past. A presumptive diagnosis of drug-induced inflammation was made, and it was elected to observe him without additional treatment. Within 6 weeks, all signs of ocular inflammation had resolved without treatment and without sequelae.
Discussion
Dabrafenib and trametinib are inhibitors of the MAP kinase pathway, a pathway that ultimately leads to cellular proliferation. MAP kinase pathway inhibitors have been under investigation in the treatment of a number of tumour types, including melanoma, and the aim of combining several MAP kinase inhibitors in a patient is to improve response rates, to delay resistance and to reduce drug toxicity, since lower doses of each drug can be used [4].
Some drugs in this class have been associated with ocular side effects in up to 27% of patients [3]. The most serious reported side effect is retinal vein occlusion [5], but uveitis has not been previously reported to the best of our knowledge. The mechanism behind these ocular side effects remains unclear, but it has been suggested that MAP kinase inhibition can lead to an inflammatory response with consequent breakdown of the blood-retinal barrier [6]. This could potentially compromise ocular immune privilege, generating autoimmune uveitis, thus providing an explanation for the findings in our case [7, 8].
Disclosure Statement
The authors have no conflicts of interest.
Acknowledgements
S.R.J.T. was supported by the UK National Institute of Health Research. The sponsor or funding organization had no role in the design or conduct of this research. This study was approved by the Royal Surrey County Hospital R&D Department (12DEV0010).
References
- 1.Adamina M, Joerger M. Dose-toxicity models in oncology. Expert Opin Drug Metab Toxicol. 2011;7:201–211. doi: 10.1517/17425255.2011.543674. [DOI] [PubMed] [Google Scholar]
- 2.Renouf DJ, Velazquez-Martin JP, Simpson R, Siu LL, Bedard PL. Ocular toxicity of targeted therapies. J Clin Oncol. 2012;30:3277–3286. doi: 10.1200/JCO.2011.41.5851. [DOI] [PubMed] [Google Scholar]
- 3.Leijen S, Middleton MR, Tresca P, Kraeber-Bodere F, Dieras V, Scheulen ME, Gupta A, Lopez-Valverde V, Xu ZX, Rueger R, et al. Phase I dose-escalation study of the safety, pharmacokinetics, and pharmacodynamics of the MEK inhibitor RO4987655 (CH4987655) in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4794–4805. doi: 10.1158/1078-0432.CCR-12-0868. [DOI] [PubMed] [Google Scholar]
- 4.Menzies AM, Long GV, Murali R. Dabrafenib and its potential for the treatment of metastatic melanoma. Drug Des Devel Ther. 2012;6:391–405. doi: 10.2147/DDDT.S38998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Noll R, Leijen S, Neuteboom GH, Beijnen JH, Schellens JH. Effect of inhibition of the FGFR-MAPK signaling pathway on the development of ocular toxicities. Cancer Treat Rev. 2013;39:664–672. doi: 10.1016/j.ctrv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Huang W, Yang AH, Matsumoto D, Collette W, Marroquin L, Ko M, Aguirre S, Younis HS. PD0325901, a mitogen-activated protein kinase kinase inhibitor, produces ocular toxicity in a rabbit animal model of retinal vein occlusion. J Ocul Pharmacol Ther. 2009;25:519–530. doi: 10.1089/jop.2009.0060. [DOI] [PubMed] [Google Scholar]
- 7.de Smet MD, Taylor SR, Bodaghi B, Miserocchi E, Murray PI, Pleyer U, Zierhut M, Barisani-Asenbauer T, LeHoang P, Lightman S. Understanding uveitis: the impact of research on visual outcomes. Prog Retin Eye Res. 2011;30:452–470. doi: 10.1016/j.preteyeres.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Tempest-Roe S, Joshi L, Dick AD, Taylor SR. Local therapies for inflammatory eye disease in translation: past, present and future. BMC Ophthalmol. 2013;13:39. doi: 10.1186/1471-2415-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]