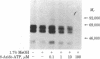
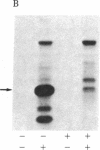
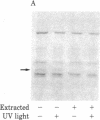
Abstract
A photoaffinity ATP ligand is used to identify the protein kinase present in acetylcholine receptor-enriched membranes from Torpedo californica. Incubation of these membranes with 8-azido-[alpha-32P]ATP and subsequent irradiation with UV light resulted in covalent labeling of a major band of Mr 43,000. Alkali-stripped membranes that show a selective reduction in the Mr 43,000 polypeptide also show a corresponding reduction in incorporation of photoaffinity label. In addition, the neutralized alkaline extract also showed one band at Mr 43,000 when labeled with the photoaffinity ligand. After alkali extraction, endogenous protein kinase activity decreased in the membranes in proportion to the loss of Mr 43,000 peptide. Moreover, the alkaline extract was able to phosphorylate casein in an exogenous assay system. These results suggest that a Mr 43,000 polypeptide in acetylcholine receptor-enriched membranes is the acetylcholine receptor kinase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayley H., Knowles J. R. Photoaffinity labeling. Methods Enzymol. 1977;46:69–114. doi: 10.1016/s0076-6879(77)46012-9. [DOI] [PubMed] [Google Scholar]
- Davis C. G., Gordon A. S., Diamond I. Specificity and localization of the acetylcholine receptor kinase. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3666–3670. doi: 10.1073/pnas.79.11.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J., Blanchard S. G., Wu W., Miller J., Strader C. D., Hartig P., Moore H. P., Racs J., Raftery M. A. Purification of Torpedo californica post-synaptic membranes and fractionation of their constituent proteins. Biochem J. 1980 Mar 1;185(3):667–677. doi: 10.1042/bj1850667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehner S. C., Gulbrandsen V., Hyman C., Jeng A. Y., Neubig R. R., Cohen J. B. Immunofluorescence localization at the mammalian neuromuscular junction of the Mr 43,000 protein of Torpedo postsynaptic membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5230–5234. doi: 10.1073/pnas.78.8.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. S., Davis C. G., Milfay D., Kaur J., Diamond I. Membrane-bound protein kinase activity in acetylcholine receptor-enriched membranes. Biochim Biophys Acta. 1980 Aug 4;600(2):421–431. doi: 10.1016/0005-2736(80)90445-9. [DOI] [PubMed] [Google Scholar]
- Gordon A. S., Milfay D., Davis C. G., Diamond I. Protein phosphatase activity in acetylcholine receptor-enriched membranes. Biochem Biophys Res Commun. 1979 Apr 13;87(3):876–883. doi: 10.1016/0006-291x(79)92039-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lo M. M., Garland P. B., Lamprecht J., Barnard E. A. Rotational mobility of the membrane-bound acetylcholine receptor of Torpedo electric organ measured by phosphorescence depolarisation. FEBS Lett. 1980 Mar 10;111(2):407–412. doi: 10.1016/0014-5793(80)80838-6. [DOI] [PubMed] [Google Scholar]
- Moore H. P., Hartig P. R., Raftery M. A. Correlation of polypeptide composition with functional events in acetylcholine receptor-enriched membranes from Torpedo californica. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6265–6269. doi: 10.1073/pnas.76.12.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubig R. R., Krodel E. K., Boyd N. D., Cohen J. B. Acetylcholine and local anesthetic binding to Torpedo nicotinic postsynaptic membranes after removal of nonreceptor peptides. Proc Natl Acad Sci U S A. 1979 Feb;76(2):690–694. doi: 10.1073/pnas.76.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Wennogle L. P., Changeux J. P. Factors regulating the susceptibility of the acetylcholine receptor protein to heat inactivation. FEBS Lett. 1979 Dec 15;108(2):489–494. doi: 10.1016/0014-5793(79)80595-5. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Lindenmayer G. E., Allen J. C. The sodium-potassium adenosine triphosphatase: pharmacological, physiological and biochemical aspects. Pharmacol Rev. 1975 Mar;27(01):3–134. [PubMed] [Google Scholar]
- St John P. A., Froehner S. C., Goodenough D. A., Cohen J. B. Nicotinic postsynaptic membranes from Torpedo: sidedness, permeability to macromolecules, and topography of major polypeptides. J Cell Biol. 1982 Feb;92(2):333–342. doi: 10.1083/jcb.92.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennogle L. P., Changeux J. P. Transmembrane orientation of proteins present in acetylcholine receptor-rich membranes from Torpedo marmorata studied by selective proteolysis. Eur J Biochem. 1980 May;106(2):381–393. doi: 10.1111/j.1432-1033.1980.tb04584.x. [DOI] [PubMed] [Google Scholar]