Abstract
The purpose of this study was to measure the effect of wrist position on the relative motion of the middle finger flexor digitorum superficialis (FDS) tendon, subsynovial connective tissue (SSCT), median nerve, and flexor retinaculum during simulated active finger motion. The relative motion of each tissue was measured by fluoroscopy in 10 human cadavers. Measurements were obtained for wrist positions of neutral (0 degree extension), 30 and 60 degrees of flexion, and 30 and 60 degrees of extension. The shear strain index (SSI) was defined as the difference in motion between two tissues (tendon, SSCT, or nerve) divided by tendon excursion, expressed as a percentage. The motion of the tendon, SSCT, and nerve in the 60 degree flexed position was significantly less than the motion in all other wrist positions (p < 0.001). The SSI at 60 degrees of flexion for tendon–SSCT and tendon–nerve were significantly increased compared with all other positions (p < 0.001). Because the SSCT and tendon are physically connected, a decrease in SSCT motion relative to the tendon would increase the shear strain on the SSCT with tendon motion. Thus, this result suggests that the SSCT may be predisposed to shear injury from activity done in 60 degrees of wrist flexion.
Keywords: carpal tunnel, subsynovial connective tissue (SSCT), median nerve, fluoroscopy, human cadaver, flexor tendon, kinematic
Carpal tunnel syndrome (CTS), a pressure-induced neuropathy of the median nerve, is one of the most common clinical problems encountered by hand surgeons. In addition to the median nerve, the carpal tunnel contains nine flexor tendons, namely, the flexor digitorum profundus (FDP) and superficialis (FDS) to each finger, and the flexor pollicis longus (FPL). These tendons are surrounded by a multilayered fibrovascular tissue, commonly termed the subsynovial connective tissue (SSCT).1–3
The most characteristic histological finding in patients with CTS is noninflammatory fibrosis of the SSCT.4–6 Although some have suggested that these changes in the SSCT might be the cause rather than an effect of CTS,7,8 this remains controversial.
Carpal tunnel pressure increases with wrist flexion or extension in both normal controls and CTS patients.9 Finger loading increases carpal tunnel pressure in normal subjects as well as in a cadaver model.10–13 Wrist flexion and extension also decrease the space available for the median nerve at carpal tunnel.14 These changes are more pronounced in CTS patients. However, how these mechanical factors might generate the pathological changes seen in the SSCT remains unknown.
The longitudinal excursion of the median nerve with wrist motion in CTS patients is decreased compared to that in studies in cadavers with no antecedent history of CTS.15 The excursion of the SSCT during finger motion in CTS patients is also decreased in comparison to studies in cadavers with no antecedent history of CTS.16 These reports suggest that the SSCT is more tightly tethered both to tendon and median nerve in CTS patients than it is in normal hands.
Further study might elucidate the role of SSCT fibrosis in CTS. Because a proposed mechanism for the fibrosis is shear injury,17 and because certain wrist positions are implicated in at least some cases of CTS,18–20 it would be helpful to know the normal shear strains between tendon and SSCT in various wrist positions. Therefore, in this study, we measured the relative motion of flexor tendon, SSCT, median nerve, and flexor retinaculum in different wrist positions in normal human cadaver specimens.
MATERIALS AND METHODS
The experimental protocol was reviewed and approved by our institutional review board. Ten fresh frozen human cadaver (five male, five female, aged 45–91 years, mean age 78.3) upper extremities specimens were used. A medical record review was performed on each cadaver donor to obtain clinical and demographic data. Cadaver specimens were excluded if there was a history of CTS or other peripheral nerve disease, or if there was a history of conditions potentially associated with CTS, such as diabetes, thyroid disease, rheumatoid arthritis, osteoarthritis, gout, hemodialysis, sarcoidosis, amyloidosis, or traumatic injuries to the ipsilateral arm.
The upper extremities were amputated approximately 15 cm proximal to the wrist joint and were thawed at room temperature prior to testing. A custom designed external fixator was used to fix the wrist in the required position. Two screws were inserted into the index metacarpal bone at the radial side of the hand, and two screws were inserted into the distal radius. A skin incision was made longitudinally to expose the middle finger flexor digitorum superficialis (FDS) tendon from the muscle tendon junction to the proximal end of the finger flexor sheath, with the flexor retinaculum and bursa intact. A small window (5-mm diameter) was made in the flexor retinaculum to expose the middle finger FDS tendon, median nerve, and SSCT. Metal markers (9291K12, McMaster-Carr, Chicago, IL) were inserted into the middle finger FDS tendon and median nerve. A third marker was glued on the surface of the SSCT. The fourth marker was glued to the flexor retinaculum. The carpal tunnel was otherwise left undisturbed. The specimen was mounted in a custom fixture by clamping the proximal ends of the radius and ulna bones. Each arm was mounted palmar side up. The proximal ends of the finger FDS tendons were fixed with sutures to Dacron cords and connected to a mechanical actuator. A 100-g weight was attached to the each fingertip to maintain finger extension (Fig. 1). A fluoroscopy unit (BV 25, Scopofix MDPM, Philips) was positioned to observe marker motion in the lateral view. After positioning, a fifth marker was glued to the skin on the ulnar side of the wrist, positioned so as to line up with the palmar edge of the scaphoid or trapezium in the lateral fluoroscopic view. This marker was used to reproduce alignment of the specimen after changes in wrist position.
Figure 1.
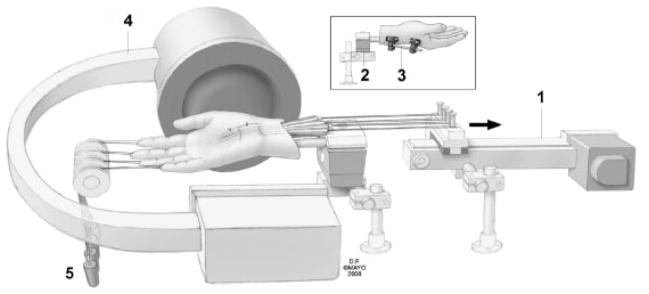
Schematic drawing of experimental setup: 1. Actuator; 2. custom fixture; 3. external fixator; 4. fluoroscopy; 5. weight. Copyright© Mayo Foundation; used with permission.
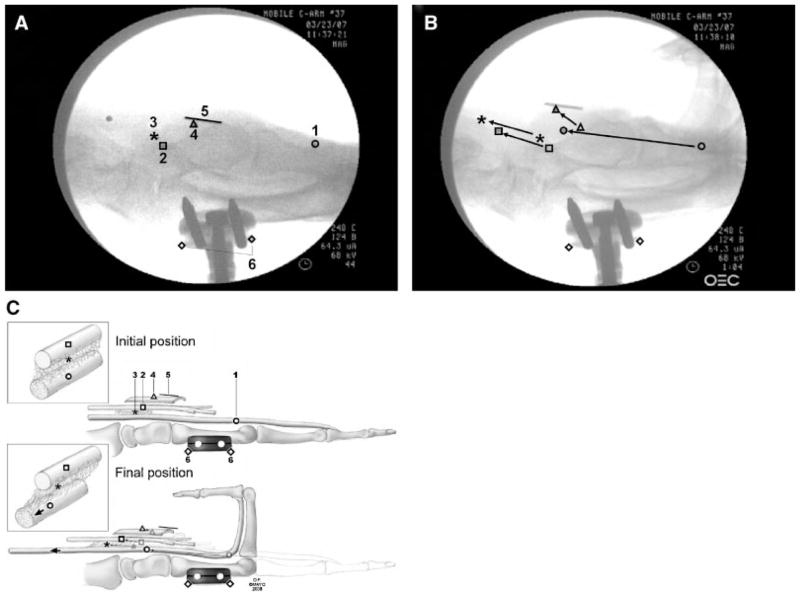
Testing was randomly performed in five wrist positions: neutral (0 degrees extension, N), 30 and 60 degrees of flexion (F30, F60), and 30 and 60 degrees of extension (E30, E60). The four FDS tendons (index, middle, ring, little finger) were pulled together proximally by the actuator against the weight at a rate of 2.0 mm/s for 20 s. This motion of the tendons toward the actuator was regarded as finger flexion (simulated fist). The motions of the four markers were recorded from the lateral view fluoroscopy using a digital video camera (DCR-TRV350, Sony, Japan) (Fig. 2A–C). Data were collected after an initial conditioning run. A 10-mm ruler was included in the camera field, so that the data measured with the video camera could be converted into a distance figure. The movement of each marker (tendon, SSCT, nerve, retinaculum) was digitized with Analyze Software (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). In addition, the left and right edges of the fixator were digitized as reference points.
Figure 2.
Example of video frames used to measure marker displacement: (A) initial position and (B) final position. (C) Schematic drawing of initial–final position: 1. tendon; 2. median nerve; 3. SSCT; 4. flexor retinaculum; 5. ruler (10 mm); and 6. the reference points. Copyright© Mayo Foundation; used with permission.
Digitized coordinate data (X, Y) for the tendon, SSCT, nerve, and retinaculum were processed using a custom Matlab program (The Mathworks, Inc, Natick, MA). All X and Y coordinate data were converted from pixels to millimeters using the pixel per mm conversion obtained from the imaged ruler. The coordinates for the tendon, SSCT, nerve, and retinaculum were normalized relative to the external fixator to correct for any translational or rotational motion of the image or specimen during data. Finally, the coordinates of the tendon, SSCT, nerve, and retinaculum were transformed to a new coordinate system such that the new X axis was aligned with the motion direction of the tendon, as defined by a linear polynomial fit to the tendon time series data. The Y axis was defined to be perpendicular to this polynomial fit line. Proximal and distal motions were defined as positive and negative motion, respectively, along the X axis. Palmar and dorsal motions were defined as positive and negative motion, respectively, along the Y axis. For the SSCT, tendon, nerve, and retinaculum, total distance and distance vectors along the X and Y axes were calculated. In addition, to estimate shear strains between structures, the relative motions of the tendon, SSCT, and nerve were compared using a shear strain index (SSI), defined as the ratio of the difference in X axis motion between two tissues divided by tendon X axis motion, expressed as a percentage. Thus, for example, the tendon–SSCT SSI would be defined as: [(tendon × axis motion − SSCT × axis motion)/tendon × axis motion] × 100%.
Statistical Analysis
Marker motion was compared for different wrist positions. A generalized linear model was used to analyze the variables. All results were expressed as mean ± standard error of the mean (SEM). The wrist position factors were considered significantly different when the p-value was <0.05 in a full model setting. A post hoc pairwise comparison was adapted using Scheffe’s test criteria for 10 combinations for the wrist position factor (Neutral vs. Flex 60, …, and Flex 60 vs. Ext 60). Thus, p-values <0.005 (0.05/10) were considered significant for each wrist position combination. All analyses were conducted using SAS version 9.1 STAT software GLM procedure (SAS Institute, Cary, NC).
RESULTS
For tendon motion, there were significant increases for the E30 and E60 positions and decreases for the F30 and F60 positions in both total and X axis motion compared to the neutral position (Table 1). There was a significant increase in Y axis motion for the F30 and F60 positions compared to the E60 position.
Table 1.
Marker Motion
| Absolute Distance (mm) | Tendon | SSCT | Nerve | Retinaculum |
|---|---|---|---|---|
| Total | ||||
| E60 | 36.3 (±1.2) a | 11.5 (±2.3) a | 8.3 (±1.8) ab | 3.5 (±0.9) ab |
| E30 | 34.7 (±1.7) b | 11.2 (±2.2) a | 8.4 (±1.6) ab | 3.9 (±1.5) ab |
| N | 33.5 (±1.3) b | 11.9 (±2.3) a | 9.3 (±2.5) a | 4.7 (±1.9) a |
| F30 | 31.6 (±1.5) c | 9.7 (±2.3) a | 7.5 (±2.1) b | 5.6 (±1.3) c |
| F60 | 30.7 (±1.4) c | 6.7 (±2.0) b | 5.8 (±2.3) c | 5.3 (±1.5) ac |
| X axis (the direction of tendon excursion) | ||||
| E60 | 36.3 (±1.2) a | 11.4 (±2.4) ab | 8.2 (±1.9) ab | 3.1 (±0.8) a |
| E30 | 34.7 (±1.7) b | 11.0 (±2.3) ab | 8.1 (±1.7) ab | 3.5 (±1.4) ab |
| N | 33.5 (±1.3) b | 11.8 (±2.3) a | 9.1 (±2.6) a | 4.4 (±1.8) b |
| F30 | 31.6 (±1.5) c | 9.3 (±2.4) b | 7.2 (±2.1) b | 4.5 (±1.4) b |
| F60 | 30.7 (±1.4) c | 6.0 (±2.2) c | 5.5 (±2.2) c | 4.1 (±1.6) ab |
| Y axis (normal to the direction of tendon excursion) | ||||
| E60 | 0.1 (±0.5) a | 0.1 (±1.7) a | 0.9 (±1.1) a | 1.5 (±0.8) a |
| E30 | 0.7 (±0.4) ab | 1.0 (±1.6) ab | 1.6 (±1.6) a | 1.6 (±0.7) a |
| N | 0.4 (±0.5) ab | 0.8 (±1.6) ab | 1.4 (±0.9) a | 1.4 (±0.9) a |
| F30 | 1.0 (±0.8) b | 2.4 (±1.3) b | 1.9 (±1.0) a | 3.1 (±1.0) b |
| F60 | 1.1 (±0.5) b | 2.3 (±1.8) b | 0.9 (±1.9) a | 3.2 (±1.2) b |
Different letters indicate significant differences (p < 0.005).
For SSCT motion, there was a significant decrease in both total and X axis motion for the F60 position compared to all other positions. There was a significant increase in Y axis motion for the F30 and F60 positions compared to the E60 position.
For median nerve motion, there was a significant decrease in both total and X axis motion for the F60 position compared to all other positions. There was no significant difference in median nerve Y axis motion for any position.
For retinaculum motion, there was a significant decrease in both total and X axis motion for the E60 position compared to the neutral position. There was a significant increase in Y axis motion for the F30 and F60 positions compared to the N, F30, and F60 positions.
The SSI between tendon and SSCT showed a significant increase in the F60 position compared to all other positions (Table 2). The SSI between tendon and nerve showed a significant increase in the F60 position compared to all other positions, as well as for the F30 and E60 positions compared to the N position. The SSI between SSCT and nerve showed a significant decrease in the SSI in the F60 position compared to the N, E30, and E60 positions.
Table 2.
Shear Strain Index (SSI)
| Shear Strain Index (%) | Tendon–SSCT | Tendon–Nerve | SSCT–Nerve |
|---|---|---|---|
| E60 | 68.7 (±6.3) a | 77.5 (±5.2) a | 8.7 (±6.7) a |
| E30 | 68.3 (±5.8) a | 76.7 (±4.4) ab | 8.4 (±6.1) a |
| N | 64.9 (±6.6) a | 72.9 (±7.6) b | 7.9 (±4.8) a |
| F30 | 70.4 (±7.5) a | 77.4 (±6.3) a | 6.9 (±6.8) ab |
| F60 | 80.3 (±7.1) b | 82.2 (±7.2) c | 1.8 (±6.2) b |
Differfent letters indicate significant differences (p < 0.005).
DISCUSSION
In this study, we investigated the relative motion of the SSCT within the carpal tunnel in different wrist positions and showed that the SSCT motion relative to tendon motion is least when the wrist was in 60 degrees of flexion. These results also show that tendon motion in wrist flexion generates higher shear strain in the SSCT. Osamura et al.21 measured shear strains in human carpal tunnel SSCT. They reported that maximum shear stress occurred at a shear strain of 30% in normal cadavers (comparable to our specimens) and 11% in the SSCT of patients with CTS. Although their method was different than ours (they looked at a dissected tendon with its SSCT and stressed the SSCT to failure), we believe that the SSI we observed, especially in the F60 position, likely exceeds the normal shear strain of the SSCT.
Zhao et al.22 showed in a cadaver study that wrist position affected tendon gliding resistance, which was highest when the wrist was in 60 degrees of flexion. If the gliding resistance is higher, then a greater force must be applied to the tendon to initiate motion. In addition, an in vivo study showed that FDS tendon force for active finger flexion was significantly higher in wrist flexion than in the neutral position.23 This greater loading in flexion is consistent with clinical studies, which showed that carpal tunnel pressure is increased with flexion. Thus, it is reasonable to assume that the increased shear strain in this position, which we report here, is accompanied by increased loading and, hence, shear stress. This may further predispose the SSCT to injury.
An exact comparison between studies of the excursion and strain of the median nerve is difficult due to differences in starting positions, range of motion, and applied force. Szabo et al.24 reported that, in human cadavers, median nerve excursion during finger motion ranged from 9–14 mm. Hough et al.15 reported that the excursion ranged from 11.2–12.5 mm in an in vivo ultrasound study. Our data showed 9.3 (±2.5) mm of median nerve total excursion in the neutral position, a similar value. In addition, we have shown that median nerve excursion is significantly decreased in wrist flexion.
Bay et al.25 used a nerve/tendon excursion ratio index to investigate the median nerve excursion in different wrist positions. They showed that the nerve/tendon excursion ratio was greatest in extension for the region proximal to the carpal tunnel. Goldstein et al.26 demonstrated decreased median nerve strain distal to the carpal tunnel, with greater nerve strain with extension and less with flexion. Combined, these two reports suggest that strain in the median nerve is increased proximal to the carpal tunnel and decreased distal to it; one possible explanation could be increased friction within the carpal tunnel, consistent with data presented by Zhao et al.22 Our results complement these studies by showing that the shear strain in the median nerve increases with both wrist flexion and extension.
The flexor retinaculum is an important pulley and helps maintain the transverse carpal arch.27,28 Our study presents a method to measure the motion of the flexor retinaculum in relation to tendon motion in a cadaver study. Our model can also be applied, using Y axis motion, to the study of various techniques that have been proposed to evaluate and treat bowstringing of the flexor tendons after carpal tunnel surgery.
The strengths of our study are that we simultaneously measured the motion of tendon, nerve, SSCT, and retinaculum in two dimensions. To evaluate the relative motion of the tendon, the X axis was defined using a regression line. We were thus able to estimate the strain in the SSCT, which is fixed to the tendon and which has been implicated in the etiology of CTS.29
There are several limitations of our study. We only measured the relative motion of the middle finger FDS tendon. We chose this tendon because the middle finger FDS tendon is anatomically closest to the median nerve. Other tendons might have different relative motions. The relative motion was measured with the tendons moving at a speed of 2 mm/s. Other speeds were not tested. An increased speed may demonstrate different relative motion, especially as the tissues studied are likely to be viscoelastic. Rate dependent changes in relative motion should be studied, and we plan to do such an analysis in the future. Finally, the excursion we chose, 40 mm, is at the upper limit of possible FDS excursion physiologically.30–32 The motion that we evaluated is likely to be most consistent with that seen with forceful gripping. However, it is just that type of action that has been associated with CTS.33 Thus, we believe our choice was a reasonable one. Finally, we only measured motion in two dimensions. Significant motion likely occurs in the transverse plane as well.34 Again, we plan to study three dimensional motion in the future.
In conclusion, we assessed the relative motion of SSCT, tendon, and nerve in different wrist positions. We demonstrated that 60 degrees of wrist flexion maximizes the shear strain in the SSCT. Future studies will be directed toward assessing the effect of rate of motion on SSCT shear, as well as in analyzing shear in three dimensions. When compared to data on SSCT stress,21 these results may be useful in estimating the risk of SSCT injury due to shear in various combinations of wrist position and tendon excursion. The data may also be useful in validating ultrasound methods35,36 of assessing motion of these structures.
Acknowledgments
The project described was supported by NIH/NIAMS Grant AR49823. The authors would like to thank Mr. Stephen Cha for help with the statistical analysis and Mr. David Factor for the artwork.
References
- 1.Guimberteau JC. The sliding system. Vascularized flexor tendon transfers. In: Guimberteau JC, editor. New ideas in hand flexor tendon surgery. Pessac, France: Institut Aquitain De La Main; 2001. [Google Scholar]
- 2.Lundborg G, Myrhage R. The vascularization and structure of the human digital tendon sheath as related to flexor tendon function. An angiographic and histological study. Scand J Plast Reconstr Surg. 1977;11:195–203. doi: 10.3109/02844317709025518. [DOI] [PubMed] [Google Scholar]
- 3.Ettema AM, Amadio PC, Zhao C, et al. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am. 2004;86:1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs PC, Nathan PA, Myers LD. Synovial histology in carpal tunnel syndrome. J Hand Surg Am. 1991;16:753–758. doi: 10.1016/0363-5023(91)90208-s. [DOI] [PubMed] [Google Scholar]
- 5.Neal NC, McManners J, Stirling GA. Pathology of the flexor tendon sheath in the spontaneous carpal tunnel syndrome. J Hand Surg Br. 1987;12:229–232. doi: 10.1016/0266-7681_87_90020-9. [DOI] [PubMed] [Google Scholar]
- 6.Nakamichi K, Tachibana S. Histology of the transverse carpal ligament and flexor tenosynovium in idiopathic carpal tunnel syndrome. J Hand Surg Am. 1998;23:1015–1024. doi: 10.1016/s0363-5023(98)80009-9. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong TJ, Castelli WA, Evans FG, et al. Some histological changes in carpal tunnel contents and their biomechanical implications. J Occup Med. 1984;26:197–201. [PubMed] [Google Scholar]
- 8.Lluch AL. Thickening of the synovium of the digital flexor tendons: cause or consequence of the carpal tunnel syndrome? J Hand Surg Br. 1992;17:209–212. doi: 10.1016/0266-7681(92)90091-f. [DOI] [PubMed] [Google Scholar]
- 9.Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63:380–383. [PubMed] [Google Scholar]
- 10.Cobb TK, Cooney WP, An KN. Aetiology of work related carpal tunnel syndrome: the role of lumbricle muscleand tool size on carpal tunnel pressures. Ergonomics. 1996;39:103–107. doi: 10.1080/00140139608964437. [DOI] [PubMed] [Google Scholar]
- 11.Corenfjord M, Sato K, Olmarker K, et al. A model for chronic nerve root compression studies. Presentation of a porcine model for controlled, slow-onset compression with analyses of anatomic aspects, compression onset rate, and morphologic and neurophysiologic effects. Spine. 1997;22:946–957. doi: 10.1097/00007632-199705010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Seradge H, Jia YC, Owens W. In vivo measurement of carpal tunnel pressure in the functioning hand. J Hand Surg Am. 1995;20:855–859. doi: 10.1016/S0363-5023(05)80443-5. [DOI] [PubMed] [Google Scholar]
- 13.Werner R, Armstong TJ, Bir C. Intracarpal canal pressures: the role of finger, hand, wrist and forearm position. Clin Biomech. 1997;12:44–51. doi: 10.1016/s0268-0033(96)00044-7. [DOI] [PubMed] [Google Scholar]
- 14.Bower JA, Stanisz GJ, Keir PJ. An MRI evaluation of carpal tunnel dimensions in healthy wrists: implications for carpal tunnel syndrome. Clin Biomech. 2006;21:816–825. doi: 10.1016/j.clinbiomech.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Hough AD, Moore AP, Jones MP. Reduced longitudinal excursion of the median nerve in carpal tunnel syndrome. Arch Phys Med Rehabil. 2007;88:569–576. doi: 10.1016/j.apmr.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Ettema AM, Zhao C, Amadio PC, et al. Gliding characteristics of flexor tendon and tenosynovium in carpal tunnel syndrome: A pilot study. Clin Anat. 2007;20:292–299. doi: 10.1002/ca.20379. [DOI] [PubMed] [Google Scholar]
- 17.Moore A, Wells R, Ranney D. Quantifying exposure in occupational manual tasks with cumulative trauma disorder potential. Ergonomics. 1991;34:1433–1453. doi: 10.1080/00140139108964888. [DOI] [PubMed] [Google Scholar]
- 18.Szabo RM, Chidgey LK. Stress carpal tunnel pressures in patients with carpal tunnel syndrome and normal patients. J Hand Surg Am. 1989;14:624–627. doi: 10.1016/0363-5023(89)90178-0. [DOI] [PubMed] [Google Scholar]
- 19.Stål M, Hansson GA, Moritz U. Wrist positions and movements as possible risk factors during machine milking. Appl Ergon. 1999;30:527–533. doi: 10.1016/s0003-6870(99)00015-0. [DOI] [PubMed] [Google Scholar]
- 20.Fung BK, Chan KY, Lam LY, et al. Study of wrist posture, loading and repetitive motion as risk factors for developing carpal tunnel syndrome. Hand Surg. 2007;12:13–18. doi: 10.1142/S0218810407003341. [DOI] [PubMed] [Google Scholar]
- 21.Osamura N, Zhao C, Zobitz ME, et al. Biomechanical evaluation of subsynovial connective tissue in carpal tunnel syndrome. J Biomech. doi: 10.1016/j.clinbiomech.2007.07.009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao C, Ettema AM, Osamura N, et al. Gliding characteristics between flexor tendons and surrounding tissues in the carpal tunnel: A biomechanical cadaver study. J Orthop Res. 2007;25:185–190. doi: 10.1002/jor.20321. [DOI] [PubMed] [Google Scholar]
- 23.Kursa K, Lattanza L, Diao E, et al. In vivo flexor tendon forces increase with finger and wrist flexion during active finger flexion and extension. J Orthop Res. 2006;24:763–769. doi: 10.1002/jor.20110. [DOI] [PubMed] [Google Scholar]
- 24.Szabo RM, Bay BK, Sharkey NA, et al. Median nerve displacement through the carpal canal. J Hand Surg Am. 1994;19:901–906. doi: 10.1016/0363-5023(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 25.Bay BK, Sharkey NA, Szabo RM. Displacement and strain of the median nerve at the wrist. J Hand Surg Am. 1997;22:621–627. doi: 10.1016/S0363-5023(97)80118-9. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein SA, Armstrong TJ, Chaffin DB, et al. Analysis of cumulative strain in tendons and tendon sheaths. J Biomech. 1987;20:1–6. doi: 10.1016/0021-9290(87)90261-2. [DOI] [PubMed] [Google Scholar]
- 27.Netscher D, Mosharrafa A, Lee M, et al. Transverse carpal ligament: its effect on flexor tendon excursion, morphologic changes of the carpal canal, and on pinch and grip strengths after open carpal tunnel release. Plast Reconstr Surg. 1997;100:636–642. doi: 10.1097/00006534-199709000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Kline SC, Moore JR. The transverse carpal ligament. An important component of the digital flexor pulley system. J Bone Joint Surg Am. 1992;74:1478–1485. [PubMed] [Google Scholar]
- 29.Ugbolue UC, Hsu W-H, Goitz RJ, et al. Tendon and nerve displacement at the wrist during finger movements. Clin Biomech. 2005;20:50–56. doi: 10.1016/j.clinbiomech.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Wehbé MA, Hunter JM. Flexor tendon gliding in the hand. Part II. Differential gliding. J Hand Surg Am. 1985;10:575–579. doi: 10.1016/s0363-5023(85)80086-1. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan EB. Functional and surgical anatomy of the hand. 2. Philadelphia: Lippincott; 1965. p. 12. [Google Scholar]
- 32.Wehbé MA, Hunter JM. Flexor tendon gliding in the hand. Part I. In vivo excursions. J Hand Surg Am. 1985;10:570–574. doi: 10.1016/s0363-5023(85)80085-x. [DOI] [PubMed] [Google Scholar]
- 33.Hagberg M, Morgenstern H, Kelsh M. Impact of occupations and job tasks on the prevalence of carpal tunnel synrome. Scand J Work Environ Health. 1992;18:337–345. doi: 10.5271/sjweh.1564. [DOI] [PubMed] [Google Scholar]
- 34.Nakamichi K, Tachibana S. Transverse sliding of the median nerve beneath the flexor retinaculum. J Hand Surg Br. 1992;17:213–216. doi: 10.1016/0266-7681(92)90092-g. [DOI] [PubMed] [Google Scholar]
- 35.Ettema AM, Belohlavek M, Zhao C, et al. High-resolution ultrasound analysis of subsynovial connective tissue in human cadaver carpal tunnel. J Orthop Res. 2006;24:2011–2020. doi: 10.1002/jor.20252. [DOI] [PubMed] [Google Scholar]
- 36.Oh S, Belohlavek M, Zhao C, et al. Detection of differential gliding characteristics of the flexor digitorum superficialistendon and subsynovial connective tissue using color Doppler sonographic imaging. J Untrasound Med. 2007;26:149–155. doi: 10.7863/jum.2007.26.2.149. [DOI] [PubMed] [Google Scholar]