Summary
The broaden application of adoptive T-cell transfer has been constrained by the technical abilities to isolate and expand antigen-specific T cells potent to selectively kill tumor cells. With the recent progress in the design and manufacturing of cellular products, T cells used in the treatment of malignant diseases may be regarded as anticancer biopharmaceuticals. Genetical manipulation of T cells has given T cells desired specificity but also enable to tailor their activation and proliferation potential. Here, we summarize the recent developments in genetic engineering of T-cell-based biopharmaceuticals, covering criteria for their clinical application in regard to safety and efficacy.
KeyWords: Adoptive cell transfer, Chimeric antigen receptor, CAR, T-cell receptor, TCR, Clinical trial
The Concept of T cells as an Anticancer Biopharmaceutical
When T cells are produced and used for the treatment of malignant diseases, they may be regarded as an anticancer biopharmaceutical. A biopharmaceutical is a pharmaceutical product that is biological in nature or derived from biological sources and is also produced by a biopharmaceutical industrial company [1].
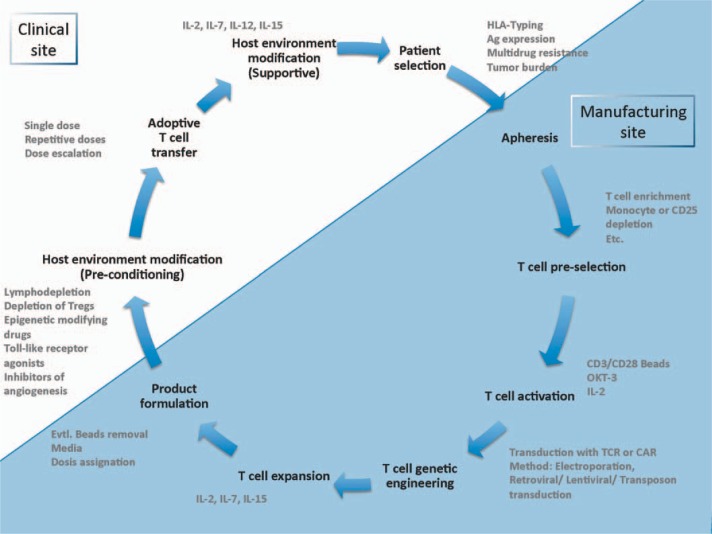
Here we review the optimization of T-cell-based biopharmaceuticals by genetic engineering, criteria for their clinical application, and the evaluation of safety and efficacy aspects in clinical trials (fig. 1).
Fig. 1.
Life cycle of biopharmaceutical T-cell-based drug.
Biological Role of T Cells in Cancer Defense Alternative: Role of T cells in Cancer Defense and an Adoptive T-Cell Transfer in Cancer Treatment
In 1909, Paul Ehrlich proposed that the immune defense system can identify and eliminate nascent tumor cells [2]. Since then tumor immunology has indeed shown that most cancer cells carry overexpressed tumor-associated or tumor-specific antigens that are not present on healthy cells. Moreover, there is now experimental evidence unambiguously showing that the immune system can and often does prevent tumors from developing, and thus plays a strong protective role against cancer [3]. In 1941, Landsteiner and Chase [4] showed that delayed hypersensitivity could be transferred between mice using cells from the sensitized donor. Two years later, Gross et al. [5] demonstrated that syngeneic mice immunized against tumors can reject ensuing tumor challenge. Since the beginning of the 20th century, tumor immunology has shown that most cancer cells carry overexpressed tumor-associated or tumor-specific antigens that are not present on healthy cells, opening up the possibility of successful application of an adoptive T-cell transfer. Moreover, there is now experimental evidence unambiguously showing that the immune system can and often does prevent tumors from developing, and thus plays a strong protective role against cancer [3].
With the identification of T-cell growth factor in 1976, the possibilities for in vitro cultivation of T cells have risen dramatically [6]. In 1988, in vitro expanded, autologous tumor-infiltrating lymphocytes were used to treat patients with metastatic melanoma [7]. Furthermore, through the discovery of the role of lymphodepletion, the efficiency of adoptively transferred T cells has been significantly augmented [8]. In order to create T cells with desired specificity, genetic engineering methods have been applied, resulting in generation of T-cell receptors (TCRs) and chimeric antigen receptors (CARs). The first successful adoptive transfer of genetically modified T cells has been performed by Morgan and colleagues in 2006 [9]. The most spectacular use of CAR technology has been demonstrated by the targeting CD19 molecule expressed on B cells. The first report on an application of this approach was published in 2010 [10].
Demonstration of Clinical Effectiveness
For tumors of the lymphohematopoietic system, allogeneic stem cell transplantation has added a successful immunological (and mainly T-cell-based) approach to the repertoire of anticancer therapies. In its context, the use of donor T cells to cure recurrent leukemia has, for the first time, revealed the power of cellular anticancer therapies [11]. In certain diseases, like chronic myelocytic leukemia, cure rates of up to 80% have been achieved by this method [12].
Although successful, classical donor T-cell transfer has significant limitations, it still requires parallel grafting of allogeneic stem cells and is very unspecific in its targets, a situation often resulting in severe graft-versus-host disease (GvHD) [13]. Moreover, for most diseases even the transfer of high numbers of unspecific allogeneic T cells is not sufficiently effective to eradicate the tumor [13]. Therefore, attempts have been undertaken to generate more specific T cells with higher cytotoxicity against defined tumor antigens, either by selection from the natural or induced repertoire or by transfer of receptor genes.
Concerning, adoptive T-cell transfer (ACT) for the treatment of solid tumors, the successes have been more modest. Nevertheless, it has been demonstrated that tumor-infiltrating lymphocytes (TILs) can be successfully isolated, expanded in vitro, and re-infused, leading up to an overall response rate of 51%.
Technological Progress in T-Cell Engineering
For a long time the generation of tumor-reactive T cells was based on selection and subsequent expansion of T cells with defined antigen reactivity. Since this approach turned out to be very difficult and time-consuming, methods were developed to retarget the specificity of T cells to any chosen tumor antigen by the genetic transfer of an antigen-specific receptor.
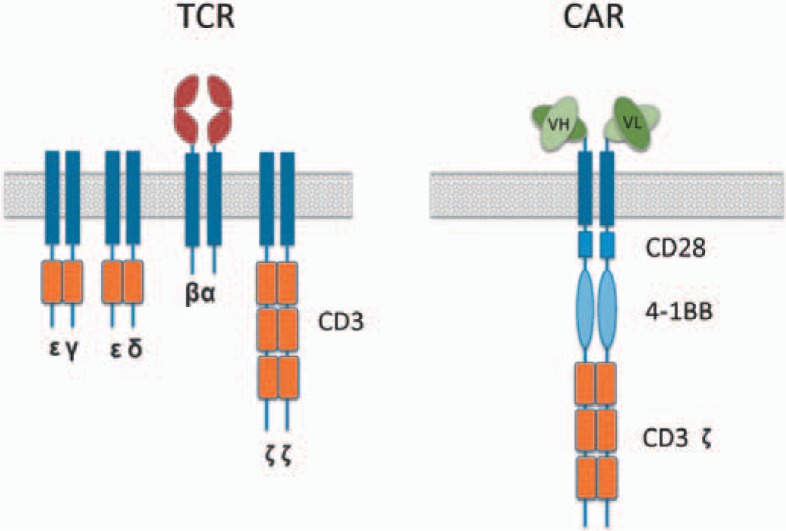
Currently, two approaches for redirecting T-cell specificity are employed (fig 2):
Fig. 2.
Schematic diagram of TCR and 3rd generation CAR.
i. Gene modification with TCRs, in which variable α- and β-chains are cloned from T cells with specificity against a tumor antigen [14]
ii. The introduction of CARs recognizing tumor antigens through single-chain variable fragments (scFvs) isolated from antigen-specific monoclonal antibodies (mAbs) linked to intracellular signaling domains [15, 16].
T-Cell Antigen Receptor Technology
The TCR is a heterodimer formed by the pairing of an α-and a β-chain and is associated with the CD3 complex. The transfer of gene sequences carrying the information for TCR-α and TCR-β chains allows the stable expression of a TCR complex with high affinity for a defined tumor antigen (either from rare human clones or from mice following immunization) into a new T cell. This T cell will have both an endogenous (natural) and an exogenous (introduced) tumor antigen-specific TCR. Both receptors can interact with specific peptides presented by HLA molecules. Subsequent cytolysis of antigen-bearing tumor cells is mediated either by perforin or Fas ligand.
The TCR approach was the first demonstration of redirected T-cell specificity [17] and has the following advantages:
i) It relies on the natural way of T-cell function.
ii) It has a low risk of cytokine release syndrome through low avidity activation.
iii) Transduced cells are showing a low immunogenicity.
iv) Mutated intracellular proteins can be targeted.
The major difficulties which TCR technology has to overcome were low cell surface expression of TCRs and so-called ‘mispairing’, which occurs by formation of TCRs formed by of one endogenous and one transduced TCR chain.
The following techniques were used to address these issues:
i) TCR-α/TCR-β vector configuration.
ii) Codon optimization.
iii) Murinization of human TCR-constant regions.
iv) Cysteine modification ‘knob-in-hole’ modification. v) Zinc-finger nucleases.
In order to enhance the transduction efficiency and to limit mispairing as well as the risk of insertional mutagenesis, the current viral vectors link both TCR-α and TCR-β chains with either the 2A peptide sequence or a less efficient internal ribosomal entry site sequence [18].
The cell surface expression of the transduced TCR can also be enhanced by codon optimization. This method replaces infrequently used codons with synonymous codons frequently encountered in the human genome. As shown by van Loenen et al. [19], this approach can be used to optimize both expression of TCR and suicide gene.
The next approach to minimize mispairing of TCR-α and TCR-β chain is murinization. Replacement of α- and β-constant domains with murine sequences results in a higher dimerization when compared to fully human TCRs. It has been postulated that this is because of a stronger association of murine TCRs with human CD3 molecules. The presence of foreign residues can induce a generation of human anti-mouse antibodies and result in life-threatening immunogenicity, as described by Maus et al. [20]. This risk can be considerably reduced by applying a minimal murinization approach. As shown by Sommermeyer and Uckert [21], exchange of sets of amino acids from murine TCR C region – if replaced with the corresponding counterparts in human TCR – led to a significant improvement of TCR cell surface expression and increased functional avidity, with only a slightly enhanced risk of inducing immunogenicity.
Another modification approach that can reduce mispairing is the introduction of cystine residues in the TCR constant domain. This results in the formation of a new intermolecular disulphide bond which not only reduces mispairing but also increases the functional avidity [22, 23].
Alternatively, a ‘knob-in-hole’ approach can be used. It incorporates an exchange of the position of two amino acids in the TCR-constant domain with naturally close steric and electrostatic interactions, and this favors pairing of the TCR-αand TCR-βchain. The ‘knob-knob’ or ‘hole-hole’ interactions, which would lead to mispairing, do not form as often due to their instability [24].
The risk of mispairing can also be reduced by application of zinc-finger nucleases. As shown by Provasi et al. [25], introduction of zinc-finger nucleases can promote a disruption of endogenous TCR-β and TCR-α chain genes, which when accompanied by the simultaneous delivery of tumor-specific TCR, can result in elimination of mispairing.
The methods described prove that low TCR cell surface expression as well as mispairing can be efficiently addressed. Since there is currently no clear clinical evidence for TCR mispairing-induced autoreactivity, the strategies applied in clinical trials seem to be sufficient in order to manage the risk of mispairing.
Chimeric Antigen Receptor Technology
The second genetic modification strategy that aims at redirecting T-cell specificity is the introduction of antibody-based extracellular receptor structures [5].
These structures can bind tumor antigen via antibody-derived complementary-determining regions (CDRs) that are coupled to an activating intracellular cytoplasmic domains. T cells bearing a CAR will induce tumor cell apoptosis using the same mechanisms as ordinary T cells. In comparison with TCR technology, this concept has major advantages:
i) It allows easy access to new receptors.
ii) It initiates a reliable high potency signal.
iii) It is HLA-independent.
iv) There is no antigen processing required.
v) There is no competition for CD3.
While the first-generation of CARs consisted only of an scFv against a target cell surface antigen and the cytoplasmic CD3 ζ-chain signaling domain, second- and third generation CARs harbor different intracellular signaling domains from various co-stimulatory protein receptors (e.g. CD28, 41BB, ICOS) which were added to the cytoplasmic tail of the CAR. Second-generation CARs, which were expanded over longer periods of time, demonstrated an improvement in cytokine secretion and showed enhanced effector function in resting human T cells. In clinical trials, in vivo expansion of the CAR-modified T cells was more than 1,000-fold [26].
The major disadvantage of T-cell biopharmaceuticals based on third-generation CARs is a massive cytokine release induced by cells activated through low-avidity ‘off-target’ binding. In some cases, signal threshold may be reduced to a level where an activation of T cells can occur without the presence of triggering antigens [27].
Another disadvantage of CAR-engineered T cells is their immunogenicity since the scFv portion of the CAR complex is generally mouse-derived, which may result in immune responses and clearance of CAR-engineered T cells.
To enhance flexibility and dimerization of the receptor, the scFv is linked to the trans-membrane domain via an extracellular linker domain such as the immunoglobulin (Ig)G Fc hinge region or extracellular CD8 section. There is some evidence that these domains can engage other cells such as macrophages or natural killer (NK) cells by binding via the Fc receptor, leading to a pro-inflammatory response irrespective of CAR binding [28].
Actually, there is no commonly accepted CAR configuration, and multiple formats of CAR signaling domain are used for clinical trials, each showing potential advantages in vitro. However, it seems that the introduction of 4–1BB (CD137) domains has significantly enhanced CAR-transduced T-cell survival and function, possibly by induction of the anti-apoptotic protein Bcl-x [29].
Technological Progress in Antigen Identification
There are a number of different approaches for identifying target antigens and TCRs appropriate for T-cell-based immunotherapy. ‘Reverse immunology’ has been applied for the prediction of tumor-associated antigens by screening sequences of selected proteins for peptides with high binding affinity to HLA molecules. A complementary approach relies on the isolation of HLA-associated peptides from target cells, followed by sequencing using chromatography fractionation and mass spectrometric analysis.
Most tumor-associated antigens that have been identified by these techniques are also expressed, albeit at lower density, in normal tissues [30]. Autologous T cells targeting these epitopes are usually of low affinity, since all high-affinity clones have been deleted during thymic selection to avoid autoimmunity.
One approach to isolate high-avidity T-cell clones is to use mice, constructed to express the human TCR repertoire. Such mice can be used to generate TCRs that are specific for human tumor antigens [31].
Mice with HLA class I transgene and the entire human TCR-αβ gene loci whose T cells express a diverse human TCR repertoire have successfully been used to generate T cells against the Melan-A melanoma antigen. Vaccination of these mice with tumor-specific molecules will allow us to identify new tumor-specific TCRs with high affinity more easily.
Another approach is to isolate high-avidity T-cell clones from HLA-mismatched donors. This approach circumvents the problem of tolerance by using the natural repertoire of T cells from an HLA-different donor. The major problem is that allogeneic epitopes not related to the HLA-presented peptide are also targeted.
Biological and Immunological Acceptance Criteria for T-Cell Biopharmaceuticals
Antigen-Related Acceptance Criteria
The optimal case is a target antigen which has the following properties:
i) It is immunogenic.
ii) It is completely tumor-specific with no significant expression on normal tissues.
iii) It is highly expressed on all tumor cells (including tumor stem cells).
iv) It is essential for survival or proliferation of the tumor cell.
v) It has multiple epitopes.
vi) It is expressed on the tumor surface.
Theoretically, fusion proteins (like bcr/abl) generated by cancer-specific mutations could be examples of such targets. However, such target antigens are relatively rare and are in most cases patient-specific, a finding that makes generic targeting of antigens with common receptors difficult. Moreover, they are generally expressed on the cell surface. The cancer-testis antigen (CTA) family members also represents tumor-associated antigen candidates which are expressed by a wide range of malignancies and found only in germ cell tissues but not in other normal tissues [32]. The problem with CTA is that they are infrequently expressed on tumor cells and thus presumably not essential for the survival of a tumor cell. Therefore, tumor escape would emerge as a frequent problem.
For clinical applications, it is not necessary that all of the above-mentioned criteria are fulfilled. The importance of each criterion will depend on the balance between aspects of safety, reliability and effectiveness in a given clinical situation.
Criteria that Determine the Clinical Efficacy of Modified T Cells
Among other more general criteria, the specific functional quality of a T-cell biopharmaceutical product is determined by
i) Its ability to lyse tumor cells expressing a particular marker.
ii) The affinity with which the introduced receptor binds its antigen.
iii) The level of receptor expression on the cell surface (in vitro and in vivo).
iv) The in vivo expansion and persistence of the T cells.
v) The lack of off-target toxicities.
There are numerous assays available to assess the capacity of T cells to react against tumor cells in vitro. However, it is important to differentiate between assays accessing the recognition of a target cell (e.g, IFN-γ release assay) and those measuring the killing of a target cell (e.g, chromium release assay). Full functionality is only assured if both the afferent (tumor recognition) as well as the efferent function (tumor kill) are operative. Assays to measure T-cell activation upon exposure to patient-derived tumor tissue may help to determine the individual in vitro effectiveness [33].
The most important issue with regard to the evaluation of T-cell-based biopharmaceuticals is the assessment of off-target toxicity. Obviously, this is a very critical point for a product that usually has no self-limiting properties. For CARs, it is relatively easy to determine whether or not the antibody shows cross-reactivity and binds to the surface of tissues not expressing the targeted antigen. Similar tests for TCR-based biopharmaceuticals are more difficult to establish because a bank of vital tissue and cell samples (with different HLA types) would be required to test for cross-reactivity against peptides presented in the context of all relevant HLA types.
Design and Engineering of Anticancer T-Cell Biopharmaceuticals
Gene Transduction Methodology
Two vector systems – retroviral or lentiviral vectors – can be used to transfer TCR- or CAR-coding genes into T cells. However, so far, it is mostly γ-retroviral vectors that have been used in clinical trials [34].
Retroviral Vectors
Retroviral vectors yield a high level of stable transgene expression through integration into the transcriptionally active site of the host genome. The major advantage of retroviral vector application is permanent gene expression and long-term experience in the clinical trials. The disadvantage of this system, is that transduction can be performed only on efficiently dividing T cells.
Lentiviral Vectors
In contradiction to retroviral vectors, lentiviral vectors are capable of integrating also into non-dividing cells. The transduction of relatively undifferentiated T cells should lead to improved function in vivo. But since pre-activation of T cells yields higher transduction rates, in most retroviral and lentiviral transduction protocols the use of anti-CD3 antibody, CD3/CD28 magnetic beads, or artificial antibody-producing cells can play a crucial role. Similar to retroviral vectors, transduction with lentiviral vectors can lead to permanent gene expression due to integration into host genome. Another advantage of lentiviral vectors is a lower risk of damaging insertions; however, a benign integration bias without oncogenic selection has recently been reported [35].
Transposons
Transposon systems such as Sleeping Beauty 100X (SB100X) or piggyBac (PB) are new attractive methods for a stable non-viral genetic modification of T cells. The Sleeping Beauty transposon system [36] integrates randomly into the human T-cell genome [37] and presents an alternative to retroviral vectors [30]. In contrast to the viral vectors, transposons do not have an intrinsic capacity to cross the cellular membranes. Therefore, they have to be delivered into the cells either by different non-viral strategies or by vector systems. Transposons have the following advantages:
i) Transduced cells show high gene expression.
ii) They do not require cell pre-activation.
iii) The method is simple and inexpensive.
iv) They have a relatively large cargo capacity. v) They have a low immunogenicity.
Having reached the comparable effectiveness of viral vectors, the production of a new generation of hyperactive transposon systems, like SB100x or PB, is less costly and less time-consuming. The major disadvantage of the system is that it is not yet tested clinically and little is known about its oncogenic potential in vivo. However, a GMP-qualified SB transposon system [38] has been approved for a gene therapy clinical trial (NCT01497184) by the Food and Drug Administration, and enrolment began in 2011.
Approaches to Enhance Effectiveness
In vitro Approaches to Modify Other T-Cell Features
Genetic engineering can also be employed to modify other features of T cells. As shown by Liu and Rosenberg [39] using transduction with exogenous human IL-2 gene, T cells can significantly increase their proliferation capacity. Moreover, in vivo persistence can be enhanced by over-expression of anti-apoptotic proteins like Bcl-2 or Bcl-xL [40]. Furthermore, introduction of dominant-negative transforming growth factor-β (TGF-β) type II receptor was shown to be a sufficient strategy for overcoming the immunosuppressive effect of TGF-β [41]. Also homing to tumor sites can be enhanced via introduction of specific chemokine receptors such as CXCR2 [42] or CCR4 [43].
Activation of T Cells
There is no doubt that culture conditions before, during, and directly after gene transfer affect the subsequent in vivo properties of T cells. The addition of high doses of IL-2 to culture media induces differentiation towards late effector state T cells. Co-stimulation with IL-7 and IL-15 may direct T cells towards an earlier differentiation phenotype which could result in greater expansion and persistence in vivo [44, 45].
The pre-selection of EBV- or CMV-specific T cells offers the theoretical advantage of optimal and continuous co-stimulation through their native virus-specific TCRs, at least in in patients with latent viral infection. Although attractive, the concept has not been proven in clinical trials so far, and it is very likely that other conditions are still of critical importance, even if EBV- or CMV-specific cells are preselected.
Pre-Selection of T-Cell Subsets for Gene Transfer
So far, most clinical trials used genetically engineered T cells that have been generated from unselected peripheral blood T cells, thus containing an unpredictable mixture of different lymphocyte subsets. Only few studies used immunomagnetic selection of CD8+ lymphocytes to restrict the T-cell pool to CD8+ naive (TN), central memory (TCM) and effector memory (TEM) populations.
The differentiation status of genetically engineered T cells and the addition of supportive cytokines during cultivation period can influence in vivo survival of adoptively transfused cells. In the past, most of the clinical trials used TEMs. This was a result of cell culture technologies which yielded a rapid differentiation into late-stage effector cells. Although in vivo TEMs show more cytotoxicity when compared to TCMs, they do not show long-term tumor surveillance. As demonstrated by Berger et al. [46] in a primate model, TEMs derived from adoptively transferred TCMs were able to persist in blood and could thereby migrate to bone marrow where they preserved their ability to differentiate into both phenotypes.
As reported by Louis et al. [47], the usage of CARs with central memory phenotype and high percentage of CD4+ T cells resulted in induction of complete tumor responses in patients with an active neuroblastoma. Moreover, administered CAR T cells expanded and persisted at a low level; this situation was associated with a longer survival. Gattinoni et al. s[48] broadened the understanding of T-cell subsets by identifying stem cell memory T cells (TSCMs). Due to their robust capacity of self-renewal and generation of TEMs, TCMs and effector T cells, TSCMs appear to be very attractive for adoptive cell transfer.
In recent years, intensive efforts have been made to improve the manufacturing protocols and to implement the newest discoveries in order to improve the survival and anti-tumor capacity of cell products. As shown by Cieri et al. [45], utilization of a CD3/CD28 activation procedure as well as the addition of IL-7 and IL-15 can efficiently generate TSCM population.
Gene transfer is performed usually after unspecific activation step with OKT-3 (which promote CD8 proliferation) or CD3/CD28 (higher CD4 subset). The cells were usually cultivated with high doses of IL-2, which directed T-cell differentiation towards late state effectors.
Summing up, the recent technological advances in cell design open new perspectives for adoptive cell therapy. TCR technology enables HLA-dependent (mostly HLA.A2) recognition. The recent advances in cell design including inter alia murinization, cystein modification, and knob-in-hole modification have led to a decrease in mispairing and a reduced risk of life-threatening on- and off-target toxicity. In contrast, CAR-modified T cells and target tumor-associated antigens which are expressed on the cell surface include different proteins, carbohydrates, and glycolipids. Moreover, they are not HLA-dependent and therefore applicable for off-the-shelf use. The risk of generating undesired autoimmunity or GvHD in the case of CARs is minimal. Independently from the genetical modification, the integration systems using γ-retroviruses or lentiviruses may be soon replaced by cheaper alternatives such as transposon systems.
Modifying the Host Environment by Preconditioning Before T-Cell Transfer
An understanding of interactions between a tumor and its microenvironment is crucial not only during oncogenesis or tumor progression but also for the design of anti-tumor therapies. It has become clear that optimizing cell engineering is not the only issue that has to be addressed. Environmental variables, including presence of i) FOXP3+ regulatory T cells (Tregs), ii) myeloid-derived suppressor cells (MDSCs) or iii) tumor-associated macrophages (TAMs) as well as iv) secretion of T-cell suppressive cytokines like TGF-β and IL-10 can be of outstanding importance for the success of adoptive T-cell transfer.
As shown by Dudley et al. [49], the efficacy of adoptive T-cell transfer of autologous TILs can be enhanced by intensification of host preparative lymphodepletion. Various lymphodepleting regimens have been developed and the depletion of T regulatory cells has proposed to be beneficial for the expansion of the transferred T-cell product. The reduction of MDSCs, a decrease in endogenous lymphocyte competition for cytokines, access to antigen-presenting cells, and engagement of toll-like receptors on antigen presenting cells are additional factors that have been suggested to contribute to the beneficial effect of chemotherapy prior to an adoptive T-cell transfer.
There are various regimes in use; generally non-myeloablative chemotherapy regimens consist of drugs with specific activity against lymphocytes like cyclophosphamide and fludarabine which are applied, sometimes, in combination with low-dose irradiation. Gemcitabine, a nucleoside analogue, has also been suggested as a beneficial immunomodulator prior to T-cell transfer since it was reported that it increases the expression of HLA class I and selectively kills myeloid suppressor cells, thereby enhancing T-cell-mediated antitumor immunity. Depletion of Tregs can also be achieved by monoclonal antibodies with specificity against CD25 such as danileukine diftitoxin (Ontak™).
Furthermore, because of the enhancement of tumor antigen expression epigenetically modifying drugs like azacitidine (AZA) or decitabine (DAC) could be effectively combined with application of genetically modified T cells. As shown by Cruz et al. [50] in vitro, efficacy of T-cell therapy against MAGE-A4 in relapsed Epstein-Barr virus-negative Hodgkin's lymphoma can be enhanced by combination therapy with epigenetic modifying drugs.
Due to their potent adjuvant effects and promising first results in vaccination trials [51], Toll-like receptor agonists have also been suggested as supportive treatment in the context of T-cell transfer.
Moreover, according to recent studies by Dings et al. [52], administration of inhibitors of angiogenesis can promote leukocyte infiltration into tumor tissue by up-regulating endothelial cell adhesion molecules, an effect that could also be exploited for the optimization of T-cell transfer.
Modifying the Host Environment by Supportive Treatment after T-Cell Transfer
The host environment can be also modified after adoptive T-cell transfer. Systemic administration of high-dose IL-2 has often been used in clinical protocols in order to increase the survival of administrated T cells [53]. Unfortunately, due to significant toxicity in form of vascular leakage syndrome, its application has been limited. The administration of other cytokines such as IL-7, IL-12 [54], IL-15 [55], and IFN-γ could also enhance T-cell effector and memory function, and its application potential in the clinical context is currently being evaluated.
Techniques to Ensure Safety
Introduction of Suicide Genes
With increasing effectiveness of genetically modified T cells, the risk of severe side effects caused by on- and off-target toxicity also has risen. Genetic solutions of this safety concern are based on the concept of on-demand cell destruction by application of a substance which can switch on a suicide gene and destroy the T-cell-caused toxicity. Among the variety of suicide gene approaches, herpes simplex virus thymidine kinase (HSV-TK) is still accepted as reference strategy [56]. HSV-TK-based cell elimination results from phospho rylation of the pro-drug by thymidine kinase, which interrupts DNA elongation and causes apoptosis. This strategy is currently under investigation in a phase III clinical trial in patients undergoing haploidentical stem cell transplantation.
Another suicide gene strategy was also tested in the context of haploidentical stem cell transplantation [57]. The introduced safety switch, the inducible caspase 9 (iCasp9), has been constructed by fusing human caspase 9 and a modified human FK-binding protein, which allows conditional dimerization [58]. Upon exposure to a synthetic dimerizing drug (AP1903), the inducible iCasp9 becomes activated and leads to the rapid death of cells expressing the construct.
Application of CD20 [59] has also been proposed as suicide gene strategy. So far, this strategy has not been applied in the clinic. The major obstacle for broader use is transient depletion of B cells. Furthermore, Sato et al. [60] proposed usage of a mutated human thymidilate kinase as a safety switch. However, this approach seems to be less efficient than the other strategies in direct comparison [61].
Anticancer T-Cell Biopharmaceuticals in Clinical Trials
Clinical Trial Design
General Issues Concerning Tumor and Patient Population
The following issues should be addressed in the design of a clinical trial:
i) Tumor evaluation criteria (sensitivity to T-cell-induced cell death, immunogenicity).
ii) Tumor incidence (high prevalence).
iii) Tumor growth characteristics (slowly proliferating).
iv) Portfolio of alternative treatment strategies (no alternative treatments).
v) Clinical need in a particular disease situation (late disease stage).
The successful adoptive T-cell transfer is only possible in patients where immunogenic tumor cell death can be induced [62] and where, as a consequence, an antitumor immune response can be elicited. Since not all tumors fulfill these requirements, there is an urgent need to define and standardize reliable biomarkers and feasible tests in order to determine the sensitivity of tumor entities towards T-cell-mediated cytotoxicity. If the defects that underlie insufficient antitumor immune responses can be corrected by exogenous intervention [63], these aspects should be included in order to expand the number of tumor entities eligible for T-cell-based immunotherapies.
These criteria have been applied in most trials. A good example is the use of CAR T cells directed against CD19 [26]. B lymphocytes are highly immunogenic, reside in the same anatomical location as T cells, and express a number of receptors that facilitate T-cell function such as co-stimulatory receptor ligands. The prevalence of B-cell neoplasms is relatively high, and there are B-cell malignancies with a low proliferation rate (chronic lymphocytic leukemia (CLL), low-grade lymphomas); patients can be included at late stages of the disease or if alternative treatment strategies (allogeneic transplantation) are not feasible.
Dosing and Administration Schedule of the T-Cell Product
There are different possibilities how the dose of a T-cell-based biopharmaceutical product can be declared. Either the total number of T cells or the number of gene-modified cells expressing the TCR or CAR can be given. Cell numbers of stem cell grafts and allogeneic donor T-cell infusions are always given on a per kg recipient body weight basis.
For early phase I/II trials, we suggest to calculate the dose based on the total number of T cells per kg body weight, because such studies which take into consideration mainly safety aspects and side effects of the biopharmaceutical are not restricted to receptor-expressing cells. However, several studies have normalized the T-cell number to number of gene-modified T cells. Consequently, patients received different total T-cell doses but the same number of gene-modified T cells [64].
Definition of the Clinical Setting
The following issues concerning the clinical setting should be considered in the design of a clinical trial:
i) Individual tumor evaluation.
ii) Supportive treatment to enhance effects.
iii) Supportive treatment to reduce side effects.
Even if a malignant disorder is exactly defined by morphology, immunophenotype, and molecular genetics, the manifestation of the individual tumor can be extremely heterogeneous. Moreover, cells of the tumor specimen of a particular patient can be very heterogeneous. Therefore, the evaluation of the tumor acceptance criteria should also take place on an individual basis. The ideal situation includes assays for tumor recognition (immunogenicity) and sensitivity towards T-cell-mediated (perforin) lysis. Other patient-specific evaluation criteria are dynamics of tumor proliferation (e.g. clinical course, tumor markers, phenotypic proliferation marker) and tumor sensitivity towards chemotherapy or other immunotherapies (mAbs). The appropriate definition of tumor characteristics is not only important for patient selection and inclusion criteria but also forms the basis for understanding differences in response and will help to further improve immunotherapeutic approaches.
In allogeneic stem cell transplantation, the transfer of cells is preceded by chemoradiotherapy known as conditioning. Conditioning has three goals:
i) To ‘make space’ for the newly administered cells.
ii) To reduce the number of host lymphocytes to prevent alloreactivity and rejection.
iii) To induce tumor cell apoptosis in order to reduce the number of tumor cells and to increase their immunogenicity.
All three aspects are also very important for the application of T-cell-based biopharmaceuticals. To make ‘space’ means to reduce the number of lymphocytes (including Tregs) which compete with the newly transferred cells for homeostatic cytokines. Since relevant immune reactivity especially against CARs can be anticipated, the immunosuppressive effects of conditioning could also help to prevent host reactivity against immunogenic parts of the T-cell product and to enhance proliferation and persistence. Moreover, cells which potentially suppress effector T cells, such as Tregs, MDSCs and TAMs, should also be reduced. Finally, the reduction of tumor cell mass and the induction of tumor apoptosis are beneficial because it positively shifts the balance between tumor and effector cell number and promotes antigen presentation, engagement of toll-like receptors, and the local secretion of pro-inflammatory cytokines.
With regard to supportive treatments in order to reduce side effects of T-cell transfer, prophylactic and therapeutic aspects have to be considered. As is known from studies with other immunologically based treatment approaches like IMiDs, a prognosis concerning the magnitude of the induced effect is difficult to make, and massive tumor destruction with subsequent potentially lethal tumor lysis syndrome can occur. Therefore, careful monitoring of markers for tumor destruction (lactate dehydrogenase) and renal function as well as prophylactic application of allopurinol and adequate hydration is essential to prevent fatal outcomes.
The second clinical problem that has to be anticipated, is an anaphylactic reaction [20], most likely directed against xenogeneic proteins of the transferred T-cell product. Due to sensitization against these antigens, the risk of an anaphylactic shock increases after the second or following administrations, and application of the product should take place in an environment where patient monitoring and equipment for adequate emergency interventions are ensured.
The third critical side effect of T-cell-based therapeutics is the induction of a cytokine release syndrome which can often not easily be discriminated from an anaphylactic reaction since major aspects of clinical manifestation (hypotension) are similar. Prophylactic administration of steroids could reduce the intensity of both anaphylactic reactions and cytokine release syndrome but could negatively influence the effectiveness of the transferred cells; therefore, steroids usually are not administered. Standards for immediate diagnosis and treatment for all three major side effects should be incorporated into the study protocol.
Evaluation of Safety
Adverse effects are categorized as on-target (also referred to as target-related, exaggerated pharmacology or mechanism-based) or off-target effects (as a result of unspecific modulation of other targets) [65]. Skin rash can be regarded as an on-target toxicity of biopharmaceuticals targeting a commonly expressed epidermal antigen, whereas an anaphylactic reaction evoked by its xenogeneic components is an example for off-target toxicity. The discrimination between on- and off-target toxicity is important because high or unacceptable off-target toxicity should be a subject of further improvements in cell engineering and production, whereas excessive or life-threatening on-target toxicity raises general questions concerning the choice of the antigen. However, in some cases the level of on-target toxicity can be reduced by changing the dose and application schedule of the biopharmaceutical.
For the evaluation of new clinical approaches employing genetically engineered T-cell products, more general aspects related to off-target toxicity should be addressed first, since they form the basis for any clinical application, regardless of the antigen targeted by the specific receptor. It is beyond the scope of this article to describe known or suspected on-target adverse effects for each receptor/antigen pair that has been, is, or will be used in clinical trials. As explained earlier, extensive in vitro and in vivo data from experimental systems and human studies dealing with this aspect constitute a central part of the particular biopharmaceutical dossier.
Transgene Integration
Long terminal repeats of the viral vector system can increase the expression not only of the transduced but also of neighboring genes. Inserted near an oncogene, retroviral vectors may thus drive oncogenesis [66]. Clinical trials have revealed that murine leukemia virus-based vectors can cause leukemia, most probably by increasing the expression level of oncogenes in the neighborhood of insertion sites [67].
However, all oncogenic events have occurred during gene transfer to stem cells, and it seems that mature lymphocytes harbor only a very low risk for insertional mutagenesis and are resistant to retroviral transformation [68]. Retroviral vectors are clinically used for many years, and long-term safety data are available over a time span of more than 10 years. There was no evidence of adverse events in patients with persistent CAR+ cells after this period who received T cells transduced with retroviral vectors [69]. There is not such a large data base for lentiviral vectors but their safety in the context of TCR and CAR gene transfer is also considered to be very high.
Non-viral transfer of TCR and CAR genes with non-integrating plasmid or mRNA is usually not an alternative because of its short-term transgene expression and low efficacy. However, the temporary receptor expression may be useful for safety testing.
For retro-transposon systems, such as the described PB or SB100X, there is so far only limited experience with regard to transfer rates and stability of expression. Although this technique may offer a feasible and cost-effective alternative to viral transduction systems, it is too early to widely use transposon systems, until safety data of the first clinical trials are available.
Safety of TCR T-Cells
Results concerning safety data of clinical trials with TCR-modified T-cells are summarized in table 1. The first clinical trial employing the TCR transduction approach to produce T-cell-based biopharmaceuticals was performed by the Rosenberg group in patients with metastatic melanoma [9]. It targeted the MART-1 antigen and demonstrated the feasibility and lack of short-term toxicity as well as the ability of adoptively transferred TCR-modified T cells to persist in vivo without significant side effects. In the subsequent trial using a different MART-1 clone and gp100-specific TCR genes with enhanced affinities, 29 of the 36 patients exhibited a widespread erythematous skin rash. Histology revealed necrotic keratinocytes and infiltrating CD8+ T cells [70]. Moreover, some patients developed hearing loss (10 of the 20 DMF5 patients) and anterior uveitis (11 of the 20 patients receiving MART-1 TCR-transduced T cells and 4 of 16 patients receiving the gp-100 TCR-transduced T cells) which can be also qualified as on-target toxicity on normal melanocytes expressed in eye and ear.
Table 1.
Serious adverse events in clinical trials assessing the safety and efficacy of adoptive cell transfer of TCR-modified T cells in cancer patients
| Antigen | Tumor | Toxicities | Effectiveness | Persistence | Comments | Reference |
|---|---|---|---|---|---|---|
| MART 1 | melanoma | erythematous skin rash grade 1–2 (14/20), ear (hearing loss) grade 1–2 (2/20), grade 3 (8/20); uveitis grade 1–2 (11/20) | 6PR/20 | >1% tetramer-positive T cells 20/20 (method: flow cytometry); persistence of tumor reactive genetically modified T cells11/20 (method: IFN-γ ELISPOT) | symptoms similar to Vogt-Koyanagi-Harada diseases | [70] |
| gp100 | melanoma | erythematous skin rash grade 1–2 (15/16), ear (hearing loss) grade 1–2 (4/16), grade 3 (1/16), uveitis grade 1–2 (4/16) | 3PR/16 | >1% tetramer -positive T-cells 20/20 (method: flow cytometry); persistence of tumor reactive genetically modified T-cells 7/16 (method: IFN-γ ELISPOT) | symptoms similar to Vogt-Koyanagi-Harada diseases | [70] |
| CEA | colorectal cancer | colitis grade 3 (3/3) | 1–2PR/3 | 3/3 1 month after ACT (method: flow cytometry, IFN-γ ELISPOT) | [71] | |
| MAGE-A3 |
|
acute cardiac failure (2/2); cytokine release syndrome, death (2/2) | not evaluable | robust expansion of genetically modified T cells 2/2 (method: quantitative PCR assay) | TCR modified by site-directed mutagenesis in the TCR CDR2 region | [72] |
| MAGE-A3/A9/A12 |
|
neurological toxicity: seizures, coma, TIA (4/9), death (2/9) | 4 PR,1CR/9 | 9/9 1 month after ACT (method: flow cytometry) | TCR was modified by site-directed mutagenesis in the TCR CDR3 region | [74] |
In a recent clinical trial [71] including patients with metastatic colorectal cancer who received high-avidity CEA TCR-modified T cells, all three patients developed severe inflammatory colitis. This has also to be interpreted as on-target toxicity as a result of transduced T cells recognizing the CEA expressed in normal colonic mucosa.
Other recently reported lethal cases of off-target toxicities in TCR trials include cardio-[72, 73] and neurotoxicity [74]. They may be caused by in vitro mutagenesis and subsequent cross-reactivity, to the titin [72] and to several MAGE family members other than MAGE-A3 as well as an unrelated protein expressed in the brain [74].
Most safety concerns involving off-target toxicity were related to mispairing, since data from a mouse model have suggested that mispairing of transferred TCR with endogenous TCR could lead to potentially lethal autoreactivity [75]. In a clinical setting, mechanisms and symptoms should be similar to GvHD (caused by HLA-matched T cells acting against minor antigens in an unpredictable manner). Fortunately, so far such symptoms have not been described. Even after lymphodepleting chemotherapy, which increases the risk of autoimmunity and GvHD, there was no evidence of GvHD in 106 patients using seven different antitumor TCRs in the human TCR gene trials at the National Cancer Institute.
Safety of CAR T Cells
Safety data of recent clinical trials with CAR-modified T cells are summarized in table 2. In a recent clinical trial with autologous T cells modified to express a CD19-targeted CAR, a patient with CLL developed fever, hypotension, and rapidly progressing dyspnea 20 h after infusion. The symptoms are relatively unspecific, and an autopsy failed to reveal an obvious cause of death. The patient was heavily pre-treated, and there were some signs pointing towards an infectious problem, finally resulting in sepsis [76]. However, it seems also possible that these symptoms were related to off-target toxicity of the transferred biopharmaceutical since problems associated with T-cell-induced cytokine release could result in similar symptoms.
Table 2.
Serious adverse events in clinical trials assessing the safety and efficacy of adoptive cell transfer of CAR-modified T cells in cancer patients
| Antigen | Tumor | Toxicities ≥ grade 3 | Effectiveness | Persistence | Comments | Reference |
|---|---|---|---|---|---|---|
| Carbonic anhydrase IX | renal cell carcinoma | liver toxicity due to the expression in biliary epithelium | no objective clinical responses/ 3+11 | limited functional peripheral persistence up to day 74 (method: flow cytometry) | adoptively transferred CAR T cells induce anti-anti–CAIX-CAR antibodies and anti-CAIX-CAR cellular immunity 9/9 | [82, 94] |
| L1-CAM | neuroblastoma | lymphopenia (2/6), neutropenia (1/6), bacteremia (2/6), interstitial pneumonitis (1/6) | 1CR, 1SD/ 6 | at day 7: 4/6, at day 14 3/5, max. up to day 56 (method: quantitative PCR) | no significant transgene-specific immune responses detected | [92] |
| ERBB2 | colorectal cancer | ARDS, hypotension, cardiac arrest, death | no objective clinical responses/1 | at day 5: autopsy, presence of vector-containing cells in multiple tissues, with highest levels in the lung and abdominal/mediastinal lymph nodes (method: quantitative PCR assay) | highly active, anti-ERBB2 directed T cells probably recognized ERBB2 expressed by normal lung cells, released inflammatory cytokines (including TNF-α and IFN-γ) causing pulmonary toxicity | [77] |
| CD19 | lymphoma | long-term depletion of healthy B cells (8/8), capillary leak syndrome (4/8); bacteremia (1/8), acute renal failure (3/8), pancytopenia (8/8), hypotension (4/8), death (1/8) | 6/8 PR, 1CR, 1 not evaluable | (method: quantitative PCR assay) | [88] | |
| Mesothelin | mesothelioma | anaphylactic shock, cardiac arrest | 1PR/1 | not described | CAR-modified T cells triggered anaphylaxis most likely by inducing an IgE antibody specific for the murine-based antibody sequences present in them | [20] |
An adverse event in a patient with colon cancer who received T cells modified with a HER2-specific CAR and CD28 plus 4–1BB was also associated with pulmonary toxicity. However, dyspnea and shock symptoms were present immediately (within 15 min) and in association with very high cytokine levels. A cardiac arrest occurred, and the patient died 4 days later. The authors favor the hypothesis that an on-target cytokine storm has resulted from the recognition of low levels of the antigen on lung tissue through HER2-specific CARs [77]. However, symptoms and high cytokine levels could also be a result of off-target toxicity, i.e. an anaphylactic reaction, as explained above.
In all other trials using T cells transduced with first- and second-generation CARs (mostly directed against B-cell antigens), no severe side effects have been reported [78, 79]. However, especially T cells transduced with first-generation CARs often failed to persist, limiting the number of patients bearing CAR-modified T cells with long-term follow up.
On-Target Toxicity
As already explained, it is beyond the scope of this review to analyze on-target toxicities for all antigens that have been used or suggested for use in clinical trials. But a few examples should demonstrate the importance of an intensive elaboration of this issue during the planning phase of a trial and, of course, before starting pharmaceutical production.
Although CD19 is one of the most frequently used targets in the recent CAR trials [80, 81], it illustrates perfectly that an efficient target antigen must not fulfill all of the criteria of an ideal antigen. The on-target toxicity of anti-CD19 CAR-modified T cells, namely severe and long-lasting B-lymphocyte depletion, is widely considered to be acceptable for patients suffering from an otherwise untreatable malignant disease. Moreover, from experience with lymphodepleting antibodies like rituximab there are well described ways how to handle increased infection risks with appropriate prophylactic and preemptive treatment strategies.
The example of CD19 demonstrates that severe and even life-threatening on-target toxicities can be accepted in a particular clinical setting. Similarly – providing two more examples – gut toxicity of Her2/neu-specific CAR-modified T cells [77] and liver toxicity of T cells transduced with CARs specific for carbonic anhydrase IX [82], have to be judged on the background of the general and individual clinical situation. As for every conventional drug, the magnitude and probability of side effects, as well as expected severe adverse events, have to be determined as accurately as possible and finally have to be outweighed against the expected clinical benefit for each individual patient. For the ethical approval of a study protocol, statistical calculations, based on the same variables, are necessary.
Evaluation of Effectiveness
An increasing number of clinical trials is not only addressing safety issues but also includes questions of effectiveness as secondary endpoints. The supplemental tables 1 and 2 (available at http://content.karger.com/ProdukteDB/produkte.asp?doi=357163) give an overview of recently terminated and ongoing trials with TCR- and CAR-modified T cells.
After first reports with rather moderate anti-tumor responses in clinical trials with genetically modified T cells, the first encouraging results are now accumulating. In the first trial with MART-1 TCR-transduced T cells, the transferred cells persisted in the blood for longer than 1 year and retained their target-specific responsiveness in 2 out of the 17 patients; this was associated with a partial clinical response [9]. A subsequent trial used a different MART-1 clone and gp100-specific TCR genes with enhanced affinities [70]. The results of this trial also indicated a correlation between clinical efficacy (an objective response was observed in 9 of 36 patients) and the persistence of the infused genetically modified T cells.
As reported by Louis et al. [83], GD2-specific CAR-modified T cells may persist up to 192 weeks after and adoptive cell transfer in patients with neuroblastoma. Survival of genetically modified cells was concordant with the percentage of CD4 and TCMs present in the administered T-cell product. In conclusion, GD2 CAR-modified T cells could induce complete remission in patients with neuroblastoma. Moreover, the administrated genetically modified cells proved to be able to persist in vivo at low level, which was associated with longer survival.
The first successful treatment of a non-melanoma tumor using TCR-modified T cells, have been reported by Robbins et al. [84]. In this clinical trial, patients with melanoma or synovial cell sarcoma were treated with NY-ESO-1-specific TCR-transduced T cells. In 4 of 6 patients with synovial cell sarcoma and 5 of 11 patients with melanoma, objective clinical responses were observed. Moreover, 2 of 11 patients with melanoma demonstrated complete remissions that persisted after 1 year. A partial response was observed in 1 patient with synovial cell sarcoma.
Recently, the meaningful results of 6 clinical trials targeting CD19+ malignancies with CAR-modified T cells have been reported [10, 85, 86, 87, 88, 89, 90]. In detail, 2 out of 3 patients with CLL, treated at the University of Pennsylvania, achieved a complete response, and the 3 showed a partial response [10, 85].
As reported by the group at the National Cancer Institute, out of 8 patients with CLL, follicular lymphoma and marginal zone lymphoma, one achieved complete remission, another stable disease and the remaining evaluable patients reached partial remissions. Furthermore, the group at the Memorial Sloan-Kettering Cancer Center reported on the results of treatment of 8 patients with CLL and 1 patient with B-cell acute lymphoblastic leukemia, demonstrating stable disease in 2 and substantial lymph node reduction in 1 of those CLL patients. [87]
Similar to other anti-cancer therapies, achievement of only partial response or the suffering of an early relapse are common phenomena following adoptive T-cell transfer. This is possibly due to the presence of resistance mechanism or development of escape mechanisms by the tumor, making it obvious, that adoptive T-cell transfer alone is often insufficient for total tumor eradication. Nevertheless, since complete responses are possible [10], we must anticipate the most likely escape or resistance mechanism in order to make T-cell-based biopharmaceuticals more efficient.
In vivo Persistence of Genetically Engineered T Cells
Lack of persistence of genetically modified T cells seems to be one of the major reasons of failure of adoptive cell transfer approaches [79, 82, 91, 92].
Large, well-established tumors can only be eradicated by a sufficient number of genetically modified cells. Based on the results of CD19 trials, it appears that the degree of treatment response is inversely proportional to the tumor burden. Therefore, patients with lowest tumor mass achieved the best clinical response [16]. Furthermore, in the majority of cases, an appropriate effector-to-target ratio in vivo can only be reached by proliferation of administered cells. There are several factors that influence the survival of transferred cells: the differentiation status of engineered T cells, the alteration of the host environment, and the presence of supportive cytokines. Providing that in vivo conditions are favorable and the transferred cells are robust, T cells can persist even up to 9 years [93]. But, as shown by Lamers et al, presence of powerful B-cell [82] or T-cell responses [94] can efficiently neutralize target cell recognition.
Summing up, it is always difficult to ascertain factors, which influence the persistence of genetically modified cells. But since it becomes more and more evident that the effector-to-target ratio plays a crucial role in the eradication of the tumor, the approaches to increase the proliferation potential of T cells have to be intensified alongside with efforts to modify the host environment.
Conclusions
There is an intense interest in harnessing immune mechanisms to control cancer. The development of promising novel T-cell therapies, making use of the potent T cells and new technologies to target them against tumor antigens, will open the door to a new field of biopharmaceuticals. The clinical value of redirected T cells as an effective and easily applicable cancer immunotherapy still has to be demonstrated. But the substantial tumor regression seen in animal models and first clinical trials impressively demonstrates the potential of these next-generation cell products. Besides all enthusiasm, for the design of clinical trials and the long-term acceptance of genetic cell engineering it is important to continuously address all relevant safely issues discussed in this review. Only the combination of high-quality products, professional risk management, and well-designed clinical trials will successfully ensure the transfer of redirected T-cell therapies from the bench to bedside.
Disclosure Statement
The authors declare that they have no competing interests.
Acknowledgments
The authors thank Alessandro Lorusso, for his help with analyzing the clinical trials database and for critical reading of this paper.
References
- 1.Rader RA. (Re)defining biopharmaceutical. Nat Biotechnol. 2008;26:743–51. doi: 10.1038/nbt0708-743. [DOI] [PubMed] [Google Scholar]
- 2.Ehrlich P. Über den jetzigen Stand der Karzinomforschung. Ned Tijdschr Genees. 1909;5:273–290. [Google Scholar]
- 3.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 4.Landsteiner K, Chase MW. Studies on the sensitization of animals with simple chemical compounds: IX. Skin sensitization induced by injection of conjugates. J Exp Med. 1941;73:431–438. doi: 10.1084/jem.73.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 8.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, Maric I, Raffeld M, Nathan D-AN, Lanier BJ, Morgan RA, Rosenberg SA. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A, Verdonck L, Niederwieser D, van Rhee F, Mittermueller J, de Witte T, Holler E, Ansari H, European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 12.Kolb H-J. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 13.Bar M, Sandmaier BM, Inamoto Y, Bruno B, Hari P, Chauncey T, Martin PJ, Storb R, Maloney DG, Storer B, Flowers MED. Donor lymphocyte infusion for relapsed hematological malignancies after allogeneic hematopoietic cell transplantation: prognostic relevance of the initial CD3+ T cell dose. Biol Blood Marrow Transplant. 2013;19:949–957. doi: 10.1016/j.bbmt.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson E, Ghorashian S, Stauss H. Improving TCR gene therapy for treatment of haematological malignancies. Adv Hematol. 2012;2012:404081. doi: 10.1155/2012/404081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med. 2012;14:405–415. doi: 10.1002/jgm.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davila ML, Brentjens R, Wang X, Rivière I, Sadelain M. How do CARs work? Early insights from recent clinical studies targeting CD19. Oncoimmunology. 2012;1:1577–1583. doi: 10.4161/onci.22524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163:507–513. [PubMed] [Google Scholar]
- 18.Leisegang M, Engels B, Meyerhuber P, Kieback E, Sommermeyer D, Xue S-A, Reuss S, Stauss H, Uckert W. Enhanced functionality of T cell receptor-redirected T cells is defined by the transgene cassette. J Mol Med (Berl) 2008;86:573–583. doi: 10.1007/s00109-008-0317-3. [DOI] [PubMed] [Google Scholar]
- 19.van Loenen MM, de Boer R, Hagedoorn RS, Jankipersadsing V, Amir AL, Falkenburg JHF, Heemskerk MHM. Multi-cistronic vector encoding optimized safety switch for adoptive therapy with T-cell receptor-modified T cells. Gene Ther. 2013;20:861–867. doi: 10.1038/gt.2013.4. [DOI] [PubMed] [Google Scholar]
- 20.Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X, Zhao Y, Kalos M, June CH. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res. 2013;1:26–31. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sommermeyer D, Uckert W. Minimal amino acid exchange in human TCR constant regions fosters improved function of TCR gene-modified T cells. J Immunol. 2010;184:6223–6231. doi: 10.4049/jimmunol.0902055. [DOI] [PubMed] [Google Scholar]
- 22.Cohen CJ, Li YF, El-Gamil M, Robbins PF, Rosenberg SA, Morgan RA. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 2007;67:3898–3903. doi: 10.1158/0008-5472.CAN-06-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuball J, Dossett ML, Wolfl M, Ho WY, Voss R-H, Fowler C, Greenberg PD. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109:2331–2338. doi: 10.1182/blood-2006-05-023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voss R-H, Willemsen RA, Kuball J, Grabowski M, Engel R, Intan RS, Guillaume P, Romero P, Huber C, Theobald M. Molecular design of the Cαβ interface favors specific pairing of introduced TCRαβ in human T cells. J Immunol. 2008;180:391–401. doi: 10.4049/jimmunol.180.1.391. [DOI] [PubMed] [Google Scholar]
- 25.Provasi E, Genovese P, Lombardo A, Magnani Z, Liu P-Q, Reik A, Chu V, Paschon DE, Zhang L, Kuball J, Camisa B, Bondanza A, Casorati G, Ponzoni M, Ciceri F, Bordignon C, Greenberg PD, Holmes MC, Gregory PD, Naldini L, Bonini C. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med. 2012;18:807–815. doi: 10.1038/nm.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramos CA, Dotti G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin Biol Ther. 2011;11:855–873. doi: 10.1517/14712598.2011.573476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hombach A, Hombach AA, Abken H. Adoptive immunotherapy with genetically engineered T cells: modification of the IgG1 Fc ‘spacer’ domain in the extracellular moiety of chimeric antigen receptors avoids ‘off-target’ activation and unintended initiation of an innate immune response. Gene Ther. 2010;17:1206–1213. doi: 10.1038/gt.2010.91. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y, Wang QJ, Yang S, Kochenderfer JN, Zheng Z, Zhong X, Sadelain M, Eshhar Z, Rosenberg SA, Morgan RA. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183:5563–5574. doi: 10.4049/jimmunol.0900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maiti SN, Huls H, Singh H, Dawson M, Figliola M, Olivares S, Rao P, Zhao YJ, Multani A, Yang G, Zhang L, Crossland D, Ang S, Torikai H, Rabinovich B, Lee DA, Kebriaei P, Hackett P, Champlin RE, Cooper LJN. Sleeping beauty system to redirect T-cell specificity for human applications. J Immunother. 2013;36:112–123. doi: 10.1097/CJI.0b013e3182811ce9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L-P, Lampert JC, Chen X, Leitao C, Popović J, Müller W, Blankenstein T. Transgenic mice with a diverse human T cell antigen receptor repertoire. Nat Med. 2010;16:1029–1034. doi: 10.1038/nm.2197. [DOI] [PubMed] [Google Scholar]
- 32.Smith HA, McNeel DG. The SSX family of cancer-testis antigens as target proteins for tumor therapy. Clin Dev Immunol. 2010;2010:150591. doi: 10.1155/2010/150591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroten C, Kraaij R, Veldhoven JLM, Berrevoets CA, den Bakker MA, Ma Q, Sadelain M, Bangma CH, Willemsen RA, Debets R. T cell activation upon exposure to patient-derived tumor tissue: a functional assay to select patients for adoptive T cell therapy. J Immunol Methods. 2010;359:11–20. doi: 10.1016/j.jim.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Scholler J, Brady TL, Binder-Scholl G, Hwang W-T, Plesa G, Hege KM, Vogel AN, Kalos M, Riley JL, Deeks SG, Mitsuyasu RT, Bernstein WB, Aronson NE, Levine B. L, Bushman FD, June CH. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;4:132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biffi A, Bartolomae CC, Cesana D, Cartier N, Aubourg P, Ranzani M, Cesani M, Benedicenti F, Plati T, Rubagotti E, Merella S, Capotondo A, Sgualdino J, Zanetti G, von Kalle C, Schmidt M, Naldini L, Montini E. Lentiviral vector common integration sites in preclinical models and a clinical trial reflect a benign integration bias and not oncogenic selection. Blood. 2011;117:5332–5339. doi: 10.1182/blood-2010-09-306761. [DOI] [PubMed] [Google Scholar]
- 36.Singh H, Manuri PR, Olivares S, Dara N, Dawson MJ, Huls H, Hackett PB, Kohn DB, Shpall EJ, Champlin RE, Cooper LJN. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68:2961–2971. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang X, Guo H, Tammana S, Jung Y-C, Mellgren E, Bassi P, Cao Q, Tu ZJ, Kim YC, Ekker SC, Wu X, Wang SM, Zhou X. Gene transfer efficiency and genome-wide integration profiling of Sleeping Beauty, Tol2, and piggyBac transposons in human primary T cells. Mol Ther. 2010;18:1803–1813. doi: 10.1038/mt.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh H, Figliola MJ, Dawson MJ, Olivares S, Zhang L, Yang G, Maiti S, Manuri P, Senyukov V, Jena B, Kebriaei P, Champlin RE, Huls H, Cooper LJN. Manufacture of clinical-grade CD19-specific T cells stably expressing chimeric antigen receptor using Sleeping Beauty System and artificial antigen presenting cells. PLoS One. 2013;8:e64138. doi: 10.1371/journal.pone.0064138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu K, Rosenberg SA. Transduction of an IL-2 gene into human melanoma-reactive lymphocytes results in their continued growth in the absence of exogenous IL-2 and maintenance of specific antitumor activity. J Immunol. 2001;167:6356–6365. doi: 10.4049/jimmunol.167.11.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalbasi A, Shrimali RK, Chinnasamy D, Rosenberg SA. Prevention of interleukin-2 withdrawal-induced apoptosis in lymphocytes retrovirally cotransduced with genes encoding an antitumor T-cell receptor and an antiapoptotic protein. J Immunother. 2010;33:672–683. doi: 10.1097/CJI.0b013e3181e475cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Q, Yang X, Pins M, Javonovic B, Kuzel T, Kim S-J, Parijs LV, Greenberg NM, Liu V, Guo Y, Lee C. Adoptive transfer of tumor-reactive transforming growth factor-beta-insensitive CD8+ T cells: eradication of autologous mouse prostate cancer. Cancer Res. 2005;65:1761–1769. doi: 10.1158/0008-5472.CAN-04-3169. [DOI] [PubMed] [Google Scholar]
- 42.Kershaw MH, Wang G, Westwood JA, Pachynski RK, Tiffany HL, Marincola FM, Wang E, Young HA, Murphy PM, Hwu P. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum Gene Ther. 2002;13:1971–1980. doi: 10.1089/10430340260355374. [DOI] [PubMed] [Google Scholar]
- 43.Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, Heslop HE, Brenner MK, Dotti G, Savoldo B. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavalieri S, Cazzaniga S, Geuna M, Magnani Z, Bordignon C, Naldini L, Bonini C. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 2003;102:497–505. doi: 10.1182/blood-2003-01-0297. [DOI] [PubMed] [Google Scholar]
- 45.Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, Bondanza A, Bordignon C, Peccatori J, Ciceri F, Lupo-Stanghellini MT, Mavilio F, Mondino A, Bicciato S, Recchia A, Bonini C. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121:573–584. doi: 10.1182/blood-2012-05-431718. [DOI] [PubMed] [Google Scholar]
- 46.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, Liu H, Wu M-F, Gee AP, Mei Z, Rooney CM, Heslop HE, Brenner MK. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cruz CR, Gerdemann U, Leen AM, Shafer JA, Ku S, Tzou B, Horton TM, Sheehan A, Copeland A, Younes A, Rooney CM, Heslop HE, Bollard CM. Improving T-cell therapy for relapsed EBV-negative Hodgkin lymphoma by targeting upregulated MAGE-A4. Clin Cancer Res. 2011;17:7058–7066. doi: 10.1158/1078-0432.CCR-11-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–233. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 52.Dings RPM, Vang KB, Castermans K, Popescu F, Zhang Y, Oude Egbrink MGA, Mescher MF, Farrar MA, Griffioen AW, Mayo KH. Enhancement of T-cell-mediated antitumor response: angiostatic adjuvant to immunotherapy against cancer. Clin Cancer Res. 2011;17:3134–3145. doi: 10.1158/1078-0432.CCR-10-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helms MW, Prescher JA, Cao Y-A, Schaffert S, Contag CH. IL-12 enhances efficacy and shortens enrichment time in cytokine-induced killer cell immunotherapy. Cancer Immunol Immunother. 2010;59:1325–1334. doi: 10.1007/s00262-010-0860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ullrich E, Bonmort M, Mignot G, Jacobs B, Bosisio D, Sozzani S, Jalil A, Louache F, Bulanova E, Geissman F, Ryffel B, Chaput N, Bulfone-Paus S, Zitvogel L. Trans-presentation of IL-15 dictates IFN-producing killer dendritic cells effector functions. J Immunol. 2008;180:7887–7897. doi: 10.4049/jimmunol.180.12.7887. [DOI] [PubMed] [Google Scholar]
- 56.Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, Ponzoni M, Rossini S, Mavilio F, Traversari C, Bordignon C. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 57.Di Stasi A, Tey S-K, Dotti G, Fujita Y, KennedyNasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, Liu H, Cruz CR, Savoldo B, Gee AP, Schindler J, Krance RA, Heslop HE, Spencer DM, Rooney CM, Brenner MK. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Straathof KC, Pulè MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, Heslop HE, Spencer DM, Rooney CM. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Introna M, Barbui AM, Bambacioni F, Casati C, Gaipa G, Borleri G, Bernasconi S, Barbui T, Golay J, Biondi A, Rambaldi A. Genetic modification of human T cells with CD20: a strategy to purify and lyse transduced cells with anti-CD20 antibodies. Hum Gene Ther. 2000;11:611–620. doi: 10.1089/10430340050015798. [DOI] [PubMed] [Google Scholar]
- 60.Sato T, Neschadim A, Konrad M, Fowler DH, Lavie A, Medin JA. Engineered human tmpk/AZT as a novel enzyme/prodrug axis for suicide gene therapy. Mol Ther. 2007;15:962–970. doi: 10.1038/mt.sj.6300122. [DOI] [PubMed] [Google Scholar]
- 61.Marin V, Cribioli E, Philip B, Tettamanti S, Pizzitola I, Biondi A, Biagi E, Pule M. Comparison of different suicide-gene strategies for the safety improvement of genetically manipulated T cells. Hum Gene Ther Methods. 2012;23:376–386. doi: 10.1089/hgtb.2012.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kepp O, Galluzzi L, Martins I, Schlemmer F, Adjemian S, Michaud M, Sukkurwala AQ, Menger L, Zitvogel L, Kroemer G. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30:61–69. doi: 10.1007/s10555-011-9273-4. [DOI] [PubMed] [Google Scholar]
- 63.Obeid M, Panaretakis T, Tesniere A, Joza N, Tufi R, Apetoh L, Ghiringhelli F, Zitvogel L, Kroemer G. Leveraging the immune system during chemotherapy: moving calreticulin to the cell surface converts apoptotic death from ‘silent’ to immunogenic. Cancer Res. 2007;67:7941–7944. doi: 10.1158/0008-5472.CAN-07-1622. [DOI] [PubMed] [Google Scholar]
- 64.Davila ML, Brentjens R, Wang X, Rivière I, Sadelain M. How do CARs work? Early insights from recent clinical studies targeting CD19. Oncoimmunology. 2012;1:1577–1583. doi: 10.4161/onci.22524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rudmann DG. On-target and off-target-based toxicologic effects. Toxicol Pathol. 2013;41:310–314. doi: 10.1177/0192623312464311. [DOI] [PubMed] [Google Scholar]
- 66.Varmus HE, Quintrell N, Ortiz S. Retroviruses as mutagens: insertion and excision of a nontransforming provirus alter expression of a resident transforming provirus. Cell. 1981;25:23–36. doi: 10.1016/0092-8674(81)90228-2. [DOI] [PubMed] [Google Scholar]
- 67.Stein S, Ott M. G, Schultze-Strasser S, Jauch A, Burwinkel B, Kinner A, Schmidt M, Krämer A, Schwäble J, Glimm H, Koehl U, Preiss C, Ball C, Martin H, Göhring G, Schwarzwaelder K, Hofmann W-K, Karakaya K, Tchatchou S, Yang R, Reinecke P, Kühlcke K, Schlegelberger B, Thrasher AJ, Hoelzer D, Seger R, von Kalle C, Grez M. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat Med. 2010;16:198–204. doi: 10.1038/nm.2088. [DOI] [PubMed] [Google Scholar]
- 68.Newrzela S, Cornils K, Li Z, Baum C, Brugman MH, Hartmann M, Meyer J, Hartmann S, Hansmann M-L, Fehse B, von Laer D. Resistance of mature T cells to oncogene transformation. Blood. 2008;112:2278–2286. doi: 10.1182/blood-2007-12-128751. [DOI] [PubMed] [Google Scholar]
- 69.Scholler J, Brady TL, Binder-Scholl G, Hwang W-T, Plesa G, Hege KM, Vogel AN, Kalos M, Riley JL, Deeks SG, Mitsuyasu RT, Bernstein WB, Aronson NE, Levine BL, Bushman FD, June CH. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;4:132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, Lee CC, Restifo NP, Schwarz SL, Cogdill AP, Bishop RJ, Kim H, Brewer CC, Rudy SF, VanWaes C, Davis JL, Mathur A, Ripley RT, Nathan DA, Laurencot CM, Rosenberg SA. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan D-A. N, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM, Cimino PJ, Binder-Scholl GK, Smethurst DP, Gerry AB, Pumphrey NJ, Bennett AD, Brewer JE, Dukes J, Harper J, Tayton-Martin HK, Jakobsen BK, Hassan NJ, Kalos M, June CH. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G, Bossi G, Vuidepot A, Powlesland AS, Legg A, Adams KJ, Bennett AD, Pumphrey NJ, Williams DD, Binder-Scholl G, Kulikovskaya I, Levine BL, Riley JL, Varela-Rohena A, Stadtmauer EA, Rapoport AP, Linette GP, June CH, Hassan NJ, Kalos M, Jakobsen BK. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. 2013;5:197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry RM, Phan GQ, Hughes MS, Kammula US, Miller AD, Hessman CJ, Stewart AA, Restifo NP, Quezado MM, Alimchandani M, Rosenberg AZ, Nath A, Wang T, Bielekova B, Wuest SC, Akula N, McMahon FJ, Wilde S, Mosetter B, Schendel DJ, Laurencot CM, Rosenberg SA. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bendle GM, Linnemann C, Hooijkaas AI, Bies L, de Witte MA, Jorritsma A, Kaiser ADM, Pouw N, Debets R, Kieback E, Uckert W, Song J-Y, Haanen JBAG, Schumacher TNM. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 2010;16:565–570. doi: 10.1038/nm.2128. 1p following 570. [DOI] [PubMed] [Google Scholar]
- 76.Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18:666–668. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O, Olszewska M, Bernal Y, Pegram H, Przybylowski M, Hollyman D, Usachenko Y, Pirraglia D, Hosey J, Santos E, Halton E, Maslak P, Scheinberg D, Jurcic J, Heaney M, Heller G, Frattini M, Sadelain M. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kohn DB, Dotti G, Brentjens R, Savoldo B, Jensen M, Cooper LJ, June CH, Rosenberg S, Sadelain M, Heslop HE. CARs on track in the clinic. Mol Ther. 2011;19:432–438. doi: 10.1038/mt.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cooper LJN, Jena B, Bollard CM. Good T cells for bad B cells. Blood. 2012;119:2700–2702. doi: 10.1182/blood-2011-12-398719. [DOI] [PubMed] [Google Scholar]
- 82.Lamers CHJ, Sleijfer S, Vulto AG, Kruit WHJ, Kliffen M, Debets R, Gratama JW, Stoter G, Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 83.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, Liu H, Wu M-F, Gee AP, Mei Z, Rooney CM, Heslop HE, Brenner MK. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee C-CR, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, Rosenberg SA. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O, Olszewska M, Bernal Y, Pegram H, Przybylowski M, Hollyman D, Usachenko Y, Pirraglia D, Hosey J, Santos E, Halton E, Maslak P, Scheinberg D, Jurcic J, Heaney M, Heller G, Frattini M, Sadelain M. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, Yang JC, Kammula US, Devillier L, Carpenter R, Nathan D-AN, Morgan RA, Laurencot C, Rosenberg SA. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR, Forman SJ. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16:1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, Liu H, Grilley B, Rooney CM, Heslop HE, Brenner MK, Dotti G. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ, Gopal AK, Pagel JM, Lindgren CG, Greenberg PD, Riddell SR, Press OW. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, Meechoovet HB, Bautista C, Chang W-C, Ostberg JR, Jensen MC. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 93.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, Bollard CM, Liu H, Wu M-F, Rochester RJ, Amrolia PJ, Hurwitz JL, Brenner MK, Rooney CM. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–35. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lamers CHJ, Willemsen R, van Elzakker P, van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J, Oosterwijk E, Sleijfer S, Debets R, Gratama JW. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2001;117:72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]