Abstract
Growth rates (µ) of abundant microzooplankton species were examined in field experiments conducted at ambient sea temperatures (−1.8–9.0°C) in the Barents Sea and adjacent waters (70–78.5°N). The maximum species-specific µ of ciliates and athecate dinoflagellates (0.33–1.67 d−1 and 0.52–1.14 d−1, respectively) occurred at temperatures below 5°C and exceeded the µmax predicted by previously published, laboratory culture-derived equations. The opposite trend was found for thecate dinoflagellates, which grew faster in the warmer Atlantic Ocean water. Mixotrophic ciliates and dinoflagellates grew faster than their heterotrophic counterparts. At sub-zero temperatures, microzooplankton µmax matched those predicted for phytoplankton by temperature-dependent growth equations. These results indicate that microzooplankton protists may be as adapted to extreme Arctic conditions as their algal prey.
Introduction
A recent decline in sea ice cover over the Arctic, with the largest losses in the Eurasian sector, has resulted in areas of open water stretching from the shelves into the deep basins [1], [2]. Changes in the cryosphere can be gradual or abrupt [3], but they have cascading effects through polar ecosystems, including food web structure and elemental cycling pathways [4]. For example, 5°C might be a temperature threshold for Arctic marine ecosystems to become net heterotrophic [5]. Specific predictions about the trajectories of food web changes are complicated by the non-linear nature of their responses to climate change and, therefore, require a detailed knowledge of their key components and linkages to dynamic processes. These considerations warrant interest in the effects of climate change on microbial plankton because even minor effects at the base of food webs could be amplified through trophic chains [6].
Plankton growth rate is a fundamental biological property and governs species composition, productivity, and carbon transformations in pelagic systems [7]. Therefore, knowledge of growth rates of individual species and their assemblages is critical to understanding food web responses to climate change. Increasing sea temperatures will likely have different effects on growth rates of different functional and taxonomic groups within pelagic communities, including microzooplankton. The resulting compositional changes may, in turn, alter food web structure and trophic interactions [8]. For example, Rose and Caron [9] hypothesized that microzooplankton growth would be more constrained by low temperatures than phytoplankton growth. This hypothesis is based on growth-temperature curves extrapolated from laboratory cultures maintained at higher temperatures. However, field observations indicate that polar microzooplankton might be adapted to their extreme environment [10]. Landry and Calbet [11] suggested that mean instantaneous growth rates for microzooplankton in the ocean should be generally comparable to those of their phytoplankton prey based on biomass ratios. This assumption corresponds to earlier observations in temperate and tropical waters (e.g., [12]). However, the dearth of direct measurements of microzooplankton growth rates at low temperatures restricts our ability to extrapolate these estimates to polar systems.
In the Arctic, microzooplankton potential growth rates were examined at non-ambient temperatures in Disko Bay, Greenland [10], the Barents Sea [13], and a Spitsbergen fjord [14]. To date, the only experimental study of Arctic microzooplankton growth rates at sub-zero temperatures reported elevated rates for heterotrophic dinoflagellates as a group (up to 1.17 d−1; [15]). Clearly, more experimental data on microzooplankton growth and production rates in the Arctic are needed before we can predict and model their responses to climate change. Thus, the primary goal of the present study was to estimate growth potential of polar microzooplankton with a special emphasis on mixotrophic taxa. Specific objectives were to (1) determine growth rates of dominant microzooplankton species across a natural range of sea temperature variation in the Barents Sea; and (2) compare these rates to those based on published equations for ciliates and dinoflagellates. Although this study was not designed specifically to test the Rose and Caron hypothesis, we also compared the measured microzooplankton growth rates with phytoplankton growth rates predicted by several published temperature-growth equations.
Materials and Methods
Ethics Statement
Permission to sample in the Spitsbergen coastal waters was obtained from Svalbard authorities by the University of Tromsø, Norway (UiT). No specific permissions for other locations/activities were required. The field studies did not involve endangered or protected species.
Study Locations
The Barents Sea is a large (1.4 million km2, average depth 230 m, maximum depth 500 m) polar shelf sea. It is the only Arctic region that remains unfrozen throughout the year up to 74–75°N [16], due to inflowing warm water masses of the Atlantic drift from the southwest [17]. The warm and more saline Atlantic water (AtW) subducts under the cold and fresher Arctic water (<0°C, ArW), which flows through the opening between Svalbard, Franz Josef Land, and Novaya Zemlia, and forms the distinct Polar Front between 74 and 76°N [18]. In addition to the Atlantic drift, the southwestern section of the shelf is affected by the coastal Nordcapp current.
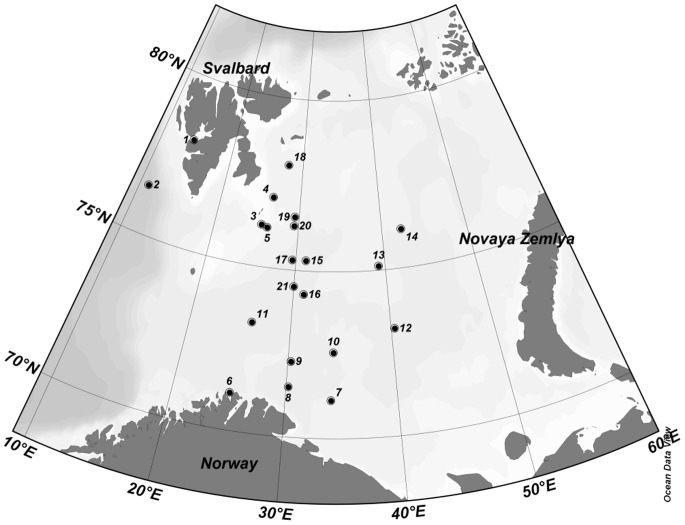
Twenty one field experiments were conducted at stations located between 70°N and 79°N and 11°E and 43°E in May 2010, August-September 2010, and June 2011 (Fig. 1) aboard the R/V Helmer Hanssen (formerly Jan Mayen) and R/V G.O. Sars. These expeditions, organized by the UiT and the Institute of Marine Research (Bergen, Norway), crossed the Polar Front from AtW in the south to ArW in the north. The UiT cruises (May 2010 and June 2011) also included the marginal ice zone and seasonal sea ice floes between Hopen Island and Kong Karls Land. In addition, experiments were conducted in Isfjorden, Spitsbergen, and the eastern slope of the Greenland Sea in May 2010.
Figure 1. The study area with experimental station locations (corresponding to Table 1).
Field Sampling
Prior to sampling, all glass-ware, plastic containers, and tubing were soaked in 10% HCl and rinsed with copious amounts of deionized water and then seawater. Gloves were used whenever handling experimental containers. At each station, water temperature, salinity and raw fluorescence were measured using a Seabird 911 Plus CTD system equipped with a fluorometer. Seawater was collected in 5 L Niskin bottles from the fluorescence maximum depth (deep chlorophyll maximum, DCM) and carefully syphoned into a 20 L polycarbonate carboy using submerged silicone tubing. The carboy was immediately transported in an insulated cooler to a shipboard temperature-controlled cold room, which was adjusted to match (±1°C) the sea surface temperature. Additionally, sea ice cores were collected for Experiment (Exp.) 4.
Experimental Manipulations
All manipulations were conducted under dim light, and samples were stored in a closed cooler whenever not being handled. The collected water was added carefully to triplicate 0.6 L Nalgene clear glass bottles. Additionally, in Exp. 4, the bottom 10 cm of sea ice was melted in unfiltered seawater at a 1∶8 ice to water (v/v) ratio to avoid osmotic shock mortality among protists [19], [20]. Growth experiments were run in conjunction with microzooplankton and copepod grazing experiments. Therefore, the samples were amended with dissolved nutrients to final concentrations of 16 µM N (KNO3+NH4Cl; 15∶1 based on N) and 1 µM P (K2HPO4). To avoid damaging delicate microzooplankton and altering phytoplankton composition, samples were not screened prior to incubation [15]. Instead, larger zooplankton such as copepods were removed using a glass pipette and headband flashlight. Post-incubation screening indicated that this technique was effective in removing mesozooplankton. The bottles were closed with Nalgene caps lined with Corning PTFE-faced silicone septa to prevent air headspace and screened with neutral density filters to mimic 25% surface irradiance.
Sample Incubation
Bottles were incubated in a deck incubator with running surface seawater for 24 h. Temperature in the incubator was monitored manually using a handheld digital thermometer. During most experiments, temperature remained within ±0.5°C of the initial sea temperature (Table 1) since the ship was either at the station or moving within the same water mass. The only exception was Exp. 16 in September 2010, when the ship was called to port earlier than expected and crossed several temperature fields on the way back. Sea surface temperatures ranged from 4.6°C at the beginning of the incubation to 9.0°C at the end of this experiment. During the May 2010 cruise, the incubator included a plankton wheel, which was set at ca. 0.25 revolutions per minute [21]. During the other two cruises, experimental bottles were rotated periodically by hand to prevent phytoplankton settling.
Table 1. Experimental dates and conditions.
| Exp | Date | Sea T°C | Incubator T°C | Sampling Depth m | Chl µg L−1 |
| 1 | 04/05/2010 | −1.3 | −1.2 | 17 | 1.64 |
| 2 | 05/05/2010 | 2.2 | 2.0 | 10 | 0.11 |
| 3 | 07/05/2010 | −1.3 | −1.3 | 10 | 3.19 |
| 4 | 08/05/2010 | −1.8 | −1.8 | 2 | 3.52 |
| 5 | 09/05/2010 | 0.3 | 0.1 | 35 | 1.37 |
| 6 | 24/08/2010 | 8.6 | 8.6 | 10 | 1.91 |
| 7 | 26/08/2010 | 7.2 | 7.2 | 10 | 1.82 |
| 8 | 27/08/2010 | 7.4 | 7.6 | 30 | 1.58 |
| 9 | 30/08/2010 | 7.5 | 7.5 | 20 | 1.20 |
| 10 | 01/09/2010 | 5.6 | 5.6 | 10 | 1.77 |
| 11 | 02/09/2010 | 7.0 | 7.0 | 25 | 0.79 |
| 12 | 04/09/2010 | 4.2 | 4.5 | 20 | 2.18 |
| 13 | 07/09/2010 | 3.1 | 3.1 | 20 | 0.67 |
| 14 | 09/09/2010 | 2.4 | 2.4 | 20 | 0.38 |
| 15 | 10/09/2010 | 4.9 | 4.9 | 20 | 0.10 |
| 16 | 12/09/2010 | 4.6 | 4.6–9 | 20 | 1.61 |
| 17 | 21/06/2011 | 4.0 | 5.0 | 35 | 1.86 |
| 18 | 22/06/2011 | −1.8 | −1.2 | 30 | 5.19 |
| 19 | 24/06/2011 | −0.5 | −0.8 | 44 | 1.32 |
| 20 | 26/06/2011 | 0.0 | 1.2 | 38 | 1.37 |
| 21 | 27/06/2011 | 4.0 | 4.8 | 20 | 3.00 |
Legend: T – temperature, Chl – chlorophyll a. Experiment sequential numbers correspond to Figure 1.
Sample Collection and Preservation
Microzooplankton samples were collected from whole water treatments at the beginning and end of experiments, preserved in 2% (final concentration) acid Lugol’s iodine, stored at 4°C in 125 mL opaque containers, and post-fixed with 1% (final concentration) formaldehyde after 24 h. An additional set of triplicate plankton samples was fixed with 1% (final concentration) formaldehyde and stored as described above. For chlorophyll a (Chl) analysis, 250–500 mL of seawater was filtered onto 0.2 µm 47 mm nylon membrane filters, which were shock-frozen and stored in liquid N2. The samples were transported to the shore-based facility in insulated coolers with added cold packs.
Sample Processing
In the laboratory, Chl was extracted in 90% acetone for 24 h at −20°C and measured using the non-acidic method [22] on a Turner Designs TD-700 fluorometer. Microzooplankton were settled onto Utermöhl chambers and counted under an Olympus IX-70 inverted microscope equipped with differential interference contrast (DIC), fluorescence, and a digital camera. The entire surface area of a chamber was scanned at 200×. Protists were identified tentatively to the lowest possible taxonomic level consulting Bérard-Therriault et al. [23], Kofoid and Campbell [24], Kofoid and Swezy [25], Matishov al. [26], Scott and Marchant [27], Steidinger and Tangen [28], and Strüder-Kypke et al. [29].
At least 40 individual cells within each abundant taxon were sized with an eyepiece micrometer at 400–600×. All ciliates were included in the counts, whereas dinoflagellates <15 µm in maximum dimension were not [30]. The smallest abundant ciliates in this study were ca. 15 µm, whereas dinoflagellates extended into the nanoplankton range. Microzooplankton biovolumes were calculated from their linear dimensions by approximating geometric shapes [31] and converted to carbon [32]. Tintinnid volumes were calculated based on their cell dimensions. Since iodine fixation masks photopigments, the formaldehyde-fixed samples were settled as described above, and ciliates and dinoflagellates were examined for the presence of chloroplasts using DIC and red autofluorescence of Chl (Olympus U-MSWG filter cube). This combination allowed simultaneous visualization of pigmented and non-pigmented cellular structures and allocation of microzooplankton into heterotrophs and mixotrophs (here pigmented ciliates and dinoflagellates). Recent literature indicates that all plastidic genera found in this study are capable of phagotrophy [33]–[36]. Some of the formalin-fixed cells were also post-stained with DAPI to visualize their nuclei for taxonomic purposes.
Rate Calculations
Microzooplankton instantaneous population growth rates (µ, d−1) were determined from the initial (n0) and final (nt) abundances of each morpho-species and incubation time (t, d) assuming exponential growth (µ = ln (nt/n0)/t). Temperature dependency of protist growth was described using the Q10 coefficient (i.e., the factorial rate increase due to a temperature increase of 10°C, Q10 = (µ1/µ2)10/(t2−t1), where µ1 and µ2 are growth rates determined at temperatures t1 and t2, respectively).
Rate Comparison
The observed µ were compared to predicted maximum specific growth rates (µpred) of ciliates and dinoflagellates using allometric equations available in the literature (Table 2, [9], [10], [12], [37], [38], [39], [41], [45], [46], [88], [89]). With the exception of two studies, which used natural plankton from the Kattegat [37] and Disko Bay, Greenland [10], these equations were based on the growth rates of cultured protists at 4 to 20°C and included their own temperature coefficients. Some of these equations yielded apparent “negative growth rates” (i.e., population decline) at sub-zero temperatures [38], [39]. Therefore, the rates calculated using these equations for 10°C were converted to ambient temperatures using a Q10 of 2.8 [40]. The same Q10 coefficient was applied to equations for Disko Bay ciliates and dinoflagellates grown at 1.4°C [10]. The dinoflagellate and phytoplankton size-dependent growth equations were also converted to ambient temperatures using a Q10 of 1.58 [41]. In addition, the observed µ of microzooplankton were log2-transformed and compared with the temperature-dependent µpred of herbivorous microzooplankton [9] and phytoplankton [42]–[44]. The Bissinger et al. equation [42] is based on a larger data set (n = 1,501 vs. 162 in Eppley [43]), relies on quantile regression analysis vs. fitting the upper envelope by eye, and includes some data obtained at low temperatures. Nevertheless, all three phytoplankton growth-temperature equations share the same slope and differ only by their intercepts. The original Eppley [43] equation expressed growth in doublings d−1. Therefore, growth rates calculated with the latter formula were converted to µ (d−1) by multiplying doublings by log2.
Table 2. Published relationships between plankton growth rates (µ, d−1), temperature (T, °C), and cell volume (V, µm3) unless noted otherwise.
| Equation | Source | Remarks |
| Ciliates | ||
| Log2 µ = (1.52 Log2T) −0.27 Log2V −1.44 | Muller and Geller [38] | |
| Log2 µ = 0.1438T −0.3285 Log2V −1.3815 | Montagnes et al. [88] | V = µm3 10−3 |
| Log2 µ = 0.85 Log2 T −0.08 Log2V −1.34 | Perez et al. [39] | |
| µ = 3.18 V−0.243exp (0.095T) | Nielsen and Kiørboe [37], [45] | |
| µ = 0.1248 V −0.331 | Levinsen et al. [10] | µ (h−1) |
| Dinoflagellates | ||
| Log10 µ = −0.51295−0.243631 Log10V | Hansen [89] | µ (h−1) |
| µ = 0.0479 V−0.25 | Nielsen and Kiørboe [37] | µ (h−1) |
| after Levinsen and Nielsen [46] | ||
| µ = 2.26C−0.18 | Tang [41] | C = pg C |
| Log10 µ = 0.14−0.15 Log10C | Banse [12] | C = pg C |
| Herbivorous Protists | ||
| Log2 µ = 0.10T −1.0 | Rose and Caron [9] | |
| Phytoplankton | ||
| Log10 µ = 0.0275T −0.07 | Eppley [43] | (doublings d−1) |
| µ = 0.81 e0.0631T | Bissinger et al. [42] | |
| µ = 0.97 e0.0633T | Brush et al. [44] | |
| µ = 3.45C−0.21 | Tang [41] | C = pg C |
Statistical Analyses
Rare taxa (here, less than 20 cells L−1 in the initial sample) were excluded from calculations to avoid statistically unreliable rate estimates. In several cases we settled additional Lugol-fixed samples, which were originally collected for phytoplankton analysis. Only those experiments where effect size was large enough (Cohen’s D >0.5) are reported. Effect size was estimated using SPSS. Standard error (± SE) is used as a measure of dispersion throughout the text. Experimental and field data were analyzed via quartile box plots, Student’s t-test, analysis of variance (ANOVA), Tukey’s multiple comparison of means, Pearson product-moment correlations, and linear regression using Minitab 16.
Results
Environmental Data
Sea surface temperatures ranged from −1.8°C in ArW under ice in May and June to 9.0°C in the Nordcapp current-influenced AtW off the coast of Finnmark in August (Table 1) and were inversely related to latitude (r = −0.91, p<0.001). Three shelf regions were distinguished based on sea temperature: ArW (below 0°C, average −1.30±0.24°C ), AtW (0–5°C, 3.41±0.74°C), and AtW>5 (above 5°C, 7.22±0.40°C).
Chl ranged from 0.10 µg L−1 on the Atlantic side of the Polar Front in September 2010 to 5.19 µg L−1 under the ice in June 2011. The average Chl during the study period was 1.74±0.27 µg l−1 (3.07±0.72, 1.40±0.31, 1.51±0.18 in ArW, AtW, and AtW>5, respectively). In Exp. 4, the ice cores contained chains of the diatom Nitzschia frigida. Under the ice, phytoplankton communities were dominated by large, chain-forming diatom species (Thalassiosira spp, Fragilariopsis cylindrus, Rhizosolenia styliformis), whereas most open water communities consisted primarily of nanoplankton-sized diatoms and nano- and picoflagellates. In June 2011, the colonial prymnesiophyte Phaeocystis pouchetii was also abundant in some samples collected in the marginal ice zone. Ciliate and dinoflagellate (>15 µm, Fig. 2) abundances ranged from 0.41 to 13.6 cells 103 L−1 and 0.44 to 7.26 cells 103 L−1, respectively.
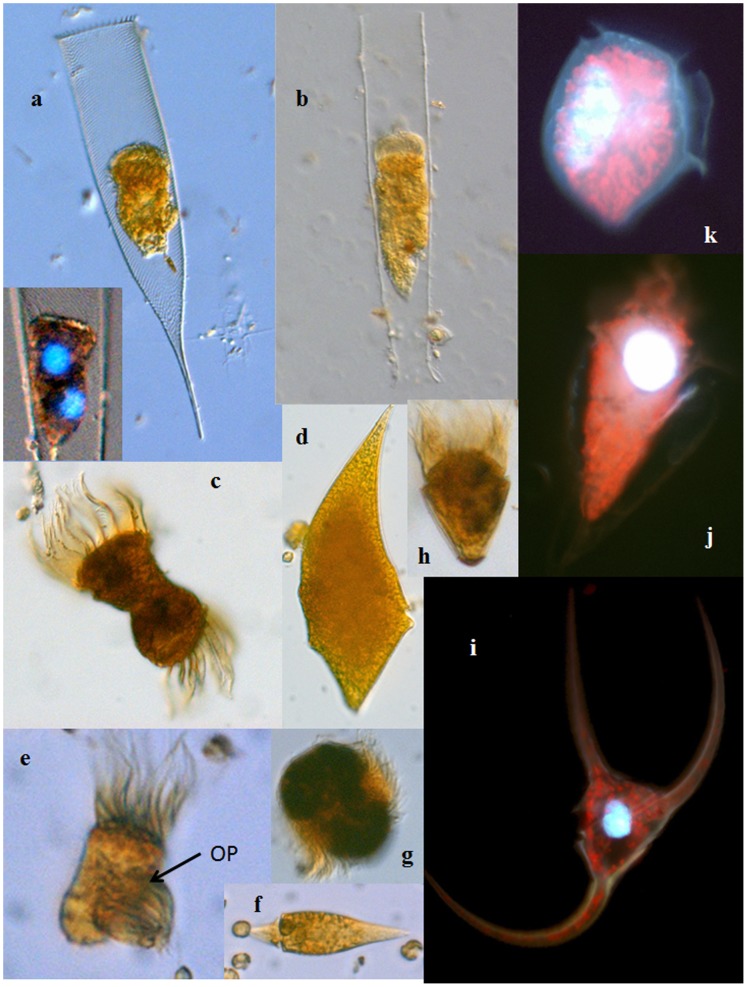
Figure 2. Common microzooplankton from the Barents Sea: (a) Parafavella gigantea, 250 µm (insert shows a mitotic cell stained with DAPI); (b) Leprotintinnus pellucidus, 300 µm; (c) cytokinetic Strombidium sp. 40 µm; (d) Gyrodinium spirale 120 µm; (e) cytokinetic Lohmaniella oviformis 22 µm, OP = oral primordium; (f) Amphidinium sphenoides 35 µm; (g) Mesodinium rubrum 35 µm; (h) Strombidium constrictum 40 µm; (i) Ceratium arcticum 200 µm; (j) Strombidium conicum 75 µm; (k) Dinophysis norvegica 65 µm.
Images are not to scale. (a–h) Lugol’s-fixed cells under DIC; (i–k) DAPI and Chl autofluorescence.
Species-Specific Growth Rates
Growth rates were measured for 14 species of aloricate ciliates, 2 species of tintinnids, 7 species of athecate dinoflagellates, and 8 species of thecate dinoflagellates (n = 163, Table 3). No single species grew in all experiments, but several taxa exhibited growth across nearly the entire temperature gradient (Amphidinium sphenoides, Balanion comatum, Gymnodinium arcticum, G. simplex, Gyrodinium pingue, Gyrodinium spirale, Leegaardiella sol, Mesodinium pulex, M.rubrum, Strombidium conicum, S. epidemum, S. lynii). The fastest species-specific growth rates (µmax) of microzooplankton in this study were measured at 4.5°C in September 2010 (Exp. 12), although several ciliates achieved their highest growth in ArW under the ice at −1.3°C. In two experiments (5 and 9), ciliates failed to grow. Overall, µmax of ciliates ranged from 0.33 d−1 for Lohmaniella oviformis to 1.67 d−1 for M. rubrum. The µmax of dinoflagellates varied between 0.52 d−1 (Gymnodinium heterostriatim) and 1.14 d−1(G. simplex).
Table 3. Growth rates and volumes of common microzooplankton species in the Barents Sea.
| Length | Volume (µm3 10−3) | Growth rate (µ, d−1) | T (°C) max µ | n | ||
| Species | (µm) | max | min | |||
| Ciliates | ||||||
| Uronema sp. | 15.0 | 0.79 | 0.68 | 0.21 | −1.8 | 3 |
| Cyclotrichium sphaericum | 55.0 | 87.1 | 0.84 | 0.35 | −1.3 | 3 |
| Parafavella gigantea | 70.0 | 74.2 | 0.46 | 0.40 | −1.3 | 2 |
| Strombidium cf. coronatum | 65.0 | 18.5 | 0.74 | 0.33 | −1.3 | 2 |
| Leegaardiella sol | 33.0 | 18.8 | 0.89 | 0.29 | −0.5 | 4 |
| Strombidium epidemum | 20.0 | 2.36 | 0.97 | 0.14 | −0.5 | 3 |
| Lohmaniella oviformis | 21.5 | 3.80 | 0.33 | 0.10 | 1.2 | 5 |
| Strombidium sp. | 22.0 | 2.95 | 0.80 | 0.54 | 2.0 | 3 |
| Parafavella obtusangula | 60.0 | 28.3 | 0.96 | – | 4.5 | 1 |
| Laboea strobila | 120 | 157 | 0.71 | 0.19 | 4.5 | 3 |
| Mesodinium pulex | 15.0 | 1.77 | 0.83 | 0.08 | 4.5 | 9 |
| Mesodinium rubrum | 35.0 | 16.5 | 1.67 | 0.28 | 4.5 | 10 |
| Strombidium cf. lynni | 30.0 | 8.17 | 1.06 | 0.07 | 4.5 | 7 |
| Strombidium wulfii | 47.0 | 15.3 | 1.31 | 0.53 | 4.5 | 5 |
| Balanion comatum | 20.0 | 2.35 | 0.72 | 0.20 | 6.8 | 7 |
| Strombidium conicum | 26.3 | 0.83 | 0.51 | 6.8 | 4 | |
| Dinoflagellates | ||||||
| Gymnodinium arcticum | 22.0 | 2.59 | 0.66 | 0.13 | −1.3 | 14 |
| Gyrodinium spirale | 100 | 83.8 | 0.76 | 0.11 | −1.3 | 7 |
| Gymnodinium heterostriatum | 75.0 | 91.6 | 0.52 | 0.23 | 0.1 | 4 |
| Dinophysis norvegica | 70.0 | 74.2 | 0.72 | 0.12 | 4.5 | 5 |
| Dinophysis rotundata | 40.0 | 18.8 | 0.81 | 0.11 | 4.5 | 3 |
| Gymnodinium simplex | 15.0 | 0.63 | 1.14 | 0.40 | 4.5 | 4 |
| Protoperidinium bipes | 28.0 | 8.18 | 0.90 | 0.13 | 4.5 | 6 |
| Torodinium sp. | 25.0 | 2.95 | 0.69 | 0.18 | 5.0 | 3 |
| Amphidinium sphenoides | 30.0 | 3.53 | 0.84 | 0.06 | 6.8 | 9 |
| Gymnodinium sp. | 40.0 | 18.8 | 0.76 | 0.12 | 6.8 | 11 |
| Gyrodinium pellucidum | 25.0 | 6.29 | 0.84 | 0.34 | 7.0 | 3 |
| Ceratium arcticum | 200 | 113 | 0.75 | 0.39 | 7.2 | 6 |
| Protoperidinium depressum | 100 | 335 | 0.63 | 0.19 | 7.5 | 6 |
| Gyrodinium pingue | 40.0 | 8.38 | 0.59 | 0.16 | 7.6 | 6 |
| Scripsiella trochoidea | 20.0 | 4.23 | 0.80 | 0.33 | 8.6 | 5 |
LEGEND: n = number of incubations, where the rates were measured.
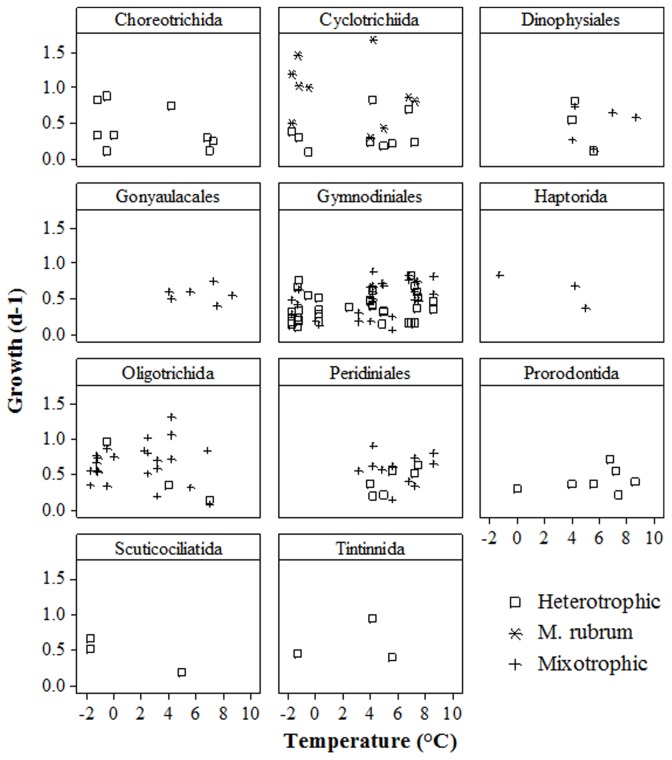
The average Q10 for microzooplankton µmax at −1.3°C and 4.5°C (Table 3) was 1.64 (1.79 for ciliates and 1.48 for dinoflagellates). For M. rubrum, Q10 was 1.36 within the same temperature range. The µ values did not correlate with temperature (p = 0.5) or cell volume (p = 0.5 and 0.17, respectively) in either phylum. However, all ciliates except Strombidium conicum reached their µmax at temperatures <5°C, whereas half of the measured µmax for dinoflagellates occurred in AtW>5. This trend also held for the entire data set when microzooplankton were arranged into taxonomic orders (Fig. 3). The thecate dinoflagellates from the orders Dinophysiales, Gonyaulacales, and Peridiniales grew only in AtW>5, whereas most ciliates grew more slowly at these temperatures than in the ice-covered ArW waters. Athecate dinoflagellates in the order Gymnodiniales grew in most experiments. Neither heterotrophic nor mixotrophic species from this order displayed temperature growth dependency.
Figure 3. Microzooplankton growth rates within taxonomic orders across a temperature gradient.
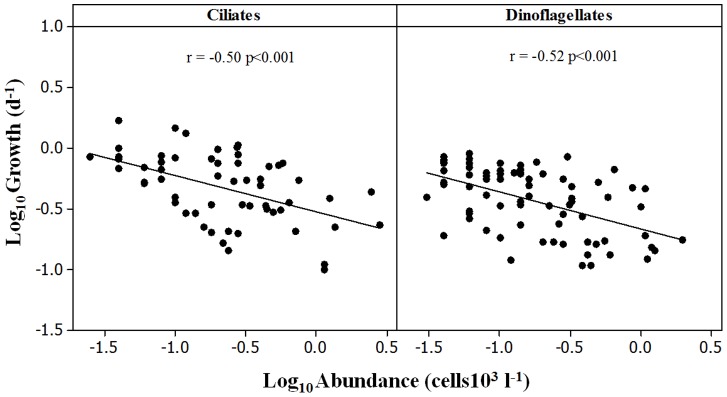
The average µ of mixotrophic ciliates (excluding the fast growing M. rubrum) exceeded those of their heterotrophic counterparts in both ArW (0.62±0.05 d−1 vs. 0.47±0.07 d−1) and AtW (0.66±0.08 d−1 vs. 0.40±0.05 d−1). The same trend was found for dinoflagellates in AtW (0.55±0.03 d−1 vs. 0.41±0.05 d−1), whereas their average growth rates did not differ (0.34±0.08 d−1 vs. 0.33±0.05 d−1) in ArW. In both ciliates and dinoflagellates, species-specific growth rates were inversely related to the initial abundance (Fig. 4).
Figure 4. The relationship between the initial abundance and growth rates of microzooplankton species in bottle experiments.
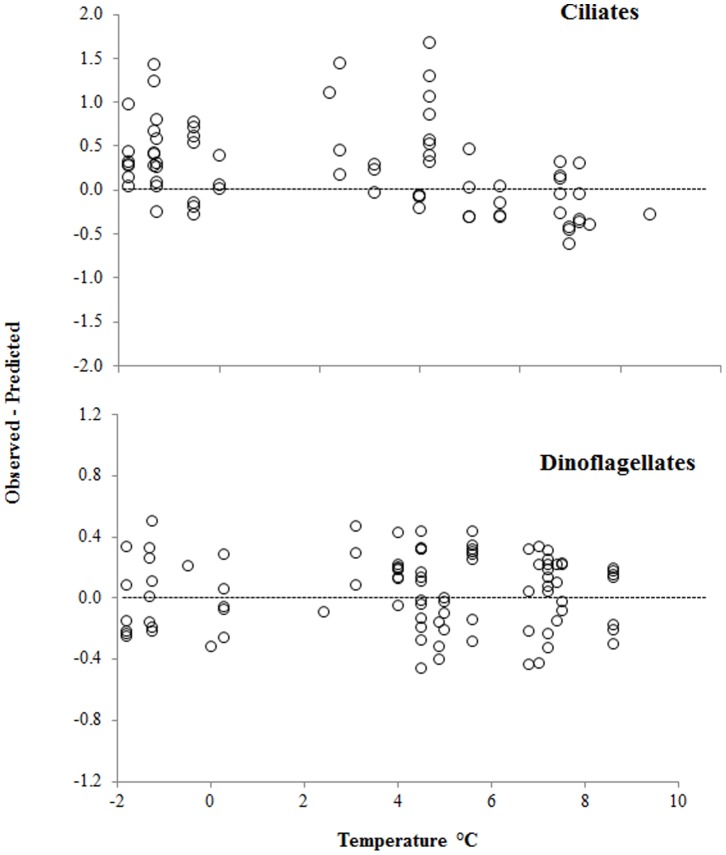
Comparison with Predicted Size- and Temperature-dependent Growth Rate
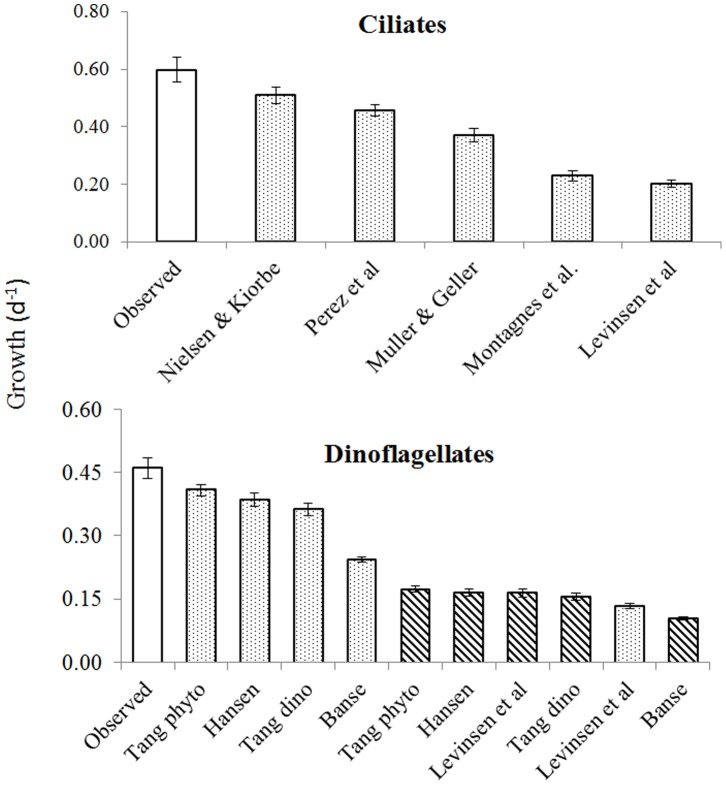
The average µpred estimated according to Nielsen and Kiørboe [37], [45] was the only one that overlapped with the average observed µ for ciliates (Fig. 5; Tukey’s test). However, these authors specifically excluded small ciliates from their equation. Therefore, the µ for species under 20 µm were compared with the next nearest µpred based on the Pérez et al. [39] equation in subsequent analyses. The Tang [41] equation for phytoplankton adjusted for ambient temperature using a Q10 of 1.58 was the closest fit for the observed µ of dinoflagellates, whereas all tested dinoflagellate formulae underestimated the observed µ (p<0.001) within the given cell size and sea temperature range.
Figure 5. Comparison of the average observed and predicted growth rates.
The equations used for growth rate calculations are presented in Table 2. Open bars indicate observed rates, striped bars indicate calculated rates corrected using Q10 = 2.8, dotted bars show Q10 = 1.58.
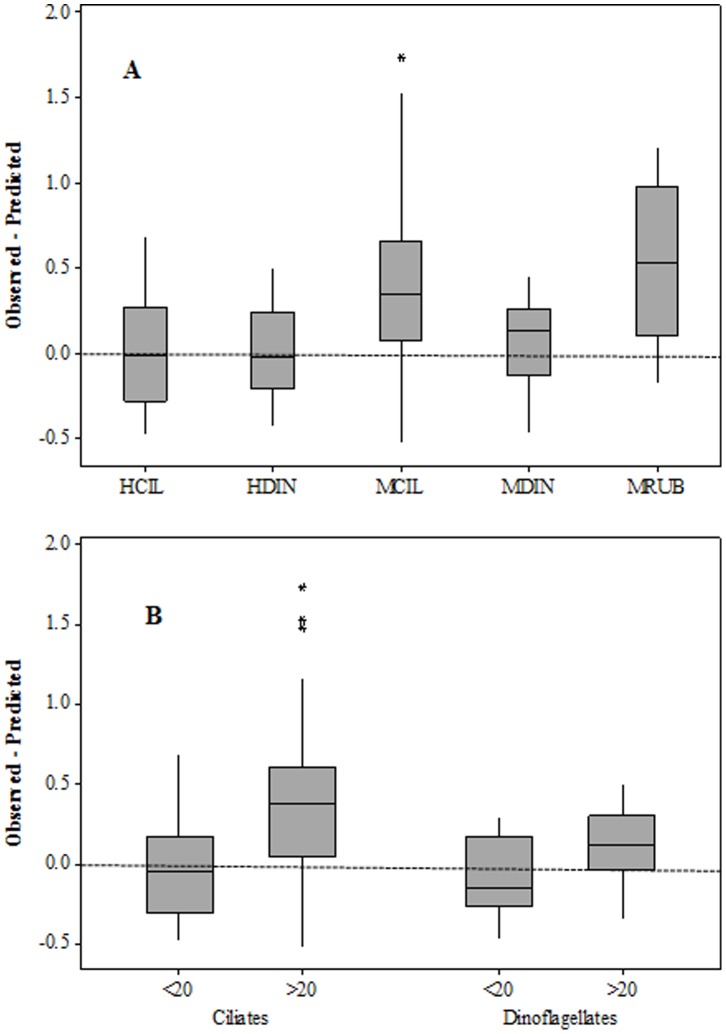
Based on the above two formulae for ciliates, the observed species-specific µ were equal to or exceeded µpred at temperatures <5°C and lower than predicted in AtW>5 (Fig. 6a). This trend was not apparent in dinoflagellates, which exceeded µpred across the entire temperature range (Fig. 6b). Regardless of temperature, the median values of heterotrophic taxa µ were equal to their µpred, whereas mixotrophic taxa, especially ciliates, grew faster than predicted (Fig. 7a). Likewise, large cells from both phyla grew faster than predicted, whereas the smaller ones did not (Fig. 7b).
Figure 6. Differences between observed species-specific and predicted growth rates of ciliates and dinoflagellates across a temperature gradient.
Predicted rates were calculated based on equations from Nielsen and Kiørboe [37], [45], Perez et al. [39], and Tang [41] for ciliates >20 µm, ciliates <20 µm, and phytoplankton, respectively.
Figure 7. Average differences between observed species-specific and predicted growth rates from Figure 5 separated into microzooplankton functional-taxonomic groups (A) and size-taxonomic groups (B).
HCIL = heterotrophic ciliates, HDIN = heterotrophic dinoflagellates, MCIL = mixotrophic ciliates, MDIN = mixotrophic ciliates, MRUB = Mesodinium rubrum.
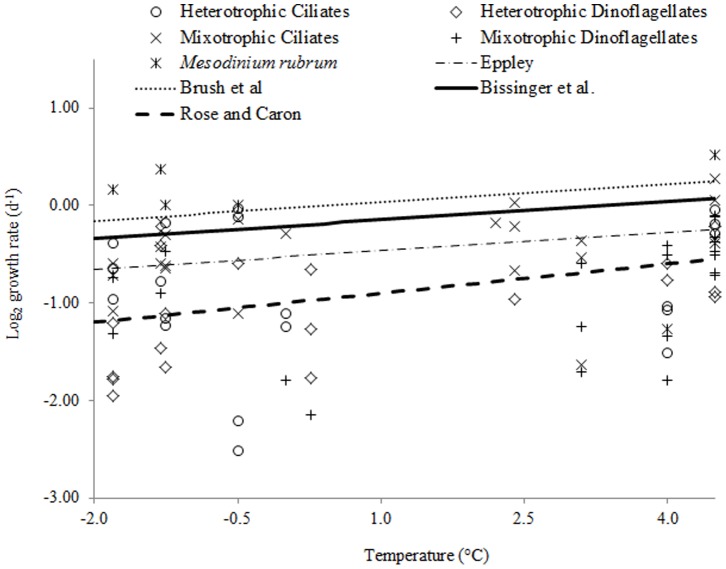
Microzooplankton grew at rates that were close or equal to the highest phytoplankton µmax estimates between −1.8 and 4.5°C (Fig. 8). At temperatures <0°C, nearly 50% and 78% of the observed growth rates of microzooplankton exceeded the values predicted by Eppley [43] and Rose and Caron [9], respectively. With increasing temperatures, these ratios decreased to 21% and 52% between 0 and 5°C, and 0% and 22% at >5°C, respectively. The only species that exceeded the growth rates predicted by Brush et al. [44] was M. rubrum.
Figure 8. Observed species-specific growth rates of microzooplankton at temperatures below 5°C and predicted temperature-dependent growth rates of phytoplankton and herbivorous microzooplankton based on published equations from Table 2.
Discussion
Few attempts have been made to measure microzooplankton growth rates in the Arctic due to logistical and methodological constraints. To our knowledge, this is the first field study to experimentally examine species-specific growth of polar microzooplankton across a broad natural temperature gradient. Maximum in situ growth rates of ciliates and dinoflagellates observed at sub-zero sea temperatures confirm the hypothesis that they are well adapted to their cold environment [10]. At the same time, many microzooplankton species from the Barents Sea appear to be eurythermal within the experimental temperature range. Although thecate dinoflagellate growth was restricted primarily to AtW>5, most ciliates and gymnodiniid dinoflagellates had wide temperature tolerances for growth. Eurythermy was also suggested for polar autotrophic protists [47]. It remains to be explored, however, whether the microzooplankton morphospecies described in this study are genetically divergent in the Arctic and Atlantic waters. For example, distinct polar clades were found within chlorophytes [48], [49].
For the experiment where incubation temperature increased from 4.6°C to 9°C in less than 24 h (Exp. 16), microzooplankton neither declined precipitously nor grew significantly faster than in most other experiments. This observation supports the potential rates obtained under altered conditions in previous Arctic studies. For example, ciliates from the −1.8°C ice-covered Arctic waters in the Barents Sea were incubated at 5±2°C without prior acclimation and grew at 0.47 to 1.38 d−1 [13]. Likewise, ciliates and dinoflagellates grew at rates up to 0.54 d−1 and 0.72 d−1, respectively, in samples collected from 0.5°C water in a Svalbard fjord and incubated at 2°C in the dark [14]. In Disko Bay, Greenland, ciliates and dinoflagellates collected from 3–7°C seawater were incubated at 1.4°C and achieved µ up to 0.3 d−1 and 0.49 d−1, respectively [10]. Combined, these observations indicate that Arctic microzooplankton can be resilient to abrupt and significant disturbances to their physical environment.
Observed vs. Predicted Growth
Nearly 60% of the measured growth rates of microzooplankton in this study (70% at temperatures below 5°C) exceeded predictions based on temperature-extrapolated data from laboratory cultures. The main source of discrepancy between the observed and predicted µmax appears to be the lack of culture data at the low temperature end. It is revealing, however, that a field-derived equation [37] provided a closer match to the observed rates of ciliates than those based on laboratory cultures. Thus, low temperature adaptations in Arctic microzooplankton may offer only a partial explanation for the observed vs. predicted rate mismatch, since all ciliate equations used in this study shared essentially the same Q10. Culture studies offer indisputable advantages, such as the ability to control growth conditions and isolate specific factors. However, they may select for clones that are acclimated to grow on specific food sources under laboratory conditions, which may not be optimum. For example, the highest growth rate recorded for M. rubrum in culture was 0.52 d−1 [35], whereas the µmax of its polar strain did not exceed 0.2 d−1 [50]. However, a wild population of M. rubrum achieved a µmax of 4.2 d−1 during the initial stages of a “red water” event in the Columbia River estuary [51]. Further, steady food supply and stable conditions in culture may not necessarily elicit a maximum growth response in microzooplankton. Under natural conditions, protists are adapted to survive on fluctuating and spatially heterogeneous resources [52], [53]. As a result, selection may favor those clones that can achieve their intrinsic maxima rapidly, when growth conditions improve. Therefore, in situ experiments might yield different and, probably, more reliable inferences of underlying temperature relationships for natural populations than data derived from laboratory experiments with individual cultures [8].
Mixotrophic Growth
Rapid growth rates of mixotrophs in this study contrast with data from the Mediterranean Sea, where mixotrophic oligotrichs grew more slowly than their heterotrophic counterparts [39]. Mixotrophy can be a response to oligotrophic conditions, especially in larger cells, because photosynthetic carbon could cover a significant fraction of their metabolism due to lower volume-specific respiration rates [54]. However, large mixotrophic oligotrichs, such as Laboea strobila, dominated under phytoplankton bloom conditions in the Bering Sea [55]. Given the same prey concentration, a mixotrophic oligotrich grew faster under luxury light [56], whereas the proportion of phagotrophic and autotrophic-derived carbon in a mixotrophic dinoflagellate diet changed dynamically in response to light conditions and food availability [57].
In some experiments in this study, mixotrophic microzooplankton could have taken full advantage of the abundant prey and 24 h insolation. Samples collected from Chl maxima were exposed to irradiance levels that were approximately double their ambient levels, assuming a light attenuation coefficient of 0.09 m−1 [58]. Improvements in the light environment were even stronger for samples collected from under the ice. It is likely, however, that mixotrophic species encounter ambient growth conditions similar to those simulated in our experiments. Mixotroph abundance often peaked in the upper part of the mixed layer in this and previous studies in the Barents Sea [59], [60], and the DCM was within the range of their diel vertical migration [61], [62].
Microzooplankton vs. Phytoplankton Growth
Our data do not support the Rose and Caron contention [9] that microzooplankton growth is more limited by low temperature than that of phytoplankton. This lack of congruence does not necessarily disprove their hypothesis but suggests that it should be approached with caution until more in situ rate data are collected at polar temperatures for both herbivores and phytoplankton. In fact, in another set of experiments these authors reported that ingestion rates of Antarctic ciliates were not constrained by low temperatures [63]. It should be noted that the temperature-dependent growth equation for herbivorous protists in the original Rose and Caron study included only cultured heterotrophs grown at temperatures >4°C, whereas Arctic microzooplankton endure much lower sea temperatures and usually include a large mixotrophic component [59], [64]–[67]. Nevertheless, the observed µmax of heterotrophic ciliates and dinoflagellates in the present study were equal to predicted phytoplankton µmax at temperatures below 4°C. Further, the average species-specific growth rate of microzooplankton below 0°C was nearly identical to the average µmax of diatom isolates from the Barents Sea grown at −0.5°C (0.50±0.02 d−1, [68]).
Simultaneous measurements of microzooplankton and phytoplankton growth rates at ambient temperatures in the Arctic are scarce, yet they provide support for the above conclusion. Microzooplankton and phytoplankton average net growth rates based on one year of weekly biomass records in Disko Bay were comparable [64]. These authors noted that microzooplankton responded almost instantly to the spring diatom bloom and increased 100 fold from their winter minimum, despite sub-zero temperatures and predation by Calanus. In the Fram Strait, the heterotrophic dinoflagellate growth rate of 1.17 d−1 at −1.2°C [15] also exceeds predicted phytoplankton µmax at this temperature based on Bissinger et al. [42]. Finally, microzooplankton growth rates calculated from their herbivory rates in dilution experiments exceeded those of phytoplankton in the Bering Sea at −1.6–4°C [69]. Although the latter estimates depend on a C:Chl ratio assumption and did not include mixotrophic dinoflagellates, it is likely that they were at least equal to phytoplankton growth. It should be noted that Arctic phytoplankton also may grow faster than predicted based on temperature. For example, diatoms grew as fast as 0.83 d−1 and 1.49 d−1 at 0°C and 5°C, respectively, in the Greenland Sea [15].
The slope of the Bissinger et al. equation yields a Q10 of 1.88, which is somewhat higher than Q10 coefficients based on phytoplankton growth in cultures (1.58 for 5–25°C, Tang 1995) and microzooplankton growth in this study (1.63 for −1.3–4.5°C). The latter value is also much lower than a Q10 of 2.6 estimated by Nielsen and Kiørboe for ciliate growth rates observed between 5 and 20°C in temperate waters [37]. Differences between these coefficients likely result from different temperature intervals in our and previous studies and are inherent in the Q10 approach. However, these differences may be meaningless if growth responses to changing temperature are linear rather than exponential as suggested by Montanges et al. [70]. These authors criticized the application of two-point Q10 to growth estimates for introducing a systematic error and neglecting the underlying complexities of the process. Instead, they proposed a solution where planktonic protists, including ciliates and diatoms, respond to temperature linearly with a single slope (0.07 d−1).
If we scale the average µmax of herbivorous ciliates grown at 20°C (2.50±0.07 d−1, [9]) to −0.5°C using the above slope, the resulting rate of 1.06 d−1 will be similar to the observed rate (0.97 d−1) for the heterotrophic oligotrich ciliate Strombidium epidemum in this study. Thus the rates predicted by the linear model appear to match the observed microzooplankton growth rates more closely than those based on the published, non-linear (Q10) models. Further, the linear model corresponds to the idea that herbivorous protists respond to temperature similarly to autotrophs. However, our data cannot be used to support or reject either of the above approaches because the growth rates measured at >5°C in this study were apparently constrained by factors other than temperature.
The Effect of Biotic Factors
As noted by Caron and Rose [71], the temperature-growth relationship plays itself out in nature together with several other factors, which affect the growth of phototrophs and heterotrophs. Resource availability is central among these factors. The negative relationship between protist species-specific growth rates and their initial abundances in the present study suggests that some of them may have reached their carrying capacity. Similar abundance-growth relationships were found for M. rubrum [51] and bloom-forming phytoplankton [72]. Prey availability also superseded temperature effects on microzooplankton dynamics in several field studies in Arctic and boreal waters [69], [73], [74]. Further, phytoplankton prey composition had pronounced effects on the growth and feeding rates of cultured Antarctic ciliates [63]. Total Chl may be too crude a measure to describe the specific resource requirements of individual microzooplankton species. However, heterotrophic ciliate average µ in the Barents Sea differed between samples with Chl <2 and >2 µg L−1 (0.37±0.06 and 0.55±0.06 d−1, respectively, p<0.05).
The faster growth of larger cells in this study apparently contradicts the allometric scaling equations (Table 2), which predict a continuous decrease of mass-specific growth rate with increasing size. Such deviations are not unusual in field experiments [37], [75], [76] and could be due to different growth conditions for protists depending on their size. Incubation experiments with natural plankton often yield net growth estimates for microzooplankton due to intraguild predation within their communities (e.g., [76]–[78]). For example, two small-sized ciliates, Balanion comatum and Lohmaniella oviformis, grew more slowly than predicted across a temperature gradient (75% of µmax vs. 190% average for all ciliates and 122% for dinoflagellates) and did not correlate with their own initial abundance in our study. This lack of relationship may indicate that they were kept below their carrying capacity by predators or competitors. In the Fram Strait, small ciliates occasionally grew in diluted samples, where their encounter rate with potential predators was reduced, but not in whole seawater samples [15].
Predation could have also restricted ciliate growth at warmer temperatures (i.e., in AtW>5) in this study. Large tintinnids, such as Parafavella gigantea and thecate dinoflagellates, which are known to prey on ciliates and dinoflagellates [33], [79]–[81], were abundant in the southwestern Barents Sea during this study. Specifically, dinoflagellates from the genus Dinophysis prey on M. rubrum in search of kleptoplasts acquired previously by this ciliate from its cryptophyte prey [82]–[84]. In the absence of large diatoms, heterotrophic dinoflagellates, such as Protoperidinium spp., could have switched to ciliate prey. Copepod nauplii and the larvacean Oikopleura, which occurred in some of the samples collected in the Atlantic waters, can feed on and compete for food with ciliates [85], [86]. The lack of statistical difference between microzooplankton growth rates in the presence and absence of micro-metazoans (p = 0.6) does not exclude the possibility that metazoan grazing may have affected ciliates directly or indirectly in some of our experiments.
Caution should be exercised when applying the maximum species-specific growth rates observed in this study at the community level. On average, actively growing populations comprised 37% of total microzooplankton (43±9.8% and 35±10% in ArW and AtW, respectively). The rest of microzooplankton either declined or, in many cases, did not change significantly during the 24 h incubations. It is not surprising that only part of their community increased in most experiments in this study. Due to their competitive and/or predator-prey interactions and different resource requirements, multiple populations comprising microzooplankton may oscillate out of phase, whereas short-term incubations provide only a snapshot of these dynamics. The importance of a species-specific approach cannot be overemphasized in field growth rate experiments. Counting microzooplankton into size classes or broad taxonomic categories can mask dynamic processes within their communities and often yields net community growth rates of ∼0 in bottle experiments (i.e., growth and loss terms appear nearly balanced). Such equilibrium is unlikely to persist for any extended period of time in natural Arctic communities, where copepods can consume a large fraction of microzooplankton standing stocks daily (e.g., [74], [87]).
Conclusions
The results of this study support the idea that microzooplankton play a major role in carbon cycling in the Arctic. These protists appear to be capable of growing as fast as their phytoplankton prey at extreme polar temperatures and demonstrate a remarkable ecological plasticity and resilience to environmental perturbations. Our data suggest that dynamic processes regulating plankton structure and function in the Arctic may be more complex than currently understood and will require additional field and laboratory research.
Acknowledgments
The authors are grateful to Paul Wassmann, Marit Reigstad, Lena Seuthe, Camilla Svensen, Jostein Røttingen, Francisco Moore, Konstantin Solovyev, and the captains and crews of the R/Vs Helmer Hanssen (Jan Mayen) and G.O. Sars for their logistical support and field assistance. David Caron, George McManus, Diane Stoecker, Mark McCarthy, and two anonymous reviewers provided helpful discussion and criticisms of the manuscript.
Funding Statement
This study was supported by the National Science Foundation (Award ARC-0909372). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kwok R, Rothrock DA (2009) Decline in Arctic sea ice thickness from submarine and ICES at records: 1958–2008. Geophysical Research Letters 36, L15501, doi:10.1029/2009GL039035
- 2.Pabi S, van Dijken GL, Arrigo KR (2008) Primary production in the Arctic Ocean, 1998–2006. Journal of Geophysical Research Oceans 113, C08005, doi:10.1029/2007JC004578
- 3. Duarte CM, Lenton TM, Wadhams P, Wassmann P (2012) Abrupt climate change in the Arctic. Nature Climate Change 2: 60–62. [Google Scholar]
- 4. Wassmann P, Reigstad M (2011) Future Arctic Ocean seasonal ice zones and implications for pelagic-benthic coupling. Oceanography 24: 220–231. [Google Scholar]
- 5. Holding JM, Duarte CM, Arrieta JM, Vaquer-Suyner R, Coello-Camba A, et al. (2013) Experimentally determined temperature thresholds for Arctic plankton community metabolism. Biogeosciences 10: 357–370. [Google Scholar]
- 6. Sarmento H, Montoya JM, Vazquez-Dominguez E, Vaque D, Gasol JM (2010) Warming effects on marine microbial food web processes: how far can we go when it comes to predictions? Philosophical Transactions of the Royal Society Biological Sciences 365: 2137–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith KL, Kaufmann RS (1999) Long-term discrepancy between food supply and demand in the deep Eastern North Pacific. Science 284: 1174–1177. [DOI] [PubMed] [Google Scholar]
- 8. Chen BZ, Landry MR, Huang B, Liu H (2012) Does warming enhance the effect of microzooplankton grazing on marine phytoplankton in the ocean? Limnology and Oceanography 57: 519–526. [Google Scholar]
- 9. Rose JM, Caron DA (2007) Does low temperature constrain the growth rates of heterotrophic protists? Evidence and implications for algal blooms in cold waters. Limnology and Oceanography 52: 886–895. [Google Scholar]
- 10. Levinsen H, Nielsen TG, Hansen BW (1999) Plankton community structure and carbon cycling on the Western Coast of Greenland during thestratified summer situation. II. Heterotrophic dinoflagellates and ciliates. Aquatic Microbial Ecology 16: 217–232. [Google Scholar]
- 11. Landry M, Calbet A (2004) Microzooplankton production in the oceans. ICES Journal of Marine Science 61: 501–507. [Google Scholar]
- 12. Banse K (1982) Cell volumes, maximal growth-rates of unicellular algae and ciliates, and the role of ciliates in the marine pelagial. Limnology and Oceanography 27: 1059–1071. [Google Scholar]
- 13. Hansen BW, Jensen F (2000) Specific growth rates of protozooplankton in the marginal ice zone of the central Barents Sea during spring. Journal of the Marine Biological Association of the United Kingdom 80: 37–44. [Google Scholar]
- 14. Seuthe L, Rokkan Iversen K, Narcy F (2011a) Microbial processes in a high-latitude fjord (Kongsfjorden, Svalbard): II. Ciliates and dinoflagellates. Polar Biology 34: 751–766. [Google Scholar]
- 15. Calbet A, Saiz E, Almeda R, Movilla JI, Alcaraz M (2011) Low microzooplankton grazing rates in the Arctic Ocean during a Phaeocystis pouchetii bloom (summer 2007): fact or artifact of the dilution technique? Journal of Plankton Research 33: 687–701. [Google Scholar]
- 16. Smolyar I, Adrov N (2003) The quantitative definition of the Barents Sea Atlantic Water: mapping of the annual climatic cycle and interannual variability. ICES Journal of Marine Science 60: 836–845. [Google Scholar]
- 17. Loeng H (1991) Features of the physical oceanographic conditions of the Barents Sea. Polar Research 10: 5–18. [Google Scholar]
- 18.Nikiforov EG, Shpaikher AO (1980) Patterns of large-scale variability in the hydrological cycle of the Arctic Ocean. Hydrometeoizdat, Leningrad, 269 p. (in Russian).
- 19. Ikavalko J, Gradinger R (1997) Flagellates and heliozoans in the Greenland Sea ice studied alive using light microscopy. Polar Biology 17: 473–481. [Google Scholar]
- 20. Garrison DL, Buck KR (1986) Organism losses during ice melting - a serious bias in sea ice community studies. Polar Biology 6: 237–239. [Google Scholar]
- 21. Bundy MH, Vanderploeg HA, Lavrentyev PJ, Kovalcik PA (2005) The importance of microzooplankton versus phytoplankton to copepod populations during late winter and early spring in Lake Michigan. Canadian Journal of Fisheries and Aquatic Sciences 62: 2371–2385. [Google Scholar]
- 22. Welschmeyer NA (1994) Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnology and Oceanography 39: 1985–1992. [Google Scholar]
- 23.Bérard-Therriault L, Poulin M, Bossé L (1999) Guide d'identification du phytoplancton marin de l'estuaire et du Golfe du Saint-Laurent incluant également certains protozoaires. Ottawa: Publication special canadienne des sciences halieutiques et aquatique 128. 387 p.
- 24. Kofoid CA, Campbell AS (1929) A conspectus of the marine and freshwater ciliata belonging to the suborder Tintinnoinea, with description of new species principally from the Agassiz expedition to the Eastern Tropical Pacific 1904–1905. University of California Publications Zoology 34: 1–264. [Google Scholar]
- 25.Kofoid CA, Swezy O (1921) The free-living unarmored dinoflagellata. Berkeley, California: University of California press. 506 p.
- 26.Matishov GG, al e (2000) Biological atlas of the arctic seas: plankton of the Barents and Kara Seas. NOAA International Ocean Atlas series. Volume 2.
- 27.Scott FJ, Marchant HJ (2005) Antarctic marine protists. Australian biological resources study, Australia. Antarctic Division. 563 p.
- 28.Steidinger K, Tangen K (1996) Dinoflagellates. In Tomas CR, editor. Identifying marine phytoplankton. Academic Press, San Diego. 387–584 p.
- 29.Strüder-Kypke MC, Kypke ER, Agatha S, Warwick J, Montagnes DJS (2001) The user-friendly key to coastal planktonic ciliates. The University of Liverpool.
- 30. Møller EF, Nielsen TG, Richardson K (2006) The zooplankton community in the Greenland Sea: Composition and role in carbon turnover. Deep Sea Research Part I: Oceanographic Research Papers 53: 76–93. [Google Scholar]
- 31. Sun J, Liu DY (2003) Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of Plankton Research 25: 1331–1346. [Google Scholar]
- 32. Menden-Deuer S, Lessard EJ (2000) Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnology and Oceanography 45: 569–579. [Google Scholar]
- 33. Jeong HJ, Yoo YD, Kim JS, Seong KA, Kang NS, et al. (2010) Growth, feeding and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Science Journal 45: 65–91. [Google Scholar]
- 34. Stoecker DK, Johnson MD, de Vargas C, Not F (2009) Acquired phototrophy in aquatic protists. Aquatic Microbial Ecology 57: 279–310. [Google Scholar]
- 35. Yih W, Kim HS, Jeong HA, Myung G, Kim YG (2004) Ingestion of cryptophyte cells by the marine photosynthetic ciliate Mesodinium rubrum . Aquatic Microbial Ecology 36: 165–170. [Google Scholar]
- 36. Myung G, Yih W, Kim HS, Park JS, Cho BC (2006) Ingestion of bacterial cells by the marine photosynthetic ciliate Myrionecta rubra . Aquatic Microbial Ecology 44: 175–180. [Google Scholar]
- 37. Nielsen TG, Kiørboe T (1994a) Regulation of zooplankton biomass and production in a temperate, coastal ecosystem. 2. Ciliates. Limnology and Oceanography 39: 508–519. [Google Scholar]
- 38. Muller H, Geller W (1993) Maximum growth-rates of aquatic ciliated protozoa - the dependence on body size and temperature reconsidered. Archiv Fur Hydrobiologie 126: 315–327. [Google Scholar]
- 39. Pérez MT, Dolan JR, Fukai E (1997) Planktonic oligotrich ciliates in the NW Mediterranean:growth rates and consumption by copepods. Marine Ecology Progress Series 155: 89–101. [Google Scholar]
- 40. Hansen PJ, Bjornsen PK, Hansen BW (1997) Zooplankton grazing and growth: Scaling within the 2–2,000-µm m body size range. Limnology and Oceanography 42: 687–704. [Google Scholar]
- 41. Tang EPY (1995) The allometry of algal growth-rates. Journal of Plankton Research 17: 1325–1335. [Google Scholar]
- 42. Bissinger JE, Montagnes DJS, Sharples J, Atkinson D (2008) Predicting marine phytoplankton maximum growth rates from temperature: Improving on the Eppley curve using quantile regression. Limnology and Oceanography 53: 487–493. [Google Scholar]
- 43. Eppley RW (1972) Temeprature and phytoplankton growth in the sea. Fishery Bulletin 70: 1063–1010–1085. [Google Scholar]
- 44. Brush MJ, Brawley JW, Nixon SW, Kremer JN (2002) Modeling phytoplankton production: problems with the Eppley curve and an empirical alternative. Marine Ecology Progress Series 238: 31–45. [Google Scholar]
- 45. Nielsen TG, Kiørboe T (1994b) Regulation of zooplankton biomass and production in a temperate, coastal ecosystem. 2. Ciliates. Limnology and Oceanography 39: 1423. [Google Scholar]
- 46. Levinsen H, Nielsen TG (2002) The trophic role of marine pelagic ciliates and heterotrophic dinoflagellates in arctic and temperate coastal ecosystems: A cross-latitude comparison. Limnology and Oceanography 47: 427–439. [Google Scholar]
- 47. Teoh ML, Phang SM, Chu WL (2013) Response of antarctic, temperate, and tropical microalgae to temperature stress. Journal of Applied Phycology 25: 285–297. [Google Scholar]
- 48. Lovejoy C, Vincent WF, Bonilla S, Roy S, Martineau MJ, et al. (2007) Distribution, phylogeny, and growth of cold-adapted picoprasinophytes in arctic seas. Journal of Phycology 43: 78–89. [Google Scholar]
- 49. Eddie B, Krembs C, Neuer S (2008) Characterization and growth response to temperature and salinity of psychrophilic, halotolerant Chlamydomonas sp. ARC isolated from Chukchi Sea ice. Marine Ecology Progress Series 354: 107–117. [Google Scholar]
- 50. Moeller HV, Johnson MD, Falkowski PG (2011) Photoacclimation in the phototrophic marine ciliate Mesodinium rubrum (Ciliophora). Journal of Phycology 47: 324–332. [DOI] [PubMed] [Google Scholar]
- 51. Herfort L, Peterson TD, Campbell V, Futrell S, Zuber P (2011) Myrionecta rubra (Mesodinium rubrum) bloom initiation in the Columbia River estuary. Estuarine Coastal and Shelf Science 95: 440–446. [Google Scholar]
- 52. Menden-Deuer S, Fredrickson KA (2010) Structure-dependent, protistan grazing and its implication for the formation, maintenance and decline of plankton patches. Marine Ecology Progress Series 420: 57–71. [Google Scholar]
- 53. Paffenhöfer GA, Sherr BF, Sherr EB (2007) From small scales to the big picture: persistence mechanisms of planktonic grazers in the oligotrophic ocean. Marine Ecology 28: 243–253. [Google Scholar]
- 54. Dolan JR, Perez MT (2000) Costs, benefits and characteristics of mixotrophy in marine oligotrichs. Freshwater Biology 45: 227–238. [Google Scholar]
- 55. Olson MB, Strom SL (2002) Phytoplankton growth, microzooplankton herbivory and community structure in the Southeast Bering Sea: insight into the formation and temporal persistence of an Emiliania huxleyi bloom. Deep Sea Research Part II: Topical Studies in Oceanography 49: 5969–5990. [Google Scholar]
- 56. McManus GB, Schoener DM, Haberlandt K (2012) Chloroplast symbiosis in a marine ciliate: ecophysiology and the risks and rewards of hosting foreign organelles. Frontiers in Microbiology 3: 321–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Riisgaard K, Hansen PJ (2009) Role of food uptake for photosynthesis, growth and survival of the mixotrophic dinoflagellate Dinophysis acuminata . Marine Ecology Progress Series 381: 51–62. [Google Scholar]
- 58.Sakshaug E, Johnsen GH, Volent Z (2009) Light. In Sakshaug E, Johnsen GH, Kovacs KM, editors. Ecosystem Barents Sea. Tapir Academic Press. 117–138 p.
- 59. Putt M (1990) Abundance, chlorophyll content and photosynthetic rates of ciliates in the Nordic Seas during summer. Deep Sea Research Part I: Oceanographic Research Papers 37: 1713–1731. [Google Scholar]
- 60. Rat'kova TN, Wassmann P (2002) Seasonal variation and spatial distribution of phyto- and protozooplankton in the central Barents Sea. Journal of Marine Systems 38: 47–75. [Google Scholar]
- 61. Crawford DW, Lindholm T (1997) Some observations on vertical distribution and migration of the phototrophic ciliate Mesodinium rubrum ( = Myrionecta rubra) in a stratified brackish inlet. Aquatic Microbial Ecology 13: 267–274. [Google Scholar]
- 62. Ji RB, Franks PJS (2007) Vertical migration of dinoflagellates: model analysis of strategies, growth, and vertical distribution patterns. Marine Ecology Progress Series 344: 49–61. [Google Scholar]
- 63. Rose JM, Fitzpatrick E, Wang AN, Gast RJ, Caron DA (2013) Low temperature constrains growth rates but not short-term ingestion rates of antarctic ciliates. Polar Biology 36: 645–659. [Google Scholar]
- 64. Levinsen H, Nielsen TG, Hansen BW (2000a) Annual succession of marine pelagic protozoans in Disko Bay, West Greenland, with emphasis on winter dynamics. Marine Ecology Progress Series 206: 119–134. [Google Scholar]
- 65. Seuthe L, Topper B, Reigstad M, Thyrhaug R, Vaquer-Sunyer R (2011b) Microbial communities and processes in ice-covered arctic waters of the Northwestern Fram Strait (75 to 80 degrees N) during the vernal pre-bloom phase. Aquatic Microbial Ecology 64: 253–266. [Google Scholar]
- 66. Sime-Ngando T, Gosselin M, Juniper SK, Levasseur M (1997) Changes in sea-ice phagotrophic microprotists (20–200 µm) during the sping algal bloom, Canadian Arctic Archipelago. Journal of Marine Systems 11: 163–172. [Google Scholar]
- 67. Booth BC, Lewin J, Postel JR (1993) Temporal variation in the structure of autotrophic and heterotrophic communities in the subarctic Pacific. Progress in Oceanography 32: 57–99. [Google Scholar]
- 68. Gilstad M, Sakshaug E (1990) Growth rates of 10 diatom species from the Barents Sea at different irradiances and day lengths. Marine Ecology Progress Series 64: 169–173. [Google Scholar]
- 69.Sherr EB, Sherr BF, Ross C (2013) Microzooplankton grazing impact in the Bering Sea during spring sea ice conditions. Deep Sea Research Part II: Topical Studies in Oceanography.
- 70. Montagnes DJS, Kimmance SA, Atkinson D (2003) Using Q10: Can growth rates increase linearly with temperature? Aquatic Microbial Ecology 32: 307–313. [Google Scholar]
- 71. Caron DA, Rose JM (2008) The metabolic theory of ecology and algal bloom formation (Reply to comment by Lopez-Urrutia). Limnology and Oceanography 53: 2048–U2010. [Google Scholar]
- 72. Irigoien X, Flynn KJ, Harris RP (2005) Phytoplankton blooms: a 'loophole' in microzooplankton grazing impact? Journal of Plankton Research 27: 313–321. [Google Scholar]
- 73. Kjaeret AH, Naustvoll LJ, Paasche E (2000) Ecology of the heterotrophic dinoflagellate genus Protoperidinium in the inner Oslofjord (Norway). Sarsia 85: 453–460. [Google Scholar]
- 74. Levinsen H, Turner JT, Nielsen TG, Hansen BW (2000b) On the trophic coupling between protists and copepods in arctic marine ecosystems. Marine Ecology Progress Series 204: 65–77. [Google Scholar]
- 75. Strom SL, Olson MB, Macri EL, Mordy CW (2006) Cross-shelf gradients in phytoplankton community structure, nutrient utilization, and growth rate in the coastal Gulf of Alaska. Marine Ecology Progress Series 328: 75–92. [Google Scholar]
- 76. Franzè G, Modigh M (2013) Experimental evidence for internal predation in microzooplankton communities. Marine Biology 160: 3103–3112. [Google Scholar]
- 77. First MR, Miller HL, Lavrentyev PJ, Pinckney JL, Burd AB (2009) Effects of microzooplankton growth and trophic interactions on herbivory in coastal and offshore environments. Aquatic Microbial Ecology 54: 255–267. [Google Scholar]
- 78. Modigh M, Franzè G (2009) Changes in phytoplankton and microzooplankton populations during grazing experiments at a Mediterranean coastal site. Journal of Plankton Research 31: 853–864. [Google Scholar]
- 79. Hansen PJ (1991) Dinophysis - a planktonic dinoflagellate genus which can act both as a prey and a predator of a ciliate. Marine Ecology Progress Series 69: 201–204. [Google Scholar]
- 80. Stoecker DK, Evans GT (1985) Effects of protozoan herbivory and carnivory in a microplankton food web. Marine Ecology Progress Series 25: 159–167. [Google Scholar]
- 81. Smalley GW, Coats DW (2002) Ecology of the red-tide dinoflagellate Ceratium furca: Distribution, mixotrophy, and grazing impact on ciliate populations of Chesapeake Bay. Journal of Eukaryotic Microbiology 49: 63–73. [DOI] [PubMed] [Google Scholar]
- 82. Carvalho WF, Minnhagen S, Granéli E (2008) Dinophysis norvegica (Dinophyceae), more a predator than a producer? Harmful Algae 7: 174–183. [Google Scholar]
- 83. Sjoqvist CO, Lindholm TJ (2011) Natural co-occurrence of Dinophysis acuminata (Dinoflagellata) and Mesodinium rubrum (Ciliophora) in thin layers in a coastal inlet. Journal of Eukaryotic Microbiology 58: 365–372. [DOI] [PubMed] [Google Scholar]
- 84. Kim S, Kang YG, Kim HS, Yih W, Coats DW, et al. (2008) Growth and grazing responses of the mixotrophic dinoflagellate Dinophysis acuminata as functions of light intensity and prey concentration. Aquatic Microbial Ecology 51: 301–310. [Google Scholar]
- 85. Lombard F, Eloire D, Gobet A, Stemmann L, Dolan JR (2010) Experimental and modeling evidence of appendicularian–ciliate interactions. Limnology and Oceanography 55: 77–90. [Google Scholar]
- 86. Turner JT, Levinsen H, Nielsen TG, Hansen BW (2001) Zooplankton feeding ecology: grazing on phytoplankton and predation on protozoans by copepod and barnacle nauplii in Disko Bay, West Greenland. Marine Ecology Progress Series 221: 209–219. [Google Scholar]
- 87. Campbell RG, Sherr EB, Ashjian CJ, Plourde S, Sherr BF, et al. (2009) Mesozooplankton prey preference and grazing impact in the Western Arctic Ocean. Deep Sea Research Part II: Topical Studies in Oceanography 56: 1274–1289. [Google Scholar]
- 88. Montagnes DJS, Lynn DH, Roff JC, Taylor WD (1988) The annual cycle of heterotrophic planktonic ciliates in the waters surrounding the Isles of Shoals, Gulf of Maine - an assessment of their trophic role. Marine Biology 99: 21–30. [Google Scholar]
- 89. Hansen PJ (1992) Prey size selection, feeding rates and growth dynamics of heterotrophic dinoflagellates with special emphasis on Gyrodinium spirale . Marine Biology 114: 327–334. [Google Scholar]