Abstract
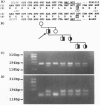
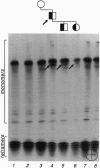
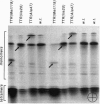
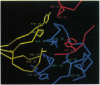
The most frequent form of inherited amyloidoses is associated with mutations in the transthyretin (TTR) gene coding for 127-amino acid residues of four identical, noncovalently linked subunits that form a pair of dimers in the plasma protein complex. Amyloid fibrils containing the variant and to a lesser extent the wild-type form of the TTR molecule are deposited in various organs, including peripheral nerves and the myocardium, with polyneuropathy and cardiomyopathy as major clinical manifestations. So far, more than 40 distinct amino acid substitutions distributed throughout the TTR sequence over 30 positions have been found to be correlated with an increased amyloidogenicity of TTR. Most of these amyloidogenic amino acid substitutions are suspected to alter the conformation and stability of the monomer. Here we identify and characterize by protein and DNA analysis a novel amyloidogenic Val-20 to Ile mutation in a German three-generation family. The index patient suffered from severe amyloid cardiomyopathy at the age of 60. Conformational stability and unfolding behavior of the Ile-20 monomer in urea gradients was found to be almost indistinguishable from that of wild-type TTR. In contrast, tetramer stability was significantly reduced in agreement with the expected change in the interactions between the two opposing dimers via the side chain of Ile-20. Our observations provide strong evidence for the view that amyloidogenic amino acid substitutions in TTR facilitate the conversion of tetrameric TTR complexes into those conformational intermediates of the TTR folding pathway that have an intrinsic amyloidogenic potential.
Full text
PDF

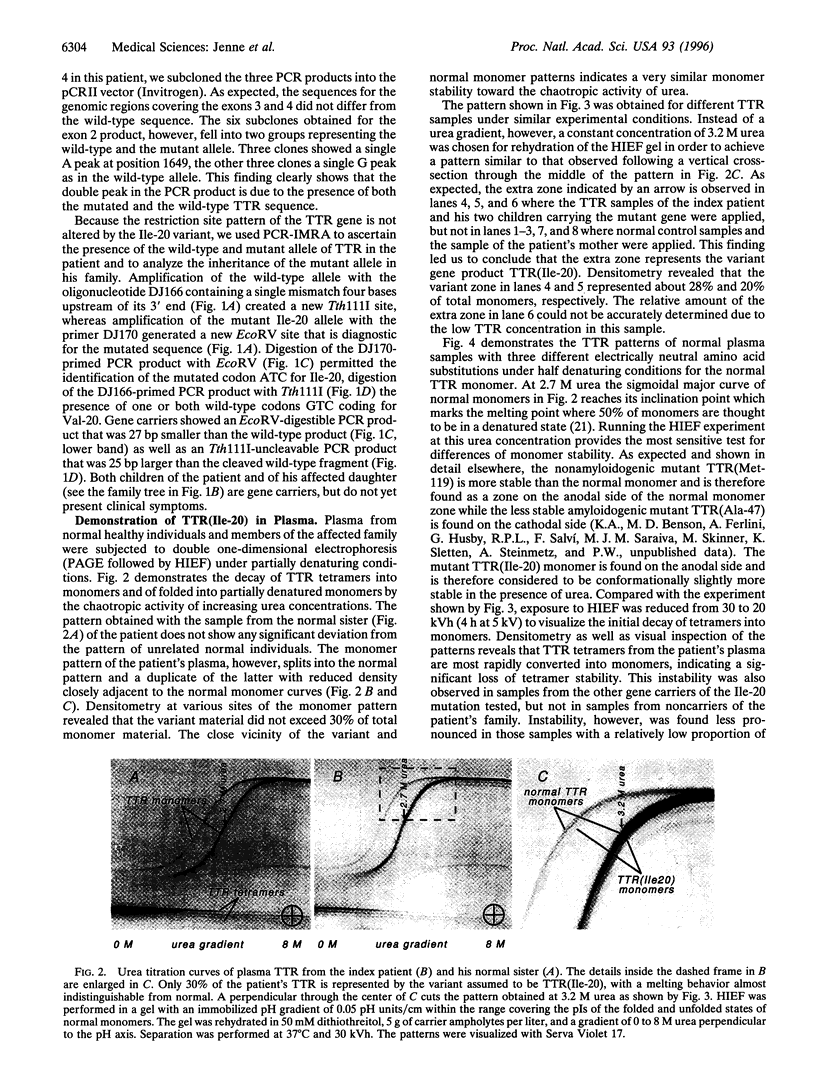
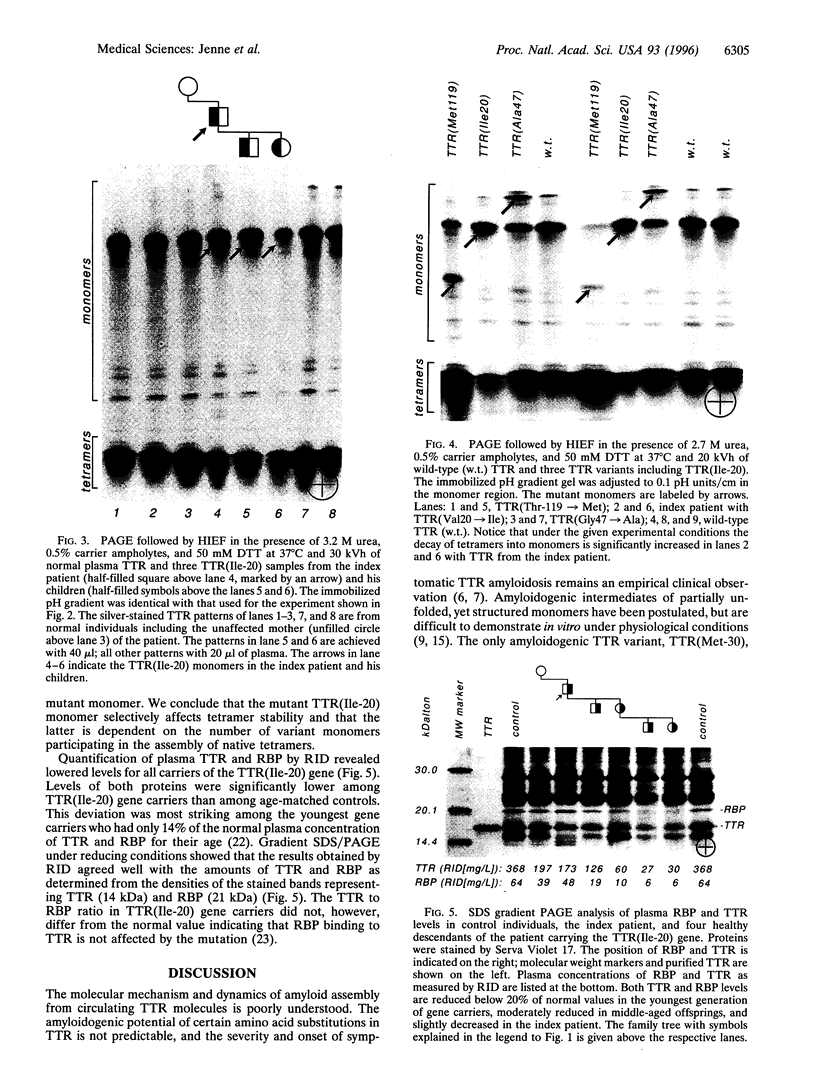


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson M. D. Familial amyloidosis. J Intern Med. 1992 Dec;232(6):525–527. doi: 10.1111/j.1365-2796.1992.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Berni R., Malpeli G., Folli C., Murrell J. R., Liepnieks J. J., Benson M. D. The Ile-84-->Ser amino acid substitution in transthyretin interferes with the interaction with plasma retinol-binding protein. J Biol Chem. 1994 Sep 23;269(38):23395–23398. [PubMed] [Google Scholar]
- Blake C. C., Geisow M. J., Oatley S. J., Rérat B., Rérat C. Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 A. J Mol Biol. 1978 May 25;121(3):339–356. doi: 10.1016/0022-2836(78)90368-6. [DOI] [PubMed] [Google Scholar]
- Bond J. S., Rojas K., Overhauser J., Zoghbi H. Y., Jiang W. The structural genes, MEP1A and MEP1B, for the alpha and beta subunits of the metalloendopeptidase meprin map to human chromosomes 6p and 18q, respectively. Genomics. 1995 Jan 1;25(1):300–303. doi: 10.1016/0888-7543(95)80142-9. [DOI] [PubMed] [Google Scholar]
- Christmanson L., Betsholtz C., Gustavsson A., Johansson B., Sletten K., Westermark P. The transthyretin cDNA sequence is normal in transthyretin-derived senile systemic amyloidosis. FEBS Lett. 1991 Apr 9;281(1-2):177–180. doi: 10.1016/0014-5793(91)80387-i. [DOI] [PubMed] [Google Scholar]
- Colon W., Kelly J. W. Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry. 1992 Sep 15;31(36):8654–8660. doi: 10.1021/bi00151a036. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Detection of folding intermediates using urea-gradient electrophoresis. Methods Enzymol. 1986;131:156–172. doi: 10.1016/0076-6879(86)31040-1. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Steinrauf L. K., Braden B. C., Liepnieks J., Benson M. D., Holmgren G., Sandgren O., Steen L. The x-ray crystal structure refinements of normal human transthyretin and the amyloidogenic Val-30-->Met variant to 1.7-A resolution. J Biol Chem. 1993 Feb 5;268(4):2416–2424. [PubMed] [Google Scholar]
- Ingenbleek Y., Young V. Transthyretin (prealbumin) in health and disease: nutritional implications. Annu Rev Nutr. 1994;14:495–533. doi: 10.1146/annurev.nu.14.070194.002431. [DOI] [PubMed] [Google Scholar]
- Kyle R. A., Linos A., Beard C. M., Linke R. P., Gertz M. A., O'Fallon W. M., Kurland L. T. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992 Apr 1;79(7):1817–1822. [PubMed] [Google Scholar]
- McCutchen S. L., Colon W., Kelly J. W. Transthyretin mutation Leu-55-Pro significantly alters tetramer stability and increases amyloidogenicity. Biochemistry. 1993 Nov 16;32(45):12119–12127. doi: 10.1021/bi00096a024. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Harrington M. G. "Ultrasensitive" silver stains: their use exemplified in the study of normal human cerebrospinal fluid proteins separated by two-dimensional electrophoresis. Clin Chem. 1984 Dec;30(12 Pt 1):1938–1942. [PubMed] [Google Scholar]
- Monaco H. L., Rizzi M., Coda A. Structure of a complex of two plasma proteins: transthyretin and retinol-binding protein. Science. 1995 May 19;268(5213):1039–1041. doi: 10.1126/science.7754382. [DOI] [PubMed] [Google Scholar]
- Patestos N. P., Fauth M., Radola B. J. Fast and sensitive protein staining with colloidal acid violet 17 following isoelectric focusing in carrier ampholyte generated and immobilized pH gradients. Electrophoresis. 1988 Sep;9(9):488–496. doi: 10.1002/elps.1150090908. [DOI] [PubMed] [Google Scholar]
- Reilly M. M., Adams D., Booth D. R., Davis M. B., Said G., Laubriat-Bianchin M., Pepys M. B., Thomas P. K., Harding A. E. Transthyretin gene analysis in European patients with suspected familial amyloid polyneuropathy. Brain. 1995 Aug;118(Pt 4):849–856. doi: 10.1093/brain/118.4.849. [DOI] [PubMed] [Google Scholar]
- Saraiva M. J. Transthyretin mutations in health and disease. Hum Mutat. 1995;5(3):191–196. doi: 10.1002/humu.1380050302. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Yoshioka N., Takagi Y., Sakaki Y. Structure of the chromosomal gene for human serum prealbumin. Gene. 1985;37(1-3):191–197. doi: 10.1016/0378-1119(85)90272-0. [DOI] [PubMed] [Google Scholar]
- Steinrauf L. K., Hamilton J. A., Braden B. C., Murrell J. R., Benson M. D. X-ray crystal structure of the Ala-109-->Thr variant of human transthyretin which produces euthyroid hyperthyroxinemia. J Biol Chem. 1993 Feb 5;268(4):2425–2430. [PubMed] [Google Scholar]
- Terry C. J., Damas A. M., Oliveira P., Saraiva M. J., Alves I. L., Costa P. P., Matias P. M., Sakaki Y., Blake C. C. Structure of Met30 variant of transthyretin and its amyloidogenic implications. EMBO J. 1993 Feb;12(2):735–741. doi: 10.1002/j.1460-2075.1993.tb05707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki T., Mita S., Maeda S., Araki S., Shimada K. Structure of the human prealbumin gene. J Biol Chem. 1985 Oct 5;260(22):12224–12227. [PubMed] [Google Scholar]
- Waits R. P., Yamada T., Uemichi T., Benson M. D. Low plasma concentrations of retinol-binding protein in individuals with mutations affecting position 84 of the transthyretin molecule. Clin Chem. 1995 Sep;41(9):1288–1291. [PubMed] [Google Scholar]