Abstract
The prognostic impact of BRAF-V600 tumor mutations in stage I/II melanoma patients has not yet been analyzed in detail. We investigated primary tumors of 437 patients diagnosed between 1989 and 2006 by Sanger sequencing. Mutations were detected in 38.7% of patients and were associated with age, histological subtype as well as mitotic rate. The mutational rate was 36.7% in patients with disease-free course and 51.7% in those with subsequent distant metastasis (p = 0.031). No difference in overall survival (p = 0.119) but a trend for worse distant-metastasis-free survival (p = 0.061) was observed in BRAF mutant compared to BRAF wild-type patients. Independent prognostic factors for overall survival were tumor thickness, mitotic rate and ulceration. An interesting significant prognostic impact was observed in patients with tumor thickness of 1 mm or less, with the mutation present in 6 of 7 patients dying from melanoma. In conclusion, no significant survival differences were found according to BRAF-V600 tumor mutations in patients with primary melanoma but an increasing impact of the mutational status was observed in the subgroup of patients with tumor thickness of 1 mm or less. A potential role of the mutational status as a prognostic factor especially in this subgroup needs to be investigated in larger studies.
Introduction
The mitogen-activated protein kinase (MAPK) signaling pathway is constitutively activated by BRAF-V600 tumor mutations and leads to enhanced mitotic activity [1], [2]. Blocking in BRAF-V600 mutant patients by specific inhibitors leads to a high rate of clinical responses and an improved survival of melanoma patients [3]–[5]. Nevertheless, the prognostic relevance of BRAF mutations in the natural course of disease is controversial [6]–[20]. A trend towards worse survival of metastatic patients with BRAF mutation was found in three patient cohorts [7]–[9]. Similarly, a worse prognosis of metastatic patients with BRAF or NRAS tumor mutations [10] and of patients with BRAF mutant tumors after treatment with temozolomide and bevacizumab [11] was reported before. In contrast, Edlundh-Rose et al. did not find any association between the tumor NRAS or BRAF genotype and survival in a metastatic setting [12]. Two independent studies reported that a BRAF tumor mutation is an unfavorable prognostic factor for stage III patients after resection of loco-regional metastases [13], [14] but others failed to show any negative association with outcome in a similar clinical situation [15]. In non-metastasized patients with primary melanoma, no impact on prognosis was observed thus far in four studies including up to 115 patients [10], [16]–[18]. A recently published meta-analysis of four studies including mainly metastatic patients reported an 1.7-fold increased risk of dying from melanoma for BRAF mutant patients relative to wild-type patients [21].
The aim of the present study was to investigate the prognostic impact of BRAF-V600 tumor mutations in patients with non-metastasized cutaneous melanoma after excision of the primary tumor.
Materials and Methods
Ethics statement
All patients had given their written informed consent to have their data recorded by the Central Malignant Melanoma Registry (CMMR). This study was approved by the Ethics Committee, University of Tübingen (approval 413/2012BO2).
Patients
Patients with invasive cutaneous melanoma treated at the University Department of Dermatology in Tübingen, Germany, were identified in the Central Malignant Melanoma Registry (CMMR) database [22]. All patients with initial excision between 1989 and 2006 and available formalin-fixed paraffin-embedded tissue of the primary tumor were included. Data obtained for each patient were gender, age, and the date and cause of death, if applicable. Moreover, time points of initial diagnosis, occurrence of the first distant metastasis, and last follow-up were collected. Histopathologic data of the primary melanoma comprised Breslow's tumor thickness, Clark's level of invasion, ulceration, subtype (superficial spreading melanoma [SSM], nodular melanoma, lentigo maligna melanoma [LMM], acral lentiginous melanoma [ALM]), and mitoses per mm2. Only patients with non-metastasized primary cutaneous melanoma at time of initial diagnosis were included (stages I and II).
Sequencing
Microdissection of formalin-fixed paraffin-embedded tumor tissue was performed to obtain at least 50% tumor cells. After digestion by proteinase K an amplicon containing the BRAF codon 600 was amplified by a polymerase-chain-reaction (PCR) assay using forward primer 5′-tcataatgcttgctctgatagga-3′ and reverse primer 5′-ccaaaaatttaatcagtgga-3′. PCR products were analyzed on an agarose gel and purified using USB® ExoSAP-IT® (Affymetrix, Santa Clara, CA). Sanger sequencing was performed in reverse direction and sequences were analyzed with Mutation Surveyor Version 3.20 (SoftGenetics, State College, PA). For all samples which could not be clearly classified as mutant or wild-type, PCR and sequencing was repeated.
Statistics
The survival times were calculated as follows: Overall survival from the date of the initial diagnosis to the date of last follow-up or death; stage IV survival from the first occurrence of distant metastasis to the date of last follow-up or death; distant metastasis-free survival (DMFS) from the date of the initial diagnosis to the time point of the first occurrence of distant metastasis. Only deaths due to melanoma were considered, whereas patients who died from other cause were censored at the date of death. In three patients who died due to melanoma, the exact date of first occurrence of distant metastases was not available, and the date of distant metastasis was estimated to be 9 months before the melanoma related death, which is the median overall survival time. Estimates of cumulative survival probabilities according to Kaplan-Meier were described together with 95%-confidence intervals and compared using log rank tests. Cox regression analyses were used to determine the independent effects of prognostic factors. All variables were considered in Cox regression analyses and patients with missing data were excluded. Models were established using backward and forward stepwise procedures. Remaining non-significant factors were assessed for potential confounding effects. Changes in the estimates of factors in a model by more than 5% were taken as indicative for confounding. Results of the Cox regression models were described by hazard ratios (HR) together with 95%-confidence intervals, and p-values were based on the Wald test. All Chi square tests were performed 2-sided using Fisher's exact tests. Throughout the analysis, p-values of less than 0.05 were considered statistically significant. All analyses were carried out using SPSS Version 21 (IBM SPSS, Chicago, Illinois, USA).
Results
Patients
437 of 451 patients (97%) with successful sequencing were further analyzed. Median age was 57 years (interquartile range 46–67 years) and 52.8% were male. The stage at initial diagnosis according to AJCC was IA in 38.2%, IB in 42.8%, IIA in 12.2%, IIB in 5.1%, IIC in 1.8% of patients, and unknown in two cases because mitotic rate was not available. During follow-up, 58 of 437 patients (12.6%) developed distant metastasis and 52 (11.9%) died from melanoma. Median follow-up was 93 months. None of the patients received treatment with BRAF or MEK inhibitors during follow up. A BRAF-V600 tumor mutation was detected in 169 patients (38.7%). 150 patients (88.8%) had V600E, 18 (10.6%) V600K, and 1 (0.6%) V600R mutations.
Clinicopathologic associations according to mutational status
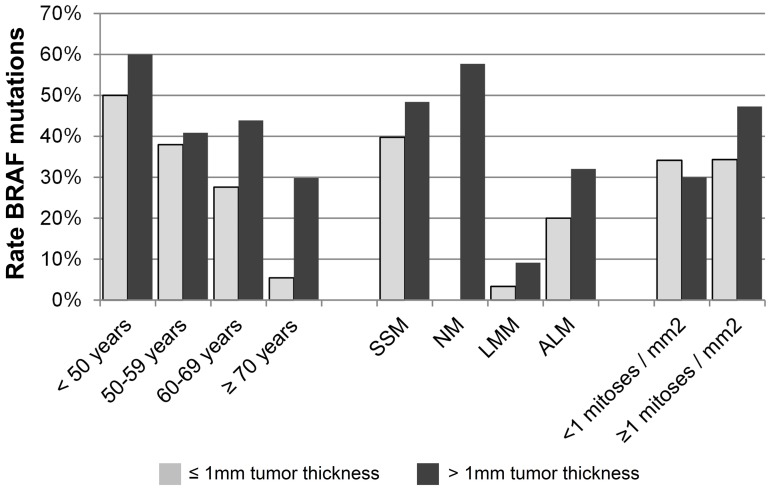
Associations between the rate of BRAF tumor mutations and demographic, clinical, or histopathologic characteristics are presented in Table 1. The BRAF mutational status was strongly associated with age. While the rate of BRAF mutant melanoma was 75% in patients younger than 30 years (n = 15) it was only 19% in patients aged 70 years or more (n = 84). This inverse correlation between BRAF-V600 mutations and age was stronger among patients with a tumor thickness of 1 mm or less (p<0.001) compared to those with thick primary melanomas (p = 0.034, Figure 1, Table 2). Furthermore, an association with the histological subtype was observed (p<0.001). The majority of nodular melanomas were BRAF-V600 mutants (57%); the rate was also high in patients with SSM (43%) but lower in ALMs (30%). In contrast, a BRAF-V600 mutation was rarely observed in LMM (6%). The detection of at least 1 mitosis/mm2 was associated with mutant BRAF in the entire cohort of patients (p = 0.038) and in patients with a tumor thickness of more than 1 mm (p = 0.046) but not in those with thin primary melanomas (p = 1.000, Table 2). No association with tumor BRAF mutations was observed for gender, Clark level, ulceration, and tumor thickness. The mutational rate was 36.7% in 379 patients with disease-free course during follow-up and 51.7% in 58 patients with subsequent distant metastasis (p = 0.031).
Table 1. Association of BRAF mutation status with clinicopathological parameters.
| Characteristic | Rate Mutant | Mutant | Wild type | p-value1 |
| All patients | 38.7% | 169 | 268 | |
| Gender | 0.844 | |||
| Male | 39.3% | 90 | 139 | |
| Female | 38.0% | 79 | 129 | |
| Age | <0.001 | |||
| <50 years | 53.4% | 70 | 61 | |
| 50–59 years | 39.3% | 42 | 65 | |
| 60–69 years | 35.7% | 41 | 74 | |
| ≥70 years | 19.0% | 16 | 68 | |
| Ulceration | 0.338 | |||
| Yes | 45.7% | 21 | 25 | |
| No | 37.9% | 148 | 243 | |
| Tumor thickness | 0.190 | |||
| ≤0.50 mm | 30.2% | 29 | 67 | |
| 0.51–0.75 mm | 33.0% | 30 | 61 | |
| 0.76–1.00 mm | 46.2% | 24 | 28 | |
| 1.01–2.00 mm | 43.8% | 60 | 77 | |
| 2.01–4.00 mm | 42.2% | 19 | 26 | |
| >4.00 mm | 43.8% | 7 | 9 | |
| Histological subtype | <0.001 | |||
| SSM | 43.0% | 141 | 187 | |
| NM | 57.7% | 15 | 11 | |
| LMM | 5.8% | 3 | 49 | |
| ALM | 30.0% | 9 | 21 | |
| Clark level | 0.277 | |||
| I–III | 36.2% | 88 | 155 | |
| IV or V | 41.8% | 81 | 113 | |
| Mitoses/mm2 | 0.038 | |||
| <1 | 33.2% | 72 | 145 | |
| ≥1 | 43.0% | 93 | 123 |
p-values are results of Chi-square tests.
Figure 1. Rate of BRAF-V600 mutations in patients with tumor thickness of 1 mm or less (grey bars) or more than 1 mm (black bars) according to age (left), histological subtype (middle), and mitotic rate (right).
SSM – superficial spreading melanoma; NM – nodular melanoma; LMM – lentigo maligna melanoma; ALM – acral lentiginous melanoma.
Table 2. Association of BRAF mutational status with clinicopathological parameters stratified according to tumor thickness.
| Tumor thickness ≤1 mm (n = 239) | Tumor thickness >1 mm (n = 198) | |||||||
| Characteristic | Rate Mutant | Mutant | Wild type | p-valuea | Rate Mutant | Mutant | Wild type | p-valuea |
| All patients | 34.7% | 83 | 156 | 43.4% | 86 | 112 | ||
| Gender | 0.419 | 0.253 | ||||||
| Male | 32.2% | 39 | 82 | 47.2% | 51 | 57 | ||
| Female | 37.3% | 44 | 74 | 38.9% | 35 | 55 | ||
| Age | <0.001 | 0.034 | ||||||
| <50 years | 50.0% | 43 | 43 | 60.0% | 27 | 18 | ||
| 50–59 years | 37.9% | 22 | 36 | 40.8% | 20 | 29 | ||
| 60–69 years | 27.6% | 16 | 42 | 43.9% | 25 | 32 | ||
| ≥70 years | 5.4% | 2 | 35 | 29.8% | 14 | 33 | ||
| Ulceration | 0.545 | 0.605 | ||||||
| Yes | 0.0% | 0 | 2 | 47.7% | 21 | 23 | ||
| No | 35.0% | 83 | 154 | 42.2% | 65 | 89 | ||
| Tumor thickness | 0.143 | 1.000 | ||||||
| ≤0.50 mm | 30.2% | 29 | 67 | |||||
| 0.51–0.75 mm | 33.0% | 30 | 61 | |||||
| 0.76–1.00 mm | 46.2% | 24 | 28 | |||||
| 1.01–2.00 mm | 43.8% | 60 | 77 | |||||
| 2.01–4.00 mm | 42.2% | 19 | 26 | |||||
| >4.00 mm | 43.8% | 7 | 9 | |||||
| Histological subtype | <0.001 | 0.001 | ||||||
| SSM | 39.7% | 81 | 123 | 48.4% | 60 | 64 | ||
| NM | 0 | 0 | 57.7% | 15 | 11 | |||
| LMM | 3.3% | 1 | 29 | 9.1% | 2 | 20 | ||
| ALM | 20.0% | 1 | 4 | 32.0% | 8 | 17 | ||
| Clark level | 0.135 | 0.159 | ||||||
| I–III | 32.7% | 66 | 136 | 53.7% | 22 | 19 | ||
| IV or V | 45.9% | 17 | 20 | 40.8% | 64 | 94 | ||
| Mitoses/mm2 | 1.000 | 0.046 | ||||||
| <1 | 34.1% | 57 | 110 | 30.0% | 15 | 35 | ||
| ≥1 | 34.3% | 24 | 46 | 47.3% | 69 | 77 | ||
p-values are results of Chi-square tests.
Survival Analysis
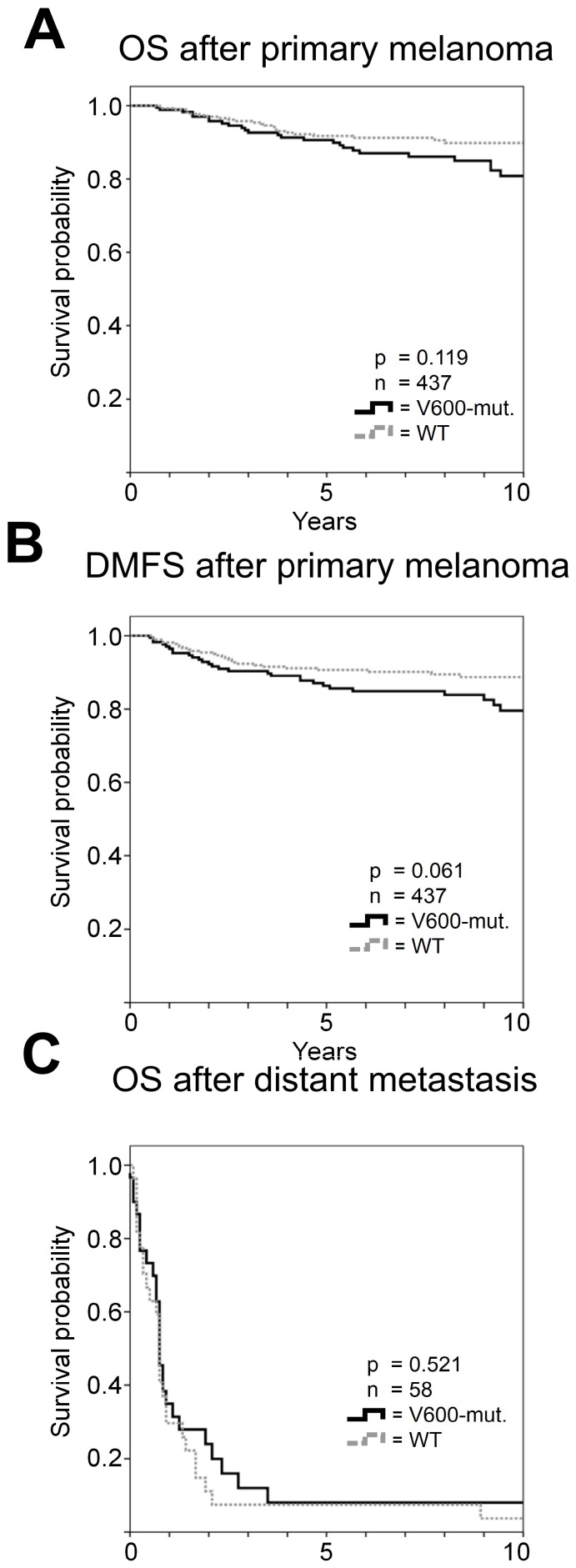
In univariate analysis, tumor thickness, Clark level, ulceration, histopathologic subtype, and mitotic rate were associated with overall survival (all p<0.001). The factors indicating worst prognosis with 10-year survival rates below 50% were a tumor thickness of at least 4 mm (42.9%) and the presence of ulceration (45.1%). In contrast, less than 1 mitosis/mm2 and a tumor thickness of 1 mm or less were associated with more than 95% survival probability ten years after initial diagnosis. In Cox regression analysis tumor thickness, ulceration and mitotic rate independently predicted survival (Table 3). A tumor thickness of greater than 4 mm or greater than 2 mm had strongest negative impact on overall survival with a hazard ratio (HR) of 4.7 (p = 0.035) or 4.6 (p = 0.010), respectively, followed by ulceration (HR 3.6, p<0.001) and a rate of at least 1 mitosis/mm2 (HR 2.9, p = 0.028). No association of overall survival with the tumor BRAF-V600 mutational status was observed (p = 0.119; Figure 2A). No differences in overall survival were detected according to age or gender.
Table 3. Analysis of overall survival.
| Univariate analysis | Cox Regression analysis3 | ||||||||
| Factor | n | % | % Dead | 10 Years survival rate (%) [95% CI1] | p-value2 | Hazard Ratio [95%-CI1] | p-value | ||
| All patients | 437 | 100.0 | 11.9 | 86.2 | [82.5; 89.9] | ||||
| Gender | 0.484 | ||||||||
| Male | 229 | 52.4 | 13.1 | 85.4 | [80.1; 90.7] | ||||
| Female | 208 | 47.6 | 10.6 | 89.4 | [84.9; 93.9] | ||||
| Age | 0.199 | ||||||||
| <50 years | 131 | 30.0 | 9.9 | 88.4 | [81.9; 94.8] | ||||
| 50–59 years | 107 | 24.5 | 12.1 | 86.6 | [79.7; 93.5] | ||||
| 60–69 years | 115 | 26.3 | 11.3 | 87.7 | [80.8; 94.5] | ||||
| ≥70 years | 84 | 19.2 | 15.5 | 78.2 | [62.9; 93.4] | ||||
| BRAF -V600 Mutations | 0.119 | ||||||||
| Wildtype | 268 | 61.3 | 9.7 | 89.8 | [85.9; 93.7] | ||||
| V600 Mutation | 169 | 38.7 | 15.4 | 80.9 | [73.6; 88.2] | ||||
| Ulceration | <0.001 | ||||||||
| Not ulcerated | 391 | 89.5 | 7.2 | 91.3 | [87.8; 94.8] | 1 | |||
| Ulcerated | 46 | 10.5 | 52.2 | 45.1 | [29.8; 60.4] | 3.6 | [1.9; 6.9] | <0.001 | |
| Histopathologic subtype | <0.001 | ||||||||
| SSM | 328 | 75.2 | 8.8 | 89.9 | [86.2; 93.6] | ||||
| Nodular | 26 | 6.0 | 42.3 | 52.3 | [30.9; 73.7] | ||||
| LMM | 52 | 11.9 | 5.8 | 91.5 | [81.7; 100.0] | ||||
| ALM | 30 | 6.9 | 26.7 | 70 | [49.0; 91.0] | ||||
| Missing data | 1 | ||||||||
| Clark level | <0.001 | ||||||||
| Level I–III | 243 | 55.6 | 5.8 | 93.3 | [89.4; 97.2] | ||||
| Level IV–V | 194 | 44.4 | 19.6 | 77.5 | [70.8; 84.2] | ||||
| Tumor thickness primary | <0.001 | ||||||||
| ≤1.00 mm | 239 | 54.7 | 2.9 | 95.6 | [91.9; 99.3] | 1 | |||
| 1.01–2.00 mm | 137 | 31.4 | 13.9 | 84.6 | [77.7; 91.5] | 1.9 | [0.7; 5.4] | 0.236 | |
| 2.01–4.00 mm | 45 | 10.3 | 40.0 | 56.6 | [40.5; 72.7] | 4.6 | [1.4; 14.5] | 0.010 | |
| >4.00 mm | 16 | 3.7 | 50.0 | 42.9 | [15.3; 70.5] | 4.7 | [1.1; 19.6] | 0.035 | |
| Mitoses/mm 2 | <0.001 | ||||||||
| <1 | 217 | 50.1 | 2.8 | 96.5 | [93.3; 99.7] | 1 | |||
| ≥1 | 216 | 49.9 | 20.8 | 76.5 | [69.9; 83.0] | 2.9 | [1.1; 7.6] | 0.028 | |
| Missing data | 4 | ||||||||
95%-CI = 95% confidence interval;
p-values are results of log rank tests excluding cases with missing values.
Cox Regression analysis was performed in 430 patients.
4 Patients had unknown mitotic rate and in 3 cases censoring occurred before the first event was observed; the model was adjusted for the confounding effects of age, gender, histological subtype, BRAF-V600 mutations, and Clark's level of invasion.
Figure 2. Univariate survival analysis according to BRAF-V600 mutational status.
No differences in overall survival (A) but a trend for unfavorable distant metastases-free survival (B) was observed in patients with tumor BRAF mutations. Survival after occurrence of distant metastasis was not different according to the tumor BRAF mutational status (C).
There was a trend for unfavorable DMFS in patients with BRAF mutant vs. wild-type melanoma (p = 0.061; Figure 2B). 17.8% of patients with BRAF mutant tumors but only 10.4% wild-type melanoma patients progressed to stage IV during observation (p = 0.031).
The median overall survival time according to Kaplan Meier after development of distant metastases was 9 months and was not associated with BRAF mutational status according to Kaplan-Meier (p = 0.521; Figure 2C). There was no difference in overall survival (p = 0.141) or DMFS (p = 0.251) between 150 patients with V600E mutations compared to 19 patients with V600K or V600R mutations.
Survival stratified according to tumor thickness
Next, we separately performed the survival analysis for 239 patients with a tumor thickness not exceeding 1 mm and those 198 with tumor thickness larger than 1 mm (Table 4).
Table 4. Overall survival stratified according to tumor thickness.
| Factor | ≤1 mm Tumor thickness | >1 mm Tumor thickness | ||||||||||||||
| Univariate analysis | Cox Regression analysisc | Univariate analysis | Cox Regression analysisd | |||||||||||||
| n | % | 10 Years survival rate (%) [95%-CIa] | p-valueb | Hazard Ratio [95%-CIa] | p-value | n | % | 10 Years survival rate (%) [95%-CIa] | p-valueb | Hazard Ratio [95%-CIa] | p-value | |||||
| All patients | 239 | 100.0 | 95.6 | [91.9; 99.3] | 198 | 100.0 | 75.1 | [68.4; 81.8] | ||||||||
| Gender | 0.987 | 0.537 | ||||||||||||||
| Male | 121 | 50.6 | 95.4 | [89.9; 100.0] | 108 | 54.5 | 74.4 | [65.4; 83.4] | ||||||||
| Female | 118 | 49.4 | 95.7 | [90.6; 100.0] | 90 | 45.5 | 75.7 | [65.3; 86.1] | ||||||||
| Age | 0.410 | 0.300 | ||||||||||||||
| <50 years | 45 | 22.7 | 80.8 | [68.7; 92.8] | 86 | 36.0 | 92.5 | [85.0; 99.9] | ||||||||
| 50–59 years | 49 | 24.7 | 71.1 | [57.5; 84.6] | 58 | 24.3 | na | na | ||||||||
| 60–69 years | 57 | 28.8 | 78.8 | [67.4; 90.2] | 58 | 24.3 | 96.3 | [89.2; 100.0] | ||||||||
| ≥70 years | 47 | 23.7 | 64.1 | [38.9; 89.3] | 37 | 15.5 | 96.6 | [89.9; 100.0] | ||||||||
| BRAF-V600 mutations | 0.013 | 0.994 | ||||||||||||||
| Wildtype | 156 | 65.3 | 99.2 | [97.6; 100.0] | 1 | 112 | 56.6 | 76.8 | [68.2; 85.4] | |||||||
| V600 Mutation | 83 | 34.7 | 95.1 | [89.4; 100.0] | 11.6 | [1.2; 111.8] | 0.034 | 86 | 43.4 | 73 | [62.4; 83.6] | |||||
| Ulceration | 0.926 | <0.001 | ||||||||||||||
| Not ulcerated | 237 | 99.2 | 95.6 | [91.9; 99.3] | 154 | 77.8 | 84.5 | [77.8; 91.2] | 1 | |||||||
| Ulcerated | 2 | 0.8 | na | 44 | 22.2 | 43.3 | [28.0; 58.6] | 4.2 | [2.2; 8.1] | <0.001 | ||||||
| Histological subtype | 0.831 | 0.015 | ||||||||||||||
| SSM | 204 | 85.4 | 95.6 | [91.7; 99.5] | 124 | 62.9 | 80.7 | [73.3; 88.1] | ||||||||
| Nodular | 0 | 0.0 | na | 26 | 13.2 | 52.3 | [30.9; 73.7] | |||||||||
| LMM | 30 | 12.6 | 95.7 | [87.3; 100.0] | 22 | 11.2 | 85.9 | [66.5; 105.3] | ||||||||
| ALM | 5 | 2.1 | na | 25 | 12.7 | 63.5 | [38.8; 88.2] | |||||||||
| Clark level | 0.206 | 0.687 | ||||||||||||||
| Level I–III | 202 | 84.5 | 96.3 | [92.6; 100.0] | 41 | 20.7 | 79.3 | [66.6; 92.0] | ||||||||
| Level IV–V | 37 | 15.5 | 91.8 | [80.2; 100.0] | 157 | 79.3 | 74.2 | [66.6; 81.8] | ||||||||
| Tumor thickness | 0.132 | <0.001 | ||||||||||||||
| ≤0.50 mm | 96 | 40.2 | 97.4 | [93.9; 100.0] | ||||||||||||
| 0.51–0.75 mm | 91 | 38.1 | 97.2 | [91.9; 100.0] | ||||||||||||
| 0.76–1.00 mm | 52 | 21.8 | 87.5 | [73.2; 100.0] | ||||||||||||
| 1.01–2.00 mm | 137 | 69.2 | 84.6 | [77.7; 91.5] | 1 | |||||||||||
| 2.01–4.00 mm | 45 | 22.7 | 56.6 | [40.5; 72.7] | 2.5 | [1.3; 4.9] | 0.009 | |||||||||
| >4.00 mm | 16 | 8.1 | 42.9 | [15.3; 70.5] | 2.8 | [1.2; 6.5] | 0.021 | |||||||||
| Mitoses/mm2 | 0.001 | 0.021 | ||||||||||||||
| <1 | 167 | 70.5 | 98.6 | [95.9; 100.0] | 1 | 50 | 25.5 | 89.5 | [79.3; 99.7] | |||||||
| ≥1 | 70 | 29.5 | 87.6 | [76.2; 99.0] | 17.9 | [1.7; 187.8] | 0.016 | 146 | 74.5 | 71.0 | [62.9; 79.1] | |||||
95%-CI = 95% confidence interval;
p-values are results of log rank tests excluding cases with missing values.
2 patients had unknown mitosis and in 3 cases censoring occurred before the first event was observed; the model was adjusted for the confounding effects of tumor thickness (n = 234).
2 patients had unknown mitosis rate and in 1 cases censoring occurred before the first event was observed; the model was adjusted for the confounding effects of mitotic rate and BRAF-V600 mutations (n = 195).
na = not available.
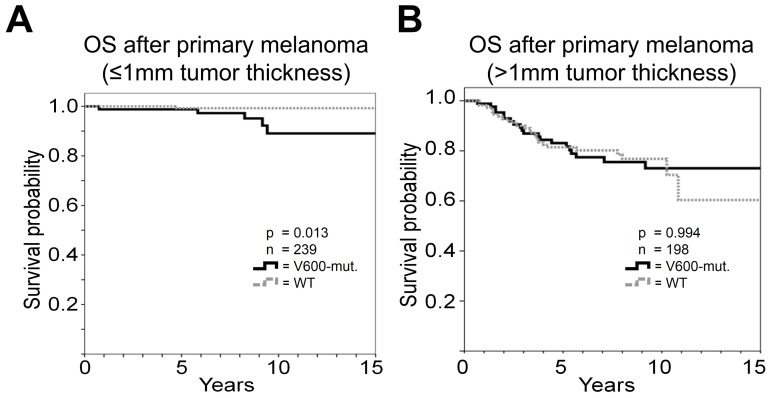
In patients with thin primary melanomas an association with overall survival was observed for the mitotic rate. The 10-year survival rate for patients with less than 1 mitosis/mm2 was 98.6% in contrast to 87.6% for the others (p<0.001); this factor had the highest impact in Cox regression analysis (HR 17.9; p = 0.016). The detection of BRAF mutations was likewise significantly associated with unfavorable survival (p = 0.013; Figure 3A) and represented an additional independent prognostic factor for melanoma patients with thin primary tumors (HR 11.6; p = 0.034). In patients with thick primary melanoma ulceration, sub-classification according to tumor thickness, rate of mitosis, and histological subtype were associated with survival but only ulceration (HR 4.2; p<0.001) and tumor thickness greater than 2 mm (HR 2.5; p = 0.009) or 4 mm (HR 2.8; p = 0.021) remained independent prognostic factors according to Cox regression analysis. Tumor BRAF mutations were not associated with survival (Figure 3B) in these patients with thick primary melanomas.
Figure 3. Kaplan-Meier analysis of overall survival (OS) of patients stratified according to tumor thickness for BRAF-V600 mutant (BRAF-mut.) vs. wild-type (WT) patients with tumor thickness ≤1 mm (A) or with tumor thickness >1 mm (B).
The difference in DMFS according to the BRAF mutational status was also limited to patients with tumor thickness of 1 mm or smaller (p = 0.011) and not evident in those with thicker primary melanomas (p = 0.745).
Discussion
No prognostic impact of BRAF-V600 mutations on overall survival was observed for the entire cohort of 437 non-metastasized melanoma patients in our study. According to Cox regression analysis, we could reproduce all established prognostic factors considered in the AJCC classification with mitotic rate, tumor thickness and ulceration being independently relevant for prognosis of stage I/II patients [23]. A tumor thickness greater than 2 mm or 4 mm (HR 4.6; p = 0.010 or HR 4.7; p = 0.035, respectively) and ulceration (HR = 3.6; p<0.001) were the most important prognostic factors, as already established [24], [25].
Our findings are in agreement with four other studies which investigated the prognostic impact of BRAF-V600 tumor mutations in small cohorts of non-metastasized patients and failed to report any relevance of the mutational status [10], [16]–[18].
We observed a higher rate of BRAF mutations in patients with SSM compared to other histopathologic subtypes. This correlation was also found in a meta-analysis which included 36 prior studies and additionally described the localization of the primary melanoma in non-chronically sun-damaged skin as a factor associated with a high rate of BRAF mutations [26]. In our study, we did not include data on early-life UV-exposure, which was reported to correlate with the BRAF mutational rate [27], but a higher rate of mutations in young patients independent of UV-exposure was observed by us, as well as by others [7], [8].
In contrast to non-metastasized patients, the prognostic relevance of BRAF mutations has been reported previously for patients with distant metastasis [7], [8], [11]. In the study of Long et al., overall survival after development of distant metastasis was reduced in BRAF mutant patients, while there was no difference in DMFS [7]. In contrast, in our study we observed no difference in stage IV survival according to the mutational status but a strong trend (p = 0.061) for an impaired DMFS in BRAF mutant patients. Similar results were reported by Edlundh-Rose et al. who analyzed 214 metastasized patients [12].
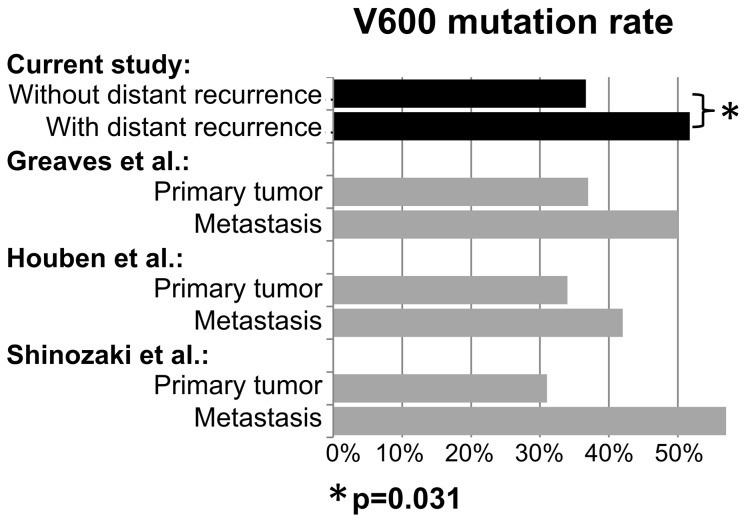
In addition to differences in DMFS, the higher mutational rate in 58 stage I/II patients who developed distant metastases during follow-up compared to 379 patients without subsequent distant recurrence provides further evidence that a BRAF-V600 mutation may indicate an increased risk of developing distant metastasis (51.7% versus 36.7%; p = 0.031). Lower rates of BRAF-V600 mutations had also been previously reported after analysis of primary tumors compared to metastasis (Figure 4) but was explained by the acquisition and accumulation of BRAF mutant tumor cells during the course of disease [10], [17], [28]. This explanation is in contrast to recent publications reporting a high proportion of patients with consistent mutation patterns when comparing pairs of primary tumors and metastases of the same individuals [29], [30] and the differences in the rate of BRAF mutations in primary tumors if stratified according to disease outcome in the current study.
Figure 4. Rate of BRAF-V600 tumor mutations according to disease outcome.
A significantly lower rate of BRAF-V600 tumor mutations was observed in 382 patients who did not develop distant metastasis during follow-up (upper black solid bar - w/o distant metastasis) compared to 55 stage I/II patients who had distant recurrences in the further course of disease (black solid bar - with distant metastases) in our study (36.6% versus 52.7%; p = 0.026). A similar difference in mutational rate was reported in prior studies comparing the mutational rate in metastases of late-stage melanoma patients and primary tumors of stage I/II patients.
The conflicting results for DMFS can also be explained by patient selection in prior studies, which limited the analysis of DMFS to patients, who had already developed distant metastasis [7]. In the current study, which represents the largest analysis of the prognostic impact of BRAF mutations in non-metastasized melanoma patients thus far, we retrospectively analyzed the BRAF status in patients who had not been selected on the basis of their later disease course or outcome. The strong trend for a worse DMFS in BRAF mutant patients observed in our cohort is completely lost, if the analysis is restricted to patients who developed distant metastasis in their later course of disease (data not shown).
The conflicting results for stage IV survival might also be explained by a potential patient selection bias. In some prior retrospective prognostic studies using already available institutional data from mutational testing it has to be assumed that the BRAF V600 status was tested due to the intention to treat with a BRAF- or MEK inhibitor at least in a subset of patients (e.g. [9]). But in order to analyze the treatment-unrelated “natural” impact of BRAF-V600 tumor mutations only patients with confirmed BRAF-mutations who finally did not receive subsequent inhibitor treatment can be considered. Reasons among others for non-treatment with inhibitors in BRAF-V600 mutant patients could be exclusion criteria in the frame of clinical studies (e.g. elevated LDH or occurrence of brain metastases), decrease of performance status or early death due to disease progression. Therefore these patients might represent a cohort biased towards worse prognosis.
We included a majority of patients (n = 239) with tumor thickness of 1 mm or less. In the 7th edition of the AJCC staging classification, ulceration and mitotic rate are considered for classification purposes in these patients [31]. Even if prognosis is generally considered good, between 5% and 10% eventually die from melanoma [31]. Additional prognostic markers are therefore desirable for this large subgroup, representing more than 40% of all stage I/II patients [31]. We showed that BRAF-V600 mutations in melanoma cells represent a prognostic factor indicating worse distant metastasis-free and overall survival of non-metastasized patients with a tumor thickness of 1 mm or less. These are results of a subgroup analysis and have to be interpreted with caution, as it is based on a small number of events. On the other hand the present study is the first which focused on low risk patients. Only Shinozaki et al. [17] included a limited number of patients (n = 19) with a tumor thickness of less than 1 mm, and a selection towards thick primary melanomas was likewise evident in other previous studies performed in non-metastatic patients. Our results may provide a rationale to analyze the prognostic impact of BRAF mutations in non-metastasized low risk patients in larger studies.
In conclusion, no significant survival differences were found according to BRAF-V600 tumor mutations in patients with primary melanoma but an increasing impact of the mutational status was observed in the subgroup of patients with tumor thickness of 1 mm or less. A potential role of the mutational status as a prognostic factor especially in this subgroup needs to be investigated in larger studies.
Acknowledgments
We thank Graham Pawelec for editorial assistance.
Funding Statement
The authors have no support or funding to report.
References
- 1. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, et al. (2002) Mutations of the BRAF gene in human cancer. Nature 417: 949–954. [DOI] [PubMed] [Google Scholar]
- 2. Satyamoorthy K, Li G, Gerrero MR, Brose MS, Volpe P, et al. (2003) Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res 63: 756–759. [PubMed] [Google Scholar]
- 3. Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, et al. (2012) Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 367: 107–114. [DOI] [PubMed] [Google Scholar]
- 4. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, et al. (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364: 2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, et al. (2010) Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363: 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Si L, Kong Y, Xu X, Flaherty KT, Sheng X, et al. (2012) Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer 48: 94–100. [DOI] [PubMed] [Google Scholar]
- 7. Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, et al. (2011) Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol 29: 1239–1246. [DOI] [PubMed] [Google Scholar]
- 8. Menzies AM, Haydu LE, Visintin L, Carlino MS, Howle JR, et al. (2012) Distinguishing clinicopathologic features of patients with V600E and V600K BRAF-mutant metastatic melanoma. Clin Cancer Res 18: 3242–3249. [DOI] [PubMed] [Google Scholar]
- 9. Jakob JA, Bassett RL Jr, Ng CS, Curry JL, Joseph RW, et al. (2012) NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 118: 4014–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Houben R, Becker JC, Kappel A, Terheyden P, Brocker EB, et al. (2004) Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog 3: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. von Moos R, Seifert B, Simcock M, Goldinger SM, Gillessen S, et al. (2012) First-line temozolomide combined with bevacizumab in metastatic melanoma: a multicentre phase II trial (SAKK 50/07). Ann Oncol 23: 531–536. [DOI] [PubMed] [Google Scholar]
- 12. Edlundh-Rose E, Egyhazi S, Omholt K, Mansson-Brahme E, Platz A, et al. (2006) NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res 16: 471–478. [DOI] [PubMed] [Google Scholar]
- 13. Mann GJ, Pupo GM, Campain AE, Carter CD, Schramm SJ, et al. (2013) BRAF Mutation, NRAS Mutation, and the Absence of an Immune-Related Expressed Gene Profile Predict Poor Outcome in Patients with Stage III Melanoma. J Invest Dermatol 133: 509–517. [DOI] [PubMed] [Google Scholar]
- 14. Moreau S, Saiag P, Aegerter P, Bosset D, Longvert C, et al. (2012) Prognostic value of BRAF(V(6)(0)(0)) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol 19: 4314–4321. [DOI] [PubMed] [Google Scholar]
- 15. Ellerhorst JA, Greene VR, Ekmekcioglu S, Warneke CL, Johnson MM, et al. (2011) Clinical correlates of NRAS and BRAF mutations in primary human melanoma. Clin Cancer Res 17: 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maldonado JL, Fridlyand J, Patel H, Jain AN, Busam K, et al. (2003) Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst 95: 1878–1890. [DOI] [PubMed] [Google Scholar]
- 17. Shinozaki M, Fujimoto A, Morton DL, Hoon DS (2004) Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res 10: 1753–1757. [DOI] [PubMed] [Google Scholar]
- 18. Akslen LA, Angelini S, Straume O, Bachmann IM, Molven A, et al. (2005) BRAF and NRAS mutations are frequent in nodular melanoma but are not associated with tumor cell proliferation or patient survival. J Invest Dermatol 125: 312–317. [DOI] [PubMed] [Google Scholar]
- 19. Chang DZ, Panageas KS, Osman I, Polsky D, Busam K, et al. (2004) Clinical significance of BRAF mutations in metastatic melanoma. J Transl Med 2: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar R, Angelini S, Czene K, Sauroja I, Hahka-Kemppinen M, et al. (2003) BRAF mutations in metastatic melanoma: a possible association with clinical outcome. Clin Cancer Res 9: 3362–3368. [PubMed] [Google Scholar]
- 21. Safaee Ardekani G, Jafarnejad SM, Tan L, Saeedi A, Li G (2012) The prognostic value of BRAF mutation in colorectal cancer and melanoma: a systematic review and meta-analysis. PLoS One 7: e47054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lasithiotakis KG, Petrakis IE, Garbe C (2010) Cutaneous melanoma in the elderly: epidemiology, prognosis and treatment. Melanoma Res 20: 163–170. [DOI] [PubMed] [Google Scholar]
- 23. Thompson JF, Soong SJ, Balch CM, Gershenwald JE, Ding S, et al. (2011) Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. J Clin Oncol 29: 2199–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, et al. (2001) Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 19: 3622–3634. [DOI] [PubMed] [Google Scholar]
- 25. Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, et al. (2001) Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 19: 3635–3648. [DOI] [PubMed] [Google Scholar]
- 26. Lee JH, Choi JW, Kim YS (2011) Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. Br J Dermatol 164: 776–784. [DOI] [PubMed] [Google Scholar]
- 27. Thomas NE, Edmiston SN, Alexander A, Millikan RC, Groben PA, et al. (2007) Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev 16: 991–997. [DOI] [PubMed] [Google Scholar]
- 28. Greaves WO, Verma S, Patel KP, Davies MA, Barkoh BA, et al. (2013) Frequency and spectrum of BRAF mutations in a retrospective, single-institution study of 1112 cases of melanoma. J Mol Diagn 15: 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Colombino M, Capone M, Lissia A, Cossu A, Rubino C, et al. (2012) BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J Clin Oncol 30: 2522–2529. [DOI] [PubMed] [Google Scholar]
- 30. Capper D, Berghoff AS, Magerle M, Ilhan A, Wohrer A, et al. (2012) Immunohistochemical testing of BRAF V600E status in 1,120 tumor tissue samples of patients with brain metastases. Acta Neuropathol 123: 223–233. [DOI] [PubMed] [Google Scholar]
- 31. Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, et al. (2009) Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27: 6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]