Abstract
To move molecules across the nuclear envelope they have to overcome the selective barrier of the nuclear pore which is formed by nucleoporins with FG repeats. For this, they are chaperoned by shuttling receptors that interact with FG nups thereby passing the barrier with an unresolved mechanism. We explored the molecular binding and dissociation of this process using single molecule force spectroscopy showing that no energetic cost is required for translocation.
Keywords: AFM, Force Spectroscopy, Nuclear Pore Complex, Nucleoporin, Recognition
Macromolecular transport between the cell nucleus and cytoplasm is gated by the nuclear pore complex (NPC). The NPC [1] is a 65–125 MDa ring-structure that spans the nuclear envelope [2] (Figure 1). It has 30 distinct proteins or nucleoporins (nups) in multiple copies totaling ~400–500 nups per NPC.[3, 4] Based on hydrophobicity and size,[5–7] it gates nucleocytoplasmic traffic of macromolecules >30 kDa or ~3 nm diameter, including most nuclear proteins and ribonucleoproteins.[8] Those shuttling cargos expose transport signals (i.e. short AA sequences) that are selectively captured by shuttling karyopherins (kaps),[9] to gain passage across the NPC. For this, the kaps continuously permeate the NPC gate by interacting with so-called FG nups thereby escorting molecules of different shapes and sizes across the barrier.[10]
Figure 1.
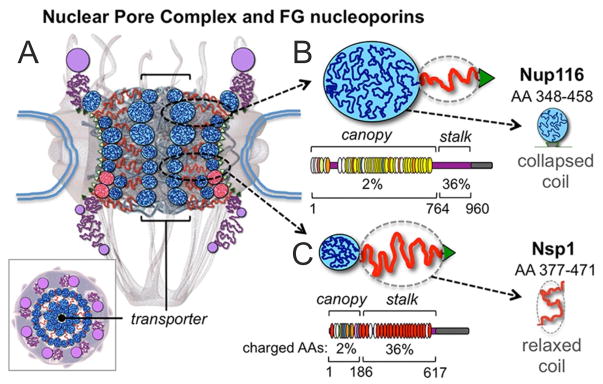
Diagrams of the NPC and its disordered FG nups. (A) Cross-section featuring the NPC ring-scaffold (center), cytoplasmic fibers (top), and nuclear basket (bottom). Intrinsically-disordered FG nups are outlined in blue (collapsed coils) and red (extended coils) lines forming the transporter/plug gate structure in the NPC channel [11]. A top view is also shown (bottom left). (B) The S. cerevisiae FG nups Nup116. As a representative of ‘cohesive’ nups it primarily adopts collapsed coil/’canopy’ configurations (blue line, globule). Below FG repeats are shown as thin ovals (GLFG in yellow; FxFG in red) and the amount of charged AA is indicated. The portion of Nup116’s canopy (AA 348–458) is highlighted. (C) Nsp1 as a representative of ‘repulsive’ nups. It adopts a relaxed-to-extended ‘stalk’ region (red line) and it has a high content of charged AAs and features mainly FxFG motifs (bottom). The portion of Nsp1’s stalk (AA 377–471) is highlighted.
Approximately one third of nups (~150) in the NPC feature phenylalanine-glycine repeats in intrinsically-disordered domains (FG nups)[11] that operate to form the NPC permeability barrier,[5, 12] whereas the other nups are forming the NPC scaffold. These line the periphery and the interior of the NPC transport conduit,[12, 13] exposing thousands of FG repeats that serve as ligands for kaps for chaperoning cargoes through the NPC pore.[14–16] The arrangement of disordered FG nups within the NPC (the barrier architecture) and the mechanism(s) by which kaps permeate the barrier are unresolved.
All current models of NPC structure and function propose that interactions between kaps and nup FG repeats govern the translocation process, [3, 13, 17, 18] and that the energetics of binding are coupled to translocation since chemical energy is not consumed.[8] Two of the models [13, 17] further suggest that hydrophobic interactions between FG nups themselves are key element in the gating mechanism. Hence, it is of key interest to quantify the interaction forces between nup FG repeats, and how they compare to the binding forces between kap and nup FG repeats.
We thus directly probed the force of interaction and the molecular dissociation rate between nup FG repeats and between kap and FG repeats using molecular recognition force spectroscopy (MRFS). MRFS measures inter-molecular interaction forces between single molecules.[19, 20] It was previously used to characterize FG motif binding sites on importin (a particular kap) and their modulation by Ran-induced conformational changes by which transport direction, cargo release and recycling of kaps are regulated.[21] Also, by studying the interaction of importin with Ran, (i) alternative conformational states in the complex were observed,[22] (ii) different modes of protein activation were discriminated [23] and (iii) the interaction energy landscape roughness was determined.[24] Last, an exploration of the nano-mechanical properties of nup FG domains by MRFS led to the hypothesis that importins reversibly collapse FG domains into compact structures (thereby opening space through the permeability barrier) by capturing multiple FG repeats simultaneously.[25]
Within this study we performed MRFS experiments (Figure 2) to measure interaction forces, stoichiometry, and molecular dissociation rates between nup FG repeats; and between importin and two distinct categories of FG domains: collapsed-coils featuring a low content of charged AAs, GLFG repeats, and a ‘cohesive’ nature (they stick to each other); and relaxed-to-extended coils featuring FxFG repeats, a high content of charged AAs, and a ‘repulsive’ nature (they repel all FG domains).[5, 13] Portions of S.cerevisiae Nup116 and Nsp1 FG domains, and human importin beta, were used as representatives.[13, 15, 21, 26–28] The Nup116 FG domain (AA 348–458) is a 110 AA collapsed-coil (Figure S1), adopts ~4 nm diameter globular configurations at 30°C (Rs = 20.4 Å), has a 3% charged-AA content, and features ten FG motifs.[13] The Nsp1 FG domain (AA 377–471) is a 95 AA relaxed-coil (Figure S1) with a dynamic backbone that fluctuates widely in hydrodynamic dimensions.[13] It has a ~5.4 nm average diameter (Rs = 26.8 Å), has a 34% charged-AA content, and features five FxFG repeats. Variants of Nup116 were constructed via FG>AG substitutions to contain zero (0-FG Nup116), one (1-GLFG Nup116), or two (2-GLFG Nup116) FG repeats instead of ten (10-FG Nup116) (Figure 2 and S1). The elimination of phenylalanines shifted their structure to more dynamic configurations [13] due to a loss of intra-molecular interactions.[26]
Figure 2.
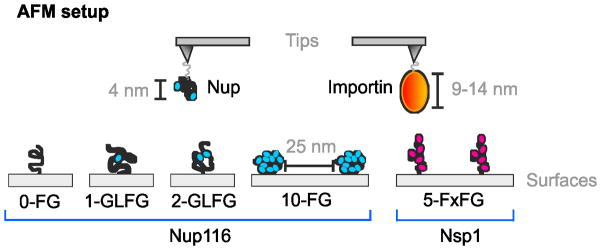
Diagram of the MRFS experimental approach. A single nup or importin molecule was immobilized at the apex of an AFM tip via a flexible 6 nm linker (Figure S2). FG domains were immobilized on flat mica surfaces (average distance ~25 nm) using the same linker.
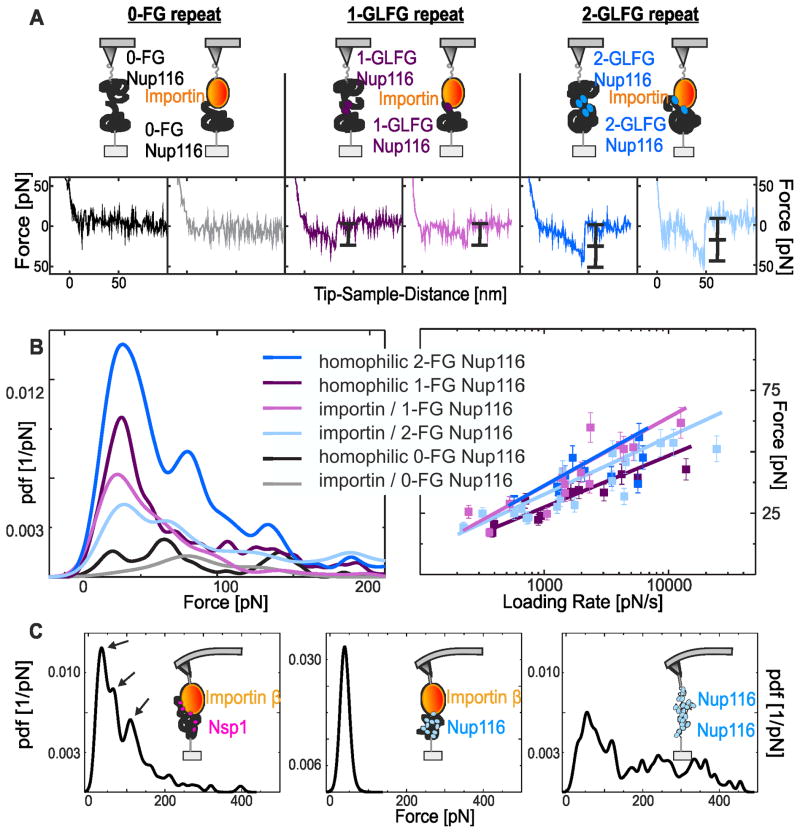
The interaction force between nup FG repeats was measured using functionalized sensor-tips and surfaces (Figure 2, S2 and S3) and recording force-distance cycles (Figure S4). The 0-FG Nup116 on the tip and mica surfaces produced only negligible interactions (Figure 3A). In contrast, the 1-GLFG Nup116 molecules formed single bonds, and the 2-GLFG Nup116 molecules formed single (not included) and double bonds with dissociation forces of ~25 pN and ~50 pN, respectively (Figure 3A). This was confirmed using statistical analyses that combined >200 force values for each experimental configuration to construct experimental probability density functions (pdfs) of interaction forces (Figure 3B). Single interactions (as the homophilic 1-GLFG Nup116 interaction) resulted in one significant peak, and double simultaneous interactions (as the homophilic 2-GLFG Nup116 interaction) in two discrete peaks. Homophilic 0-FG Nup116 interactions produced only low background signals with no distinguishable peak(s). Last, homophilic 10-FG Nup116 interactions showed broad force distributions, consistent with numerous FG repeat interactions (Figure 3C, right).
Figure 3.
MRFS analysis of nup-nup and importin-nup interactions. (A) MRFS analysis of homophilic Nup116 interactions and importin/Nup116 interactions. Homophilic 0-FG Nup116 interactions and importin/0-FG Nup116 interactions (left) showed negligible binding. Homophilic 1-GLFG Nup116 interactions and importin/1-GLFG Nup116 interactions (center) showed a single unbinding event (black bracket). Homophilic 2-GLFG Nup116 interactions and importin/2-GLFG Nup116 interactions showed two unbinding events: double-force events (right) and single-force events (not included). (B) Pdf analysis of MRFS measurements from nup/nup and importin/nup interactions. Interactions involving FG domains with zero, one and two GLFG repeats showed zero, one, and two force-maxima, respectively (left panel). Loading rate dependencies of the first force peak (right panel) yielding the kinetic off-rates and the distances of energy minimum (bound state) to maximum (unbound state) in the energy landscape (Table 1, Figure S5). (C) Pdf analysis of MRFS measurements from importin-nup interactions. Importin on the tip and 5-FxFG Nsp1 (left) or 10-FG Nup116 (center) on mica. Importin/5-FxFG Nsp1 complexes produced single-, double-, and triple-force unbinding events (arrows), whereas importin/10-FG Nup116 showed only single unbinding events. Homophilic 10-FG Nup116 interactions (right) showed multiple unbinding events and a broad force distribution.
Importin/FG nup interactions were also analyzed. The 0-FG Nup116 domain produced no interactions with importin (Figure 3A, left), and only background signals in the force distribution analyses (Figure 3B). In contrast, the 1-GLFG Nup116 molecules showed one interaction with importin, and the 2-GLFG Nup116 showed two interactions (Figure 3A). Consistently, the force distributions resulted in one and two significant peaks for the interactions, respectively (Figure 3B). Their force quanta were comparable to the ones from homophilic nup FG interactions (Figure 3B). Lastly, the 5-FxFG Nsp1 molecule achieved three distinct binding events corresponding to single, double and triple interactions; these were confirmed as separate peaks in the force pdf distributions (Figure 3C, left). Hence, at least three FxFG repeats (or two GLFG repeats; see above) could be captured simultaneously by importin. Next we tested whether importin binds collapsed-coil FG domains, such as 10-FG Nup116, similarly to relaxed-coil FG domains. Surprisingly, importin captured only one of ten FG repeats simultaneously, as evident from a single maximum in the force pdf profile (Figure 3C, center). Only one of ten FG repeats in the collapsed-coil Nup116 FG domain appeared available for importin binding at any given moment. Hence, intra-molecular FG repeat interactions in collapsed coil FG domains may dominate over interactions with importin, suggesting that importins are guided to follow paths between FG domain globules across the NPC, as proposed.[13, 26]
Variations in the loading rate (r) during MRFS experiments (Figure 3B and S5) allowed an estimation of kinetic off-rate constants (koff) and energy barrier distances (xβ) for the interactions (Table 1).[29, 30] Interactions involving a single FG repeat binding to importin, or to another nup FG repeat, showed comparable off-rates with 4–5 dissociations per second or ~200 ms bond lifetimes (Table 1). This was forty-times slower than the recorded interaction time of importin with an NPC during transit (~3–7 ms).[31] Hence, a particular mechanism like Ran-GTP triggered cargo dissociation from importin, environmental factors such as nup location and, and local molecule density variations previously reported as “highway” effect must accelerate FG repeat dissociations.[32] In the latter, kap translocation occurs on different “lanes” depending on their concentration and, kap crowding is a key element in fast transport rates at the center of the pore.
Table 1.
Kinetic parameters of nup-nup and importin-nup interactions. The koff is the kinetic off-rate constant; xβ is the energy barrier distance.
| Interactions | koff[s−1] | xβ[Å] |
|---|---|---|
| Homophilic 1-GLFG Nup116 | 5.41± 3.56 | 4.52 ± 1.29 |
| Homophilic 2-GLFG Nup116 | 4.26 ± 5.86 | 3.36 ± 1.88 |
| Importin with 1-GLFG Nup116 | 4.23 ± 1.79 | 3.39 ± 0.64 |
| Importin with 2-GLFG Nup116 | 3.99 ± 2.18 | 4.05 ± 0.79 |
| Importin with 10-FG Nup116 | 4.87 ± 1.01 | 1.14 ± 0.20 |
| Importin with 5-FxFG Nsp1 | 5.81 | 1.49 |
The koff and x β values were comparable among the single interactions of importin with Nup116 and Nsp1 FG domains (Table 1), despite these nups featuring different types of FG motifs, AA composition, and categories of disordered structure (Figure S1). Hence, all interactions appeared dominated by the common phenylalanine in the FG repeats, and not by intervening AA sequences. This was unexpected for the homophilic interactions because amyloid-like bonds involving residues between FG motifs have been reported.[33] However, such interactions require minutes to hours of incubation to form,[34] clearly beyond the timescale explored here.
In conclusion, the force distributions and loading rate dependencies (Table 1) for single nup-nup and importin-nup interactions were energetically equivalent. This key finding supports the hypothesis that importins enter and diffuse across the NPC by substituting FG repeat interactions of nups with energetically equivalent importin-FG repeat interactions. In addition, the different interaction behaviour of collapsed and extended coils with importin endorse the assumption of distinct pathways within the transport channel.
Supplementary Material
Acknowledgments
This work was supported by NIH RO1 grant GM077520 to M.R.; the Austrian Science Fund (FWF W1201 N13) to P.H.; L’Oréal Austria Scholarship “For Women in Science” to P H; Johannes Kepler
Contributor Information
Dr. Martina Rangl, Institute for Biophysics, Johannes Kepler University Linz, Gruberstr. 40, A-4020 Linz
Assist.Prof. Andreas Ebner, Institute for Biophysics, Johannes Kepler University Linz, Gruberstr. 40, A-4020 Linz
Justin Yamada, Department of Molecular, Cell and Developmental Biology, Sinsheimer Laboratories, University of California, Santa Cruz, CA 95064 USA.
Dr. Christian Rankl, Agilent Technologies Austria GmbH, Mooslackengasse 17, A-1190 Wien
Prof. Robert Tampe, Institute of Biochemistry, Biocenter, Goethe-University Frankfurt, Frankfurt, Germany
Prof. Hermann J. Gruber, Institute for Biophysics, Johannes Kepler University Linz, Gruberstr. 40, A-4020 Linz
Prof. Michael Rexach, Email: mrexach@ucsc.edu, Department of Molecular, Cell and Developmental Biology, Sinsheimer Laboratories, University of California, Santa Cruz, CA 95064 USA
Prof. Peter Hinterdorfer, Email: peter.hinterdorfer@jku.at, Institute for Biophysics, Johannes Kepler University Linz, Gruberstr. 40, A-4020 Linz
References
- 1.Feldherr CM, Kallenbach E, Schultz N. J Cell Biol. 1984;99(6):2216–22. doi: 10.1083/jcb.99.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoelz A, Debler E, Blobel G. Annu Rev Biochem. 2011;7(80):613–43. doi: 10.1146/annurev-biochem-060109-151030. [DOI] [PubMed] [Google Scholar]
- 3.Rout MP, Aitchison JD, Suprapto A, Hjertaas K, Zhao Y, Chait BT. J Cell Biol. 2000;148(4):635–51. doi: 10.1083/jcb.148.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ. J Cell Biol. 2002;158(5):915–27. doi: 10.1083/jcb.200206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel SS, Belmont B, Sante J, Rexach M. Cell. 2007;129:83–96. doi: 10.1016/j.cell.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 6.Naim B, Zbaida D, Dagan S, Kapon R, Reich Z. EMBO J. 2009;16(28):2697–705. doi: 10.1038/emboj.2009.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribbeck K, Gorlich D. EMBO J. 2002;3(21):2664–71. doi: 10.1093/emboj/21.11.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorlich D, Kutay U. Ann Rev Cell Dev Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 9.Chook Y, Suël K. Biochim Biophys Acta. 2010 E pub ahead of print. [Google Scholar]
- 10.Wente S, Rout M. Cold Spring Harb Perspect Biol. 2010;2(10):a000562. doi: 10.1101/cshperspect.a000562. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denning DP, Patel SS, Uversky V, Fink AL, Rexach M. Proc Natl Acad Sci USA. 2003;100(5):2450–5. doi: 10.1073/pnas.0437902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alber F, et al. Nature. 2007;450:695–701. doi: 10.1038/nature06405. [DOI] [PubMed] [Google Scholar]
- 13.Yamada J, et al. Mol Cell Prot. 2010;10:2205–24. doi: 10.1074/mcp.M000035-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radu A, Moore M, Blobel G. Cell. 1995;81(2):215–22. doi: 10.1016/0092-8674(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 15.Bayliss R, Littlewood T, Strawn LA, Wente SR, Stewart M. J Biol Chem. 2002;277(52):50597–606. doi: 10.1074/jbc.M209037200. [DOI] [PubMed] [Google Scholar]
- 16.Denning D, Rexach M. Mol Cell Proteomics. 2007;6(2):272–82. doi: 10.1074/mcp.M600309-MCP200. [DOI] [PubMed] [Google Scholar]
- 17.Ribbeck K, Gorlich D. EMBO J. 2001;15(20):1320–30. doi: 10.1093/emboj/20.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters R. Traffic. 2005;6(5):421–7. doi: 10.1111/j.1600-0854.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 19.Florin EL, Moy VT, Gaub HE. Science. 1994;264(5157):415–417. doi: 10.1126/science.8153628. [DOI] [PubMed] [Google Scholar]
- 20.Hinterdorfer P, Baumgartner W, Gruber HJ, Schilcher K, Schindler H. Proc Natl Acad Sci USA. 1996;93:3477. doi: 10.1073/pnas.93.8.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otsuka S, Iwasaka S, Yoneda Y, Takeyasu, Yoshimura S. Proc Natl Acad Sci USA. 2008;105(42):16101–6. doi: 10.1073/pnas.0802647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nevo R, Stroh C, Kienberger F, Kaftan D, Brumfeld V, Elbaum M, Reich Z, Hinterdorfer P. Nat Struct Biol. 2003;10(7):553–7. doi: 10.1038/nsb940. [DOI] [PubMed] [Google Scholar]
- 23.Nevo R, Brumfeld V, Elbaum M, Hinterdorfer P, Reich Z. Biophys J. 2004;87:4. doi: 10.1529/biophysj.104.041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nevo R, Brumfeld V, Kapon R, Hinterdorfer P, Reich Z. EMBO Rep. 2005;6(5):482–86. doi: 10.1038/sj.embor.7400403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim RY, Fahrenkrog B, Köser J, Schwarz-Herion K, Deng J, Aebi U. Science. 2007;318:640–643. doi: 10.1126/science.1145980. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan V, Lau E, Yamada J, Denning D, Patel S, Colvin M, Rexach M. PLoS Comp Biol. 2008;4(8):e1000145. doi: 10.1371/journal.pcbi.1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isgro TA, Schulten K. Structure. 2005;13(12):1869–79. doi: 10.1016/j.str.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Cingolani G, Petosa C, Weis K, Müller C. Nature. 1999;399(6733):221–9. doi: 10.1038/20367. [DOI] [PubMed] [Google Scholar]
- 29.Evans E, Ritchie K. Biophysical J. 1997;72(4):1541–1555. doi: 10.1016/S0006-3495(97)78802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rankl C, Kienberger F, Wildling L, Wruss J, Gruber HJ, Blaas D, Hinterdorfer P. Proc Natl Acad Sci USA. 2008;105(46):17778–83. doi: 10.1073/pnas.0806451105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang W, Musser S. J Cell Biol. 2006;174(7):951–961. doi: 10.1083/jcb.200605053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoch RL, Kapinos LE, Lim RY. Proc Natl Acad Sci USA. 2012;109(42):16911–16916. doi: 10.1073/pnas.1208440109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alder C, Frey S, Maas W, Schmidt H, Gorlich D, Baldus M. Proc Natl Acad Sci USA. 2010;107(14):6281–5. doi: 10.1073/pnas.0910163107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alberti S, Halfmann R, King O, Kapila A, Lindquist S. Cell. 2009;137(1):146–58. doi: 10.1016/j.cell.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.