Abstract
Background
Neuropathy is common in Waldenström’s macroglobulinemia (WM, an IgM-associated lymphoplasmacytic lymphoma) and in IgM-monoclonal gammopathy of undetermined significance (IgM-MGUS). Paraneoplastic or paraimmune mechanisms are thought to be involved in the pathogenesis of these neuropathies. Attempts at distinguishing WM and IgM-MGUS neuropathies are lacking especially among bone marrow (BM) confirmed patients.
Methods
Retrospective analyses were performed on BM confirmed WM (N=30) and IgM-MGUS (N=73) neuropathy patients with neurologic assessments and hematologic features.
Results
The presence of anemia and quantity of IgM monoclonal protein were significantly greater in WM. Based on multiple neurologic assessments differences were not found for: 1) length of time from neurologic symptom onset to evaluation; 2) chief complaint of painless loss of feeling in the feet, Romberg’s sign and tremor; and 3) clinical motor, sensory and reflex abnormalities. Autonomic testing was normal in both diseases. Using nerve conduction (NCS) criteria for demyelination, 62% of IgM-MGUS and 27% of WM met this criteria (p=0.013). IgM MGUS patients had greater terminal conduction slowing by ulnar residual latency calculation (<0.01). The degree of axonal loss as measured by summated compound muscle action potentials and available nerve biopsy was not significantly different between diseases.
Conclusion
Although WM and IgM-MGUS must be distinguished for hematologic prognosis and treatment, clinical neuropathy presentations of WM and IgM-MGUS are similar and likely related to comparable axonal loss in both conditions. Despite these similarities, evidence of demyelination was found by electrophysiologic studies much more commonly in IgM-MGUS. This difference may reflect varied immune mechanism(s) in the two disorders.
In the evaluation of a patient with peripheral neuropathy the discovery of a serum monoclonal protein has implications for the neurologic and hematologic diagnosis, prognosis and treatment1. Among such patients the type of neuropathy may suggest the specific underlying hematologic process. To illustrate, in a patient with a monoclonal protein having subacute painful multiple mononeuropathies the diagnosis of mixed cryoglobulinemia is likely and evaluation for viral hepatitis should be emphasized. In another patient, also with a monoclonal protein and with insidious onset of a symmetric painful autonomic, sensory motor polyneuropathy, primary amyloid light chain (AL) amyloidosis should be suspected. In a third example a monoclonal protein with insidious demyelinating polyradiculoneuropathy occurs in POEMS (Polyneuropathy, Organomegaly, Endocrinopathy, M spike and Skin changes) and should lead to search of treatable osteosclerotic myeloma2. Familiarity of these patterns, and others, leads to correct diagnosis and treatment.
Among persons having IgM-MGUS an insidious painless distal sensory ataxic neuropathy with demyelination has been felt to be so characteristic as to coin the acronym DADS (Distal, Acquired, Demyelinating, Sensory-neuropathy)3. By contrast descriptions of the neuropathy features occurring in another IgM condition, namely Waldenström’s macroglobulinemia (WM) are limited4,5. Waldenström’s macroglobulinemia is defined by the Revised European American Lymphoma (REAL) and World Health Organization (WHO) as having a bone marrow (BM) infiltrated lymphoplasmacytic lymphoma and IgM paraproteinemia6. In WM the hematologic features of anemia, hepatosplenomegaly, lymphadenopathy, and hyperviscosity have previously been reported but are variably present with current requirement of BM diagnosis7.
Some investigators have suggested there are more axonal features in the nerve conduction studies (NCS) in WM, but the diagnosis of WM did not appear to be based on current BM criteria4,5. By contrast, distal, symmetric, chronic demyelinating neuropathy is more commonly described in IgM-MGUS neuropathy patients3,8 including the reports of reduced terminal latency index (TLI), indicating relative slowing in distal motor nerve segments9–11. Tremor has also been described to be one of the possible clinical stigmata of the IgM-MGUS neuropathy12. Some studies have suggested that the existence of myelin-associated glycoprotein antibodies (anti-MAG) may explain the clinical, electrophysiologic, and histologic features of the DADS-IgM-MGUS neuropathy13–15. However, other studies of IgM neuropathy have found little or no difference between the type and severity of neuropathies in IgM patients with or without anti-MAG antibodies16,17. The MAG antibodies are commonly present in IgM amyloid neuropathy and IgM patients without neuropathy and therefore are not routinely used for diagnosis at many institutions including our own18.
We conducted this retrospective review to characterize the neuropathies associated with WM and IgM-MGUS to answer whether there are clinical, electrophysiologic and nerve biopsy findings that distinguish the neuropathies associated with WM from IgM-MGUS. In an attempt to study distal predominant involvements we use the previously published formulas to calculate the residual latency (RL) and TLI from routine NCS. The RL19 is a subtraction of the calculated latency from the measured latency. It evaluates the distal segment of the motor nerves and has been reported to be associated with the neuropathy associated with IgM-MGUS20. The TLI is the ratio between the calculated latency [distance/motor conduction velocity (MCV)] and the measured latency, i.e. distal motor latency (DML) and also assesses for selective distal conduction velocity slowing9–11,21.
Methods
Subjects
With the approval of our institutional review board, an electronic record retrieval system was used to identify patients diagnosed with either WM or IgM-MGUS, who had peripheral neuropathy and had undergone NCS testing between January, 1973 and December, 2007. Hematology and neurology consultation with complete neurologic examination by specialists working in peripheral nerve disease was required for inclusion of cases. Possible hereditary motor and sensory neuropathy cases and cases with other acquired etiologies other than IgM-associated cause were excluded22,23. This included clinical evaluation and laboratory testing for diabetes, thyroid disease, kidney disease, infectious hepatitis and alcoholic neuropathy. Detailed family histories had been obtained as part of our routine practice to exclude inherited peripheral neuropathies. Also excluded were cases of amyloidosis and POEMS syndrome. No patients had amyloid on bone marrow biopsy and none had systemic sequelae of POEMS syndrome, all having undergone metastatic bone survey in exclusion of osteosclerotic myeloma. Only patients with NCS before neurotoxic or immunosuppressant therapies were studied in both WM and IgM-MGUS.
Bone marrow biopsy, hematologic parameters (levels of hemoglobin, IgM and other monoclonal proteins, platelets, creatinine, light chains), sural nerve biopsies, autonomic testing,24,25 neurologic examinations including neuropathy impairment score (NIS)26 with individual motor, reflex, and sensory parameters were examined. Other features were also extracted from the records including presenting complaint, presence of tremor, age, sex, and length of symptoms to the time of NCS. The hematologic and clinical parameters were reviewed at the time of NCS.
Diagnosis of WM and IgM-MGUS
Bone marrow diagnosis of WM was based on the WHO classification by which lymphoplasmacytic cells must be seen diffusely without myeloma or other lymphomatous disorders present6. Diagnosis of WM or IgM-MGUS with bone marrow biopsy was made by hematologists and hematopathologists using the standard approach6. All patients had positive immunofixation for IgM-MGUS and simultaneously performed serum protein electrophoresis. We chose to include only BM confirmed IgM-MGUS to exclude inclusion of occult WM for this research study.
Nerve Conduction Study (NCS)
Nerve conduction study were performed by uniform technique by experienced and certified technicians, previously on Teca-TD20 and subsequently on Nicolet Viking with similar filter settings and electrode placement and stimulation technique27. Skin temperature was measured at the dorsum of hand or foot, which was maintained above 32°C with electrode placement and stimulation similar between all patients. Using our standard peripheral neuropathy protocol, motor nerve conductions were reviewed in three motor (peroneal, tibial and ulnar) and two sensory (sural, median) nerves available in all patients. Median motor studies were also available among 8 WM and 32 IgM-MGUS patients, and done typically in evaluation of superimposed mononeuropathy. Normal values were defined based on standards set at the electromyelogram (EMG) laboratory at Mayo Clinic, Rochester, MN, USA. Demyelination and axonal features were determined by standard criteria28 widely used in research and clinical practice, i.e. demyelination if; 1) Reduction in conduction velocity in two or more nerves; <80% of lower limit of normal (LLN) if amplitude >80% of the LLN, <70% of LLN if amplitude <80% LLN; 2) Partial conduction block or abnormal temporal dispersion in one or more nerves; <15% change in duration between proximal and distal segments or >20% drop in negative peak area or peak-to-peak amplitude between proximal and distal sites; 3) And/or presence of one of these findings; a) Prolonged distal latencies in two or more nerves; >125% of upper limit of normal (ULN) if amplitude >80%, or >150% of ULN if amplitude <80% of LLN; b) Absent F waves or prolonged minimum F wave latency; >120% of ULN if amplitude is >80% of LLN or >150% of ULN if amplitude is <80% of LLN28.
Terminal latency index was calculated on motor studies. The TLI is the ratio between the calculated latency [distance/motor conduction velocity] and the measured latency, i.e. distal motor latency21 and was determined by the previously published formula: . Motor studies with no response were not included in the calculation of TLI and RL calculations. Terminal latency index was used to compare the wrist-to-thenar muscle (distal segment) with the elbow-to-wrist conduction velocity in median nerve, the wrist-to-hypothenar muscle (distal segment) with the elbow-to-wrist conduction velocity in ulnar nerve, the ankle-to-extensor digitorum brevis muscle (distal segment) with the knee-to-ankle conduction velocity in common peroneal nerve, and the ankle-to-abductor hallucis muscle (distal segment) with the knee-to-ankle conduction velocity in posterior tibial nerve.
Residual latency considers differently the distal motor axonal slowing and was determined on available NCS attributes. Residual latency is a subtraction of the calculated latency (distance/MCV) from the measured latency (DML) and was determined by the previously published formula19: . This calculation was derived from the same NCS studied for TLI.
Ascertainment of Axonal Loss
To assess the degree of nerve fiber loss in motor and sensory nerves, percentiles were used to compare summated compound motor unit potentials (peroneal, tibial, ulnar) and summated sensory nerve action potential (sural and median) between WM and IGM-MGUS29–31. This approach was chosen in order to allow for comparison of axonal loss between individuals of different ages, heights, weights and gender correcting to a comparison of normal. Axonal involvement was also examined by needle examination standard techniques in muscles comparably examined in all patients (tibialis anterior, medial gastrocnemius, vastus medialis) using summated abnormalities of fibrillations or motor unit potential amplitudes, motor unit duration and recruitment with which a composite abnormality was created. The Mayo needle EMG classification scheme of semi-quantification using a 0–4 plus scale of each of these parameters was utilized32. Zero is normal and 4 are the most affected for fibrillations and motor unit parameters of amplitude, duration and recruitment.
In further review of neuropathic features available, sural nerve biopsies, including paraffin and epoxy embedded sections and by teased fiber preparations, were reviewed. Characterization of axonal and demyelinating features of whole sural nerve biopsy was determined using standard techniques33.
Autonomic testing
Available autonomic reflex testing including study of cardiovagal, adrenergic, and sudomotor functions were reviewed by standard techniques24,25.
Statistical analysis
Descriptive statistics were used to assess for clinical, electrophysiologic, and other features of the neuropathies of WM and IgM-MGUS. Two tailed test and p<0.05 were used to determine significant differences. Associations between the presence of WM and putative risk covariates were evaluated by Student’s t-test or Wilcoxon Rank-Sum test for continuous variables and with Chi-square test or Fisher’s exact test for nominal variables. Multiple logistic regression analysis was performed for evaluating the importance of neuropathy type in establishing the probability of developing WM with IgM. Multivariate two-step logistic regression models for the presence of WM were also performed to create cut-off values for predictive purposes of individual nerve conductions and hematologic parameters found significant in the total cohort.
Results
The disease characteristics of the 30 WM and 73 IgM-MGUS neuropathy patients are given in Tables 1–3. Among both groups, “numb toes and feet” was the most common chief complaint with “tremor” the second most common occurring in 10% or less of both groups. Romberg’s sign and presence of tremor were not significantly different between WM and IgM-MGUS. Between the two diseases, there was no significant difference in gender, age, duration of symptoms and severity of peripheral neuropathy as determined by NIS. A significant difference was not found between diseases for motor, sensory and reflex abnormalities. Autonomic reflex screens were performed on 11 WM and 26 IgM-MGUS patients and all showed normal adrenergic, cardiovagal function and only slight sudomotor abnormalities not significantly different between the two groups25.
Table 1.
Clinical characteristics of WM compared to IgM-MGUS
| WM (N=30) | IgM-MGUS (N=73) | p-value | ||
|---|---|---|---|---|
| Age (yrs.) | median 65.5, range 46–82 | median 64.0, range 31–82 | 0.821 | |
| Gender | Male Female |
22 (73%) 8 (27%) |
53 (73%) 20 (27%) |
0.939 |
| NIS at time of NCS (points) | median =17.5, range 0–75 | median =24, range 0–92 | 0.055 | |
| Chief first complaint number abnormal (percent) | “Numb toes & feet” | 28 (93%) | 66 (90%) | 0.633 |
| “Tremor” | 2 (7%) | 7 (10%) | 0.633 | |
| Romberg’s’ sign number abnormal (percent) | 9 (30%) | 29 (41%) | 0.352 | |
| Tremor by examination number abnormal (percent) | 6 (20%) | 20 (27%) | 0.432 | |
| NIS reflex abnormality | median = 6, range 0–20 | median = 8, range 0–20 | 0.543 | |
| NIS sensory abnormality | median = 7, range 2–26 | median = 10, range 2–32 | 0.352 | |
| NIS motor abnormality | median = 7, range 0–46 | median = 8, range 0–64 | 0.442 | |
| Duration (yrs.) of symptoms prior to NCS | median = 2.0, range 0.1–20.0 | median = 3.0, range 0.2–29 | 0.329 | |
NIS=Neuropathy Impairment Score; NCS= Nerve Conduction Studies
Table 3.
Assessment of axonal loss by summated motor and sensory nerve conduction amplitudes in WM, IGM-MGUS, and healthy subjects
| WM (N=30) | IgM-MGUS (N=73) | Healthy subjects | WM vs healthy subjects | IgM-MGUS vs healthy subjects | IgM-MGUS vs WM | |
|---|---|---|---|---|---|---|
| CMAPs* Percentiles of mean (range) | 8.08 (0.06 – 40.79) | 6.34 (0.03 – 85.15) | 50.92 (3.22 – 96.86) | P<0.001 | P<0.001 | p=0.419 |
| SNAPs* Percentiles of mean (range) | 0.09 (0.03 –17.23) | 0.20 (0.03 – 53.59) | 49.48 (0.70 – 98.66) | P<0.001 | P<0.001 | p=0.376 |
The mean of mean values of summed ulnar, peroneal, and tibial compound motor action potential (CMAPs) (mV) or median and sural sensory sensory nerve action potential (SNAPs)’ corrected for age, gender, height and weight as compared to reference values of healthy subjects (29–31).
Using the standard electrophysiological criteria described in the method section, demyelination was found more frequently in IgM-MGUS (62%) than in WM (27%) (p=0.001). The specialized NCS calculations (motor TLI and RL) also showed that ulnar-RL was significantly greater in IgM-MGUS (p=0.009) which is consistent with distal slowing, although ulnar-TLI, median, peroneal and tibial RL as well as TLI did not reach significant difference between these two groups. Hemoglobin, IgG and IgA levels were all significantly reduced while IgM levels were significantly elevated in WM group compared to IgM-MGUS group. The multiple logistic regression analysis showed demyelinating findings in IgM-MGUS were significant (odds ratio = 11.1, p = 0.005). In contrast, ulnar-RL did not reach significance with multivariate regression analysis, suggesting ulnar-RL may be correlated with the other factors.
The degree of axonal loss was not significantly different between IgM-MGUS and WM determined by composite score of motor and sensory CMAPs (Table 3); summated needle EMG abnormalities (Table 4); and by available nerve biopsies (Table 5) and the Figure. Summated deficits of tibialis anterior, medial gastrocnemius, vastus medialis or vastus lateralis muscles were not significantly different between IgM-MGUS and WM.
Table 4.
Assessment of axonal loss by summated needle EMG abnormalities WM vs IgM-MGUS
| WM (N=30) | IgM-MGUS (N=73) | WM vs IgM-MGUS | |||
|---|---|---|---|---|---|
| Median | Range | Median | Range | p-value | |
| Motor unit potentials* | 8 | 0–18 | 6 | 0–24 | 0.327 |
| Fibrillations* | 2 | 0–10 | 2.5 | 0–8 | 0.525 |
Summated tibialis anterior, medial gastrocnemius, vastus medialis muscle EMG abnormalities (studied in all patients) for motor unit amplitude, duration, recruitment or fibrillation abnormalities using semi quantification plus scale (0–4, 0=normal, 4=most severely affected) summed in routine needle EMG32.
Table 5.
Teased fiber nerve preparations for WM and IgM-MGUS
| WM (N=4) | IgM-MGUS (N=12) | WM vs IgM-MGUS | Healthy controls (N=14) | ||||
|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | p-value | Median | Range | |
| Normal A, B (%) | 73 | 65–82 | 50 | 25–82 | 0.016 | 85.1 | 72–95 |
| Demyelination C, D (%) | 10 | 5–20 | 11 | 1–37 | 0.800 | 0.0 | 0–2 |
| Axonal Degeneration E, H (%) | 4 | 3–5 | 4 | 2–29 | 0.700 | 0.0 | 0–3 |
| Remyelination F, G (%) | 10 | 10–10 | 16 | 3–25 | 0.480 | 13.7 | 5–28 |
| Axon loss by empty strands (number) | 30 | 15–40 | 35 | 3–45 | 0.470 | 6.0 | 0–19 |
Teased fiber types: A = normal, B = myelin wrinkling, C = demyelination, D = demyelination and remyelination, E = axonal degeneration, F = remyelination, G = myelin reduplication, H = regeneration after axonal degeneration, empty = strands with loss of fibers.
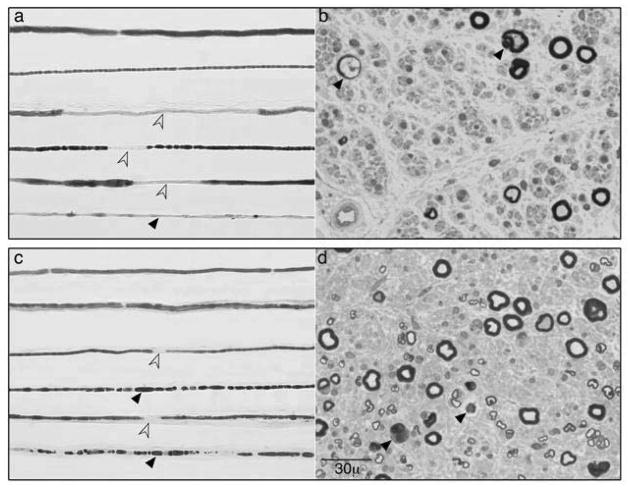
Figure.
Teased and epoxy embedded sections from representative sural nerve biopsies of a patient with IgM-MGUS (a, b) and Waldenström’s macroglobulinemia (WM) (c, d) neuropathy. Both biopsies show combination axonal fiber loss and degeneration (black arrows) with de and remyelination (open arrows). By the electrophysiologic criteria28 the shown IgM-MGUS patient had demyelinating features with axonal loss whereas the WM patient had axonal nerve conductions despite occasional demyelinating teased fibers. Both these patients presented with insidious onset sensory gait ataxia without pain or significant weakness.
Further analysis of hematologic parameters by calculating the area under the ROC curve (AUC) suggested two predictive values for WM including a hemoglobin level less than 12.6 g/dl (anemia) [71% sensitivity; 88% specificity, p<0.001] and IgM level greater than 1830 mg/dl [85% sensitivity; 93% specificity, p<0.001]. The predictability of the hematologic cut-off values were independent of nerve conduction status, these values achieved the same specificity and sensitivity in axonal IgM-MGUS and demyelinating WM. Individual nerve conduction parameters (RL, TLI) were also analyzed using AUC calculation. The only statistically significant predictive value for WM was an ulnar –RL less than 0.43 [89% sensitive; 44% specific, p=0.0311].
Discussion
The distinction of IgM-MGUS from WM is important but complex. Previous studies have shown that the prevalence of IgM-MGUS is higher in familial occurrence of WM, but there is no evidence that IgM-MGUS progresses to WM with each appearing to be a different disease from their onset34.
In this retrospective study, we found that 73% of bone marrow biopsy confirmed WM patients had only primary axonal features on nerve conductions whereas 62% of IgM-MGUS patients had demyelinating features. Despite apparent demyelinating nerve conduction differences the clinical presentations were similar. The main presenting complaint of distal sensory loss and gait ataxia and similar and not infrequent occurrence of tremor was indistinguishable. Those similar impairments and severity of impairments likely relate to the comparable amounts of axonal loss seen in both groups, i.e. a mixed demyelinating-axonal pathogenesis. The apparent axonal predominant changes in WM patients do not appear related to symptom duration. Specifically, axonal features in WM related neuropathy are unlikely a simple evolution of demyelinating to axonal features as the length of time from symptom onset did not correlate with the type of nerve conduction abnormality. The more common occurrence of demyelination in our IgM-MGUS group over WM group is consistent with earlier observations without report of bone marrow biopsy4,5.
Our findings emphasize the complexity of electrophysiologic presentations in these two primary IgM hematologic disorders. The divergent electrophysiologic findings lead to speculation that a spectrum of immunity is responsible with overlap between some. The careful exam of the other aspects of clinical presentation such as the levels of hemoglobin and IgM can also provide assistance in distinguishing WM from IgM-MGUS and deciding whether to perform bone marrow biopsy. Specifically our study showed that using the cut-off level of IgM greater than 1830 mg/dl and hemoglobin level less than 12.6 g/dl (anemia) can achieve 71% of sensitivity and 88% specificity for predicting WM cases independent of nerve conduction status. The fact that markedly elevated IgM level and anemia are more frequent in WM than in IgM-MUGS is also supported by previous study35.
The significant association of distal slowing came from only the results of the ulnar-RL in distinction of IgM-MGUS from WM. That association did not bear out with multiple regression analysis and in other nerves suggesting possible outlier statistical phenomena. Similarly we did not find reduced median-TLI significantly associated with IgM-MGUS as had been suggested in the earlier reports8,9. This difference may be due to the fact that earlier described samples used for the TLI association analysis were tested positive with anti-myelin associated glycoprotein assay, of which the occurrence may select for a specific distal phenomenon. We used retrospective study design, with this antibody not routinely tested at our institution, and therefore do not have the status of IgM antibodies against MAG,9–11 suggesting reduced TLI is not associated with patients without anti-MAG.
Conclusions
What are the practical implications of our findings? In cases where IgM monoclonal proteins are found in neuropathy patients, an axonopathy on NCS favors the diagnosis of WM over IgM-MGUS, especially if the neuropathy associates with anemia and elevated IgM concentration. Therefore, bone marrow biopsy would be more likely to yield positive WM diagnosis in the presence of axonal features. However, if the electrophysiology reveals evidence of demyelination, the decision to advance to bone marrow confirmatory diagnosis of WM should not be excluded, especially if anemia or other worrisome features are present. The decision to advance to BM should remain for those where the extent of neurologic or hematologic involvements would warrant therapeutic approaches. Based on this study, individual NCS parameters such as TLI and RL may not be as helpful, with comprehensive NCS being more helpful.
A spectrum of divergent and shared immune mechanism is implicated by both the similar and different clinical and electrophysiological features identified in WM and IgM-MGUS. Future prospective studies looking to identify the implicated varied immune paraneoplastic or paraimmune neuropathy mechanisms will be important.
Table 2.
Significant nerve conduction and hematologic differences of WM vs IgM-MGUS
| WM | IgM-MGUS | p-value | ||
|---|---|---|---|---|
| Demyelinating nerve conductions(28) | 8/30(27%) | 45/73 (62%) | 0.001 | |
| Nerve conduction study | Ulnar RL(19) | N=27 median 1.72, range 1.22–6.46 |
N=70 median 2.43, range 1.70–7.43 |
0.009* |
| Hematologic findings | Hemoglobin (gm/dl) | N=30 median 11.8, range 7.3–14.5 |
N=73 median 14.4, range 7.4–18.0 |
<0.001 |
| IgM (mg/dl) | N=30 median 3100, range 658–6360 |
N=73 median 650, range 169–2960 |
<0.001 | |
Shown are statistically significant differences: NIS =neuropathy impairment score: RL= residual latency; No response conduction responses are not included in the calculations.
Multiple regression analysis was not significant for ulnar RL.
Acknowledgments
The authors thank the Mayo Center for Translational Science Activities (CTSA), Ms. Susanna R. Stevens, and in the Peripheral Nerve Lab Ms. Jenny Davies for statistical analysis.
References
- 1.Kyle RA. Neuropathy associated with the monoclonal gammopathies. In: Noseworthy JH, editor. Neurologic therapeautics: principles and practice. London, New York: Martin Dunitz; 2003. pp. 2126–36. [Google Scholar]
- 2.Lozeron P, Adams D. Monoclonal gammopathy and neuropathy. Curr Opin Neurol. 2007 Oct;20(5):536–41. doi: 10.1097/WCO.0b013e3282ef79e3. [DOI] [PubMed] [Google Scholar]
- 3.Katz JS, Saperstein DS, Gronseth G, Amato AA, Barohn RJ. Distal acquired demyelinating symmetric neuropathy. Neurology. 2000;54:615–20. doi: 10.1212/wnl.54.3.615. [DOI] [PubMed] [Google Scholar]
- 4.Levine T, Pestronk A, Florence J, et al. Peripheral neuropathies in Waldenstrom’s macroglobulinaemia. J Neurol Neurosurg Psychiatry. 2006 Feb;77(2):224–8. doi: 10.1136/jnnp.2005.071175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldini L, Nobile-Orazio E, Guffanti A, et al. Peripheral neuropathy in IgM monoclonal gammopathy and Waldenstrom’s macroglobulinemia: a frequent complication in elderly males with low MAG-reacitve serum monoclonal component. Am J Hematol. 1994;45:25–31. doi: 10.1002/ajh.2830450105. [DOI] [PubMed] [Google Scholar]
- 6.Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003 Apr;30(2):110–5. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]
- 7.Gertz MA. Waldenstrom macroglobulinemia: a review of therapy. Am J Hematol. 2005 Jun;79(2):147–57. doi: 10.1002/ajh.20363. [DOI] [PubMed] [Google Scholar]
- 8.Kaku DA, England JD, Sumner AJ. Distal accentuation of conduction slowing in polyneuropathy associated with antibodies to myelin-associated glycoprotein and sulphated glucuronyl paragloboside. Brain. 1994 Oct;117(Pt 5):941–7. doi: 10.1093/brain/117.5.941. [DOI] [PubMed] [Google Scholar]
- 9.Lupu VD, Mora CA, Dambrosia J, Meer J, Dalakas M, Floeter MK. Terminal latency index in neuropathy with antibodies against myelin-associated glycoproteins. Muscle Nerve. 2007 Feb;35(2):196–202. doi: 10.1002/mus.20678. [DOI] [PubMed] [Google Scholar]
- 10.Capasso M, Torrieri F, Di Muzio A, De Angelis MV, Lugaresi A, Uncini A. Can electrophysiology differentiate polyneuropathy with anti-MAG/SGPG antibodies from chronic inflammatory demyelinating polyneuropathy? Clin Neurophysiol. 2002 Mar;113(3):346–53. doi: 10.1016/s1388-2457(02)00011-1. [DOI] [PubMed] [Google Scholar]
- 11.Attarian S, Azulay JP, Boucraut J, Escande N, Pouget J. Terminal latency index and modified F ratio in distinction of chronic demyelinating neuropathies. Clin Neurophysiol. 2001 Mar;112 (3):457–63. doi: 10.1016/s1388-2457(01)00469-2. [DOI] [PubMed] [Google Scholar]
- 12.Bain PG, Britton TC, Jenkins IH, et al. Tremor associated with benign IgM paraproteinaemic neuropathy. Brain. 1996 Jun;119(Pt 3):789–99. doi: 10.1093/brain/119.3.789. [DOI] [PubMed] [Google Scholar]
- 13.Gabriel JM, Erne B, Miescher GC, et al. Selective loss of myelin-associated glycoprotein from myelin correlates with anti-MAG antibody titre in demyelinating paraproteinaemic polyneuropathy. Brain. 1996;119:775–87. doi: 10.1093/brain/119.3.775. [DOI] [PubMed] [Google Scholar]
- 14.Hays AP, Latov N, Takatsu M, Sherman WH. Experimental demyelination of nerve induced by serum of patients with neuropathy and an anti-MAG IgM M-protein. Neurology. 1987 Feb;37(2):242–56. doi: 10.1212/wnl.37.2.242. [DOI] [PubMed] [Google Scholar]
- 15.Tatum AH. Experimental paraprotein neuropathy, demyelination by passive transfer of human IgM anti-myelin-associated glycoprotein. Ann Neurol. 1993 May;33(5):502–6. doi: 10.1002/ana.410330514. [DOI] [PubMed] [Google Scholar]
- 16.Gosselin S, Kyle RA, Dyck PJ. Neuropathy associated with monoclonal gammopathies of undetermined significance. Ann Neurol. 1991;30:54–61. doi: 10.1002/ana.410300111. [DOI] [PubMed] [Google Scholar]
- 17.Suarez GA, Kelley JJ. Polyneuropathy associated with monoclonal gammopathy of undetermined significance: Further evidence that IgM-MGUS neuropathies are different than IgG-MGUS. Neurology. 1993;43:1304–8. doi: 10.1212/wnl.43.7.1304. [DOI] [PubMed] [Google Scholar]
- 18.Garces-Sanchez M, Dyck PJ, Kyle RA, et al. Antibodies to myelin-associated glycoprotein (anti-Mag) in IgM amyloidosis may influence expression of neuropathy in rare patients. Muscle Nerve. 2008 Apr;37(4):490–5. doi: 10.1002/mus.20955. [DOI] [PubMed] [Google Scholar]
- 19.Kraft GH, Halvorson GA. Median nerve residual latency: normal value and use in diagnosis of carpal tunnel syndrome. Arch Phys Med Rehabil. 1983 May;64(5):221–6. [PubMed] [Google Scholar]
- 20.Radziwill AJ, Steck AJ, Renaud S, Fuhr P. Distal motor latency and residual latency as sensitive markers of anti-MAG polyneuropathy. J Neurol. 2003 Aug;250(8):962–6. doi: 10.1007/s00415-003-1128-7. [DOI] [PubMed] [Google Scholar]
- 21.Shahani B, Young R, Potts F, Maccabee P. Terminal latency index (TLI) and late response studies in motor neuron disease (MND), peripheral neuropathies and entrapment syndromes (abstract) Acta Neurol Scand. 1979;73(Supp):118. [Google Scholar]
- 22.Mauermann ML, Burns TM. Pearls and Oysters: evaluation of peripheral neuropathies. Neurology. 2009 Feb 10;72(6):e28–31. doi: 10.1212/01.wnl.0000342135.27500.df. [DOI] [PubMed] [Google Scholar]
- 23.Dyck PJ, Dyck PJB, Chalk CH. The 10 P’s: a mnemonic helpful in characterization and differential diagnosis of peripheral neuropathy. Neurology. 1992;42:14–8. doi: 10.1212/wnl.42.1.14. [DOI] [PubMed] [Google Scholar]
- 24.Low PA, Benarroch EE. Clinical autonomic disorders. Philadelphia: Wolters Kluwer; 2008. [Google Scholar]
- 25.Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc. 1993 Aug;68(8):748–52. doi: 10.1016/s0025-6196(12)60631-4. [DOI] [PubMed] [Google Scholar]
- 26.Dyck PJ, Sherman WR, Hallcher LM, et al. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol. 1980;8(6):590–6. doi: 10.1002/ana.410080608. [DOI] [PubMed] [Google Scholar]
- 27.Daube J. Clinical Neurophysiology. 1. Philadelphia: Davis; 1996. [Google Scholar]
- 28.Research criteria for diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP). Report from an Ad Hoc Subcommittee of the American Academy of Neurology AIDS Task Force. Neurology. 1991 May;41(5):617–8. [PubMed] [Google Scholar]
- 29.Dyck PJ, O’Brien PC, Litchy WJ, Harper CM, Daube JR, Dyck PJB. Use of percentiles and normal deviates to express nerve conduction and other test abnormalities. Muscle Nerve. 2001;24:307–10. doi: 10.1002/1097-4598(200103)24:3<307::aid-mus1000>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 30.Dyck PJ, Litchy WJ, Lehman KA, Hokanson JL, Low PA, O’Brien PC. Variables influencing neuropathic endpoints: the Rochester Diabetic Neuropathy Study of Healthy Subjects. Neurology. 1995 Jun;45(6):1115–21. doi: 10.1212/wnl.45.6.1115. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien PC, Dyck PJ. Procedures for setting normal values. Neurology. 1995 Jan;45(1):17–23. doi: 10.1212/wnl.45.1.17. [DOI] [PubMed] [Google Scholar]
- 32.Daube JR. Clinical neurophysiology (CD disc monogram of EMG needle examination) New York: Oxford University Press; 2009. [Google Scholar]
- 33.Dyck PJ, Dyck PJB, Giannini C, Sahenk Z, AJW, Engelstad J. Peripheral nerves. In: Graham DI, Lantos PL, editors. Greenfields Neuropathology. London: Oxford University Press; 2002. pp. 551–675. [Google Scholar]
- 34.McMaster ML, Kristinsson SY, Turesson I, Bjorkholm M, Landgren O. Novel aspects pertaining to the relationship of Waldenstrom’s macroglobulinemia, IgM monoclonal gammopathy of undetermined significance, polyclonal gammopathy, and hypoglobulinemia. Clin Lymphoma Myeloma. 2009 Mar;9(1):19–22. doi: 10.3816/CLM.2009.n.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Facon T, Brouillard M, Duhamel A, et al. Prognostic factors in Waldenstrom’s macroglobulinemia: a report of 167 cases. J Clin Oncol. 1993 Aug;11(8):1553–8. doi: 10.1200/JCO.1993.11.8.1553. [DOI] [PubMed] [Google Scholar]