Abstract
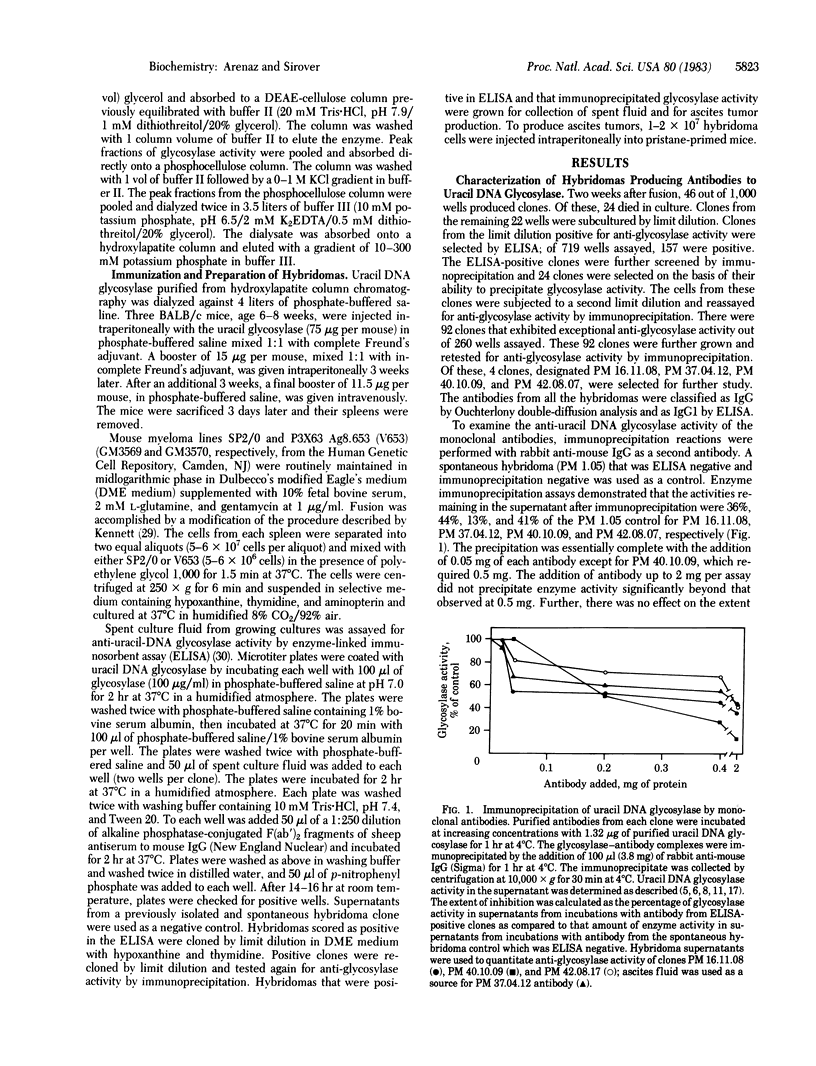
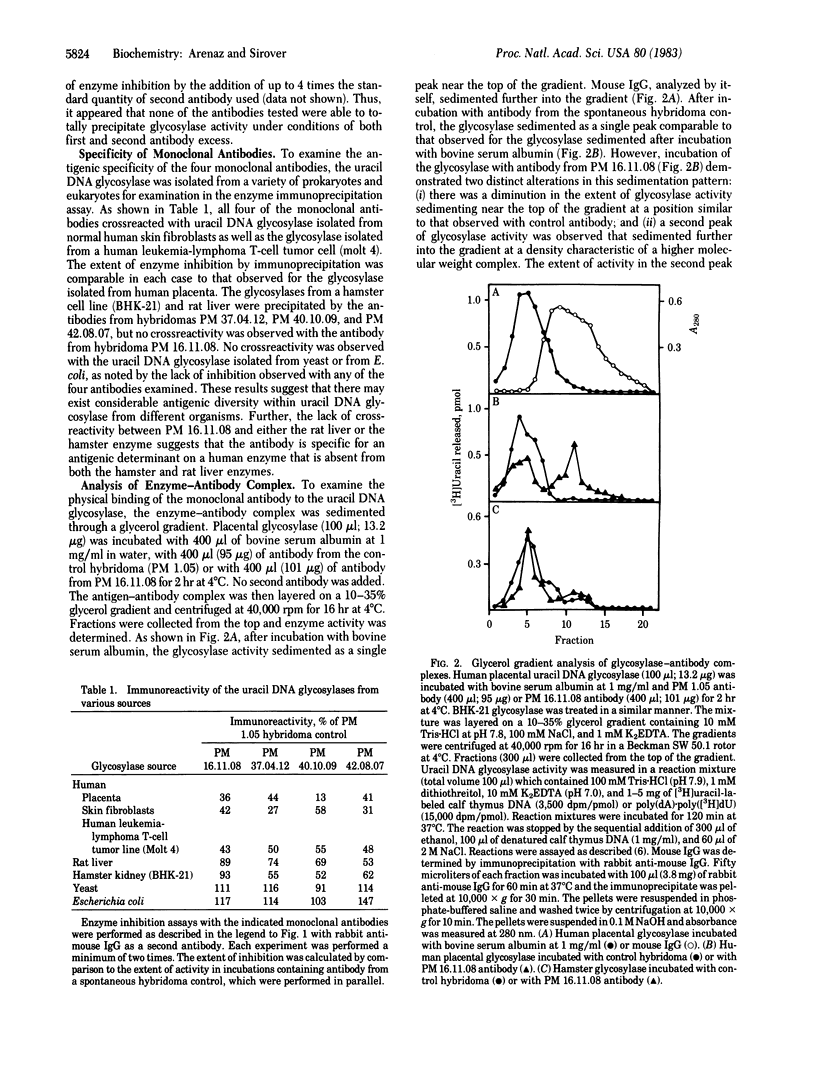
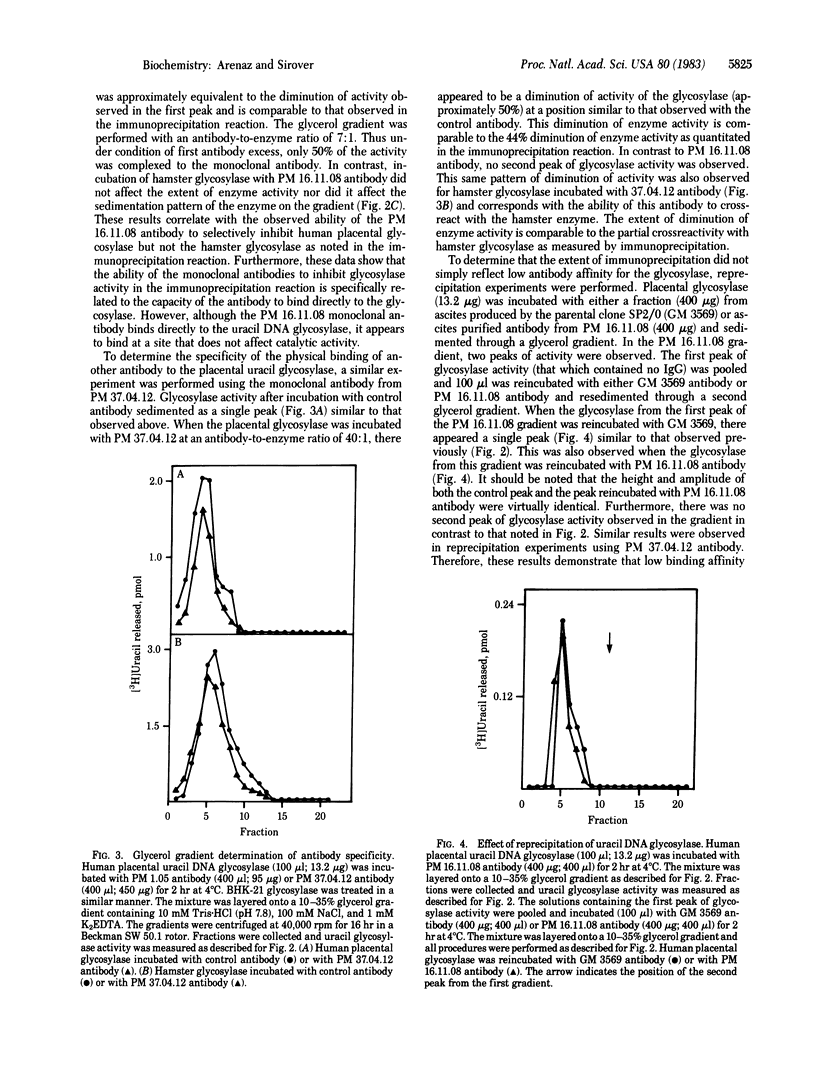
A series of monoclonal antibodies has been prepared against the base excision repair enzyme uracil DNA glycosylase isolated from human placenta. Spleen cells from BALB/c mice immunized with purified human placental uracil DNA glycosylase were fused with either P3X63 Ag8.653 or SP2/0 myeloma cells. Hybridomas producing antibodies directed against the placental glycosylase were identified in an enzyme-linked immunosorbent assay. Each positive hybridoma was cloned twice by limit dilution and tested for anti-glycosylase activity in an enzyme immunoprecipitation assay. Each of the four clones examined in detail precipitated enzyme activity in an immunoprecipitation reaction only in the presence of rabbit anti-mouse IgG as a second antibody. No anti-uracil DNA glycosylase activity was observed in a spontaneous hybridoma used as a control. Each monoclonal antibody immunoprecipitated uracil DNA glycosylases isolated from several human tissues. Partial crossreactivity was observed with rat liver glycosylase and with a hamster enzyme. In contrast, no crossreactivity was observed with yeast or Escherichia coli glycosylase. Glycerol gradient sedimentation analysis demonstrated that one of the antibodies bound to the glycosylase at a site that did not diminish its catalytic activity. A second monoclonal antibody bound at a determinant that affected catalytic activity. Analysis of antibody-glycosylase interactions suggests that human cells contain antigenically distinct glycosylase species that may be encoded by individual uracil DNA glycosylase genes. The potential use of these monoclonal antibodies in studies examining the regulation of glycosylase isoenzymes during cell proliferation in normal human cells and in cells from cancer-prone individuals is considered.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. T., Friedberg E. C. The presence of nuclear and mitochondrial uracil-DNA glycosylase in extracts of human KB cells. Nucleic Acids Res. 1980 Feb 25;8(4):875–888. [PMC free article] [PubMed] [Google Scholar]
- Aprelikova O. N., Tomilin N. V. Activity of uracil-DNA glycosylase in different rat tissues and in regenerating rat liver. FEBS Lett. 1982 Jan 25;137(2):193–195. doi: 10.1016/0014-5793(82)80347-5. [DOI] [PubMed] [Google Scholar]
- Arlett C. F., Lehmann A. R. Human disorders showing increased sensitivity to the induction of genetic damage. Annu Rev Genet. 1978;12:95–115. doi: 10.1146/annurev.ge.12.120178.000523. [DOI] [PubMed] [Google Scholar]
- Bertazzoni U., Stefanini M., Noy G. P., Giulotto E., Nuzzo F., Falaschi A., Spadari S. Variations of DNA polymerase-alpha and -beta during prolonged stimulation of human lymphocytes. Proc Natl Acad Sci U S A. 1976 Mar;73(3):785–789. doi: 10.1073/pnas.73.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertazzoni U., Stefanini M., Pedrali-Noy G., Nuzzo F., Falaschi A. Levels of DNA polymerase-alpha and beta in normal and xeroderma pigmentosum fibroblasts. Nucleic Acids Res. 1977 Jan;4(1):141–148. doi: 10.1093/nar/4.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caradonna S. J., Cheng Y. C. The role of deoxyuridine triphosphate nucleotidohydrolase, uracil-DNA glycosylase, and DNA polymerase alpha in the metabolism of FUdR in human tumor cells. Mol Pharmacol. 1980 Nov;18(3):513–520. [PubMed] [Google Scholar]
- Duker N. J., Grant C. L. Alterations in the levels of deoxyuridine triphosphatase, uracil-DNA glycosylase and AP endonuclease during the cell cycle. Exp Cell Res. 1980 Feb;125(2):493–497. doi: 10.1016/0014-4827(80)90145-7. [DOI] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Gombar C. T., Katz E. J., Magee P. N., Sirover M. A. Induction of the DNA repair enzymes uracil DNA glycosylase and 3-methyladenine DNA glycosylase in regenerating rat liver. Carcinogenesis. 1981;2(7):595–599. doi: 10.1093/carcin/2.7.595. [DOI] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Gupta P. K., Sirover M. A. Cell cycle regulation of DNA repair in normal and repair deficient human cells. Chem Biol Interact. 1981 Jul;36(1):19–31. doi: 10.1016/0009-2797(81)90026-0. [DOI] [PubMed] [Google Scholar]
- Gupta P. K., Sirover M. A. Sequential stimulation of DNA repair and DNA replication in normal human cells. Mutat Res. 1980 Sep;72(2):273–284. doi: 10.1016/0027-5107(80)90042-1. [DOI] [PubMed] [Google Scholar]
- Gupta P. K., Sirover M. A. Stimulation of the nuclear uracil DNA glycosylase in proliferating human fibroblasts. Cancer Res. 1981 Aug;41(8):3133–3136. [PubMed] [Google Scholar]
- Kuhnlein U., Lee B., Linn S. Human uracil DNA N-glycosidase: studies in normal and repair defective cultured fibroblasts. Nucleic Acids Res. 1978 Jan;5(1):117–125. doi: 10.1093/nar/5.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin M. F., Kidson C. Repair of ionizing radiation induced DNA damage in human lymphocytes. Nucleic Acids Res. 1977 Nov;4(11):4015–4022. doi: 10.1093/nar/4.11.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewensohn R., Killander D., Ringborg U., Lambert B. Increase of uv-induced DNA repair synthesis during blast transformation of human lymphocytes. Exp Cell Res. 1979 Oct 1;123(1):107–110. doi: 10.1016/0014-4827(79)90426-9. [DOI] [PubMed] [Google Scholar]
- Linsley W. S., Penhoet E. E., Linn S. Human endonuclease specific for apurinic/apyrimidinic sites in DNA. Partial purification and characterization of multiple forms from placenta. J Biol Chem. 1977 Feb 25;252(4):1235–1242. [PubMed] [Google Scholar]
- Long E. O., Dawid I. B. Repeated genes in eukaryotes. Annu Rev Biochem. 1980;49:727–764. doi: 10.1146/annurev.bi.49.070180.003455. [DOI] [PubMed] [Google Scholar]
- Mortelmans K., Friedberg E. C., Slor H., Thomas G., Cleaver J. E. Defective thymine dimer excision by cell-free extracts of xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2757–2761. doi: 10.1073/pnas.73.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses R. E., Beaudet A. L. Apurinic DNA endonuclease activities in repair-deficient human cell lines. Nucleic Acids Res. 1978 Feb;5(2):463–473. doi: 10.1093/nar/5.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Parker V. P., Lieberman M. W. Levels of DNA polymerases alpha, beta, and gamma in control and repair-deficient human diploid fibroblasts 1. Nucleic Acids Res. 1977 Jun;4(6):2029–2037. doi: 10.1093/nar/4.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen R. A., Mangia F. Ultraviolet-light-induced unscheduled DNA synthesis by resting and growing mouse oocytes. Mutat Res. 1978 Mar;49(3):425–429. doi: 10.1016/0027-5107(78)90113-6. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Perry W., Bennett R. A. Effect of partial hepatectomy on removal of O6-methylguanine from alkylated DNA by rat liver extracts. Biochem J. 1981 Jul 1;197(1):195–201. doi: 10.1042/bj1970195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remsen J. F., Cerutti P. A. Excision of gamma-ray induced thymine lesions by preparations from ataxia telangiectasia fibroblasts. Mutat Res. 1977 Apr;43(1):139–145. doi: 10.1016/0027-5107(77)90138-5. [DOI] [PubMed] [Google Scholar]
- Scudiero D., Norin A., Karran P., Strauss B. DNA excision-repair deficiency of human peripheral blood lymphocytes treated with chemical carcinogens. Cancer Res. 1976 Apr;36(4):1397–1403. [PubMed] [Google Scholar]
- Setlow R. B. Repair deficient human disorders and cancer. Nature. 1978 Feb 23;271(5647):713–717. doi: 10.1038/271713a0. [DOI] [PubMed] [Google Scholar]
- Shaper N. L., Grafstrom R. H., Grossman L. Human placental apurinic/apyrimidinic endonuclease. Its isolation and characterization. J Biol Chem. 1982 Nov 25;257(22):13455–13458. [PubMed] [Google Scholar]
- Sheridan R. B., 3rd, Huang P. C. Apurinic and/or apyrimidinic endonuclease activity in ataxia telangiectasia cell extracts. Mutat Res. 1978 Oct;52(1):129–136. doi: 10.1016/0027-5107(78)90101-x. [DOI] [PubMed] [Google Scholar]
- Sirover M. A. Induction of the DNA repair enzyme uracil-DNA glycosylase in stimulated human lymphocytes. Cancer Res. 1979 Jun;39(6 Pt 1):2090–2095. [PubMed] [Google Scholar]



