Abstract
Hyperoxia contributes to the development of bronchopulmonary dysplasia in premature infants. Earlier we observed that aryl hydrocarbon receptor (AhR)-deficient mice are more susceptible to hyperoxic lung injury than AhR-sufficient mice, and this phenomenon was associated with a lack of expression of cytochrome P450 1A enzymes. Omeprazole, a proton pump inhibitor, used in humans with gastric acid related disorders, activates AhR in hepatocytes in vitro. However, the effects of omeprazole on AhR activation in the lungs and its impact on hyperoxia-induced ROS generation and inflammation are unknown. In this study, we tested the hypothesis that omeprazole attenuates hyperoxia-induced cytotoxicity, ROS generation, and expression of monocyte chemoattractant protein-1 (MCP-1) in the human lung derived H441 cells via AhR activation. Experimental groups included cells transfected with AhR small interfering RNA (siRNA). Hyperoxia resulted in significant increases in cytotoxicity, ROS generation, and MCP-1 production, which were significantly attenuated with the functional activation of AhR by omeprazole. The protective effects of omeprazole on cytotoxicity, ROS production, and MCP-1 production were lost in H441 cells whose AhR gene was silenced by AhR siRNA. These findings support the hypothesis that omeprazole protects against hyperoxic injury in vitro via AhR activation that is associated with decreased ROS generation and expression of MCP-1.
Keywords: Omeprazole, Aryl hydrocarbon Receptor, Hyperoxic Injury, Oxidant Stress, Inflammation, Free radicals
Introduction
Supplemental oxygen is commonly administered as an important and life-saving measure in patients with impaired lung function. Although delivery of enriched oxygen relieves the immediate life-threatening consequences transiently, it may also exacerbate lung injury [1]. Excessive oxygen exposure and lung-stretching lead to increased reactive oxygen species (ROS) production and expression of proinflammatory cytokines [2]. ROS react with nearby molecules (e.g., protein, lipids, DNA, and RNA) and modify their structure and function [3], resulting in both acute and chronic pulmonary toxicities. The antioxidant defense system develops late in gestation, making preterm neonates highly susceptible to oxidative stress [4, 5]. Despite significant improvements in the neonatal intensive care management of premature neonates, bronchopulmonary dysplasia (BPD) remains the most prevalent, and one of the most serious long-term sequelae of preterm birth, affecting approximately 14,000 preterm infants born each year in United States [6, 7]. Evidence implicates hyperoxia induced generation of ROS as a major contributor in the development of BPD and its sequelae [8]. Infants with BPD are more likely to have long-term pulmonary problems, increased re-hospitalizations during the first year of life, and delayed neurodevelopment [6, 9]. Hence, there is an urgent need for improved therapies in the prevention and treatment of BPD.
The aryl hydrocarbon receptor (AhR) is a member of basic - helix – loop – helix / PER – ARNT – SIM family of transcriptional regulators [10, 11 12]. In humans, the AhR is highly expressed in the lungs, thymus, kidney and liver [13]. The AhR is predominantly cytosolic, held in a core complex comprising two molecules of 90-kDa heat shock protein and a single molecule of the co-chaperone hepatitis X-associated protein-2 [14, 15]. AhR activation results in the translocation of the cytosolic AhR to the nucleus, where it dimerizes with the AhR nuclear translocator, to form a heterodimeric transcription factor. The heterodimeric transcription factor initiates transcription of many phase I and phase II detoxification enzymes such as cytochrome P450 (CYP) 1A1, CYP1A2, glutathione S-transferase-α, NAD(P)H quinone reductase-1, UDP glucuronosyl transferase, and aldehyde dehydrogenase, which are encoded by the Ah gene locus [16-19]. We reported that mice deficient in AhR are more susceptible to hyperoxic lung injury due to lack of expression of pulmonary and hepatic CYP1A subfamily of enzymes [20, 21]. Recently, it was observed that AhR deficient mice have an increased inflammatory response in their lungs on exposure to cigarette smoke or bacterial endotoxin [22], suggesting that AhR-mediated processes suppresses inflammation. However, the impact of activated AhR on hyperoxia-induced generation of ROS and inflammation in the lungs is not clear.
Omeprazole, a substituted benzimidazole derivative, is a proton pump inhibitor that inhibits gastric acid secretion in humans [23]. It has been widely used in the management of gastric acid related disorders in humans for about 15 years [24]. Previous studies have shown that omeprazole is an activator of AhR in human and rat hepatocytes [25-28]. Whether omeprazole activates AhR in the lungs and mitigate hyperoxia-induced generation of ROS and inflammation in the lungs has not been studied. The goals of this study, therefore, were to investigate the effects of omeprazole on the activation of the AhR in a pulmonary cell line, and its impact on hyperoxia-induced injury in these lung cells in vitro. The human pulmonary adenocarcinoma cell line H441, which has both type II and Clara cell characteristics, was used in this study to test the hypothesis that omeprazole attenuates hyperoxia-induced cytotoxicity, ROS generation, and expression of monocyte chemoattractant protein-1 (MCP-1) in vitro via AhR activation.
Materials and Methods
Cell culture and treatment
H441 cells, a human lung adenocarcinoma epithelial cell line, obtained from American Type Culture Collection (Manassas, VA), were grown in RPMI −1640 medium (Invitrogen, Carlsbad, CA; 21870) containing 10% fetal bovine serum, 50 U/ml penicillin and 50 U/ml streptomycin, in 95% air and 5% CO2 at 37°C. Cells were treated with either dimethylsulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO; 276855), or omeprazole 2μM (OM2) or omeprazole 5μM (OM5) (Sigma-Aldrich, St. Louis, MO; O104) for 4 h, followed by exposure to room air or hyperoxia for up to 72 h.
Exposure of cells to hyperoxia
Hyperoxia experiments were conducted in a plexiglass, sealed chamber into which a mixture of 95% O2 and 5% CO2 was circulated continuously. The chamber was placed in a Forma Scientific water-jacketed incubator at 37°C. Once the O2 level inside the chamber reached 95%, the cells were placed inside the chamber for the desired length of time.
Determination of Functional Activation of AhR
It is widely established that functional activation of AhR results in its translocation into the nucleus, which results in transcriptional activation of a number of CYP1 genes such as CYP1A1, CYP1A2 and CYP1B1. Therefore, we determined the functional activation of AhR by analyzing the expression of nuclear AhR apoprotein and CYP1A1 apoprotein and mRNA levels.
Western Blot Assays
Whole-cell, nuclear and cytoplasmic protein extracts from the cells were obtained by using nuclear extraction kit (Active Motif, Carlsbad, CA; 40010) according to the manufacturer's instructions. β-actin and histone deacetylase class 1 (HDAC1) were used as reference proteins for the cytoplasmic and nuclear fractions, respectively. Five or 40 μg of protein extracts was separated by 10% SDS-polyacrylamide gel electrophoresis for detection of AhR and CYP1A1 apoproteins, respectively, and transferred to polyvinylidene difluoride membranes. The membranes were incubated overnight at 4°C with the following primary antibodies: anti-AhR antibody (Santa Cruz Biotechnologies, Santa Cruz, CA; sc-5579, dilution 1:500), anti-CYP1A1 antibody (gift from P.E. Thomas, Rutgers University, Piscataway, NJ, dilution 1:500), anti-β-actin antibody (Sigma-Aldrich, St. Louis, MO; A5316, dilution 1:2000), and anti-HDAC1 antibody (Santa Cruz Biotechnologies, Santa Cruz, CA; sc-7872, dilution 1:500). The primary antibodies were detected by incubation with the appropriate horseradish peroxidase-conjugated secondary antibodies. The immuno-reactive bands were detected by chemiluminescence methods and the band density was analyzed by Kodak 1D 3.6 imaging software (Eastman Kodak Company, Rochester, NY).
Real-time RT- PCR assays
Cells were grown on 6 well plates to 50-60 % confluence, after which they were treated with DMSO or omeprazole, and exposed to room air or hyperoxia. At 12, 48, and72 h of exposure, total RNA was isolated using RNeasy kit (Qiagen, Hilden, Germany; 74104) and reverse transcribed to cDNA with SuperscriptIII reverse transcriptase enzyme (Invitrogen, Carlsbad, CA; 11752). Real-time quantitative RT-PCR analysis was performed with 7900HT Fast Real-Time PCR System using SYBR Green qPCR Supermix-UDG (Invitrogen, Carlsbad, CA; 11733). The PCR reaction was performed using the indicated primers after an initial 2-minute denaturation at 94°C, followed by the indicated annealing temperatures for 10 s, and 20 s extension at 72°C. The thermal cycling step was for 40 cycles at 95°C for 15 s, and 40 cycles at 60°C for 1 minute. The annealing temperatures used were 65, 62 and 60°C for CYP1a1, AhR and OAZ1 respectively. The sequences of the primer pairs were hAhR: 5′- CACCGATGGGAAATGATACTATCC-3′ and 5′-GGTGACCTCCAGCAAATGAGTT -3′; hCYP1a1: 5'-TGGATGAGAACGCCAATGTC-3' and 5'-TGGGTTGACCCATAGCTTCT-3'; hOAZ1: 5’-AAACGCATTAACTGGCGAAC-3’ and 5’-GAACTCCAGGAGAACTGCAAA-3’. OAZ1 was used as the reference gene. The ΔΔCt method was used to calculate the fold change in mRNA expression: ΔCt = Ct (target gene) - Ct (reference gene), ΔΔCt = ΔCt (treatment) - ΔCt (control), fold change = 2(−ΔΔCt).
Cell Viability Assay
Cell viability was determined by a colorimetric assay based on the ability of viable cells to reduce the tetrazolium dye, 2, 3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carbox-anilide (XTT) (Invitrogen, Carlsbad, CA; X6493) to formazan. H441 cells were seeded onto 96-well microplates at a density of 1×104 cells per well in 100 μl of phenol red free RPMI medium (Invitrogen, Carlsbad, CA; 11835), and treated with DMSO or omeprazole, followed by exposure to room air or hyperoxia for up to 72 h. The cell viability was assessed by XTT reduction assays as outlined in the XTT Assay protocol (American Type Culture Collection, Manassas, VA). The XTT reduction activities in cells exposed to hyperoxia were expressed as a percentage of the reduction activities in corresponding cells incubated in normoxia, calculated as: (absorbance of cells exposed to normoxia)-(absorbance of cells exposed to hyperoxia)/(absorbance of cells exposed to normoxia) × 100.
Measurement of ROS generation
Intracellular level of reactive oxygen species was quantified by the ROS sensitive fluorophore 5-(and-6)-chloromethyl-2’, 7’-dichlorodihydrofluorescein diacetate (CM-H2DCF-DA) according to the manufacturer's recommendation (Invitrogen, Carlsbad, CA; C6827). Briefly, cells were grown on 6-well plates to 60-70% confluence in phenol red free RPMI-1640 medium, after which they were treated with DMSO or omeprazole, and exposed to room air or hyperoxia for up to 48 h. The cells were then incubated with 5μM CM-H2DCF-DA in PBS for 30 min at 37°C in 5% CO2. The stained cells were washed with PBS and allowed to recover in the growth media for 30 min at 37°C in 5% CO2, and then analyzed by flow cytometry on a FACScan (BD Biosciences, San Jose, CA) with the associated software (Cell Quest).
Measurement of MCP-1 production: enzyme-linked immunosorbent assay (ELISA)
The levels of MCP-1 in culture supernatants of cells treated with DMSO or omeprazole, and exposed to room air or hyperoxia for up to 72 h, were determined by an ELISA Kit according to the manufacturer's instructions (R&D Systems, Minneapolis, MN; USA).
siRNA transfections
H441 cells were seeded on 6-well plates at 50-60% confluence 24 h before transfection. Transfection was performed with either 50 nM control siRNA (Dharmacon, Lafayette, CO; d-001810) or 50 nM AhR specific siRNA (Dharmacon, Lafayette, CO; L-004990) using Lipofectamine (Invitrogen, Carlsbad, CA; 11668). After 24 h of transfection, the medium was replaced, and the cells were treated with DMSO or omeprazole, and exposed to room air or hyperoxia for 48 h. siRNA mediated knockdown of AhR was validated by determining the expression of AhR mRNA by real time RT PCR and protein by western blotting. After 48 h of exposure, cells were harvested and analyzed for functional activation of AhR, viability, ROS generation, and MCP-1 production as described above.
Analyses of data
The results were analyzed by computerized statistical package (SPSS version 19). At least three separate experiments were performed for each measurement, and the data are expressed as means ± SEM. Differences between the various groups were compared by ANOVA followed by post hoc Tukey's test. A p value of <0.05 was considered significant.
Results
In this study, we investigated the effects of omeprazole on the functional activation of AhR, and its impact on hyperoxic injury in the human lung derived H441 cells in vitro.
Omeprazole increased nuclear AhR protein levels in H441 cells
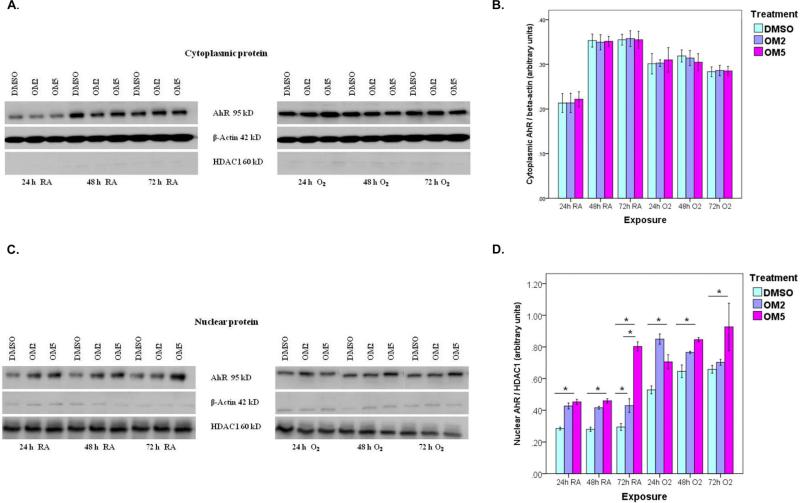
It has been observed that activation of AhR results in its translocation from the cytoplasm to the nucleus. So, we fractionated the cytoplasmic and nuclear proteins of the cell lysates and then analyzed the amounts of AhR in each fraction by western blotting. Omeprazole significantly augmented AhR apoprotein expression in the nucleus compared to controls in H441 cells exposed to room air and hyperoxia (Figs. 1 C and D). The cytoplasmic levels of AhR did not differ between omeprazole and control groups both in room air and hyperoxic conditions (Figs.1A and B). Hyperoxia independently increased nuclear localization of AhR protein in H441 cells (Figs. 1C and D).
Figure 1. Omeprazole increases nuclear translocation of AhR.
H441 cells were treated with dimethylsulfoxide (DMSO) or omeprazole (OM) at concentrations of 2μM (OM2) or 5μM (OM5), followed by exposure to room air or hyperoxia for up to 72 h. The cell lysates were separated into cytoplasmic and nuclear fractions at the indicated time points, and Western blotting was performed using anti-AhR, anti-β-actin, or anti- HDAC1 (histone deacetylase class 1) antibodies. (A). Representative western blot showing cytoplasmic AhR apoprotein expression. (B). Densitometric analysis of the cytoplasmic AhR apoprotein and β-actin. (C). Representative Western blot assay showing nuclear AhR apoprotein expression. (D). Densitometric analysis of the nuclear AhR apoprotein and HDAC1. Values are presented as means ± SEM (n=4). *P < 0.005. Data are representative of at least three independent experiments. Bars: Controls (blue), OM2 (lavender), and OM5 (purple).
Omeprazole increased CYP1A1 apoprotein and mRNA levels in H441 cells
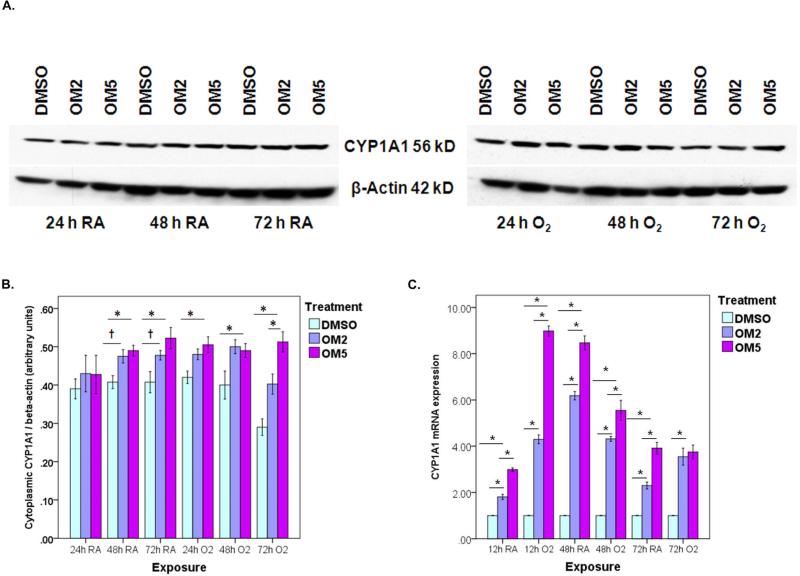
To provide further evidence on functional activation of AhR by omeprazole, we next determined the effects of omeprazole on CYP1A1 apoprotein and mRNA levels by western blot assay and real-time RT-PCR analysis, respectively. Hyperoxia increased CYP1A1 apoprotein and mRNA expression compared to corresponding room air groups at 24-h time point (Fig 2). Interestingly, the CYP1A1 apoprotein and mRNA expression decreased to below that of the corresponding room air groups in the samples exposed to 48 – 72 h hyperoxia. However, omeprazole significantly increased CYP1A1 apoprotein (Figs. 2 A and B) and mRNA (Fig. 2 C) levels in a dose-dependent manner compared to controls both in room air and hyperoxic conditions
Figure 2. Omeprazole is a transcriptional activator of the AhR target gene, cytochrome (CYP) 1A1.
H441 cells treated with DMSO, OM2 or OM5 were exposed to room air or hyperoxia for up to 72 h. (A) Cytoplasmic fractions of the cells were extracted at 24, 48 and 72 h of exposure, and Western blot assays were performed using anti-CYP1A1 and anti-β-actin antibodies. (B) CYP1A1 band intensities were quantified and normalized to β-actin. (C) RNA, isolated at 12, 48 and 72 h of exposure, was subjected to real-time RT-PCR analysis of CYP1A1 mRNA. Data are representative of at least three independent experiments. Values are presented as means ± SEM (n=4). *P < 0.005, †P < 0.05.
Omeprazole protected against hyperoxia-induced cytotoxicity in H441 cells
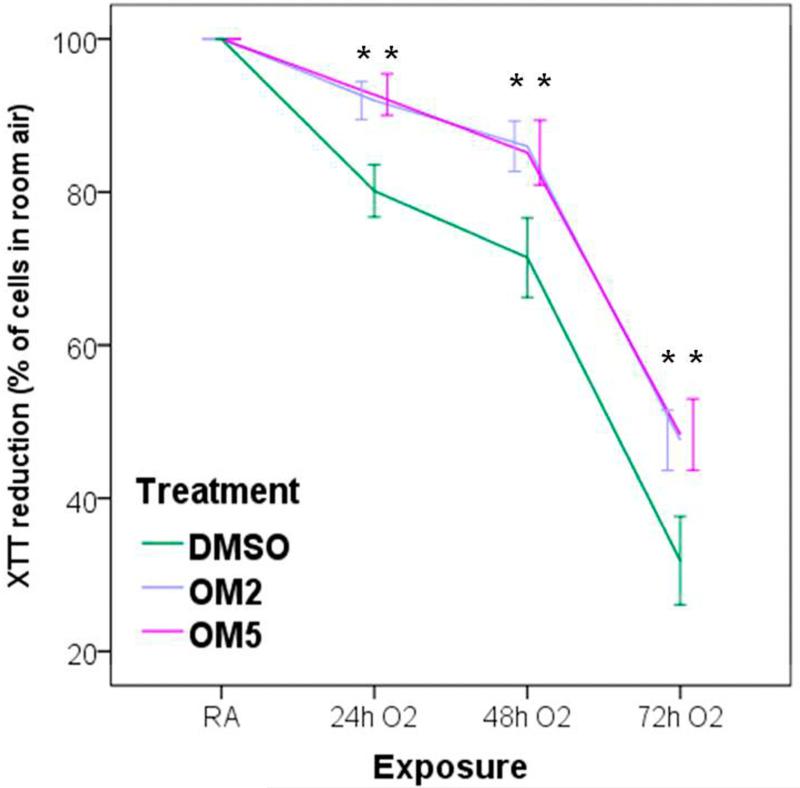
The XTT activity reflects the mitochondrial activity of the cells, and thus the absorbance measured reflects the cell viability. Exposure to hyperoxia caused cytotoxicity, as reflected by a decrease in the cellular capacities to reduce XTT, in a time-dependent manner. However, omeprazole significantly attenuated hyperoxia-induced cytotoxicity compared to controls (Fig. 3).
Figure 3. Omeprazole decreases cytotoxicity in H441 cells exposed to hyperoxia.
The viability of H441 cells treated with DMSO or OM and exposed to room air or hyperoxia was assessed by 2, 3 bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carbox-anilide (XTT) assay, at 24, 48 and 72 h of exposure. The graph shows XTT reduction activities in cells exposed to hyperoxia, expressed as a percentage of the reduction activities in corresponding cells incubated in normoxia. Values are presented as means ± SEM (n=4). Data are representative of at least three independent experiments. *P < 0.005.
Omeprazole decreased hyperoxia-induced generation of ROS in H441 cells
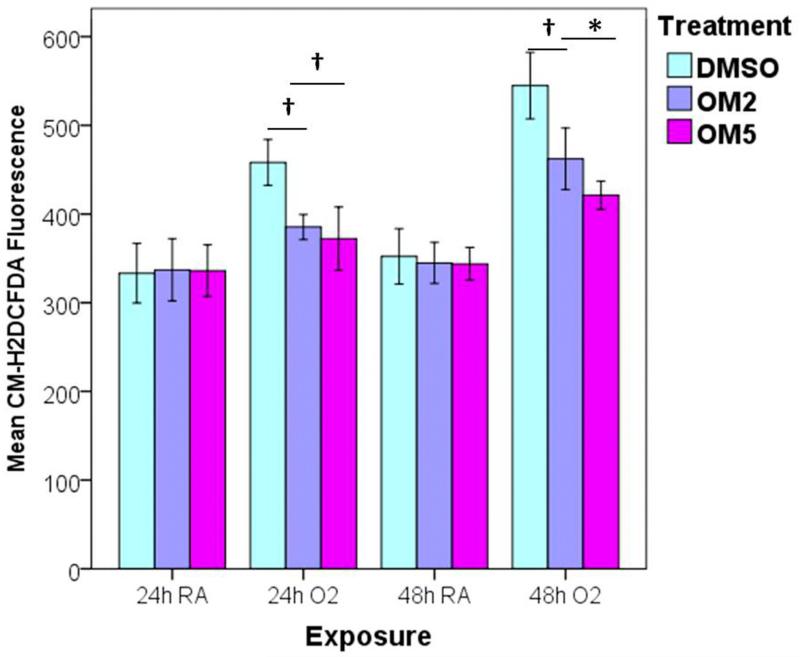
Hyperoxia-induced generation of ROS has been widely implicated in the pathogenesis of hyperoxic lung injury. To determine if omeprazole exerts its protective effects by decreasing ROS production, intracellular ROS levels were measured by flow cytometry after the cells were stained with the redox-sensitive dye CM-H2DCF-DA. Not surprisingly, hyperoxia increased ROS generation. However, omeprazole significantly decreased hyperoxia-induced generation of ROS compared to controls (Fig. 4).
Figure 4. Omeprazole attenuates ROS generation during exposure to hyperoxia.
H441 cells were treated with DMSO or OM, and exposed to room air or hyperoxia for up to 48 h. At 24 and 48 h of exposure, the oxidation of the fluorescent dye, CM-H2DCF-DA, was measured by flow cytometry. The graph represents the mean CM-H2DCF fluorescence intensity compared to corresponding room air groups for at least three independent experiments. Values are presented as means ± SEM (n=4). *P < 0.005, †P < 0.05.
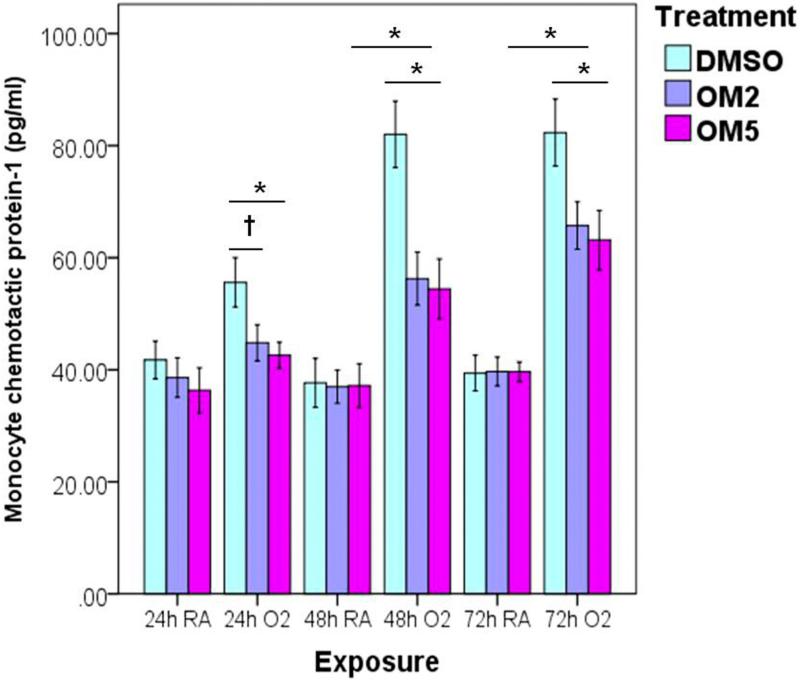
Omeprazole suppressed hyperoxia-induced production of MCP-1 in H441 cells
We determined the levels of MCP-1 in the cell culture supernatants by ELISA to evaluate the impact of omeprazole on the inflammatory response generated during hyperoxia. As expected, hyperoxia increased the secretion of MCP-1 in a time-dependent manner. Consistent with protective effects of omeprazole against hyperoxia-induced cytotoxicity and ROS generation, omeprazole significantly suppressed hyperoxia-induced production of MCP-1 (Fig. 5).
Figure 5. Omeprazole decreases hyperoxia-induced monocyte chemoattractant protein-1 (MCP-1) production.
H441 cells were pretreated with DMSO or OM at two different concentrations (2 and 5 μM) for 4 h before exposure to room air or hyperoxia for up to 72 h. At 24, 48 and 72 h of exposure, the cell-free supernatants were analyzed for MCP-1 by ELISA. The graph represents the mean MCP-1 concentrations for at least three independent experiments. Values are presented as means ± SEM (n=4). *P < 0.005, †P < 0.05.
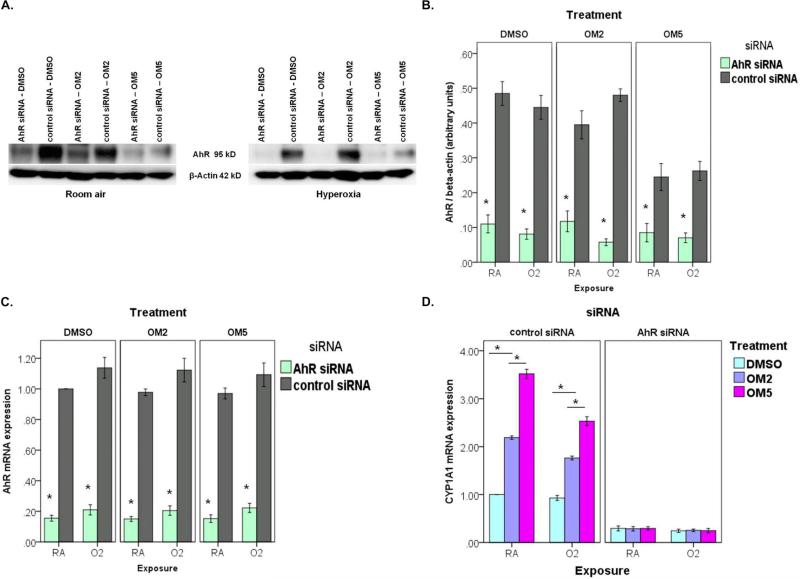
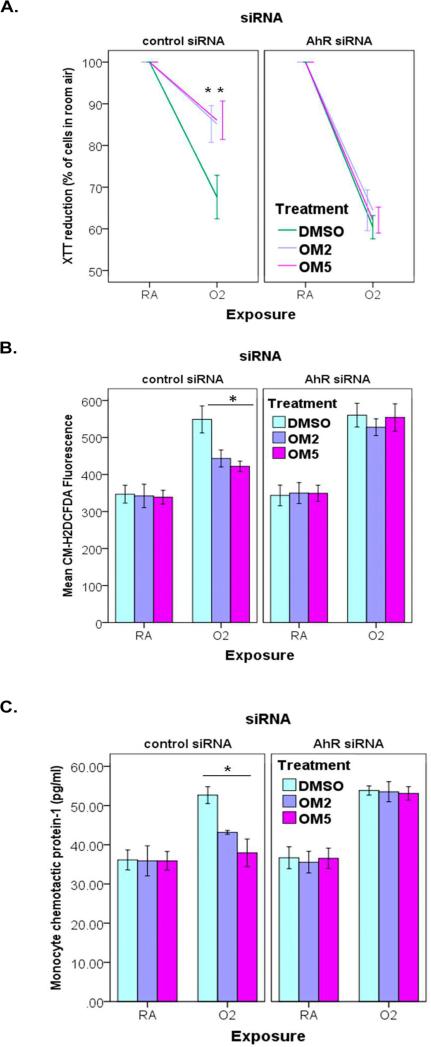
siRNA-mediated knockdown of AhR abrogates the protective effects of omeprazole against hyperoxia in human lung derived H441 cells
To ascertain whether the AhR is a crucial regulator of the protective effects of omeprazole against hyperoxic injury in human lung cells in vitro, we performed the experiments after AhR expression was downregulated with AhR siRNA. We validated the knockdown of AhR by evaluating the expression of AhR protein (Figs. 6A and B) and mRNA (Fig. 6C). As expected, AhR knockdown resulted in failure of omeprazole to induce CYP1A1, which is a downstream target gene of AhR (Fig. 6D). In AhR deficient H441 cells, omeprazole failed to protect against hyperoxia induced - cytotoxicity (Fig. 7A), ROS generation (Fig. 7B), and MCP-1 production (Fig. 7C) compared to controls.
Figure 6. Validation of siRNA mediated knockdown of AhR in H441 cells.
H441 cells were transfected with either 50 nM negative control siRNA or 50 nM AhR siRNA. Twenty-four hours after transfection, cells were treated with DMSO or OM, and exposed to room air or hyperoxia for up to 48 h. (A) At 48 h of exposure, whole-cell extract was used for Western blot analysis (A) with the anti-AhR or anti-β-actin antibodies. (B) AhR band intensities were quantified and normalized to β-actin. At 12 h of exposure, RNA was extracted for real-time RT-PCR analyses of AhR mRNA (C), and CYP1A1 mRNA (D) expression. Data are representative of at least three independent experiments. Values are presented as means ± SEM (n=4). (B,C) *P < 0.005 vs. control siRNA. (D) *P < 0.005.
Figure 7. siRNA knockdown of AhR mitigates the protective effects of omeprazole against hyperoxic injury.
(A). Assesment of cell viability by XTT assay. (B). Determination of ROS generation by measuring the MFI of CM-H2DCF-DA with flow cytometry. (C). Quantification of MCP-1 protein (pg/ml) in cell culture supernatants by ELISA. Data are representative of at least three independent experiments. Values are presented as means ± SEM (n=4). *P < 0.005 vs. control.
Discussion
The present study demonstrates that the proton pump inhibitor, omeprazole, attenuates hyperoxic injury in vitro via the AhR, which is associated with decreased ROS generation and MCP-1 production. In human lung-derived H441 cells in vitro, omeprazole-mediated protection against hyperoxic injury correlated with the functional activation of the AhR by omeprazole compared to control. Whereas, in H441 cells, whose AhR gene was silenced with AhR siRNA, the lack of omeprazole-mediated protective effects against hyperoxic injury correlated with the absence of the functional activation of the AhR.
The AhR is a versatile transcription factor that has important physiological functions in addition to its widely established role in the induction of a battery of genes involved in the metabolism of xenobiotics. Studies from our laboratory and others have reported that AhR may be a crucial regulator of oxidant stress and inflammation through the induction of several detoxifying enzymes or via “cross-talk” with other signal transduction pathways. Several in vitro studies suggest that omeprazole activates AhR in human and rat hepatocytes [25-28]. However, whether omeprazole activates AhR in the lung cells, and its impact on hyperoxic injury has not been investigated. Therefore, we conducted experiments with omeprazole in human lung derived H441 cells in vitro, both in the presence and absence of a functional AhR, to investigate whether omeprazole protects against hyperoxic injury via activation of the AhR. Functional activation of AhR results in the transcriptional activation of various target genes referred to as AhR gene battery, of which the best studied example is CYP1A1 gene [29]. Therefore, we analyzed the expression of pulmonary CYP1A1 to determine the functional activation of AhR. Strikingly, omeprazole induces an AhR-dependent gene expression in H441 cells in vitro, as evidenced by enhanced expression of CYP1A1 apoprotein (Figs. 2 A and B) and mRNA (Fig. 2 C). Our results suggest that hyperoxia on its own does cause translocation of the AhR into the nucleus (Figs. 1C and D). Although, hyperoxia for 24 h increases the expression of the AhR target gene, CYP1A (Fig. 2), with continued exposure to hyperoxia beyond 48 to 72 h, CYP1A expression declines (Fig. 2). This time period coincides with increased hyperoxia-mediated cytotoxicity (Fig. 3) and ROS generation (Fig. 4), suggesting that AhR – mediated CYP1A1 expression may protect against hyperoxic injury in H441 cells. These findings are consistent with the previous work from our laboratory [21, 30, 31]. In contrast to CYP1A1 expression, hyperoxia increased nuclear AhR apoprotein content beyond 48 to 72 h. The discrepancies in our observations between nuclear AhR apoprotein content and the AhR target gene, CYP1A1, expression beyond 48 to 72 h of hyperoxia suggest post-translational mechanisms (e.g., protein stabilization), although the exact mechanisms are unknown at this time. Although, hyperoxia beyond 48 to 72 h decreased CYP1A1 expression compared to their corresponding room air groups, CYP1A1 expression was higher in omeprazole-treated cells exposed to hyperoxia compared to room air-exposed DMSO-treated cells. These findings suggest that under hyperoxic conditions, omeprazole increased CYP1A1 expression compared to the constitutional expression seen under room air conditions. To the best of our knowledge, only one study has reported that omeprazole induces pulmonary CYP1A1 mRNA in the lungs. However, the mechanism of induction of CYP1A1 was not investigated in that study [32]. Our data provide direct evidence that pulmonary CYP1A1 is induced by omeprazole via functional activation of the AhR.
Previous studies have demonstrated that exposure to hyperoxia results in increased generation of ROS [34] and decreased cell viability [30]. Similarly, we observed increased ROS generation as determined by enhanced CM-H2DCF-DA fluorescence (Figs. 4 and 7B) and decreased cell viability as determined by decreased XTT reduction (Figs. 3 and 7A) upon exposure to hyperoxia. Importantly, studies have shown that ROS and inflammatory responses are the key mediators in the pathogenesis of hyperoxia-induced lung disorders such as BPD in preterm infants, and acute respiratory distress syndrome (ARDS) in older children and adults [8, 35, 36]. MCP-1 is a CC chemokine that is increased in the bronchoalveolar lavage fluid and plasma of infants with oxidant injury, who later on develop BPD [37]. Our study demonstrates that omeprazole attenuates hyperoxia induced: (i) cytotoxicity (Fig. 3); (ii) ROS generation (Fig. 4); and (iii) inflammation as indicated by MCP-1 expression (Fig. 5) in H441 cells in vitro. Our data suggests that omeprazole protects against hyperoxic injury by decreasing ROS and inflammation. In this study, the concentrations of omeprazole that was used were similar to the plasma concentrations documented in humans after conventional oral and intravenous administration of omeprazole [24, 33]. Omeprazole has a proven safety profile in humans for over a decade, and it is important to note that omeprazole protects against hyperoxic injury in H441 cells in vitro at concentrations similar to that documented in humans after conventional doses of omeprazole.
Our observation that omeprazole fails to protect against hyperoxic injury in H441 cells lacking AhR (Fig. 7) demonstrates that AhR plays a crucial role in omeprazole-mediated protection against hyperoxic injury. The protective effects of AhR against hyperoxic lung injury in rodents have been documented, as evidenced by (i) attenuation of hyperoxic lung injury in rodents treated with the AhR agonists, beta-naphthoflavone or 3-methylcholanthrene [38, 39] and (ii) increased susceptibility of AhR deficient rodents and human lung derived cells to hyperoxic injury [20, 21, 30]. Because the AhR regulates the induction of CYP1A enzymes that may detoxify ROS [38, 40], it is possible that suppression of these enzymes may have contributed to the failure of omeprazole to protect AhR deficient H441 cells against hyperoxic injury. Because AhR also induces other enzymes such as NAD(P)H quinone reductase, glutathione S-transferase-α, aldehyde dehydrogenase, and superoxide dismutase [41], the beneficial role of these enzymes against hyperoxic injury are not excluded. However, the reduction of ROS generation and inflammation is well established in the studies reported herein and the molecular mechanisms could well work through any one of the enzymes induced by AhR.
Interestingly, it has been postulated that the antiulcer and gastroprotective effects of omeprazole may also involve acid-unrelated mechanisms, which include inhibition of neutrophil infiltration and of oxidative tissue damage [42, 43]. To support this, several studies in vitro have revealed that omeprazole has a direct scavenging activity against oxygen free radicals and it inhibits neutrophil function [44, 45]. Thus, it is plausible that the antioxidant properties of omeprazole may be beneficial in other pathologic conditions associated with oxidative damage [46, 47]. Omeprazole has also been shown to protect against necrotizing enterocolitis in newborn rats subjected to hypoxia/reoxygenation [48]. All of these data strongly imply that omeprazole has antioxidant and anti-inflammatory properties. However, the molecular mechanism(s) of the antioxidant and anti-inflammatory effects of omeprazole remains obscure. Our results support the concept that one of the main mechanisms of omeprazole-mediated protective effects against hyperoxic injury may be related to activation of the AhR. To the best of our knowledge, this is the first study to report a novel finding that omeprazole protects against hyperoxic injury in vitro via the AhR that is associated with decreased ROS generation and expression of MCP-1.
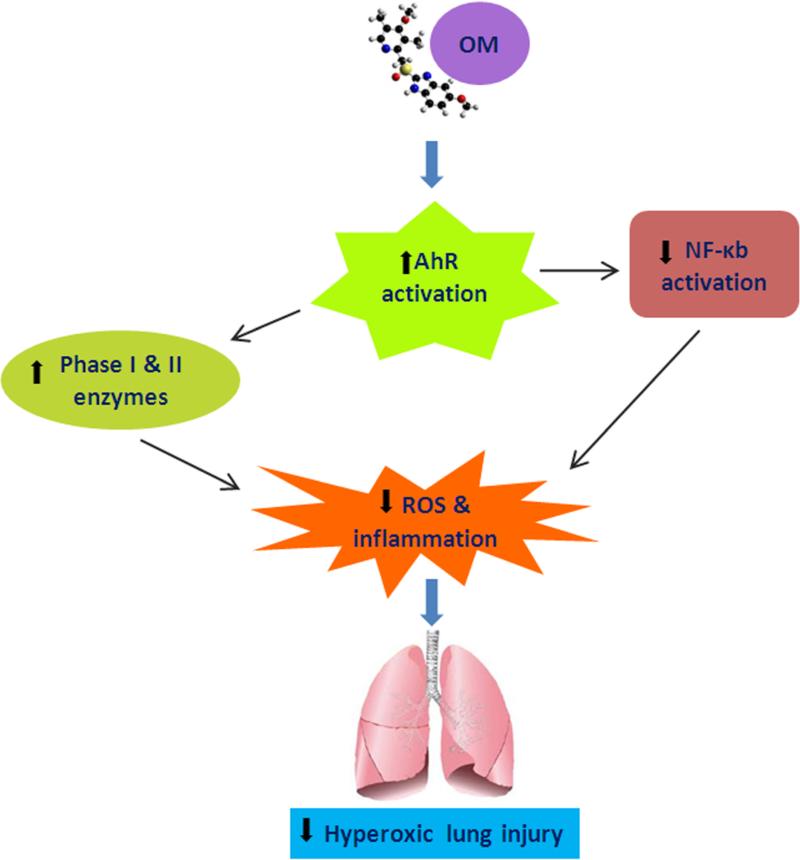
In summary, we provide evidence that omeprazole protects against hyperoxic injury in vitro via the AhR that is associated with decreased ROS generation and expression of MCP-1. We propose that the protective effects of omeprazole may be due to AhR mediated induction of enzymes such as CYP1A1 that may detoxify lipid peroxides and hydroperoxides generated by ROS, and/or due to cross talk with other transcription factors such as NF-κB that modulate hyperoxic injury (Fig. 8). Our results suggest that omeprazole is a potential therapeutic agent in the prevention and treatment of hyperoxia-induced lung disorders in humans such as BPD in premature infants and ARDS in older children and adults. Further studies are needed to investigate the safety and efficacy of omeprazole against hyperoxic lung injury in neonatal mice in vivo and in human neonatal lung derived cells in vitro. These studies may provide additional insight into the mechanism(s) of protection and the feasibility of the use of omeprazole for BPD in human premature infants.
Figure 8. Proposed mechanism(s) of omeprazole-mediated protective effects against hyperoxic lung injury.
OM activates AhR, which in turn may decrease hyperoxia-induced ROS generation and inflammation, either by inducing phase I & II detoxifying enzymes and/or by modulating NF-κB activation.
Acknowledgements
This work was supported by a grant from Division of Neonatal-Perinatal Medicine, Texas Children's Hospital to B.S. and National Institutes of Health [ES- 009132, HL-070921, HL-087174, and ES-019689] to B.M. The study sponsors had no involvement in study design, data collection, analysis and interpretation, writing of the report or decision to submit the paper for publication. We thank Esther Inman for providing technical assistance for the completion of this article.
Abbreviations
- AhR
aryl hydrocarbon receptor
- ARDS
acute respiratory distress syndrome
- BPD
bronchopulmonary dysplasia
- CM-H2DCF-DA
5-(and-6)-chloromethyl-2’, 7’-dichlorodihydrofluorescein diacetate
- CYP
cytochrome P450
- DMSO
dimethylsulfoxide
- HDAC1
histone deacetylase class 1
- MCP-1
monocyte chemotactic protein-1
- OM
omeprazole
- OM2
omeprazole 2μM
- OM5
omeprazole 5μM
- ROS
reactive oxygen species
- XTT
2, 3 bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carbox-anilide
References
- 1.Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, Lukashev D, Bittmann I, Sitkovsky MV. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 2005;3:e174. doi: 10.1371/journal.pbio.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jobe AH, Hillman N, Polglase G, Kramer BW, Kallapur S, Pillow J. Injury and inflammation from resuscitation of the preterm infant. Neonatology. 2008;94:190–196. doi: 10.1159/000143721. [DOI] [PubMed] [Google Scholar]
- 3.Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, Lee PJ, Geick A, de Fougerolles AR, Elias JA. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med. 2006;12:1286–1293. doi: 10.1038/nm1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vina J, Vento M, Garcia-Sala F, Puertes IR, Gasco E, Sastre J, Asensi M, Pallardo FV. L-cysteine and glutathione metabolism are impaired in premature infants due to cystathionase deficiency. Am J Clin Nutr. 1995;61:1067–1069. doi: 10.1093/ajcn/61.4.1067. [DOI] [PubMed] [Google Scholar]
- 5.Asikainen TM, White CW. Antioxidant defenses in the preterm lung: role for hypoxia-inducible factors in BPD? Toxicol Appl Pharmacol. 2005;203:177–188. doi: 10.1016/j.taap.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, Lemons JA, Oh W, Papile LA, Shankaran S, Stevenson DK, Tyson JE, Poole WK. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147, e141–148. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Van Marter LJ. Epidemiology of bronchopulmonary dysplasia. Semin Fetal Neonatal Med. 2009;14:358–366. doi: 10.1016/j.siny.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol. 2003;8:39–49. doi: 10.1016/s1084-2756(02)00194-x. [DOI] [PubMed] [Google Scholar]
- 9.Short EJ, Klein NK, Lewis BA, Fulton S, Eisengart S, Kercsmar C, Baley J, Singer LT. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics. 2003;112:e359. doi: 10.1542/peds.112.5.e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sogawa K, Fujii-Kuriyama Y. Ah receptor, a novel ligand-activated transcription factor. J Biochem. 1997;122:1075–1079. doi: 10.1093/oxfordjournals.jbchem.a021864. [DOI] [PubMed] [Google Scholar]
- 12.Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18:207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tirona RG, Kim RB. Nuclear receptors and drug disposition gene regulation. J Pharm Sci. 2005;94:1169–1186. doi: 10.1002/jps.20324. [DOI] [PubMed] [Google Scholar]
- 14.Denis M, Cuthill S, Wikstrom AC, Poellinger L, Gustafsson JA. Association of the dioxin receptor with the Mr 90,000 heat shock protein: a structural kinship with the glucocorticoid receptor. Biochem Biophys Res Commun. 1988;155:801–807. doi: 10.1016/s0006-291x(88)80566-7. [DOI] [PubMed] [Google Scholar]
- 15.Carver LA, Bradfield CA. Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo. J Biol Chem. 1997;272:11452–11456. doi: 10.1074/jbc.272.17.11452. [DOI] [PubMed] [Google Scholar]
- 16.Emi Y, Ikushiro S, Iyanagi T. Xenobiotic responsive element-mediated transcriptional activation in the UDP-glucuronosyltransferase family 1 gene complex. J Biol Chem. 1996;271:3952–3958. doi: 10.1074/jbc.271.7.3952. [DOI] [PubMed] [Google Scholar]
- 17.Favreau LV, Pickett CB. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem. 1991;266:4556–4561. [PubMed] [Google Scholar]
- 18.Fujisawa-Sehara A, Sogawa K, Yamane M, Fujii-Kuriyama Y. Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: a similarity to glucocorticoid regulatory elements. Nucleic Acids Res. 1987;15:4179–4191. doi: 10.1093/nar/15.10.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rushmore TH, King RG, Paulson KE, Pickett CB. Regulation of glutathione S-transferase Ya subunit gene expression: identification of a unique xenobiotic-responsive element controlling inducible expression by planar aromatic compounds. Proc Natl Acad Sci U S A. 1990;87:3826–3830. doi: 10.1073/pnas.87.10.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang W, Welty SE, Couroucli XI, Barrios R, Kondraganti SR, Muthiah K, Yu L, Avery SE, Moorthy B. Disruption of the Ah receptor gene alters the susceptibility of mice to oxygen-mediated regulation of pulmonary and hepatic cytochromes P4501A expression and exacerbates hyperoxic lung injury. J Pharmacol Exp Ther. 2004;310:512–519. doi: 10.1124/jpet.103.059766. [DOI] [PubMed] [Google Scholar]
- 21.Couroucli XI, Welty SE, Geske RS, Moorthy B. Regulation of pulmonary and hepatic cytochrome P4501A expression in the rat by hyperoxia: implications for hyperoxic lung injury. Mol Pharmacol. 2002;61:507–515. doi: 10.1124/mol.61.3.507. [DOI] [PubMed] [Google Scholar]
- 22.Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP, Sime PJ. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. Am J Pathol. 2007;170:855–864. doi: 10.2353/ajpath.2007.060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lind T, Cederberg C, Ekenved G, Haglund U, Olbe L. Effect of omeprazole--a gastric proton pump inhibitor--on pentagastrin stimulated acid secretion in man. Gut. 1983;24:270–276. doi: 10.1136/gut.24.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li XQ, Andersson TB, Ahlstrom M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32:821–827. doi: 10.1124/dmd.32.8.821. [DOI] [PubMed] [Google Scholar]
- 25.Quattrochi LC, Tukey RH. Nuclear uptake of the Ah (dioxin) receptor in response to omeprazole: transcriptional activation of the human CYP1A1 gene. Mol Pharmacol. 1993;43:504–508. [PubMed] [Google Scholar]
- 26.Backlund M, Ingelman-Sundberg M. Regulation of aryl hydrocarbon receptor signal transduction by protein tyrosine kinases. Cell Signal. 2005;17:39–48. doi: 10.1016/j.cellsig.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Murray IA, Perdew GH. Omeprazole stimulates the induction of human insulin-like growth factor binding protein-1 through aryl hydrocarbon receptor activation. J Pharmacol Exp Ther. 2008;324:1102–1110. doi: 10.1124/jpet.107.132241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshinari K, Ueda R, Kusano K, Yoshimura T, Nagata K, Yamazoe Y. Omeprazole transactivates human CYP1A1 and CYP1A2 expression through the common regulatory region containing multiple xenobiotic-responsive elements. Biochem Pharmacol. 2008;76:139–145. doi: 10.1016/j.bcp.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Whitlock JP., Jr. Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- 30.Bhakta KY, Jiang W, Couroucli XI, Fazili IS, Muthiah K, Moorthy B. Regulation of cytochrome P4501A1 expression by hyperoxia in human lung cell lines: Implications for hyperoxic lung injury. Toxicol Appl Pharmacol. 2008;233:169–178. doi: 10.1016/j.taap.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moorthy B, Nguyen UT, Gupta S, Stewart KD, Welty SE, Smith CV. Induction and decline of hepatic cytochromes P4501A1 and 1A2 in rats exposed to hyperoxia are not paralleled by changes in glutathione S-transferase-alpha. Toxicol Lett. 1997;90:67–75. doi: 10.1016/s0378-4274(96)03832-5. [DOI] [PubMed] [Google Scholar]
- 32.Wei C, Caccavale RJ, Weyand EH, Chen S, Iba MM. Induction of CYP1A1 and CYP1A2 expressions by prototypic and atypical inducers in the human lung. Cancer Lett. 2002;178:25–36. doi: 10.1016/s0304-3835(01)00809-6. [DOI] [PubMed] [Google Scholar]
- 33.Cederberg C, Thomson AB, Mahachai V, Westin JA, Kirdeikis P, Fisher D, Zuk L, Marriage B. Effect of intravenous and oral omeprazole on 24-hour intragastric acidity in duodenal ulcer patients. Gastroenterology. 1992;103:913–918. doi: 10.1016/0016-5085(92)90025-t. [DOI] [PubMed] [Google Scholar]
- 34.Budinger GR, Tso M, McClintock DS, Dean DA, Sznajder JI, Chandel NS. Hyperoxia-induced apoptosis does not require mitochondrial reactive oxygen species and is regulated by Bcl-2 proteins. J Biol Chem. 2002;277:15654–15660. doi: 10.1074/jbc.M109317200. [DOI] [PubMed] [Google Scholar]
- 35.Chabot F, Mitchell JA, Gutteridge JM, Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J. 1998;11:745–757. [PubMed] [Google Scholar]
- 36.Barazzone C, White CW. Mechanisms of cell injury and death in hyperoxia: role of cytokines and Bcl-2 family proteins. Am J Respir Cell Mol Biol. 2000;22:517–519. doi: 10.1165/ajrcmb.22.5.f180. [DOI] [PubMed] [Google Scholar]
- 37.Ambalavanan N, Carlo WA, D'Angio CT, McDonald SA, Das A, Schendel D, Thorsen P, Higgins RD. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123:1132–1141. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinha A, Muthiah K, Jiang W, Couroucli X, Barrios R, Moorthy B. Attenuation of hyperoxic lung injury by the CYP1A inducer beta-naphthoflavone. Toxicol Sci. 2005;87:204–212. doi: 10.1093/toxsci/kfi226. [DOI] [PubMed] [Google Scholar]
- 39.Mansour H, Levacher M, Azoulay-Dupuis E, Moreau J, Marquetty C, Gougerot-Pocidalo MA. Genetic differences in response to pulmonary cytochrome P-450 inducers and oxygen toxicity. J Appl Physiol. 1988;64:1376–1381. doi: 10.1152/jappl.1988.64.4.1376. [DOI] [PubMed] [Google Scholar]
- 40.Tong V, Chang TK, Chen J, Abbott FS. The effect of valproic acid on hepatic and plasma levels of 15-F2t-isoprostane in rats. Free Radic Biol Med. 2003;34:1435–1446. doi: 10.1016/s0891-5849(03)00151-5. [DOI] [PubMed] [Google Scholar]
- 41.Park EY, Rho HM. The transcriptional activation of the human copper/zinc superoxide dismutase gene by 2,3,7,8-tetrachlorodibenzo-p-dioxin through two different regulator sites, the antioxidant responsive element and xenobiotic responsive element. Mol Cell Biochem. 2002;240:47–55. doi: 10.1023/a:1020600509965. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi T, Ohta Y, Inui K, Yoshino J, Nakazawa S. Protective effect of omeprazole against acute gastric mucosal lesions induced by compound 48/80, a mast cell degranulator, in rats. Pharmacol Res. 2002;46:75–84. doi: 10.1016/s1043-6618(02)00034-8. [DOI] [PubMed] [Google Scholar]
- 43.Pozzoli C, Menozzi A, Grandi D, Solenghi E, Ossiprandi MC, Zullian C, Bertini S, Cavestro GM, Coruzzi G. Protective effects of proton pump inhibitors against indomethacin-induced lesions in the rat small intestine. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:283–291. doi: 10.1007/s00210-006-0121-y. [DOI] [PubMed] [Google Scholar]
- 44.Wandall JH. Effects of omeprazole on neutrophil chemotaxis, super oxide production, degranulation, and translocation of cytochrome b-245. Gut. 1992;33:617–621. doi: 10.1136/gut.33.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida N, Yoshikawa T, Tanaka Y, Fujita N, Kassai K, Naito Y, Kondo M. A new mechanism for anti-inflammatory actions of proton pump inhibitors--inhibitory effects on neutrophil-endothelial cell interactions. Aliment Pharmacol Ther. 2000;14(Suppl 1):74–81. doi: 10.1046/j.1365-2036.2000.014s1074.x. [DOI] [PubMed] [Google Scholar]
- 46.Halliwell B, Zhao K, Whiteman M. The gastrointestinal tract: a major site of antioxidant action? Free Radic Res. 2000;33:819–830. doi: 10.1080/10715760000301341. [DOI] [PubMed] [Google Scholar]
- 47.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(Suppl 1):S3–9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 48.Cadir FO, Bicakci U, Tander B, Kilicoglu-Aydin B, Rizalar R, Ariturk E, Aydin O, Bernay F. Protective effects of vitamin E and omeprazole on the hypoxia/reoxygenation induced intestinal injury in newborn rats. Pediatr Surg Int. 2008;24:809–813. doi: 10.1007/s00383-008-2157-1. [DOI] [PubMed] [Google Scholar]