Abstract
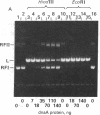
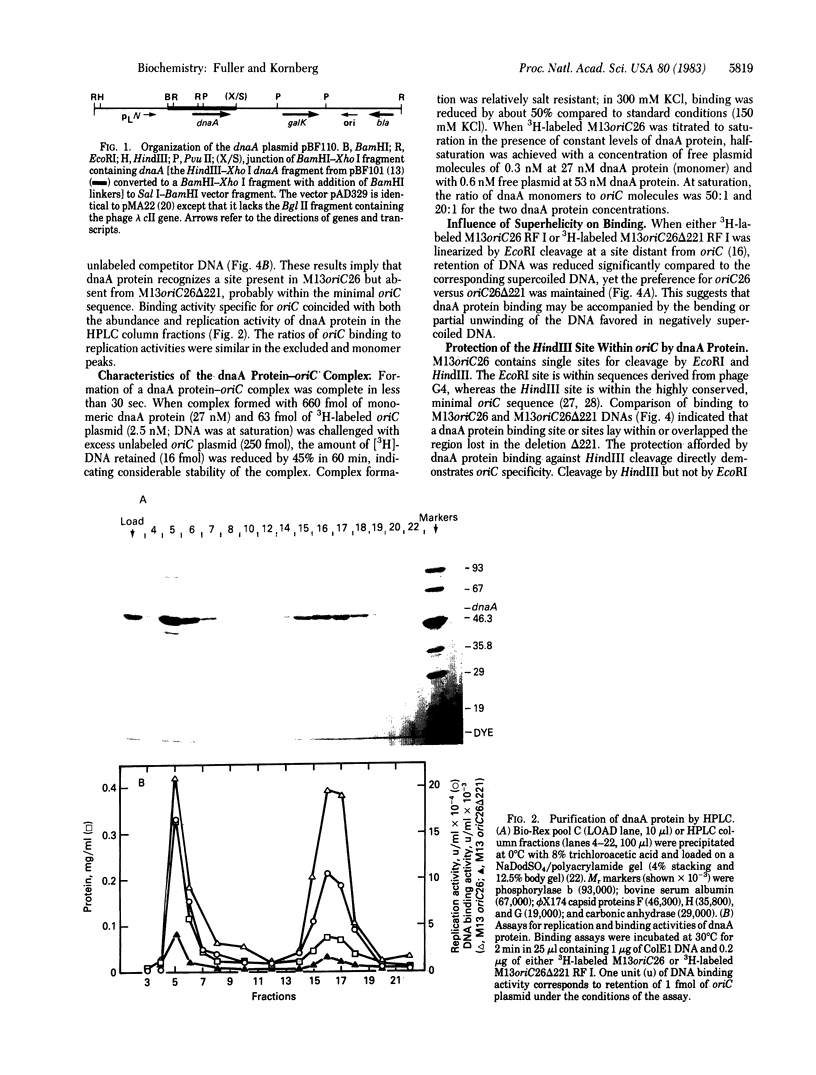
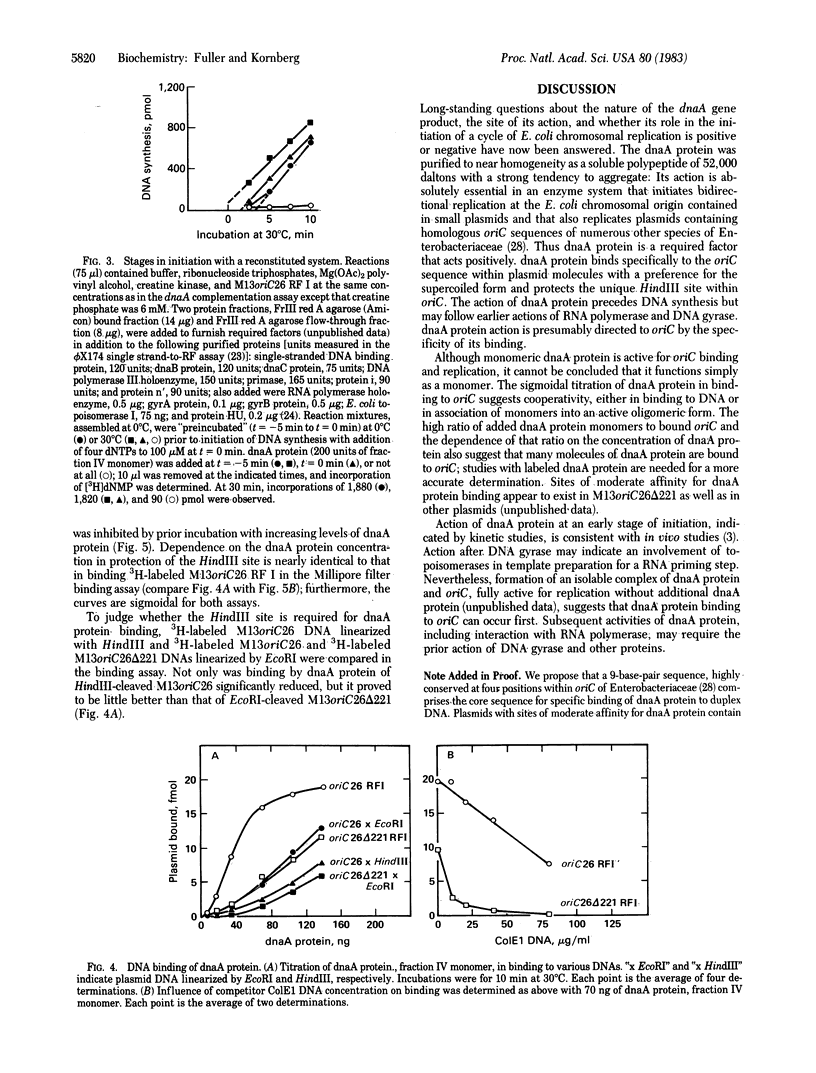
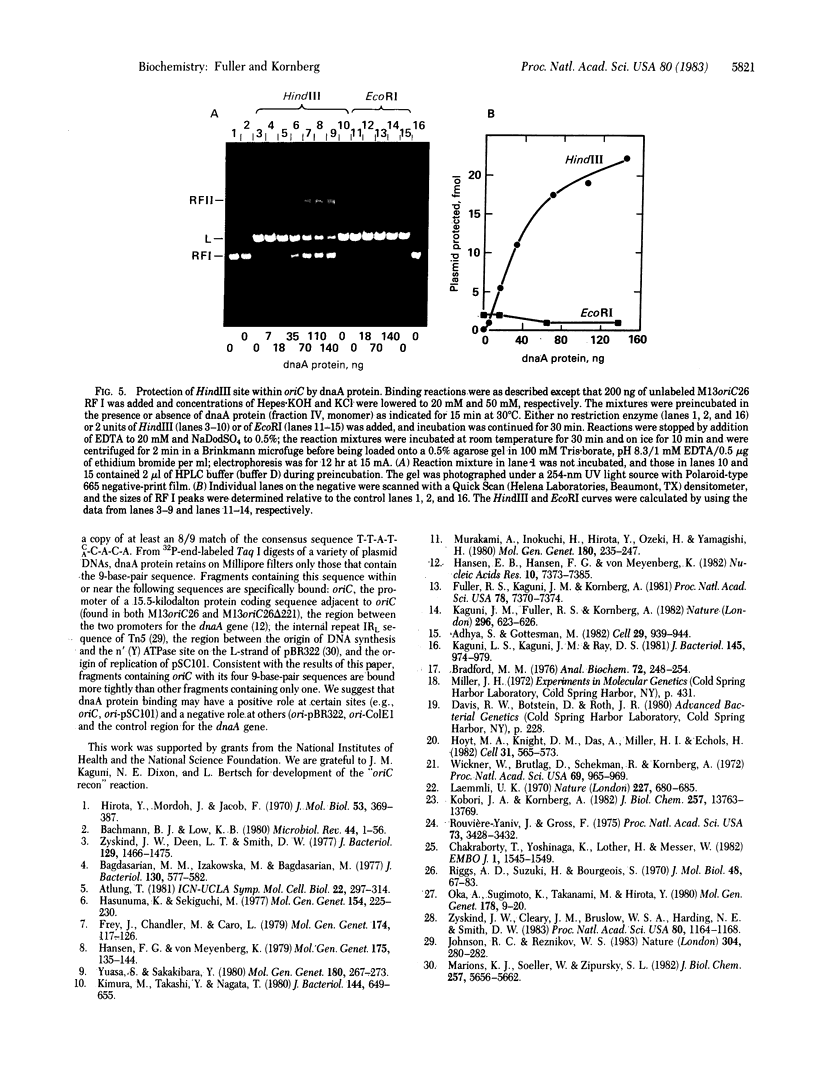
Soluble protein fractions from Escherichia coli dnaA+ cells but not dnaA temperature-sensitive cells replicate plasmids containing the E. coli chromosomal origin of replication (oriC). Complementation of these mutant fractions provided an assay for dnaA protein activity in initiation of replication at oriC. From a strain (constructed in vitro) that overproduces the dnaA protein more than 200-fold, the 52,000-dalton polypeptide was purified to near homogeneity. Although the protein tends to aggregate, monomer-sized protein purified by high-performance liquid chromatography is fully active for replication. It binds specifically and tightly to oriC in a supercoiled plasmid as judged by a Millipore filter-binding assay and by protection of the unique HindIII site within the oriC sequence. In the oriC replication reaction, dnaA protein acts at an early step preceding DNA synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Promoter occlusion: transcription through a promoter may inhibit its activity. Cell. 1982 Jul;29(3):939–944. doi: 10.1016/0092-8674(82)90456-1. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M. M., Izakowska M., Bagdasarian M. Suppression of the DnaA phenotype by mutations in the rpoB cistron of ribonucleic acid polymerase in Salmonella typhimurium and Escherichia coli. J Bacteriol. 1977 May;130(2):577–582. doi: 10.1128/jb.130.2.577-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chakraborty T., Yoshinaga K., Lother H., Messer W. Purification of the E. coli dnaA gene product. EMBO J. 1982;1(12):1545–1549. doi: 10.1002/j.1460-2075.1982.tb01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. B., Hansen F. G., von Meyenburg K. The nucleotide sequence of the dnaA gene and the first part of the dnaN gene of Escherichia coli K-12. Nucleic Acids Res. 1982 Nov 25;10(22):7373–7385. doi: 10.1093/nar/10.22.7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen F. G., von Meyenburg K. Characterization of the dnaA, gyrB and other genes in the dnaA region of the Escherichia coli chromosome on specialized transducing phages lambda tna. Mol Gen Genet. 1979 Sep;175(2):135–144. doi: 10.1007/BF00425529. [DOI] [PubMed] [Google Scholar]
- Hasunuma K., Sekiguchi M. Replication of plasmid pSC101 in Escherichia coli K12: requirement for dnaA function. Mol Gen Genet. 1977 Sep 9;154(3):225–230. doi: 10.1007/BF00571277. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Hoyt M. A., Knight D. M., Das A., Miller H. I., Echols H. Control of phage lambda development by stability and synthesis of cII protein: role of the viral cIII and host hflA, himA and himD genes. Cell. 1982 Dec;31(3 Pt 2):565–573. doi: 10.1016/0092-8674(82)90312-9. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Reznikoff W. S. DNA sequences at the ends of transposon Tn5 required for transposition. Nature. 1983 Jul 21;304(5923):280–282. doi: 10.1038/304280a0. [DOI] [PubMed] [Google Scholar]
- Kaguni J. M., Fuller R. S., Kornberg A. Enzymatic replication of E. coli chromosomal origin is bidirectional. Nature. 1982 Apr 15;296(5858):623–627. doi: 10.1038/296623a0. [DOI] [PubMed] [Google Scholar]
- Kaguni L. S., Kaguni J. M., Ray D. S. Replication of M13 oriC bacteriophages in Escherichia coli rep mutant is dependent on the cloned Escherichia coli replication origin. J Bacteriol. 1981 Feb;145(2):974–979. doi: 10.1128/jb.145.2.974-979.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Yura T., Nagata T. Isolation and characterization of Escherichia coli dnaA amber mutants. J Bacteriol. 1980 Nov;144(2):649–655. doi: 10.1128/jb.144.2.649-655.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori J. A., Kornberg A. The Escherichia coli dnaC gene product. II. Purification, physical properties, and role in replication. J Biol Chem. 1982 Nov 25;257(22):13763–13769. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marians K. J., Soeller W., Zipursky S. L. Maximal limits of the Escherichia coli replication factor Y effector site sequences in pBR322 DNA. J Biol Chem. 1982 May 25;257(10):5656–5662. [PubMed] [Google Scholar]
- Murakami A., Inokuchi H., Hirota Y., Ozeki H., Yamagishi H. Characterization of dnaA gene carried by lambda transducing phage. Mol Gen Genet. 1980;180(2):235–247. doi: 10.1007/BF00425835. [DOI] [PubMed] [Google Scholar]
- Oka A., Sugimoto K., Takanami M., Hirota Y. Replication origin of the Escherichia coli K-12 chromosome: the size and structure of the minimum DNA segment carrying the information for autonomous replication. Mol Gen Genet. 1980 Apr;178(1):9–20. doi: 10.1007/BF00267207. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Gros F. Characterization of a novel, low-molecular-weight DNA-binding protein from Escherichia coli. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3428–3432. doi: 10.1073/pnas.72.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa S., Sakakibara Y. Identification of the dnaA and dnaN gene products of Escherichia coli. Mol Gen Genet. 1980;180(2):267–273. doi: 10.1007/BF00425838. [DOI] [PubMed] [Google Scholar]
- Zyskind J. W., Cleary J. M., Brusilow W. S., Harding N. E., Smith D. W. Chromosomal replication origin from the marine bacterium Vibrio harveyi functions in Escherichia coli: oriC consensus sequence. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1164–1168. doi: 10.1073/pnas.80.5.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyskind J. W., Deen L. T., Smith D. W. Temporal sequence of events during the initiation process in Escherichia coli deoxyribonucleic acid replication: roles of the dnaA and dnaC gene products and ribonucleic acid polymerase. J Bacteriol. 1977 Mar;129(3):1466–1475. doi: 10.1128/jb.129.3.1466-1475.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]