Abstract
Unperturbed fetal development is essential for future health of an individual. Previous studies have linked diseases of aging to harmful alterations that happen during fetal development. Given the significant long-term impact that intrauterine environment has on an individual’s life, it was hypothesized that maternal stress during pregnancy will have negative effects on the offspring’s prenatal and postnatal growth. To test this, twenty-eight female and seven male Wistar rats (Rattus norvegicus) were purchased and bred to produce 176 offspring. During pregnancy, dams were randomly divided into four groups (n=7, per group) and immobilization stress induced as follows; Group 1 (GW1): immobilization stress on days 1–7 of pregnancy, Group 2 (GW2): on days 8–14, Group 3 (GW3): on days 15–21, Group 4 (Controls): left undisturbed. Maternal cortisol hormone, food intake, and weight gain were monitored during pregnancy. Pups were raised under normal laboratory conditions and sacrificed at ages: 4, 8, 12, and 16 weeks to determine the effect of prenatal stress. At necropsy, the tibia was removed and processed for histology. Differences among groups were determined by T-test or analysis of variance (ANOVA). Linear regression analysis was performed to establish the relationship between stress in utero and indicators of bone development in offspring. P values ≤ 0.05 were considered significant. Cortisol hormone levels in controls were lower than those of stressed animals. Stressed dams consumed 12.5% less food per day compared to controls. Animals in GW1 and GW2 gained less weight during pregnancy but had larger litters than did GW3 or the control group. Offspring born to GW3 were heavier compared to all other groups. GW3 offspring had a higher rate of bone formation. In conclusion, stress during pregnancy resulted in increased cortisol and reduced food intake in mothers, but faster growth and higher weight gain in offspring compared to controls.
Keywords: prenatal stress, elevated cortisol, offspring development
Fetal development is important for ensuring the future health of an individual. A number of studies have linked diseases of aging to harmful alterations that happen in critical periods during fetal development [1–3]. During pregnancy some women experience daily stress. These stresses include depression, anxiety, anger, day-to-day challenges, sudden change of environment, social isolation, and pathological conditions [4–6]. The typical somatic response to these and other similar stressors is elevation of cortisol hormone. Cortisol is known to cross the placenta and consequently influence various aspects of development in the human fetus [5]. The effects of elevated cortisol levels on the fetus may vary from defective development [7] to spontaneous abortion [8].
Timing of the stress also plays a crucial role. The outcomes are dependent on whether the mother was exposed to stress during the first, second or third trimester [6]. The first trimester is characterized by rapid organ development and therefore stress exposure in this period leads to widespread, global effects like cognitive dysfunction, heart malformation, cataracts, deafness and genital and intestinal abnormalities. In the second and third trimesters the organs mainly enlarge and undergo refinement. Stress exposure in this period leads to low birth weight, skeletal abnormalities and hearing loss [6].
Psychological stress in mothers has been found to decrease the gestation period, which results in low birth weight [9]. For example, in 1994 when an earthquake of magnitude 6.8 struck the city of Northridge, California USA, women who were pregnant at this time delivered one week earlier on average [10]. On the other hand, after the September 11, 2001, terrorist attack in the United States, pregnant women who were present at or in close proximity to the World Trade Center gave birth to babies with low birth weight, suggesting their growth in utero had been affected [11]. In a different study, maternal perception of negative life events between 24 and 29 weeks gestation was demonstrated to increase the risk of preterm birth [12].
One recently published study conducted a 10-month follow-up of behavioral reactivity to prenatal stress exposure and found it to persist throughout this period [13]. However these results were not consistent with those from an earlier study in which the most significant differences between infants born of stressed mother and those born of non-stressed mothers disappeared by the age of 4 to 5 months [14]. De Bruijin and colleagues (2009)[15] went further and tested cortisol levels of preschool children born of stressed and non-stressed mothers. Children who were prenatally exposed to high cortisol levels had higher cortisol levels when compared to non-exposed children
In animal studies, the effects of maternal stress have been shown to vary based on the type and intensity of stress applied, gender of the offspring, age, strain of the experimental subject used, the timing of stress relative to the critical developmental period of the organ under investigation and nature of the dependent variables evaluated [16]. Since most of these effects last for a long time, the results suggest that stress in utero may lead to a permanent change in gene expression, which results in permanent physiological effects to the individual. A number of animal studies have demonstrated that most defects reported in offspring due to stress in utero occur when stress is induced during the last trimester of gestation. This is the period when the gonads, reproductive tract, lymphoid organs, and the brain differentiate [16]. The results are contrary to the human studies reviewed in the previous section, in which more global defects are manifested in offspring when exposed to stress in utero in the first trimester, and lower birth weight associated in maternal stress in the third trimester.
Given the significant long-term impact that intrauterine environment has on an individual’s life [1,3,17–19], it was hypothesized that maternal prenatal stress will have a negative effect on the offspring’s development, in part as a result of exposure of the fetus to high levels of cortisol.
The objective of this study was to determine how the timing of the negative affects of prenatal stress, and presumably high cortisol levels in utero, impacts weight gain and bone development in the offspring. The hypothesis tested was that environmentally induced stress on a pregnant female has significant negative impact on the general development of her offspring, and ultimately on the offspring’s weight gain and skeletal development, despite the offspring’s postnatal environment being stress-free. This hypothesis was tested using data collected during a twelve-month experimental protocol on rats.
MATERIALS AND METHODS
Animal Experiments
All animal experiments were approved by the University of California Berkeley, Institutional Animal Care and Use Committee (IACUC). The study was carried out using a total of 211 Wistar rats (Rattus norvegicus). Twenty-eight females, sexually mature, 9–10 weeks old weighing 200–250 grams, and 7 males, established breeders, weighing 400–500 grams, were purchased from Charles River, Hollister, California, USA. The animals were allowed to acclimatize to the vivarium for one week prior to the start of experiments. The animals were bred to obtain 176 offspring.
Housing
The animals were housed in rat cages according to the guidelines developed by IACUC. The number of animals per cage was determined based on estimated weight at various ages to ensure the rats had adequate cage-floor space per animal. The animal housing room was maintained under standard laboratory conditions with 12-hour light/dark cycle, 22°C temperature and 50% relative humidity. Rats were maintained on commercial rodent chow (Rodent Diet, Cat# 2918, Teklad, Madison, WI, USA) with 0.95% calcium and 0.67% phosphorus.
Breeding of Animals for Timed Pregnancies
Before breeding, females were determined to be in estrus by behavioral observation. The female was moved to a male cage and observed for an average of 10 to 15 minutes. Estrous females were left in the male cage for 24 hours. Conception was confirmed by monitoring weight gain. Pregnant females gain about 6 grams of weight per 24 hours compared to 3 grams for non pregnant females.
Stress induction to pregnant mothers at various times of gestation
Pregnant rats were divided into four groups of 7 rats each.
Group 1 (GW1): immobilization stress on days 1–7 of pregnancy
Group 2 (GW2): immobilization stress on days 8–14 of pregnancy
Group 3 (GW3): immobilization stress on days 15–21 of pregnancy
Group 4 (Controls): left undisturbed throughout their pregnancy
Immobilization stress was administered in three, 45 minutes sessions; 9am, 1pm and 5pm. Stress was administered by placing the rats in standard rodent sized immobilization bags called DecapiCones (Braintree Scientific, Inc. Cat# DC200, Braintree, MA, USA) [20]. The immobilization bags were modified as follows: 1) the tip was snipped to allow the rat’s snout to protrude; 2) in order to minimize hyperthermia small holes were punched on the sides of the bag to increase ventilation: 3) the wide end of the bag was twisted around the tail of the animal and securely taped to conform to the individual size of the animals. The rat in the bag was placed in an empty cage. Only their tails and snouts protruded from the bag, and they did not have access to either food or water during this time. The secured animals could not make any movements other than shuffling their paws. They could not turn their heads to the side nor turn their bodies within the bags. However, they had sufficient room for their rib cage to expand with heavy breathing.
The cages containing the restrained rats were placed in a well-lit fume hood to contain the unpleasant odors emitted by stressed rats. The constant flow of air in the hood minimized hyperthermia during the procedure. During stress sessions, the animals were monitored every 15 minutes, from the time they were placed in the DecapiCone bags until they were returned to their home cages. Animals were examined for signs of strenuous breathing or body distortion into potentially painful or harmful positions. At the end of the 45 minutes, the rat was removed from the bag, and returned to its home cage. Animals were observed for the following: chromodacryorrhea, reduced feed consumption or total failure to feed, difficulty in breathing, excessive weight loss, ruffled fur, lameness, bloody vaginal discharge or abortion.
The pregnant females were weighed daily following each stress session, and every other day during the other two weeks of the pregnancy.
Fecal matter sampling
Fecal samples were collected from all 28 pregnant rats to determine whether or not the immobilization stress resulted in elevated cortisol levels. Fecal sampling was done on day 2 and 5 of pregnancy for GW1, day 9 and 12 of pregnancy for GW2, day 15 and 18 of pregnancy for GW3, and on days 2, 5, 9, 12, 15, and 18 of pregnancy for the control group. On the days that cortisol levels were monitored, the monitoring was done multiple times over the course of 24 hours. Pregnant rats were housed alone on recycled paper bedding, (Tek-fresh, Cat# 7099, Harlan Laboratories, Madison Wisconsin, USA). To sample fecal matter, fresh bedding was provided every 4 hours (at 8am, 12pm, 4pm, 8pm, and 12am). Fecal samples were then taken from the soiled bedding and stored at −80°C immediately after collection until analysis.
Extraction of Fecal Corticosterone Metabolites (CMs)
Each sample was put in an aluminum foil cup and dried in an oven at 60°C for a minimum of 4 hours. The time it took for each sample to dry varied, with some taking as long as 12 hours. Each sample was then crushed and homogenized with a mortar and pestle. An amount of 0.1g of each sample was placed in a clean test tube and 2ml of 80% methanol added. The samples were then shaken 3 times for 30 seconds each, using a hand vortex. This was followed by centrifugation at a speed of 2500g for 15 minutes. An amount of 0.5 ml of the supernatant was then transferred to a clean vial and evaporated under a gentle stream of nitrogen. These samples were then assayed by 5α-pregnanae-3β, 11β,21-triol-20-one enzyme immunoassay (EIA) [21–23]
Monitoring food intake of the pregnant dams
To determine whether immobilization stress had an effect on the caloric intake of the dams, the amount of food intake during the stress period was compared to the amount of food intake when the dams were not being stressed. This was done by measuring the weight of food placed in the cage at the beginning of a 24-hour cycle and subtracting the weight of the food at the end of the 24 hours every day during pregnancy. The cage floor was checked to ensure any spilled food pellets were collected and measured as well. This procedure did not apply to the offspring.
Rearing of the offspring
The offspring were raised to various stages of maturity and then sacrificed to determine the effect of the prenatal stress.
All of the 28 dams gave birth on day 21 or 22 of pregnancy, as is standard for rats. The litter sizes ranged from 5 to 21 (Table 1). Since a maximum of 8 pups was required per dam, culling was done for those mothers with more than 8 pups soon after delivery to standardize the litter size. This helped avoid excessive food competition among the offspring during the first three weeks of their life in which they mainly depend on breastfeeding for nutrition. The pups were weaned at 3 weeks, the mothers sacrificed, and the weanling rats housed at 4 per cage. At 6 weeks of age, the pups were housed as adults in accordance with the guidelines developed by IACUC.
Table 1.
Number of pups born in each group (Mean±SD)
| Groups of mothers n=7 per group | Mean±SD of litter size | Range of litter size | Minimum number of pups born | Maximum number of pups born |
|---|---|---|---|---|
| Control | 11.14±3.98 | 9 | 6 | 15 |
| GWI | 13.14±1.68 | 4 | 11 | 15 |
| GW2 | 13.86±1.77 | 5 | 11 | 16 |
| GW3 | 12.29±5.82 | 16 | 5 | 21 |
GWI = gestation week 1; GW2 = gestation week 2; GW3 = gestation week 3
Pups were raised under normal laboratory conditions with standard diets and sacrificed at predetermined ages: 4 weeks, 8 weeks, 12 weeks, and 16 weeks. There were 4 main pup groups defined by whether and when the mother was subjected to immobilization stress or not. Within these 4 main pup groups there were four sub-groups consisting of 11 offspring sacrificed at each stage of the predetermined stages of development.
Euthanasia and necrospy
All animals were weighed before euthanasia. The rats were euthanized by CO2 inhalation. This was followed by bilateral thoracotomy for rats over 200 grams or cervical dislocation for rats less than 200 grams, as a secondary measure to ensure the animals were dead. The right tibia was excised and cleaned. The tibia was fixed in 10% phosphate buffered formalin at 4°C for 24 hours then transferred to 70% ethanol for storage until processing [24]. All bones in solutions were stored at 4°C.
Bone Histomorphometry Measurements
This section focused on the comparison between offspring of the control mothers and offspring of GW3 mothers. The decision to initially focus on GW3 offspring was informed by the fact that this is the week during which rats’ bones mineralize. It was therefore hypothesized that maternal stress during this stage of pregnancy will have the greatest impact on the quality of the bone of the offspring.
The right proximal tibia was dehydrated in graded concentration of ethanol and xylene and embedded undecalcified in methyl methacrylate. Longitudinal sections, 4-μm thick, were obtained using a Leica RM 2265 microtome (Leica Microsystems, Nussloch GmbH, Germany) and affixed to slides coated with a 2% gelatin solution. The 4-μm sections were stained with Tetrachrome, then counterstained with Von Kossa, and mounted with Permount. The stained sections were used to measure; a) trabecular total tissue area, b) trabecular bone area, c) trabecular bone perimeter, d) osteoblast surface, e) osteoid surface f) number of osteoclasts, g) erosion surface, h) the thickness of the upper zone of the growth plate (consisting of resting and proliferating chondrocytes), and i) the thickness of the lower zone of the growth plate (consisting of the hypertrophic chondrocytes) using a light microscope (Nikon Eclipse E400, Nikon Instruments, Inc, Melville, NY, USA) at a magnification of ×20. Bone histomorphometric analyses were performed in the growth plate and a region corresponding to secondary spongiosa, 1mm distal to the growth plate. Bone histomorphometry was performed using a semi-automatic image analysis Bioquant system (Bioquant Image Analysis, Nashville, TN, USA) linked to a microscope equipped with transmitted and fluorescent light (Nikon Eclipse E400, Epi-Fluorescence Microscope, Nikon Instruments, Inc, Melville, NY, USA) [25,26].
The bone measurements, units and terminology used in histomorphometric analysis were defined and derived according to the American Society for Bone and Mineral Research (ASBMR) guidelines for bone histomorphometry [27,28]. The growth plate measurements were defined and derived according to Howell and Dean (1992) [29].
Statistical Analysis
The mean and standard deviation was calculated for all outcome variables. The differences in the means of cortisol hormone level in the stressed groups and control group were analyzed using T-test. Differences among the four maternal groups for gestational weight gain were analyzed by analysis of variance (ANOVA). Differences were considered statistically significant at p-value ≤ 0.05 and inter-group differences were determined by an F-test with the Tukey-Kramer Honestly Significant Difference test (JMP 8 Statistical Discovery. Cary, NC: SAS Institute Inc) as a post-hoc test for pair-wise comparisons.
Bone histomorphometry data, was first analyzed using a two-sample t-test to compare the male and female differences in the total tissue area of all offspring at the ages of 4, 8, 12 and 16 weeks. The total tissue area was used as a proxy for size of the tibia in this study. This first analysis was mainly designed to test whether there was a size difference between the males and females.
In the next step of analysis, the GW3 offspring were separated from control offspring and a two sample t-test used to compare the male and female differences in the total tissue area of the offspring at each age, that is, 4, 8, 12 and 16 weeks.
Subsequently, a statistical model that removes the male and female difference was used to isolate the stress effect. Also, since the number of male and female pups from the GW3 mothers and control mothers was not equal, the sample size difference between males and females had to be removed in order to compare GW3 and Control offspring.
To accomplish this, a linear regression model, which allowed the difference in mean outcome between GW3 offspring and control offspring to vary with pups’ age was used. Wald test was used to check for how in utero stress during week three of gestation affected bone development in offspring at different ages (StataCoRP. 2009. Stata; Release 11. Statistical software. College Station, TX: StataCorp LP).
| Equation 1 |
In the equation, (yi) is the outcome variable, the bone histology measurements taken, and the predictor variables are as follows, sex of the pups (X1), whether the pups were born to GW3 mothers (X2), age of the pups (X3, X4, X5), whether the prenatal stress effect was different at different ages (X6, X7, X8), and the error term (εi).
If the difference in mean outcome for GW3 offspring and control offspring did not vary with pups’ age (p > 0.10), stress effect was estimated with a model that controlled for pups’ sex and age.
| Equation 2 |
All models adjusted standard errors for correlation between pups from the same litter using the Huber-White Sandwich estimator.
These equations were developed based on the explanation of how to analyze correlated data (www.ats.ucla.edu/stat/stata/library/cpsu.htm) using the statistical program, Stata 11 (StataCoRP. 2009. Stata; Release 11. Statistical software. College Station, TX: StataCorp LP).
This study design was such that each group had pups from the same litter, which are correlated. To correctly analyze the data we had to take into account the correlation. P values ≤ 0.05 were considered significant.
RESULTS: RESULTS ON LIVE ANIMAL DATA
Maternal weight gain from pregnancy day 1 to day 21
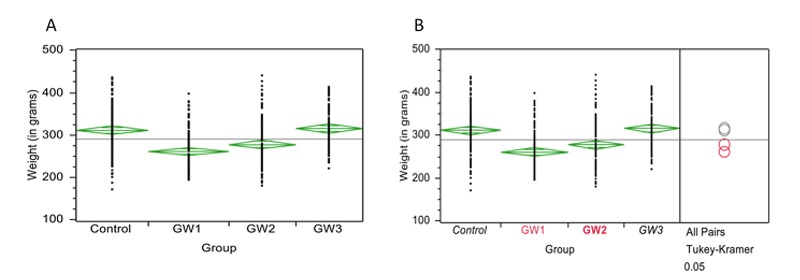
Pregnant rats in GW1 and GW2 groups gained significantly less weight over the duration of the pregnancy than did those in GW3 or in the control group (Figure 1A). The results showed the mean weight gain of the control and GW3 mothers were not significantly different from each other. The mean weight gain of GW1 and GW2 mother groups were also not significantly different from each other (Figure 1A). However the mean weight gain of the controls and GW3 mothers were significantly higher than the mean weight gain of the GW1 and GW2 mothers (p < 0.0001) (Figure 1B).
Figure 1.
Maternal weight gain during pregnancy. A. Comparison of maternal gestational weight gain in GW1, GW2, GW3 and Control mothers. The figure shows the results of one-way ANOVA of weight by maternal group. The side points of each diamond are connected by a horizontal line at the mean of each group. The top and bottom diamond points are the upper and lower 95% confidence points of each group. B. Comparison for all pairs of means of maternal gestational weight gain using Tukey-Kramer (HSD). The Tukey-Kramer (HSD) added comparison circles to the plot and statistically compared each pair of means. There is a circle for each group with a horizontal diameter that aligns with its group mean. Circles with the same color are not statistically different while those with different colors represent groups with statistically different means.
Cortisol hormone level in dams during gestation period
Cortisol hormone levels were used as a surrogate measure of stress [21–23]. In this study, cortisol hormone was quantified as micrograms per gram of feces (μg/g) [21–23].
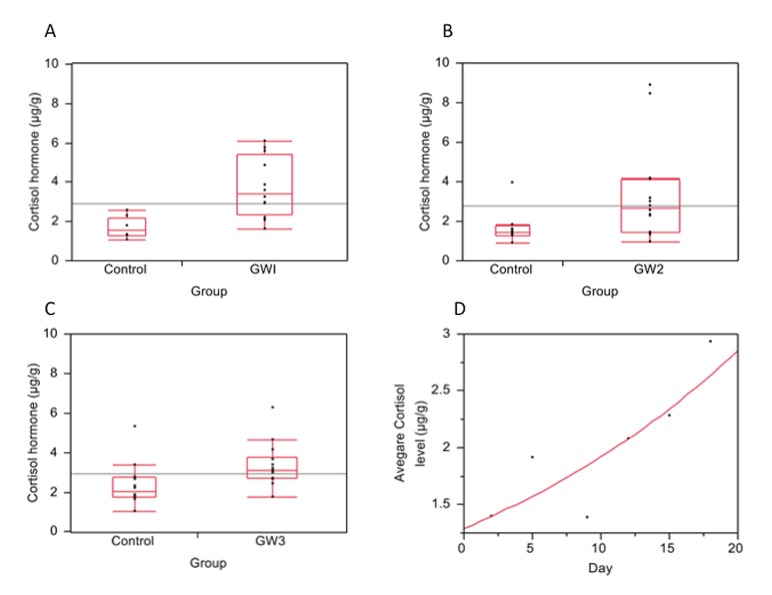
The mean cortisol hormone levels in GW1 mothers were significantly higher compared to those of control mothers in their first week of pregnancy (GW1 = 3.72μg/g, controls = 1.66μg/g, difference = 2.06μg/g; p = 0.0007) (Figure 2A).
Figure 2.
Cortisol (stress) hormone levels in dams during pregnancy. Cortisol hormone levels measured and quantified as micrograms per gram of feces (μg/g) in: A. Control mothers during pregnancy week 1 and GW1 mothers; B. Control mothers during pregnancy week 2 and GW2 mothers; C. Control mothers during pregnancy week 3 and GW3 mothers; D. Control mothers from day 1 to 20 of gestation.
The mean cortisol hormone levels in GW2 were also significantly higher compared to those of control mothers in their second week of pregnancy (GW2 = 3.34μg/g, controls = 1.73μg/g, difference = 1.62μg/g; p = 0.0205) (Figure 2B). However, the cortisol hormone level difference between the controls in week two and GW2 was less compared to the hormone level difference between the controls in week one and GW1. Similarly, the mean cortisol hormone levels in GW3 mothers were significantly higher compared to those of control mothers in their third week of pregnancy (GW3 = 3.36μg/g, controls = 2.38μg/g, difference = 0.98μg/g; p = 0.017) (Figure 2C). However, the difference in cortisol hormone level between controls and GW3 was the least compared to the difference between controls and GW1 and the difference between controls and GW2.
To further explore the apparent declining levels of difference in cortisol hormone levels between controls and stressed animals as the pregnancy advanced, the hormone levels for controls were plotted independently and found to increase significantly with the progression of the pregnancy (P = 0.0344) (Figure 2D).
However results from a pair wise comparison of mean cortisol level in control rats during the first week, second week, and third week of pregnancy using the Tukey-Kramer HSD test was not significant (p = 0.0960). A similar trend was also observed when cortisol hormone levels in stressed groups were compared (p = 0.8409).
Therefore, since increase in cortisol hormone over the course of the pregnancy in controls did not represent a significant pattern, the cortisol levels measured for all three weeks of the control group were combined as one group for the next analysis. Also, since the stressed groups were not significantly different from each other, all three groups were combined as one for the next analysis.
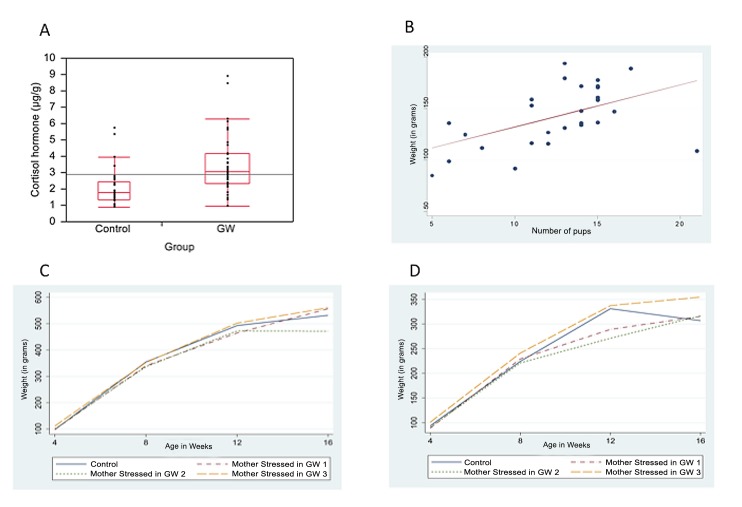
T- test results for the combined means for the control and stressed groups showed that cortisol level was significantly higher in the rats stressed during pregnancy compared to the control rats (controls = 2.12μg/g, GW = 3.46μg/g, difference = 1.35 μg/g, p = 0.0003) (Figure 3A).
Figure 3.
Overall comparison of means of cortisol hormone levels between control and stressed mothers, Litter size and Offspring weight gain. A. Overall comparison of means of cortisol hormone levels from all control mothers and all stressed mothers during pregnancy; B, Correlation between the number of pups bornand weight (in grams) gained by the mother during pregnancy; C, Mean weight (ingrams) of male offspring at age 4, 8, 12 and 16 weeks; D, Mean weight (in grams) of female offspring at age 4, 8, 12 and 16 weeks.
Food intake by the mothers during pregnancy
The stressed animals consumed 3 grams (12.5%) less food per day compared to the controls (p < 0.005). On the day before delivery all of the animals increased their food intake, almost doubling their norm (data on file).
Litter size
The number of pups born to females in GW1 and GW2 groups was greater on average than for the controls or GW3 (Table 1). However, there was much more variation in the number of pups born in the control and GW3 groups and much less variation in the number of pups born in GW1 and GW2 groups (Table 1). There was a positive correlation between maternal weight gained during pregnancy and the number of pups born (p = 0.0054) (Figures 3B).
Offspring weight gain
Both male and female offspring born to GW3 mothers were heavier compared to all the other groups (Figures 3C and 3D respectively). However, pair-wise comparison of the means in weight for each group using the Tuker-Kramer HSD test showed no statistically significant difference for males (p = 0.33) or females (p = 0.9).
RESULTS OF THE HISTOMORPHORMETRIC ANALYSIS OF THE OFFSPRING’S PROXIMAL TIBIA
As mentioned in the methods, this section focuses on the comparison between offspring of the control mothers and offspring of GW3 mothers.
Total tissue area
The combined means for all the GW3 offspring and control offspring indicated that the total tissue area in the secondary spongiosa region in males was significantly larger compared to the females at age 8, 12 and 16 weeks (p < 0.001) (Table 2).
Table 2.
Difference in means of total tissue area (T.Ar) between male and females offspring from GW3 mothers and control mothers.
| Age in weeks | 4 | 8 | 12 | 16 |
|---|---|---|---|---|
| Difference in T.Ar between male and female offspring from both GW3 and Control mothers | 0.07 (n=12) | 2.12*** (n=21) | 2.18*** (n=20) | 2.35*** (n=26) |
| Difference in T.Ar between male and female in GW3 offspring | 0.46 (n=7) | 2.05*** (n=13) | 2.89*** (n=8) | 2.65* (n=8) |
| Difference in T.Ar between male and female Control offspring | 0.41 (n=5) | 2.34* (n=8) | 1.61* (n=12) | 2.19** (n=18) |
T.Ar = tissue area; GW3 = gestation week 3;
= p < 0.05;
= p < 0.01;
= p < 0.001
In the next step of analysis where GW3 offspring were separated from control offspring. The means for GW3 offspring and control offspring indicated that in both groups, the males were significantly larger than the females at age 8, 12 and 16 weeks (p < 0.05) (Table 2).
From the above results, it is evident that total tissue area varies by sex, regardless of whether the pups were born to a GW3 mother or a control mother. Controlling for sex, there was no difference in mean total tissue area for GW3 offspring compared to control offspring at ages 4, 8, 12 and 16 weeks (Table 3).
Table 3.
Bone histomorphometry measurements performed in the proximal tibia of the offspring from the control group and the mothers subjected to immobilization stress in gestation week 3 (GW3).
| 4 weeks | 8 weeks | 12 weeks | 16 weeks | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | GW3 | Estimated difference adjusted for sex | p value | Control | GW3 | Estimated difference adjusted for sex | p value | Control | GW3 | Estimated difference adjusted for sex | p value | Control | GW3 | Estimated difference adjusted for sex | p value | |
| Number | 5 | 7 | 8 | 13 | 12 | 8 | 18 | 8 | ||||||||
| Total tissue area (mm2) | 4.55 | 4.36 | −0.18 | 0.788 | 6.27 | 6.42 | 0.4 | 0.3 | 7.03 | 6.61 | 0.14 | 0.733 | 6.77 | 7.28 | 0.22 | 0.733 |
| Bone area (mm2) | 1.06 | 1.25 | 0.11 | 0.664 | 1.4 | 2.16 | 0.82*** | 0.001 | 1.27 | 1.61 | 0.3 | 0.16 | 1.39 | 1.53 | 0.17 | 0.58 |
| Bone perimeter (mcm) | 41.39 | 38.62 | −3.77 | 0.714 | 54.18 | 60.74 | 7.4 | 0.288 | 47.27 | 44.43 | −2.51 | 0.779 | 44.53 | 47.68 | 5.02 | 0.542 |
| Osteoblast surface (mm) | 9.59 | 8.88 | −1.48 | 0.61 | 11.31 | 16.8 | 5.91 | 0.144 | 12.14 | 21.66 | 11.60* | 0.04 | 10.28 | 14.92 | 3.62 | 0.255 |
| Osteoid surface (mm) | 0.42 | 2.18 | 1.44 | 0.093 | 1.92 | 4.98 | 3.15* | 0.046 | 0.99 | 5.5 | 4.94* | 0.022 | 1.9 | 3.12 | 0.98 | 0.446 |
| N.Oc per eroded surface (N.Oc/mm) | 45.18 | 245.72 | 233.31 | 0.077 | 95.32 | 120.99 | 26.8 | 0.52 | 89.26 | 102.95 | −29.07 | 0.735 | 74.18 | 66.57 | −9.05 | 0.829 |
| Eroded surface (mm) | 1.46 | 0.92 | −0.64 | 0.074 | 0.94 | 0.81 | −0.07 | 0.597 | 1.31 | 0.87 | −0.33 | 0.359 | 0.98 | 0.72 | −0.22 | 0.329 |
| Proliferative zone growth plate (mcm) | 302.83 | 210.29 | −92.78* | 0.023 | 195.73 | 123.56 | −63.97 | 0.063 | 166.01 | 150.84 | −8.67 | 0.485 | 130.73 | 123.35 | −10.93 | 0.305 |
| Hypertrophic zone growth plate (mcm) | 179.64 | 126.15 | −48.66 | 0.244 | 88.81 | 70.4 | −15.06 | 0.405 | 75.44 | 75.02 | 1.78 | 0.735 | 62.14 | 62.62 | −0.46 | 0.89 |
GWS = gestation week 3; N.Oc = Osteoclast Number;
= p < 0.05;
= p < 0.01;
= p< 0.001
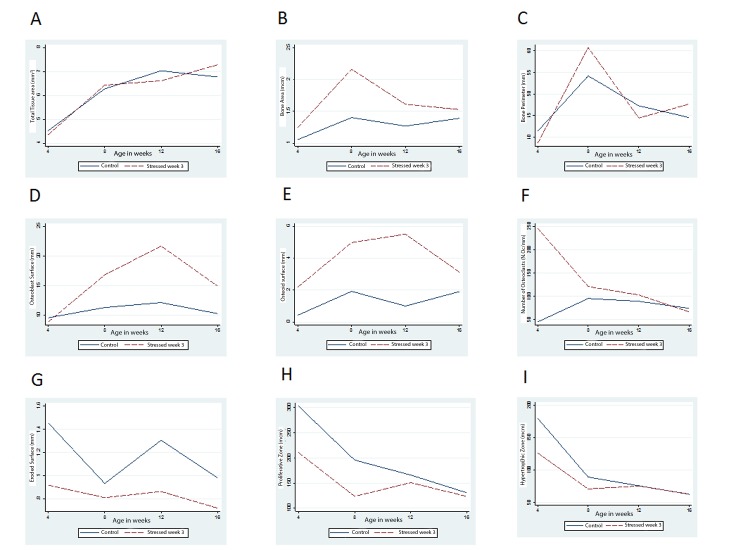
The second linear regression analysis (using equation 1) that examines how in utero stress during week three of gestation affected bone development showed that maternal prenatal stress did not affect total tissue area at different developmental stages, for GW3 offspring compared to the control offspring at age 4, 8, 12 and 16 weeks (p = 0.7200). Therefore, the pattern of bone development in GW3 offspring and control offspring did not vary significantly (Figure 4A).
Figure 4.
The changes in histomorphormetric measurements at age 4, 8, 12 and 16 weeks in GW3 offspring (dashed line) and control offspring (solid line). A. Total tissue area (mm2); B. Bone area (mcm); C. Bone perimeter (mm); D. Osteoblast surface (mm); E, Osteoid surface (mm); F. Number of osteoclasts (N.Oc/mm); G, Eroded surface (mm); H. Upper zone of the growth plate (containing resting and proliferative cells) (mcm); I. Lower zone of the growth plate (containing hypertrophic cells) (mcm).
Bone area
Mean bone area was found to be significantly higher in the GW3 offspring compared to the control offspring at the age of 8 weeks (Table 3). Also, maternal prenatal stress did not affect bone area at different developmental stages, for GW3 offspring compared to the control offspring at ages 4, 8, 12 and 16 weeks (p = 0.3301). Even though the bone area was significantly higher in GW3 offspring at the age of 8 weeks, the overall pattern of bone development in GW3 offspring and control offspring did not vary significantly (Figure 4B).
A regression analysis on the ratio of bone area to total tissue area showed no significant difference in the way maternal prenatal stress affects the ratio at different developmental stages, for GW3 offspring compared to the control offspring at ages 4, 8, 12 and 16 weeks (p = 0.4048).
Bone perimeter
Controlling for sex, there was no difference in mean bone perimeter for GW3 offspring compared to control offspring at age 4, 8, 12 and 16 weeks (Table 3).
Maternal prenatal stress in GW3 did not affect bone perimeter at different developmental stages compared to the control offspring at age 4, 8, 12 and 16 weeks (p = 0.7758). Therefore, the pattern of bone development in GW3 offspring and control offspring did not vary significantly (Figure 4C).
A regression analysis on the ratio of bone perimeter to total tissue area showed no significant difference in the way maternal prenatal stress affects the ratio at different developmental stages, for GW3 offspring compared to the control offspring at ages 4, 8, 12 and 16 weeks (p = 0.0563).
Osteoblast surface
The GW3 offspring had a significantly higher osteoblast surface compared to the control offspring at the age of 12 weeks (Table 3).
There was a significant difference in the way maternal prenatal stress affected osteoblast surface at different developmental stages for GW3 offspring compared to the control offspring at age 4, 8, 12 and 16 weeks (p = 0.0333). Therefore, the patterns of bone formation in GW3 offspring and control offspring varied significantly (Figure 4D).
A regression analysis on the ratio of osteoblast surface to bone perimeter showed a significant difference in the way maternal prenatal stress affects the ratio at different developmental stages for GW3 offspring compared to the control offspring at ages 4, 8, 12 and 16 weeks (p = 0.0415).
Osteoid surface
The mean osteoid surface was significantly higher in GW3 offspring compared to the control offspring at the ages of 8 weeks and 12 weeks (Table 3). However maternal prenatal stress in GW3 was found to have no effect on osteoid surface at different developmental stages compared to the control offspring at age 4, 8, 12 and 16 weeks (p = 0.1006). Even though the osteoid surface was significantly higher in GW3 offspring at the age of 8 weeks and 12 weeks, the overall pattern of bone development in GW3 offspring and control offspring did not vary significantly (Figure 4E). A regression analysis on the ratio of osteoid surface to bone perimeter showed no significant difference in the way maternal prenatal stress affects the ratio at different developmental stages, for GW3 offspring compared to the control offspring at ages 4, 8, 12 and 16 weeks (p = 0.1399).
Osteoclast number/erosion surface
Controlling for sex, there was no difference in the number of osteoclasts per erosion surface for GW3 offspring compared to the control offspring at age of 4, 8, 12 and 16 weeks (Table 3). Maternal prenatal stress in GW3 did not affect the number of osteoclasts per erosion surface at different developmental stages compared to the control offspring at age 4, 8, 12 and 16 weeks (p = 0.1544). Therefore, the pattern of bone development in GW3 offspring and control offspring did not vary significantly (Figure 4F).
A regression analysis on the ratio of osteoclast number to bone perimeter showed no significant difference in the way maternal prenatal stress affects the ratio at different developmental stages, for GW3 offspring compared to the control offspring at ages 4, 8, 12 and 16 weeks (p = 0.4288).
Erosion surface
Controlling for sex, there was no difference in mean bone surface for GW3 offspring as compared to control offspring at age 4, 8, 12 and 16 weeks (Table 3). Maternal prenatal stress in GW3 did not affect the erosion surface at different developmental stages compared to the control offspring at age 4, 8, 12 and 16 weeks (p = 0.4142). Therefore, the pattern of bone development in GW3 offspring and control offspring did not vary significantly (Figure 4G).
A regression analysis on the ratio of erosion surface to bone perimeter showed no significant difference in the way maternal prenatal stress affects the ratio at different developmental stages, for GW3 offspring compared to the control offspring at ages 4, 8, 12 and 16 weeks (p = 0.4288).
Resting and proliferative chondrocyte zone of the growth plate
The mean thickness of the proliferative zone of the growth plate in GW3 offspring was significantly smaller compared to the control offspring at the age of 4 weeks (Table 3).
Maternal prenatal stress was shown to significantly reduce the size of the proliferative zone of the growth plate for GW3 offspring compared to the control offspring at the ages of 4, 8, 12 and 16 weeks (p = 0.0141). The patterns of growth in the upper zone of the epiphyseal plate varied significantly between GW3 offspring and control offspring (Figure 4H).
Hypertrophic chondrocyte zone of the growth plate
Controlling for sex, there was no difference in the thickness of the hypertrophic zone of the growth plate for GW3 offspring compared to control offspring at age of 4, 8, 12 and 16 weeks (Table 3).
Maternal prenatal stress in GW3 did not affect the thickness of the hypertrophic zone of the growth plate for GW3 offspring compared to the control offspring at the ages of 4, 8, 12 and 16 weeks (p = 0.3149). The pattern of growth in the lower zone of the epiphyseal plate did not vary significantly between GW3 offspring and control offspring (Figure 4I).
DISCUSSION
As pregnancy advanced, there was a general trend of incremental weight increase in the female due to fetal growth. However there was a wide range of variation in the amount of maternal weight gained in this study that was likely dependent on the number of fetuses conceived, caloric intake, and the cortisol levels in the dams due to stress. The pregnant dams that were stressed during gestation week 1 and 2 gained significantly less weight compared to those stressed in week 3 or the controls. This was consistent with previous studies in which prenatal stress was induced by glucocorticoid injection [30] and by immobilization [31].
Results also show that stress induction resulted in significantly higher levels of cortisol hormone in stressed dams compared to the controls, a result also consistent with a previous study [31]. Given that cortisol crosses the placenta [32–34], it is plausible that it will alter the in utero environmental condition of the fetuses. While the mean cortisol hormone levels in the stressed mothers were significantly higher compared to those of controls, the average difference between the stressed groups and the controls reduced through time of gestation. Stressing the animals in gestation week 1 more significantly raised their cortisol hormone levels relative to the controls, but the difference declined during pregnancy. When considering only the control group, we find that cortisol level increases throughout gestation even without the stress of immobilization.
Cortisol concentration has been demonstrated to increase significantly during pregnancy in humans as well [35,36]. This can be attributed to production of corticotropin-releasing hormone (CRH) in the placenta, decidua, and fetal membranes [35], enlargement of maternal pituitary gland [37], and increased secretion of corticotropin by the pituitary gland [38]. An increase in estrogen hormone, which is necessary to support pregnancy and inhibit ovulation [39], also results in increased plasma cortisol. This is because the elevated estrogen levels reduce cortisol catabolism by the liver [35]. These series of events during pregnancy lead to a steady rise in cortisol hormone that peaks in the third trimester [35]. The increase in placental glucocorticoids is essential for maturation of the fetal organ system, including the liver, lung, adrenal gland, and brain, in preparation for survival outside the uterine environment [40]. It also influences the timing and onset of delivery [40].
Therefore, a slight increase in cortisol levels during gestation is necessary for proper maturation of the organ system in the fetus before birth, as well as to the timing and onset of parturition [40]. However, excess cortisol (that may result from abnormal circumstances), has deleterious effects. Such circumstances in humans include disease, under-nutrition and depression [41,42]. For lack of a better model to help us understand the long-term effects of stress that goes beyond the normal pregnancy challenge in humans, laboratory animals like rats, sheep, mice and non-human primates have been subjected to conditions that intentionally raise their cortisol hormone levels during gestation, such as restraint, immobilization, forced swimming, forced treadmill exercise, noise stress, nutrient deficiency, electric shock and administration of CRH, reviewed in [43].
Pregnancy alone also leads to increased food intake in rats [44] to meet the increasing nutritional needs of the growing fetuses. In terms of caloric intake though, while there was a general trend of increase in food intake for the pregnant dams in our study, the animals undergoing stress consumed significantly less food compared to the controls. Previous studies in which pregnant rats were injected with cortisol daily also showed that there was significant reduction in daily food intake [45]. High levels of glucocorticoids have been shown to have a catabolic effect, which increases levels of amino acids, glucose, and lipids in plasma. When the levels of these food molecules are high in plasma, the hunger signal in the brain is turned off [20,46]. The catabolic effect of glucocorticoids, as induced by the high cortisol level in stressed dams in this study, is assumed to have resulted in high circulating levels of plasma amino acids, glucose and lipids in plasma [20,46]. As a result, a stressed animals’ food intake reduces. An additional possible explanation for this phenomenon in the current study is that the stressed animals spent 1.25 hours a day without access to food (during immobilization) for one of the three weeks of their pregnancies, reducing the time of opportunity for feeding.
Not surprisingly, a positive correlation was found between maternal weight gained during pregnancy and the number of pups born. However, in contrast to this overall trend, the animals stressed in gestation week 1 and gestation week 2 had the highest average number of pups but gained the least amount of weight. Even though birth weights were not collected in this study, the weight data from the mothers during gestation showed that mothers with significantly higher cortisol levels (GW1 and GW2) had the lowest weight gain on average at the end of their gestation period and the largest average number of pups. We can therefore infer that pups born to GW1 and GW2 mothers had lower birth weight compared to the controls. Since cortisol hormone levels in these two groups were significantly higher than those of the controls the impact of stress to the mother seems to have affected fetal development at this early stage, causing them to be smaller.
Other studies report similar results. For example, social stress during early pregnancy in hamsters resulted in dams giving birth to male pups that were smaller in size compared to the controls [47]. By injecting pregnant rats with cortisol hormone Megías and colleagues (1983)[45] noted a slight reduction in litter size and individual pup weight in hormone treated dams. In human studies, maternal cortisol is known to cross the placenta and likely influence fetal development [32–34]. Elevated fetal cortisol levels, such as evident in fetuses of depressed mothers, result in delayed prenatal growth, prematurity, and low birth weight [48]. Excess cortisol alters the normal functioning of hypothalamo-pituitary-adrenal (HPA) axis, which may last for a lifetime [49].
Studies in humans have demonstrated that stress activates the excessive release of cortisol hormone, which is known to inhibit bone growth and has been shown to be a risk factor for osteoporosis in pre-menopausal women [50,51]. Previous studies have also examined the effects of corticosteroids on growing bones of rat and mice by injecting pregnant mothers with cortisone acetate during the second and third week of gestation [52–55]. In each of these studies the experimental newborns had shorter and smaller long bones, thinner metaphyseal and diaphyseal trabeculae, and abnormal calcification compared to the controls. All these differences were obvious during the first week after birth but by day 30 postnatal no morphological differences were observed in different groups.
In the current study, rather than injecting corticosteroids into the pregnant rats, a method that could prompt the body to produce higher levels of cortisol hormone itself, and therefore mimic the more natural way the mother’s body deals with stressful conditions during pregnancy was employed. The offspring were also studied for a longer time postnatally, up to the age of 16 weeks, which is a young adult age for rats. This allowed for an analysis of bone growth after the apparent catch-up growth period of 30 days postnatal as suggested by previous studies [52–55]. As such, the analysis was done on pups that were 4 to 16 weeks old. Male rats are sexually mature at 12 weeks and classified as adults at 6 months. Female rats on the other hand are sexually mature at 5–6 weeks and classified as adults at 5–6 moths.
The novel observation of our study was that, given similar environmental conditions in postnatal life, offspring born to GW3 mothers and those born to control mothers followed different trajectories in postnatal development. The GW3 offspring developed faster in terms of general body growth and bone growth compared to the control offspring. More specifically in terms of bone development, GW3 offspring had a higher bone area and a potentially higher bone formation rate indicated by a higher number of osteoblasts at the age of 12 weeks, and a larger area of the osteoid surface at the ages of 8 and 12 weeks. Even though this was contrary to expectation, it implies that these pups were growing faster than the controls. This difference manifested by the age of 8 weeks.
In addition to bone mineralization rate, this study also found a differences in how the linear growth rate of bone between GW3 offspring and control offspring. The upper zone of the growth plate, consisting of the resting and the proliferation cells, was significantly thicker in the control offspring at the age of 4 weeks compared to the GW3 offspring. The difference disappeared as soon as the animals reached the age of 8 weeks. The thickness of the growth plate reduces drastically as the animals advance in age, which is expected because it eventually closes, marking the end of the appendicular bone growth period. The results suggest that the control animals had a more active linear growth phase at the younger stage compared to experimental animals. In turn, this may indicate that in utero exposure to cortisol had a significant negative effect on the linear growth of the bone. Previous studies have suggested that corticosteroids inhibit linear growth of bone due to disturbance in the growth plate [56] and disturbance in the formation of the collagen matrix [57–60], the latter potentially mediated through reduction of osteoblastic activity. Therefore, the results demonstrate that prenatal stress significantly affected the growth plate of the offspring. Further investigation is required to understand how this may negatively affect adult skeletal size. Since the GW3 mothers reduced food intake compared to the controls, nutrient restriction and exposure to high cortisol levels likely contributed to the observed outcomes in the GW3 offspring. Having experienced a poor environment in utero the GW3 offspring may have undergone changes in their physiology and metabolism that would enable them to thrive in similar conditions during their postnatal life. An example of such a programming shift could be a more efficient metabolism in order to maximize on the scarce nutritional resources available to them. Such changes during this crucial developmental period have been demonstrated to be permanent and perhaps provide an evolutionary advantage [61].
However, if the offspring are born in an environment that is not as stressful as that experienced in utero, they encounter a mismatch from what their metabolism was programmed for. This may predispose them to metabolic disorders like diabetes at a later stage in life [62]. This phenomenon is currently known as the Thrifty Phenotype Hypotheses and was first proposed by Hales and Barker in 1992 in regard to Type II diabetes [18]. Following a number of studies, they proposed that poor nutrition in utero and during early life of an infant negatively affect the function of Beta cells of the islet of Langerhans, leading to impaired glucose tolerance [18]. This is interpreted to be an adaptation in preparation for a poor nutritional environment in postnatal life, but instead it predisposes the individual to development of Type II diabetes later in life if the poor conditions are not realized [18].
The GW3 offspring in this study were raised in similar conditions to those of the control offspring. Both groups had unlimited access to food and water, and were only handled twice a week when the cages were cleaned and food added to their feeding trays. This was done to minimize the stress from handling as much as possible. Therefore, in the relatively stress-free and nutrient sufficient environment, the GW3 offspring thrived and eventually outperformed the control offspring. They ended up having a higher average body weight, higher bone formation rate, and higher bone area. It is important to note the limitations of this study that will shape the future directions for this research track. First, the offspring birth weight was not measured and neither was sex ratio determined before culling the pups. Second, the offspring daily food intake was not monitored. And third, cortisol levels in the pups were not monitored. These data were not collected in order to avoid extra handling of the pups that would induce further stress postnatally. The handling required to collect these data would have been a confounding factor because the main objective of the study was to determine the effects of prenatal stress. Overcoming the logistical hurdle of imposing confounding stress will be an important next step in teasing apart the biology of the phenomena observed here.
In conclusion, this research demonstrated that the experience of stress during pregnancy in rats has negative consequences for both the mother and the offspring. The caloric intake in the mother is reduced, potentially due to the excess cortisol that alters the hypothalamic control of food intake. Whether or not this reduced food intake results in nutrient restriction remains to be determined, but it does leave the possibility that the offspring may have been nutrient-restricted in utero. We do know from our data that the offspring were at least exposed to high levels of cortisol. Whether both nutrient restriction and high cortisol levels of exposure, or just the cortisol exposure affected the offspring, these animals appear to be born with an altered metabolism that results in faster growth and higher weight gain compared to controls. The positive effect of this fast growth is that the offspring stressed in utero ended up with a higher bone area compared to the control.
Acknowledgments
We thank the advisory committee for this dissertation work, Prof. Leslea J. Hlusko (Chair), Prof. Tim D. White, Prof. Thomas J. Carlson, Prof. Katharine Milton and Prof. Tyrone Hayes for advice, guidance and support. We thank Prof. Maureen Lahiff for help with the statistical analysis. We thank Dr. Peter T. Gakunga and Dr. Wei Yao for technical advice and critical reading of this manuscript. This work was supported by funds from the National Science Foundation grant number BCS 0925788 DDIG to Leslea J. Hlusko and Sarah K. Amugongo; Wenner-Gren Foundation Dissertation Write-Up Fellowship and Wadsworth International Fellowship; Leakey Foundation Franklin Mosher Baldwin Fellowship; University of California Department of Integrative Biology Research Grants to Sarah K. Amugongo; University of California Museum of Paleontology (UCMP) Annie M. Alexander Fellowship; University of California Museum of Vertebrate Zoology (MVZ) Annie M. Alexander Fund.
References
- [1].Barker DJ. Intrauterine programming of adult disease. Mol Med Today. 1995;1:418–423. doi: 10.1016/s1357-4310(95)90793-9. [DOI] [PubMed] [Google Scholar]
- [2].Dennison E, Fall C, Cooper C, Barker D. Prenatal factors influencing long-term outcome. Horm Res. 1997;48(Suppl 1):25–29. doi: 10.1159/000191262. [DOI] [PubMed] [Google Scholar]
- [3].Barker DJ. The fetal and infant origins of disease. Eur J Clin Invest. 1995;25:457–463. doi: 10.1111/j.1365-2362.1995.tb01730.x. [DOI] [PubMed] [Google Scholar]
- [4].Lazinski MJ, Shea AK, Steiner M. Effects of maternal prenatal stress on offspring development: a commentary. Arch Womens Ment Health. 2008;11:363–375. doi: 10.1007/s00737-008-0035-4. [DOI] [PubMed] [Google Scholar]
- [5].Field T, Diego M. Cortisol: the culprit prenatal stress variable. Int J Neurosci. 2008;118:1181. doi: 10.1080/00207450701820944. [DOI] [PubMed] [Google Scholar]
- [6].Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Obel C, Hedegaard M, Henriksen TB, Secher NJ, Olsen J, et al. Stress and salivary cortisol during pregnancy. Psychoneuroendocrinology. 2005;30:647–656. doi: 10.1016/j.psyneuen.2004.11.006. [DOI] [PubMed] [Google Scholar]
- [8].Nepomnaschy PA, Welch KB, McConnell DS, Low BS, Strassmann BI, et al. Cortisol levels and very early pregnancy loss in humans. Proc Natl Acad Sci U S A. 2006;103:3938–3942. doi: 10.1073/pnas.0511183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- [10].Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol. 2001;184:637–642. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- [11].Berkowitz GS, Wolff MS, Janevic TM, Holzman IR, Yehuda R, et al. The World Trade Center disaster and intrauterine growth restriction. JAMA. 2003;290:595–596. doi: 10.1001/jama.290.5.595-b. [DOI] [PubMed] [Google Scholar]
- [12].Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, et al. Maternal stress and preterm birth. Am J Epidemiol. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- [13].Leung E, Tasker SL, Atkinson L, Vaillancourt T, Schulkin J, et al. Perceived maternal stress during pregnancy and its relation to infant stress reactivity at 2 days and 10 months of postnatal life. Clin Pediatr (Phila) 2010;49:158–165. doi: 10.1177/0009922809346570. [DOI] [PubMed] [Google Scholar]
- [14].de Weerth C, van Hees Y, Buitelaar JK. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Hum Dev. 2003;74:139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- [15].de Bruijn AT, van Bakel HJ, Wijnen H, Pop VJ, van Baar AL. Prenatal maternal emotional complaints are associated with cortisol responses in toddler and preschool aged girls. Dev Psychobiol. 2009;51:553–563. doi: 10.1002/dev.20393. [DOI] [PubMed] [Google Scholar]
- [16].Bellinger DL, Lubahn C, Lorton D. Maternal and early life stress effects on immune function: relevance to immunotoxicology. J Immunotoxicol. 2008;5:419–444. doi: 10.1080/15476910802483415. [DOI] [PubMed] [Google Scholar]
- [17].Morton NE. The inheritance of human birth weight. Ann Hum Genet. 1955;20:125–134. doi: 10.1111/j.1469-1809.1955.tb01362.x. [DOI] [PubMed] [Google Scholar]
- [18].Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- [19].Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 1994;86:217–222. doi: 10.1042/cs0860217. discussion 121. [DOI] [PubMed] [Google Scholar]
- [20].Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008;18:158–168. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- [21].Touma C, Palme R, Sachser N. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav. 2004;45:10–22. doi: 10.1016/j.yhbeh.2003.07.002. [DOI] [PubMed] [Google Scholar]
- [22].Touma C, Sachser N, Mostl E, Palme R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol. 2003;130:267–278. doi: 10.1016/s0016-6480(02)00620-2. [DOI] [PubMed] [Google Scholar]
- [23].Lepschy M, Touma C, Hruby R, Palme R. Non-invasive measurement of adrenocortical activity in male and female rats. Lab Anim. 2007;41:372–387. doi: 10.1258/002367707781282730. [DOI] [PubMed] [Google Scholar]
- [24].Iwaniec UT, Wronski TJ, Turner RT. Histological analysis of bone. Methods Mol Biol. 2008;447:325–341. doi: 10.1007/978-1-59745-242-7_21. [DOI] [PubMed] [Google Scholar]
- [25].Yao W, Cheng Z, Shahnazari M, Dai W, Johnson ML, et al. Overexpression of secreted frizzled-related protein 1 inhibits bone formation and attenuates parathyroid hormone bone anabolic effects. J Bone Miner Res. 2010;25:190–199. doi: 10.1359/jbmr.090719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cheng Z, Yao W, Zimmermann EA, Busse C, Ritchie RO, et al. Prolonged treatments with antiresorptive agents and PTH have different effects on bone strength and the degree of mineralization in old estrogen-deficient osteoporotic rats. J Bone Miner Res. 2009;24:209–220. doi: 10.1359/jbmr.81005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- [28].Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Howell DS, Dean DD. Biology, chemistry, and biochemistry of the mammalian growth plate. In: Coe FL, Favus MJ, editors. Disorders of Bone and Mineral Metabolism. New York: Raven Press; 1992. pp. 313–353. [Google Scholar]
- [30].O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Environmental disturbance confounds prenatal glucocorticoid programming experiments in Wistar rats. Lab Anim. 2010;44:199–205. doi: 10.1258/la.2009.009032. [DOI] [PubMed] [Google Scholar]
- [31].D'Mello A P, Liu Y. Effects of maternal immobilization stress on birth weight and glucose homeostasis in the offspring. Psychoneuroendocrinology. 2006;31:395–406. doi: 10.1016/j.psyneuen.2005.10.003. [DOI] [PubMed] [Google Scholar]
- [32].Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. Lancet. 1998;352:707–708. doi: 10.1016/S0140-6736(05)60824-0. [DOI] [PubMed] [Google Scholar]
- [33].Gitau R, Fisk NM, Glover V. Maternal stress in pregnancy and its effect on the human foetus: an overview of research findings. Stress. 2001;4:195–203. doi: 10.3109/10253890109035018. [DOI] [PubMed] [Google Scholar]
- [34].Glover V. Maternal stress or anxiety during pregnancy and the development of the baby. Pract Midwife. 1999;2:20–22. [PubMed] [Google Scholar]
- [35].Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. 2003;997:136–149. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- [36].Noguchi M. [Changes in human serum corticosterone and cortisol during pregnancy, labor and delivery] Nihon Sanka Fujinka Gakkai Zasshi. 1988;40:14–20. [PubMed] [Google Scholar]
- [37].Taylor RN. The endocrinology of pregnancy. In: Greenspan FS, Baxter JD, editors. Basic and Clinical Endocrinology. Prentice Hall; Hart-ford, CT: 2001. pp. 575–602. [Google Scholar]
- [38].Magiakou MA, Mastorakos G, Rabin D, Margioris AN, Dubbert B, et al. The maternal hypothalamic-pituitary-adrenal axis in the third trimester of human pregnancy. Clin Endocrinol, (Oxf) 1996;44:419–428. doi: 10.1046/j.1365-2265.1996.683505.x. [DOI] [PubMed] [Google Scholar]
- [39].Jones RE, Lopez KH. Human Reproductive Biology. Burlington, MA: Elsevier Academic Press; 2006. [Google Scholar]
- [40].Challis JR, Sloboda D, Matthews SG, Holloway A, Alfaidy N, et al. The fetal placental hypothalamic-pituitary-adrenal (HPA) axis, parturition and post natal health. Mol Cell Endocrinol. 2001;185:135–144. doi: 10.1016/s0303-7207(01)00624-4. [DOI] [PubMed] [Google Scholar]
- [41].Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- [42].Kajantie E. Fetal origins of stress-related adult disease. Ann N Y Acad Sci. 2006;1083:11–27. doi: 10.1196/annals.1367.026. [DOI] [PubMed] [Google Scholar]
- [43].Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58:424–430. [PMC free article] [PubMed] [Google Scholar]
- [44].Lopez Frias M, Llopis J, Montellano MA, Urbano G. Influence of gestation and hydrocortisone-acetate treatment on the nutritive utilization of protein. Nahrung. 1985;29:11–18. doi: 10.1002/food.19850290103. [DOI] [PubMed] [Google Scholar]
- [45].Megias MV, Lopez Frias M, Llopis J, Montellano MA, Urbano G. Effect of cortisol on some nutritional and biochemical parameters in pregnant rats and their foetuses. Comp Biochem Physiol A Comp Physiol. 1983;75:615–617. doi: 10.1016/0300-9629(83)90429-2. [DOI] [PubMed] [Google Scholar]
- [46].Delaere F, Magnan C, Mithieux G. Hypothalamic integration of portal glucose signals and control of food intake and insulin sensitivity. Diabetes Metab. 2010;36:257–262. doi: 10.1016/j.diabet.2010.05.001. [DOI] [PubMed] [Google Scholar]
- [47].Pratt NC, Lisk RD. Effects of social stress during early pregnancy on litter size and sex ratio in the golden hamster (Mesocricetus auratus) J Reprod Fertil. 1989;87:763–769. doi: 10.1530/jrf.0.0870763. [DOI] [PubMed] [Google Scholar]
- [48].Field T, Diego M, Hernandez-Reif M. Prenatal depression effects on the fetus and newborn: a review. Infant Behav Dev. 2006;29:445–455. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- [49].Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572:31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Eskandari F, Martinez PE, Torvik S, Phillips TM, Sternberg EM, et al. Low bone mass in premenopausal women with depression. Arch Intern Med. 2007;167:2329–2336. doi: 10.1001/archinte.167.21.2329. [DOI] [PubMed] [Google Scholar]
- [51].Cizza G, Ravn P, Chrousos GP, Gold PW. Depression: a major, unrecognized risk factor for osteoporosis? Trends Endocrinol Metab. 2001;12:198–203. doi: 10.1016/s1043-2760(01)00407-6. [DOI] [PubMed] [Google Scholar]
- [52].Ornoy A. Transplacental effects of cortisone and vitamin D 2 on the osteogenesis and ossification of fetal long bones in rats. Isr J Med Sci. 1971;7:540–543. [PubMed] [Google Scholar]
- [53].Ornoy A, Horowitz A. Postnatal effects of maternal hypercortisonism on skeletal development in newborn rats. Teratology. 1972;6:153–158. doi: 10.1002/tera.1420060206. [DOI] [PubMed] [Google Scholar]
- [54].Atkin I, Ornoy A. Transplacental effects of cortisone acetate on calcification and ossification of long bones in mice. Metab Bone Dis Relat Res. 1981;3:199–207. doi: 10.1016/0221-8747(81)90009-6. [DOI] [PubMed] [Google Scholar]
- [55].Atkin I, Ornoy A, Soskolne WA. Comparison of the effects of cortisone acetate on calcification, ossification, and resorption of fetal mice long bones--in vivo and in vitro studies. Adv Exp Med Biol. 1984;171:137–148. [PubMed] [Google Scholar]
- [56].Follis RH., Jr Effect of cortisone on growing bones of the rat. Proc Soc Exp Biol Med. 1951;76:722–724. doi: 10.3181/00379727-76-18607. [DOI] [PubMed] [Google Scholar]
- [57].Jee WS, Park HZ, Roberts WE, Kenner GH. Corticosteroid and bone. Am J Anat. 1970;129:477–479. doi: 10.1002/aja.1001290409. [DOI] [PubMed] [Google Scholar]
- [58].Peck WA, Brandt J, Miller I. Hydrocortisone-induced inhibition of protein synthesis and uridine incorporation in isolated bone cells in vitro. Proc Natl Acad Sci U S A. 1967;57:1599–1606. doi: 10.1073/pnas.57.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Storey E. Bone changes associated with cortisone administration in the rat. Effect of variations in dietary calcium and phosporus. Br J Exp Pathol. 1960;41:207–213. [PMC free article] [PubMed] [Google Scholar]
- [60].Sissons HA, Hadfield GJ. The influence of cortisone on the structure and growth of bone. J Anat. 1955;89:69–78. [PMC free article] [PubMed] [Google Scholar]
- [61].Cooper C, Harvey N, Cole Z, Hanson M, Dennison E. Developmental origins of osteoporosis: the role of maternal nutrition. Adv Exp Med Biol. 2009;646:31–39. doi: 10.1007/978-1-4020-9173-5_3. [DOI] [PubMed] [Google Scholar]
- [62].Gluckman PD, Hanson MA, Pinal C. The developmental origins of adult disease. Matern Child Nutr. 2005;1:130–141. doi: 10.1111/j.1740-8709.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]