Abstract
The utility of wild-type outer membrane vesicle (wtOMV) vaccines against serogroup B (MenB) meningococcal disease has been explored since the 1970s. Public health interventions in Cuba, Norway and New Zealand have demonstrated that these protein-based vaccines can prevent MenB disease. Data from large clinical studies and retrospective statistical analyses in New Zealand give effectiveness estimates of at least 70%. A consistent pattern of moderately reactogenic and safe vaccines has been seen with the use of approximately 60 million doses of three different wtOMV vaccine formulations. The key limitation of conventional wtOMV vaccines is their lack of broad protective activity against the large diversity of MenB strains circulating globally. The public health intervention in New Zealand (between 2004–2008) when MeNZB was used to control a clonal MenB epidemic, provided a number of new insights regarding international and public-private collaboration, vaccine safety surveillance, vaccine effectiveness estimates and communication to the public. The experience with wtOMV vaccines also provide important information for the next generation of MenB vaccines designed to give more comprehensive protection against multiple strains.
Keywords: Neisseria meningitidis, New Zealand MenB outbreak, OMV vaccines, controlling MenB epidemics, meningococcal disease
Introduction
Meningococcal septicemia and meningitis were feared even before the discovery of the meningococcus in the early nineteenth century.1-5 Meningococcal infection is associated with rapid onset of severe disease, often following initial mild symptoms and can result in fatality or permanent disability. Hence there is a high level of anxiety concerning the possibility for epidemic disease caused by virulent clones or sporadic disease, which can occur suddenly in otherwise healthy individuals.6-10 Before the antibiotic era, the mortality rate was 70–90%,4 but over the past 50 y, despite the introduction of modern antibiotics, the overall case-fatality rate has remained between 5 and 15%; with permanent disabilities affecting approximately 10–20% of survivors.11,12 Case-fatality rates are somewhat higher in patients with septicaemia, in epidemic situations and in adolescents and elderly age groups.13,14 While infants and children under five years of age are most commonly affected by invasive meningococcal disease, a second peak is observed among adolescents, especially during epidemics.8,15 Traditionally, the Sub-Saharan “meningitis belt” in Africa has been the most significant global site for meningococcal disease, in seasonal outbreaks caused by serogroup A.
Since the late twentieth century, routine vaccination against meningococcal disease has become increasingly widespread due to continuing advances in technology and increased awareness of the disease. The principle of using the capsular polysaccharide as a vaccine antigen was discovered and developed by Emil C. Gotschlich, Irvin Goldschneider and their colleagues at Walter Reed Army Institute of Research, USA, in the late 1960s.16,17 Their efforts resulted in good vaccines against serogroup A and C disease. Later serogroup Y and W vaccines were developed using the same strategy, and a quadrivalent ACYW polysaccharide vaccine was licensed in 198118,19 During the 21st century, safe and effective conjugate vaccines against serogroups A, C, Y and W were introduced to protect all age groups20-24 and a low-cost conjugate vaccine against serogroup A disease was especially developed for use in Africa.25,26 Hence, control of meningococcal serogroup B (MenB) is now the remaining challenge for the overall control of meningococcal disease worldwide.27 The MenB capsular polysaccharide has been determined unsuitable (by most research groups) for vaccine development due to low immunogenicity and the potential risk of autoimmunity.28-31 Thus, several wild-type outer membrane vesicle (wtOMV) vaccines have been used to control clonal MenB outbreaks. We review information about these wtOMV vaccines that were developed to control clonal outbreaks in Cuba, Norway and New Zealand. In addition we evaluate how these vaccines have been considered for use in other situations and to provide some helpful background for the evaluation and implementation of the new “universal” MenB vaccines in development.
A Brief Historic Journey
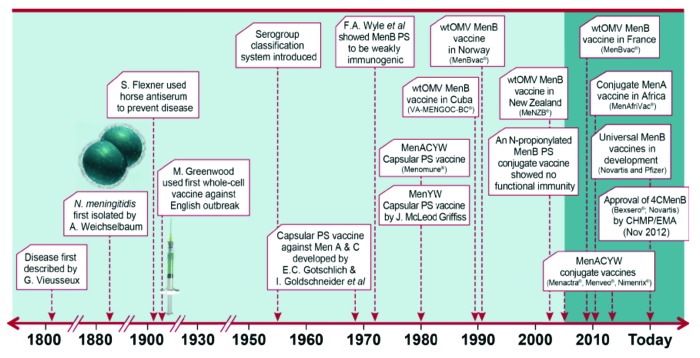
A graphical representation of the historical evolution for main events in the area of meningococcal disease and prevention can be seen in Figure 1. Although they predate the development of meningococcal conjugate vaccines by some years, the “tailor-made” wtOMV vaccines against particular outbreak strains remain the only vaccines that have shown efficacy and effectiveness against MenB disease in general use.30-32 It is important to recognize that these wtOMV vaccines were designed to target specific epidemic strains and hence not expected to be suitable for general use in most epidemiologic situations32-34 where endemic disease is caused by multiple different circulating strains. Development of the OMV-concept was pioneered during the 1970s by Wendell D. Zollinger of Walter Reed Army Institute of Research, USA, Torstein B. Helting of Behringwerke, Germany and Carl E. Frasch of the US Food and Drug Administration, USA and their respective coworkers.33,35-41 The research activity in these and subsequently other laboratories led to the development of two vaccine formulations for clinical protection trials in Cuba and Norway ;-Dr. Frasch advised on both efforts.33
Figure 1. Meningococcal disease prevention; from identification to vaccination, a brief schematic presentation of the history (adopted from a slide originally prepared by Julio Vazquez (presented at IPNC 2012, Wurzburg, Germany). References to the various events may be found in “Introduction”).
The initial wtOMV vaccine in general use VA-MENGOCOC-BC®, was developed at the Finlay Institute in Cuba to address an ongoing MenB epidemic and used in a 16-mo clinical study in 10–14 y olds between 1987 and 1989.42 The second wtOMV formulation was MenBvac® (Fig. 2), developed at the Norwegian Institute of Public Health (NIPH) to address another ongoing epidemic and used in a 29-mo efficacy trial among 13–16 y olds starting in 1988 and ending in 1991. While efficacy estimates of roughly 83% and 57% were found for the Cuban and Norwegian trials, respectively,32,42,43 the two trials were not of entirely similar design and the longer observation period for the Norwegian trial affected the efficacy outcome. Clinical data over the initial 10-mo observation period in the Norwegian trial showed 87% efficacy.32,33,44 These findings and a separate immunogenicity trial in Norway confirmed that a booster dose about one year after the primary two-dose immunization schedule resulted in better persistence of protective antibodies, thus potentially providing greater effectiveness.32,33,44-46
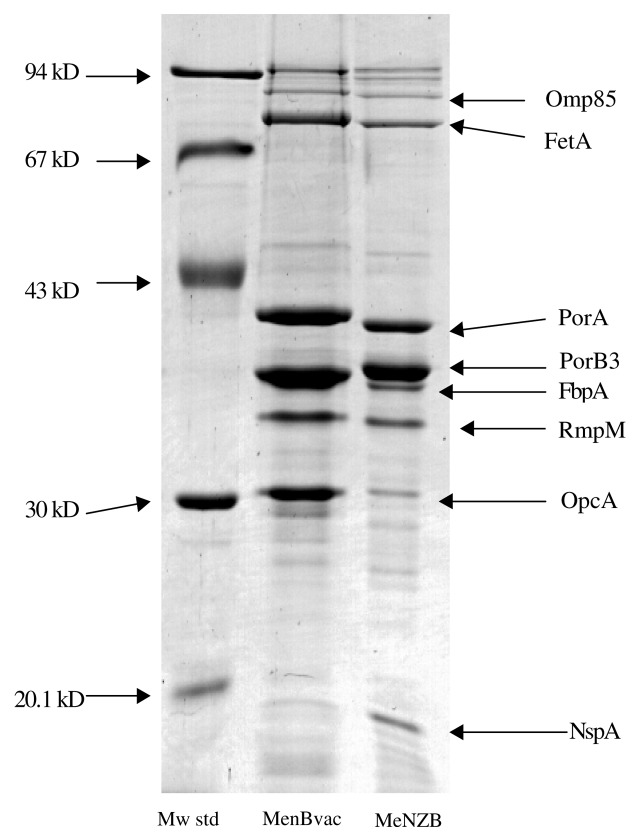
Figure 2. Main Protein Composition* of wtOMVs from MenBvac (NO) and MeNZB (NZ), visualized by CBB staining after SDS-PAGE. *The location in the gel of well0known and characterized components is indicated by their respective acronyms to the right in the figure. Omp85, out membrane protein 85; FetA, ferric enterobactin transporter (protein) A (formerly FrpB); PorA, porin protein A (formerly class 1); PorB3, porin protein B3 (formerly class 3); FbpA, ferric binding protein A; RmpA, reduction modifiable protein M (formerly class 4); OpcA, opacity protein cA (formerly class 5); NspA, Neisserial surface protein A.
Two immunogenicity and reactogenicity trials47,48 sponsored by the Ministry of Health in Iceland, the US Centers for Disease Control and Prevention (CDC), World Health Organization (WHO) and the Pan-American Health Organization (PAHO) directly compared the Cuban and Norwegian wtOMV MenB vaccines. In the two studies performed (one in Reykjavik, Iceland and the other in Santiago, Chile), both vaccines induced good functional immune responses as measured in a serum bactericidal activity test, using human complement (hSBA) against the, homologous MenB strains (in this case only the strains used to make the vaccines), but not against heterologous MenB strains (i.e., carrying a different PorA sero-subtype) as the Chilean P1.4 epidemic strain. Therefore, the monitoring committee and PAHO judged that neither wtOMV vaccine would impact the ongoing MenB clonal epidemic among infants in Chile. Immune responses to wtOMV vaccines especially in infants are largely directed toward the PorA protein; only about 10% of infants mounted a protective antibody response against the Chilean epidemic strain following vaccination with either the Cuban or Norwegian wtOMV vaccine.48 In contrast, approximately half of adult vaccinees had a protective antibody response against the Chilean epidemic strain after either of the two wtOMV vaccines, indicating a less restricted immune response in primed individuals of this age group.48 Reassuringly, both wtOMV vaccines demonstrated good functional immunity; approximately 98%, against their respective vaccine production strain in infants and older age groups, which suggested that a protein based, “tailor-made” vaccine for a defined clonal outbreak was likely to be successful in both adults and children.33,48,49 Another important lesson from these pioneering clinical trials was that a primary immunization with two doses of a wtOMV vaccine was insufficient to maintain long-term protection against MenB disease, especialy in infants.32,33,44
Clonal MenB Epidemic in New Zealand
In 1991, a substantial clonal MenB outbreak began in New Zealand. This outbreak was found to be caused by a strain with a PorA (P1.4) protein that was heterologous to that in the Cuban and Norwegian wtOMV vaccines. The magnitude and ongoing nature of this outbreak made it necessary to develop a new wtOMV vaccine.50-53 The MeNZB® vaccine (Table 1), which was based on a typical isolate, strain NZ 98/254, from the clonal outbreak,54-58 was used between 2004 and 2008 to limit the MenB epidemic. The NZ 98/254 wtOMV (used in MeNZB®) is one of four key immunogenic components of the 4CMenB (Novartis Vaccines and Diagnostics), a multi-component MenB vaccine designed to provide broad protection against most circulating MenB strains. The 4CMenB vaccine has recently been granted Marketing Authorization by the European Medicines Agency under the trade name Bexsero® (http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002333/WC500134836.pdf).59-62 Of note, in this new vaccine formulation the NZ OMV component is combined with three key recombinant protein components: factor H-binding protein (fHbp), Neisserial adhesin A, (NadA) Neisserial heparin-binding antigen (NHBA).61,63-65 Review of the New Zealand experience is particularly important because extensive safety and effectiveness evaluations were undertaken in more than one million vaccine recipients, and also because this type of vaccine component is likely to be used in additional populations. Therefore, the medical community might benefit from consolidated information without very much additional information about the other wtOMV vaccines, as presented in previous reviews. Since fHbp is also being investigated in a bivalent MenB vaccine (fHbp molecules from two different families or main variant groups),66-68 the current body of information is intended as important background information for the development of this and similar; more “universal” MenB vaccines.20,30,31
Table 1. Composition of MeNZB from strain NZ 98/254*.
| Component | Function | Quantity per mL | Quantity per dose | Reference/standard |
|---|---|---|---|---|
| OMV drug substance** | Active ingredient | 50 μg*** | 25 μg*** | Internal |
| Aluminum hydroxide | Adjuvant | 3.3 mg | 1.65 mg | Licensed as a part of a solvent in UK (Chiron SpA, PL 13767/0014) |
| NaCl | Tonicity modifying agent | 9 mg | 4.5 mg | Ph. Eur. |
| Histidine | pH buffering agent | 5 mM | 5 mM | Ph. Eur. |
| WFI | Diluent | to 1 mL | to 0.5 mL | Ph. Eur. |
*B:4:P1.7–2,4 (B:4:P1.7h,4 according to previous classification); **The OMV antigen drug substance contains wtOMV protein, lipopolysaccharide (0.05–0.15 µg/µg protein) deoxycholate (0.1–0.4 µg/µg protein) in 3% sucrose solution. The final sucrose concentration in the formulated drug product is approximately 0.1–0.3%. ***Quantity expressed as total protein amount; PorA content approx. between 13–25% of total protein (see Fig. 2).
In the present review we summarize lessons learned during the development and use of wtOMV vaccines. We have placed a particular emphasis on the history of MeNZB, including the public health intervention to fight the devastating MenB epidemic that occurred in New Zealand from the early 1990s to mid-2000s (Fig. 3). Our choice is based on the fact that this is one of the most recent events where a wtOMV vaccine has been used with success to control an outbreak of MenB disease and that the New Zealand undertaking contains a number of important lessons in addition to the vaccine development per se.
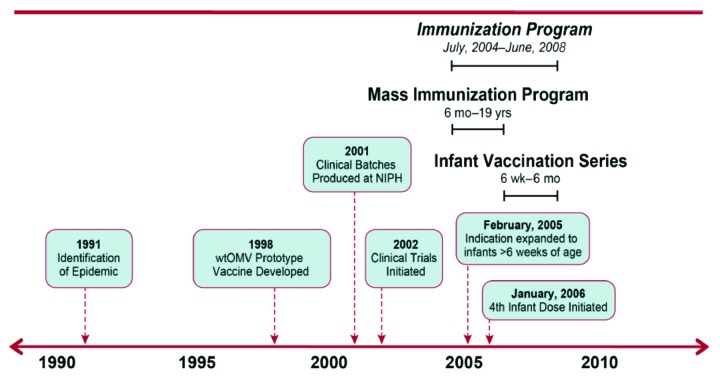
Figure 3. Schematic presentation of vaccine development and immunizationprogram for the public health intervention in New Zealand (the immunization program began in July 2004 and ended in June, 2008. The infant vaccines were given at 6 weeks and at 3, 5 and 10 mo. In January 2006, a 4th infant dose was initiated).
Partnerships to Develop MeNZB
During the 1990s, scientists and health authorities in New Zealand needed research and commercial partners to develop a vaccine for a very small market in global terms. Developing and delivering the vaccine required building partnerships and organizing extensive national and international collaborations, with all groups supporting the goal of ending the epidemic. The key organizations involved were the New Zealand Ministry of Health, the University of Auckland, the Institute of Environmental Science and Research (ESR), Chiron (now Novartis Vaccines and Diagnostics), the NIPH and the WHO. Partnerships were built on contacts established in the early years of New Zealand’s search for a vaccine and relied heavily on the goodwill of people from around the world.69
From 1998 onwards, the WHO was a key player providing a framework for progress and brought together international advisors who mentored and guided New Zealand scientists, clinicians and government officials through the process of vaccine development and approval. The WHO was also able to engage the interest of pharmaceutical companies following earlier assistance by scientific institutes in Norway, the Netherlands and Cuba.69 The WHO helped the New Zealand government select Chiron, based in Siena, Italy to manufacture MeNZB. The New Zealand Ministry of Health had the central contractual relationship with the University of Auckland who ran the clinical trials overseen by Chiron; ESR who performed the hSBA test to measure the vaccine response and partnered with international laboratories for assay validation; and finally Chiron, which worked in close partnership with NIPH, where the initial vaccine development took place. Key to the program’s success was the autonomy in decision making granted to the government and vaccine company staff members charged with leadership of the project, thereby enabling vaccine development to progress with all due urgency.
Vaccine Development of MeNZB
Initial plans for a vaccine to address the New Zealand MenB epidemic focused on novel gene technology developed by Jan T. Poolman and colleagues at the Netherland’s National Institute for Public Health and the Environment (RIVM), later the Netherlands Vaccine Institute (NVI), which allowed the design of custom recombinant OMVs enriched with selected PorA variants.69-71 However, initial clinical data in UK infants who received a recombinant OMV vaccine against multiple PorA sero-subtypes were discouraging because four doses were required to induce substantial functional immune responses; particularly against strains harboring PorA P1.4, the sero-subtype predominating in the New Zealand epidemic.71 Following a recommendation by the Expert Committee of the WHO, the New Zealand authorities decided that they would be best served by a tailor-made MenB vaccine, which was later named MeNZB®.32,33,54,72 Initial development took place at NIPH, based on previous experience developing MenBvac®, the Norwegian wtOMV vaccine.32,38,54,73,74 MeNZB was prepared from a representative epidemic case isolate, strain NZ 98/254 (cc41/44-B:4:P1.7–2,4). Fermenter growth and detergent extraction with the detergent sodium-deoxycholate was used to yield OMVs, which were then adsorbed to aluminum hydroxide. Manufacture and pre-clinical testing of MeNZB started at NIPH33 and yielded data that initially was presented at the International Pathogenic Neisseria Conference (IPNC) in Nice in 1998 and two years later at the IPNC in Oslo.74,75 The first three batches of MeNZB used in safety and immunogenicity studies were manufactured by NIPH (Table 2).33
Table 2. Primary immunogenicity assessments in clinical studies using the serum bactericidal activity test with human complement (hSBA) of MeNZB.
| Age group | Enrollment N |
Percent of vaccinees with hSBA titers ≥ 4 % (95% CI) |
Reference |
|---|---|---|---|
| Infants 6–10 weeks | 250 | 76 (70, 81) 74 (61, 84) |
Wong et al., PIDJ 2009 [87] |
| 239 | 76 (70, 81) | Oster et al., Vaccine 2007 [78] | |
| Booster dose | 51 | 82 (68, 91) | Wong et al., PIDJ 2009 [87] |
| Infants 6–8 mo | 201 | 74 (67, 80) | Oster et al., Vaccine 2005 [77] |
| 211 | 92 (87, 95) | Oster et al., Vaccine 2007 [78] | |
| 312 | 74 (68, 80) | Jackson et al., Arch Dis Child 2009 [58] | |
| Toddlers 16–24 mo | 231 | 75 (69, 80) | Oster et al., Vaccine 2005 [77] |
| 248 | 92 (87, 95) | Oster er al, Vaccine 2007 [78] | |
| 332 | 62 (56, 67) 75 (69, 80) |
Wong et al., PIDJ 2007 [79] | |
| Children 8–12 y | 485 | 76 (72, 80) | Oster et al., Vaccine 2005 [77] |
| Adults 18–50 y | 24 | 96 (79, 100) | Oster et al., Vaccine 2005 [77] |
| Adults; MeNZB compared with MenBvac | 75 | 100 (25µg dose) 87 (50µg dose) |
Thornton et al., Vaccine 2006 [56] |
As indicated above, previous experience with the Cuban and Norwegian wtOMV vaccines32,33,44,48 indicated that a three-dose primary series would most likely be necessary to increase the persistence of immune responses and to optimize the number of persons mounting a protective immune response. To make MeNZB available more quickly, an intensified clinical research program, including 17 clinical trials, was undertaken between May 2002 and November 2004. These clinical studies evaluated immunogenicity and safety outcomes in various age groups and the consistency of vaccines manufactured at different sites.76 Initial clinical trials of MeNZB in New Zealand strengthened the case for a three-dose (25 μg) primary immunization at 6-week intervals, starting at six weeks of age.77-79
The design of the clinical studies that supported licensure of MeNZB relied on knowledge gained through the development of the two other extensively tested wtOMV vaccines from Cuba and Norway. A placebo-controlled efficacy trial was deemed unethical during the ongoing epidemic, especially given evidence that the vaccine induced protective immune responses and the already known acceptable safety profile of similar vaccines.32,72 The MeNZB program used the standard serologic correlate of protection for meningococcal vaccines (a titer > 4), measured in a serum bactericidal assay using human complement (hSBA).80-82 The bactericidal test with rabbit complement (rSBA), sometimes used to evaluate polysaccharide-based vaccines, is less suitable for evaluating responses to wtOMV and other MenB vaccines.81-84 As the evaluation of functional immune response and vaccine effectiveness was of great importance57,77,78,85,86 an international collaboration was established for validation and independent control of the hSBA testing in these studies.83,84
In the initial clinical program, which included 1068 children and adults, MeNZB induced protective levels of serum antibodies and had an acceptable tolerability profile, similar to that observed with the Norwegian and Cuban wtOMV vaccines.76,77 In adults, 87% of those receiving two 25μg doses of the vaccine exhibited a 4-fold rise in serum bactericidal antibodies against the outbreak strain (NZ 98/254).87 Following a three-dose regimen of MeNZB, 74% of 6–8 mo olds and 75% of 16–24 mo-olds showed a 4-fold rise in bactericidal antibody titers against the outbreak strain.77 As expected, serum antibody titers waned after the primary series, especially in young infants, and responses to a booster dose in the second year of life were pronounced.87 See Table 2 and 3 for a complete listing of clinical trial publications.
Table 3. Publications describing effectiveness and safety outcomes of the MeNZB vaccine.
| Citation* (author/year) | Design and aims | Outcomes |
|---|---|---|
| Arnold et al., Vaccine 2011[86] | Effectiveness estimates of MeNZB in children up to 19 y of age using GEE model and NIR data through 2008. Examined waning of the immune response after 12 mo and cross-protection against other meningococci. | Effectiveness of 77–79% for ages 6 mo–19 y during 2002–2008. |
| Galloway et al., Int J Epidemiol 2009 [57] | Cohort analysis from NIR data for children aged 6 mo to < 5 y followed for 24 mo after vaccination with MeNZB. | Effectiveness was 80–85% compared with unvaccinated children in the 24 mo after eligibility for vaccination. |
| Kelly et al., Amer J Epidemiol 2007 [85] | Post licensure effectiveness of MeNZB was estimated using a GEE model and data from the NIR for children aged 6 weeks to 19 y. | Disease rates were 3.7 times higher in the unvaccinated group yielding a vaccine effectiveness of 73%. |
| O’Hallahan et al., NZMJ 2009; 122:48–59 [92] | Outcomes of the MeNZB vaccination program in New Zealand: vaccine coverage, effectiveness, safety risk management. | MenB disease decreased after MeNZB introduction. Safety outcomes were generally positive, and the vaccine exhibited a well-defined reactogenicity profile in various age groups. |
| McNicolas et al., Hum Vaccin 2007; 3:196–204 [90] | Describes intensive safety monitoring activities. | Vaccine was associated with fever outcomes in infants and injection-site pain in adolescents. These events were generally transient and self-limiting. |
*For a more complete list of publications; see the chapters “Safety Monitoring in New Zealand” and “MeNZB Program Effectiveness” in the text. **GEE, generalized estimating equation; NIR, National Immunisation Register
Licensure of MeNZB
The introduction of a new mass immunization campaign requires extensive logistical and regulatory planning. MeNZB was the first vaccine or medicinal product to be designed specifically for use in the New Zealand population. Thus, the evaluation of MeNZB was the first time national regulators in New Zealand were called upon to evaluate a totally new agent without the benefit of prior review by outside regulatory agencies.51,69,76,88 Pre-clinical documentation provided by the manufacturing team was independently reviewed by the UK’s National Institute for Biological Standards and Control (NIBSC), and clinical data were reviewed by the New Zealand Ministry of Health regulatory group, MedSafe. Regulatory reviews and clinical experience with MenBvac, the “parent” vaccine, were also considered.69
As the MenB epidemic had been ongoing for many years, MedSafe considered it particularly important to undertake certain adaptations for the clinical development program to support a more rapid introduction of MeNZB, including “licensure with provisional consent” i.e., use of the vaccine during the actual epidemic so long as additional requirements were met. Further, because earlier OMV vaccines were understood to be moderately reactogenic, specific measures were required to monitor adverse events following immunization (AEFIs) in the absence of Phase III clinical trials for MeNZB.89-91 The initial licensure was for individuals over 6 mo of age, with the vaccination program commencing in July 2004. The program was expanded to infants > 6 weeks of age in February 2005, once adequate safety data were documented. By June 2006, more than 3 million doses had been administered to individuals less than 20 y of age. In January 2006, findings of waning immunity in infants led to the addition of a fourth dose at 10 mo of age for infants who had begun the primary vaccination series before the age of 6 mo.92 The MeNZB vaccine continued to be offered to infants as part of the routine vaccination schedule until June 1, 2008.53,76
The MeNZB Vaccination Program
To supply adequate vaccine for the intended cohort of approximately 1.2 million individuals below 20 y of age, scale-up and industrial manufacturing took place at Chiron in Siena, Italy.53,69,76,86,92
The vaccination campaign rollout was staggered by geographic region, determined primarily by disease burden, the requirements of the safety monitoring program and vaccine availability. The initial rollout was in the highest risk areas, with Auckland and the northern areas being offered vaccine first. These regions were also the areas where the most intensive safety surveillance activities were undertaken.92
At the end of the mass campaign over 90% of those aged under 20 y in the highest risk area had received three doses of MeNZB, with the highest vaccine coverage achieved in Pacific children, the group which suffered disproportionately most from MenB disease.92 Nationally, 81% of those aged under 20 y received three doses of MeNZB.86 The use of a comprehensive and transparent vaccine safety assessment program, with independent oversight helped overcome potential barriers to public acceptance.52,69,88,92
Safety Monitoring in New Zealand
Post-licensure efficacy and safety evaluations were pre-specified as part of the vaccination campaign.58,69,90 For the first time in New Zealand, a nationwide immunization register was established and a comprehensive post-licensure vaccine safety program was implemented. Since multiple surveillance activities increase the ability to detect adverse events, a multi-faceted safety surveillance system was established, with an emphasis on collecting and analyzing data in as close to real time as possible. The safety surveillance program comprised the routine passive reporting system for health professionals already operating in New Zealand as well as three active systems established specifically for the MeNZB program: hospital-based surveillance, the intensive vaccine monitoring program (IVMP) and mortality surveillance. Importantly, an independent safety monitoring board (ISMB) comprising New Zealand and international members provided oversight of the safety monitoring program and reviewed all safety findings.
The hospital-based surveillance operated in the four regions where the vaccine program first started and monitored approximately 200,000 MeNZB recipients for subsequent hospital emergency department consultations or admissions. In particular, this program searched for pre-selected conditions of interest; such as acute flaccid paralysis, encephalopathy and seizures (including febrile seizures). None of the surveillance events occurred at rates in excess of the background rates to be expected in the general population.90,93-95
The IVMP actively reviewed electronic records from 35 sentinel medical centers located across New Zealand, and a cohort of approximately 10,000 children under five years of age were monitored in the six weeks following immunization.89,90 The IVMP’s findings were consistent with those from the New Zealand-wide passive reporting system routinely used by health professionals, i.e., largely localized events, somatic immune responses and mild hypersensitivity events.89,90 As observed in clinical trials with MeNZB (and other wtOMV vaccines), injection site reactions of short duration and short-term fevers were common, but did not cause serious concerns and generally did not require medical intervention.78,87,92,96 Of note, in spite of general fever being common following immunization, there was no evidence of an increase of febrile seizures.94 Finally, an analysis of mortality data from across all of New Zealand did not identify concerns related to MeNZB vaccination.90
With more than three million doses of MeNZB administered to individuals less than 20 y of age, the safety-monitoring program did not reveal any novel or unexpected safety concerns, and provided consistent evidence supporting the safety of the vaccine. The ISMB concluded that the combined results of the safety monitoring program provided confidence in the safety of MeNZB.90
Communications for the Vaccine Campaign in New Zealand
One of the critical elements of the MeNZB campaign was a comprehensive communication strategy to address questions and concerns about the epidemic and the new vaccine in a proactive, responsible way that avoided sensationalism.52,88 In 2004 the Ministry of Health invited New Zealand infectious disease specialists, pediatricians, microbiologists, public health specialists and representatives from its medicines regulatory authority, MedSafe, to a meeting where the first results of the clinical trials in toddlers were presented.97
Throughout the program’s operation, vaccine experts also sought to engage the media and also to avoid public anxiety as a result of unbalanced reporting of negative “newsworthy” incidents. District Health Boards interacted with various stakeholders and undertook local initiatives as needed. Importantly, front-line health care workers were identified to serve as key trusted communicators with parents and vaccine recipients. Substantial information was provided to health professionals, including detailed information about the expected reactogenicity of MeNZB, and presentations were given at meetings throughout the country. These steps ensured that the health sector was well informed and able to confidently champion the vaccine program. Printed information describing the expected side-effects of pain, swelling or redness at the injection site and a slight fever or headache was also given to families through schools and health care providers. As school-aged children were vaccinated at school, resources were also specifically designed for teachers to use in the classroom. Additional communication measures for the public included a toll-free number for parents to access health care providers, a dedicated website an advertising campaign. On-going communication efforts coordinated through the Ministry of Health, District Health Boards and the schools, ensured the program was able to quickly manage a range of issues52,88,92 such as anxiety-related episodes involving 50 students at one school, an influenza B epidemic coinciding with the school vaccination program, and a brief period of intense media and political scrutiny regarding the lack of Phase III trials for MeNZB.92
The introduction of MeNZB had a high level of public acceptance and there was not widespread public concern regarding the safety of the vaccine. Key to this outcome was the establishment of the ISMB. Its role in monitoring adverse events was frequently explained to the public and the media, and the ISMB Chairperson was available to answer specific media queries regarding the vaccine’s safety profile and the expanded safety monitoring program.92 This approach helped to ensure public confidence in the safety of MeNZB and facilitated the speedy resolution of any concerns that arose.
MeNZB Program Effectiveness
Since control of the epidemic was the primary objective of the MeNZB program, vaccine program effectiveness was assessed in a post introduction observational manner. Initial effectiveness, estimated using two different methodologies was 80% (95% CI-: 52.5–91.6%) for children 6 mo to less than 5 y of age57 and 73% (95% CI-: 52–85%) for all ages.57,85,86,98 However, since this was a large-scale introduction rather than a clinical placebo-controlled trial, interpretation of effectiveness was complicated by secular disease trends. In an analysis of disease prior to the vaccine campaign in 2004 showed a steady decrease in incidence between 2001 and 2004, which accelerated following implementation of the vaccination program, indicating a vaccine effect.86 Arnold and colleagues estimated overall vaccine effectiveness using Poisson-regression models adjusted for year, age, season, region, ethnicity and socioeconomic status.86 They also tested for a relationship between the number of doses and effectiveness, and for possible waning effectiveness one year after vaccination. Their approach allowed the vaccine program effect to be differentiated from a secular decrease in disease incidence. Arnold et al. estimated vaccine effectiveness of 77% (95% CI-: 62–85%) over an average period of 3.2 y following the three-dose primary series, but only 68% when potential residual confounding was considered. In partially vaccinated individuals, effectiveness was estimated to be 47% (95% CI-: 16–67%) after two doses of MeNZB. No evidence of waning protection after one year with the full three-dose immunization series could be detected.86 The adjustments for residual confounding resulted from a test for “protection” against pneumococcal disease by MeNZB. An observed dose-response relationship in the level of protection (which could not come from the vaccine itself) was interpreted as a combination of program effects and some degree of residual confounding.86 The correlation of protection and the number of doses further supports the conclusion that the observed effectiveness is vaccine related.
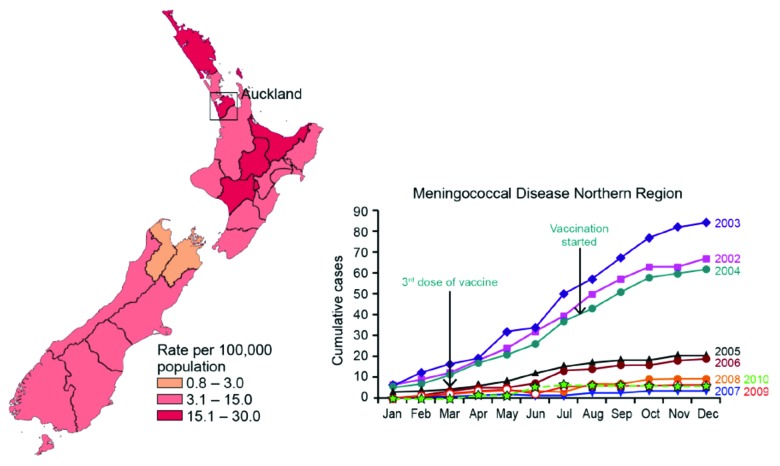
Figure 4 illustrates the decline of the MenB disease among the vaccinated and unvaccinated parts of the population below 20 y of age in New Zealand. As can be seen from the graph in Figure 4 and the data in Table 4, the epidemic was declining before the vaccine campaign started. However, a significantly more rapid decline was demonstrated among the vaccinated individuals. The effect of introducing the vaccine appeared even more dramatic analyzing the cumulative cases of meningococcal disease over the years from 2002 to 2010 in the northern region which had the highest incidence. As described above it was also here where the vaccination started, and within a year the drop in meningococcal cases was significant and by 2007 it was down to pre-epidemic rates in that region (see Fig. 5).
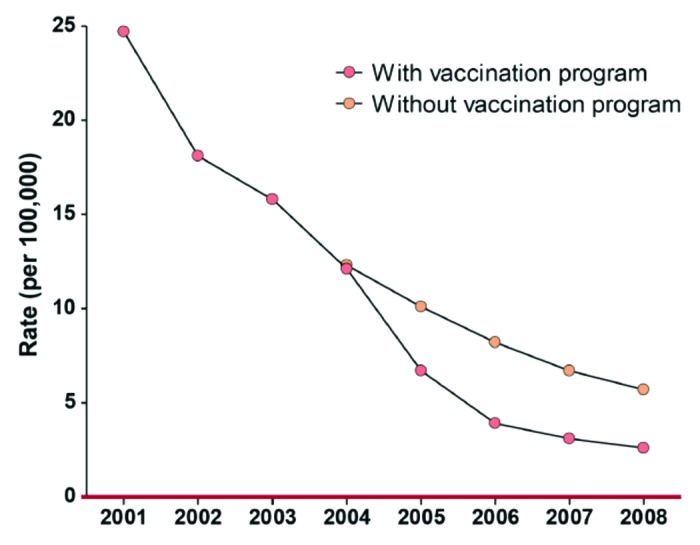
Figure 4. Rates by year of meningococcal B disease in New Zealand (ages 0–19). Diagram based on data given in Table 4.
Table 4. Distribution of cases in New Zealand from 2001–2008 and estimations of cases prevented by MeNZB (all rates are per 100,000)*.
| Year | Actual | Actual | Predicted | Predicted | Cases | Proportion | Prevented |
|---|---|---|---|---|---|---|---|
| Cases | Rate | Cases | Rate | Prevented | Prevented | Est. Total** | |
| 2001 | 285 | 24.7 | 285 | 24.7 | 0 | 0.00% | 0 |
| 2002 | 211 | 18.1 | 211 | 18.1 | 0 | 0.00% | 0 |
| 2003 | 187 | 15.8 | 187 | 15.8 | 0 | 0.00% | 0 |
| 2004 | 144 | 12.1 | 146 | 12.3 | 2 | 1.30% | 2.6 |
| 2005 | 80 | 6.7 | 120 | 10.1 | 39.8 | 33.20% | 47.9 |
| 2006 | 47 | 3.9 | 98 | 8.2 | 51.2 | 52.10% | 61.1 |
| 2007 | 37 | 3.1 | 80 | 6.7 | 43.4 | 54.00% | 50.8 |
| 2008 | 31 | 2.6 | 69 | 5.7 | 37.7 | 54.90% | 45.5 |
*Actual numbers of cases (age 0–19) and rates by year, along with predictions of numbers of cases with no vaccination program, and numbers and proportions of predicted cases that were prevented. 'Cases Prevented' is based on the number of confirmed cases seen. **'Est. Total Prevented' is the number including cases of unknown sero-subtype.
Figure 5. Cumulative number of meningococcal disease cases in the Northern Region of New Zealand from 2002 to 2010 (right; note the Significant Drop between 2004 and 2005). The left part of the figure gives a representation of the Epidemiological data from before the vaccination started (year incidence in 2002 for various regions).
It is worth noting that some protection was also observed against MenB strains other than the outbreak clone (i.e., non-P1.4) with effectiveness of 54%, (or 41% including the correction for potential residual confounding). Since the wtOMV component is not serogroup (i.e., capsule polysaccharide) specific, effectiveness was also calculated against non-MenB disease where effectiveness was found to be 56%, (or 43% corrected for residual confounding).86 These observations are particularly interesting when considering the role of the New Zealand strain wtOMV in the new multi-component vaccine 4CMenB. The wtOMV component is generally expected to protect against strains carrying the same PorA sero-subtype epitopes. However, functional immune responses are also raised against a number of other epitopes and antigenic components; like conserved parts on PorA, PorB, OpcA, Omp85, LPS and likely some other components.32,33,45,48,99,100
Discussion
Over the past 50 y, our understanding of the importance of MenB disease per se, the social impact of fear caused by the devastating effects of the disease, and the role of OMV vaccines in providing protection, has evolved substantially. We now know that wtOMV-based vaccines are most effective when used against epidemics due to a homologous or clonal strain carrying the same PorA as that present in the vaccine. When used against endemic disease or outbreaks due to a number of different strains, (heterologous epidemiologic situations), the level of effectiveness will generally be too low to rely on the effects of a conventional wtOMV vaccine alone for protection. Moreover, multiple doses of such a vaccine will be required for primary protection and a booster dose is required to ensure long-term protection, especially in those who receive their initial vaccine series as young infants.
There were more than 6,000 cases and around 250 deaths caused by meningococcal disease in New Zealand between 1991 and 2006, with approximately 80% of cases due to the epidemic clone targeted by the MeNZB vaccine.53,101 However, following the concerted efforts of an extensive international and national collaboration, including the WHO, Chiron and NIPH; the MeNZB vaccine was developed to control this specific outbreak. A substantial national mobilization in New Zealand, involving complex logistics, monitoring and communication strategies was successfully implemented to meet the public health challenge posed by the meningococcal epidemic. The mass vaccination campaign that started in July 2004 and ended in June 2006, targeted the population below 20 y of age (approximately 1.2 million persons) and resulted in a vaccine uptake of 81%.86 For the period between July 2004 and December 2008 it has been estimated that 210 cases, six deaths and 15–30 cases of severe sequelae were avoided thanks to the MeNZB vaccine.86
The New Zealand epidemic was waning before and during the rollout of MeNZB. However, the staggered introduction of the vaccine enabled year-by-year comparison of rates in vaccinated and unvaccinated populations that allowed the effectiveness of the vaccine to be estimated (see Table 4 and Fig. 4). Simultaneous modeling of invasive pneumococcal disease and the clonal outbreak strain of MenB disease suggests a degree of residual confounding that reduces the effectiveness estimate from 77% to 68%.86 Following the (cumulative) number of MenB cases in the area with the highest incidence in New Zealand (the northern region) from 2002 to 2010 also demonstrate the vaccine impact from one year (2004) to the next (2005); see Figure 5. There was also found some evidence for (lesser) cross-protection against other MenB strains.86 This observation is consistent with the findings of Tappero et al. in Chile, were they found an age-dependent, but clearly functional immune response (hSBA) against non-vaccine type strains; reflecting that other than the immune-dominant antigenic epitopes on PorA also have an effect and play a role in protection.48
The extensive general experience with wtOMV vaccines, and in particular the extensive evaluation of MeNZB in more than one million individuals, provides vital information regarding the safety and acceptability of wtOMV vaccines for widespread use. By the end of 2012 more than 60 million doses of the wtOMV vaccine type have been administered worldwide.91,102 Although these vaccines are moderately reactogenic, in New Zealand local and systemic reactions such as fever were common, but predictable and transient and did not interfere with widespread acceptance of vaccination. A very effective education program to inform parents and recipients regarding the nature of these events likely contributed to the high levels of public acceptance of this vaccine.
Unlike MeNZB, which was designed to provide protection against a clonal outbreak, 4CMenB has been formulated to provide broad-based protection and to be used for routine immunization in various regions of the world. The three recombinant protein components, active in this vaccine, were identified through a process called reverse vaccinology, which starts with the bacterial genome. The multi-component vaccine approach was considered necessary because of the labile nature of the meningococcal genome, differences in protein sequences and surface expression among various MenB strains for the proteins selected as vaccine antigens. The intrinsic ability of the meningococcus to change both through recombination and variability in the degree of surface expression of proteins creates a situation in which any single component vaccine, even if effective initially, would likely become ineffective over time as meningococcus could adapt and become resistant to that particular vaccine. A multi-component strategy severely reduces the ability of the organism to circumvent all antibodies elicited by the vaccine. Based on these observations and insights the novel multi-component vaccine 4CMenB contains four major active ingredients, including the same wtOMV as used in MeNZB.
Apart from the implications of the MeNZB experience for newer OMV containing vaccines such as 4CMenB, the New Zealand program also provides a number of other important broadly applicable public health lessons. Key factors that contributed to the success of the program were the willingness of New Zealand and international parties to make a committed and collaborative support the goal of epidemic control. Lengthy negotiations and discussions built trust and understanding between parties. Those leading the project from the New Zealand government and Chiron Vaccines were given enough autonomy to enable timely progress. The time from when the epidemic started until final identification of a candidate vaccine and subsequent manufacture was lengthy (13 y; 1991 to 2004) and some critics have indicated the need to act more expediently during similar situations in the future.103 However, from July 2001, when Chiron/NIPH was initially contracted to deliver the vaccine; the overall process of vaccine development, completion of clinical trials and vaccine licensure, through to implementation of the vaccine program was much faster than usual (3 y vs. 10–15 y). See Figure 3 for a schematic presentation of the vaccine development and immunization program.
One key lesson from the New Zealand experience is that all countries should be prepared, with regulatory mechanisms in place, to anticipate the possible rapid evaluation and introduction of a new vaccine. The recent H1N1 influenza pandemic is a case in point for all countries, but this lesson might be especially relevant for developing countries where new vaccines for malaria, typhoid and other diseases for which no prior experience in Europe or the US exists, will become available. Such situations will also require local oversight and evaluation, active surveillance, adequate epidemiology and sufficient strain characterization. In Cuba, Norway and New Zealand32 the basis for using the concept of wtOMV vaccines was the selection of a manufacturing strain that matched the clone causing the epidemic. In each case, a measured approach to vaccine evaluation and introduction was undertaken. In considering approaches to any public health emergency, there will always be a tension between the need to introduce a new intervention quickly and the need to ensure that the intervention is safe and effective. The extent and success of the post-introduction evaluation in New Zealand could provide the impetus to develop protocols for earlier introduction of interventions for public health emergencies, which are associated with contemporaneous evaluation, thus reducing the need for extensive, time consuming pre-introduction evaluations. This will most likely happen when much is already known about the vaccine or other interventions.
Conclusion
Meningococcal wtOMV vaccines have been employed for decades (since the 1980s) and administered to more than 30 million individuals. These vaccines have been effective and associated with a well-characterized and acceptable safety profile. The major limitation of these wtOMV vaccines is that their immune response provides protection mainly against strains that are homologous (i.e., harboring the same PorA, sero-subtype protein) to the outbreak strain used to develop the vaccine.32,48 This shortcoming has restricted the utility of wtOMV vaccines to large on-going epidemics, and public health benefits have been limited due to the long delay in formulation. To address these concerns and make management of MenB disease a programmatic possibility rather than an episodic event, a multi-component vaccine (4CMenB), which includes the wtOMV used in MeNZB, has recently been designed for widespread use and coverage against multiple strains and diverse epidemiological situations globally.59-63 Thus, even novel technologies in this field draw on previous experience with wtOMV vaccines. Additional knowledge and experience for use of the wtOMV concept can also be gleaned from the handling of a localized clonal outbreak in Normandy, France34,104,105 and from pre-clinical and clinical studies using so-called native OMV vaccines; where the LPS has been genetically de-toxified (lpx1-mutants), avoiding the need for detergent extraction and with overexpressed vaccine antigens naturally folded in the membrane.106-112 These various promising vaccine approaches owe much to the pioneering experiences gained by using wtOMV vaccines and in particular such large-scale public health interventions as the one that took place in New Zealand with the MeNZB vaccine.
Acknowledgments
This article is largely indebted to Lisa DeTora (Albany Medical College, New York, USA, formerly of Novartis Vaccines and Diagnostics, Cambridge, USA), for her everlasting enthusiasm and continuous editorial support. Julio Vazquez (Hospital Marques de Valdecilla, Santander, Spain) is thanked for generously providing an earlier version of Figure 1, which was further developed for this article. Giorgio Corsi (Novartis Vaccine and Diagnostics, Siena, Italy) is thanked for originally designing Figure 5. We would also like to thank Susan Myers and Gerard P. Johnson of Complete Healthcare Communications, Inc. (Chadds Ford, PA, USA), whose assistance with figure and table preparation was funded by Novartis Vaccines and Diagnostics. James Wassil (Novartis Vaccines and Diagnostics, Cambridge, USA) and Julie A. Bettinger (Vaccine Evaluation Centre, BC Children’s Hospital and the University of British Columbia, Canada) are both thanked warmly for helpful comments to earlier versions of the manuscript.
Disclosure of Potential Conflicts of Interest
J.H.: Employee of NIPH and has performed consulting activities for Wyeth Vaccines Research (now Pfizer) and Novartis Vaccines and Diagnostics; in addition served as a temporary advisor on a number of occasions for WHO and PAHO. P.O.: Employee of Sanofi Pasteur (an employee of Chiron/Novartis during the MeNZB development and vaccine implementation in New Zealand). No other conflict of interest. R.A., M.V.T., L.M.N., I.S.A.. Y.G., A.M., J.O. and E.R.: All employees of their respective organizations and with no other conflict of interest. SB: Is an employee of Center for Global Health, Cincinnati Children’s Hospital consultant for Novartis Vaccines and serve on DSMBs (Independent Data Safety Monitoring Board) for GSK and Novartis Vaccines for Global Health.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/24129
References
- 1.Vieusseux M. Memoire sur le maladie qui a regne a Geneva au printemps de 1805. J Med Clin Pharm. 1805;11:163–82. [Google Scholar]
- 2.Danielson L, Mann E. A history of a singular and very noted disease, which lately made its appearance in Medfield. Medical and Agricultural Register. 1806;1:65–9. [Google Scholar]
- 3.Weichselbaum A. Ueber die aetiologie der akuten meningitis cerebro-spinal. Fortschr Med. 1887;5:573–83, 620-6. [Google Scholar]
- 4.Flexner S. The results of the serum treatment in thirteen hundred cases of epidemic meningits. J Exp Med. 1913;17:553–76. doi: 10.1084/jem.17.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenwood M. The outbreak of cerebrospinal fever at Salisbury in 1914-15. Proc R Soc Med. 1917;10(Sect Epidemiol State Med):44–60. doi: 10.1177/003591571701001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephens DS. Uncloaking the meningococcus: dynamics of carriage and disease. Lancet. 1999;353:941–2. doi: 10.1016/S0140-6736(98)00279-7. [DOI] [PubMed] [Google Scholar]
- 7.Stephens DS. Conquering the meningococcus. FEMS Microbiol Rev. 2007;31:3–14. doi: 10.1111/j.1574-6976.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 8.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369:2196–210. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- 9.de Souza AL, Seguro AC. Two centuries of meningococcal infection: from Vieusseux to the cellular and molecular basis of disease. J Med Microbiol. 2008;57:1313–21. doi: 10.1099/jmm.0.47599-0. [DOI] [PubMed] [Google Scholar]
- 10.Tyler KL. Chapter 28: a history of bacterial meningitis. Handb Clin Neurol. 2010;95:417–33. doi: 10.1016/S0072-9752(08)02128-3. [DOI] [PubMed] [Google Scholar]
- 11.Goldacre MJ, Roberts SE, Yeates D. Case fatality rates for meningococcal disease in an English population, 1963-98: database study. BMJ. 2003;327:596–7. doi: 10.1136/bmj.327.7415.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sørensen HT, Steffensen FH, Schønheyder HC, Nielsen GL, Hansen I, Madsen KM, et al. Trend in incidence and case fatality of meningococcal disease over 16 years in Northern Denmark. Eur J Clin Microbiol Infect Dis. 1998;17:690–4. doi: 10.1007/s100960050162. [DOI] [PubMed] [Google Scholar]
- 13.Pollard AJ, Frasch C. Development of natural immunity to Neisseria meningitidis. Vaccine. 2001;19:1327–46. doi: 10.1016/S0264-410X(00)00333-9. [DOI] [PubMed] [Google Scholar]
- 14.Pollard AJ. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr Infect Dis J. 2004;23(Suppl):S274–9. [PubMed] [Google Scholar]
- 15.Gardner P. Clinical practice. Prevention of meningococcal disease. N Engl J Med. 2006;355:1466–73. doi: 10.1056/NEJMcp063561. [DOI] [PubMed] [Google Scholar]
- 16.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–26. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotschlich EC, Goldschneider I, Artenstein MS. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969;129:1367–84. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hankins WA, Gwaltney JM, Jr., Hendley JO, Farquhar JD, Samuelson JS. Clinical and serological evaluation of a meningococcal polysaccharide vaccine groups A, C, Y, and W135. Proc Soc Exp Biol Med. 1982;169:54–7. doi: 10.3181/00379727-169-41306. [DOI] [PubMed] [Google Scholar]
- 19.Griffiss JM, Brandt BL, Altieri PL, Pier GB, Berman SL. Safety and immunogenicity of group Y and group W135 meningococcal capsular polysaccharide vaccines in adults. Infect Immun. 1981;34:725–32. doi: 10.1128/iai.34.3.725-732.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan LK, Carlone GM, Borrow R. Advances in the development of vaccines against Neisseria meningitidis. N Engl J Med. 2010;362:1511–20. doi: 10.1056/NEJMra0906357. [DOI] [PubMed] [Google Scholar]
- 21.Lewis S, Sadarangani M, Hoe JC, Pollard AJ. Challenges and progress in the development of a serogroup B meningococcal vaccine. Expert Rev Vaccines. 2009;8:729–45. doi: 10.1586/erv.09.30. [DOI] [PubMed] [Google Scholar]
- 22.Gasparini R, Panatto D. Meningococcal glycoconjugate vaccines. Hum Vaccin. 2011;7:170–82. doi: 10.4161/hv.7.2.13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bröker M, Cooper B, Detora LM, Stoddard JJ. Critical appraisal of a quadrivalent CRM(197) conjugate vaccine against meningococcal serogroups A, C W-135 and Y (Menveo) in the context of treatment and prevention of invasive disease. Infect Drug Resist. 2011;4:137–47. doi: 10.2147/IDR.S12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keyserling H, Papa T, Koranyi K, Ryall R, Bassily E, Bybel MJ, et al. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch Pediatr Adolesc Med. 2005;159:907–13. doi: 10.1001/archpedi.159.10.907. [DOI] [PubMed] [Google Scholar]
- 25.Bilukha OO, Rosenstein N, National Center for Infectious Diseases, Centers for Disease Control and Prevention (CDC) Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2005;54(RR-7):1–21. [PubMed] [Google Scholar]
- 26.LaForce FM, Okwo-Bele J-M. Eliminating epidemic Group A meningococcal meningitis in Africa through a new vaccine. Health Aff (Millwood) 2011;30:1049–57. doi: 10.1377/hlthaff.2011.0328. [DOI] [PubMed] [Google Scholar]
- 27.Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344:1378–88. doi: 10.1056/NEJM200105033441807. [DOI] [PubMed] [Google Scholar]
- 28.Wyle FA, Artenstein MS, Brandt BL, Tramont EC, Kasper DL, Altieri PL, et al. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972;126:514–21. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- 29.Bruge J, Bouveret-Le Cam N, Danve B, Rougon G, Schulz D. Clinical evaluation of a group B meningococcal N-propionylated polysaccharide conjugate vaccine in adult, male volunteers. Vaccine. 2004;22:1087–96. doi: 10.1016/j.vaccine.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Granoff DM. Review of meningococcal group B vaccines. Clin Infect Dis. 2010;50(Suppl 2):S54–65. doi: 10.1086/648966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadarangani M, Pollard AJ. Serogroup B meningococcal vaccines-an unfinished story. Lancet Infect Dis. 2010;10:112–24. doi: 10.1016/S1473-3099(09)70324-X. [DOI] [PubMed] [Google Scholar]
- 32.Holst J, Martin D, Arnold R, Huergo CC, Oster P, O’Hallahan J, et al. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine. 2009;27(Suppl 2):B3–12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 33.Holst J, Feiring B, Naess LM, Norheim G, Kristiansen P, Høiby EA, et al. The concept of “tailor-made”, protein-based, outer membrane vesicle vaccines against meningococcal disease. Vaccine. 2005;23:2202–5. doi: 10.1016/j.vaccine.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 34.Taha MK, Zarantonelli ML, Alonso JM, Naess LM, Holst J, Feiring B, et al. Use of available outer membrane vesicle vaccines to control serogroup B meningococcal outbreaks. Vaccine. 2007;25:2537–8. doi: 10.1016/j.vaccine.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 35.Helting TB, Guthöhrlein G, Blackkolb F, Ronneberger H. Serotype determinant protein of Neisseria Meningitidis. Large scale preparation by direct detergent treatment of the bacterial cells. Acta Pathol Microbiol Scand C. 1981;89:69–78. [PubMed] [Google Scholar]
- 36.Tsai CM, Frasch CE, Mocca LF. Five structural classes of major outer membrane proteins in Neisseria meningitidis. J Bacteriol. 1981;146:69–78. doi: 10.1128/jb.146.1.69-78.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frasch CE. Meningococcal Vaccines: Past, Present and Future. In: Cartwright K, editor. Meningococcal Disease. New York: John Wiley & Sons, 1995: 245-83. [Google Scholar]
- 38.Frasch C, Van Alphen L, Holst J, Poolman J, Rosenqvist E. Outer membrane protein vesicle vaccines for meningococcal disease. In: Pollard AJ, Maiden MC, editors. Meningococcal vaccines: methods and protocols. Totowa, New Jersey: Humana Press, 2001: 81-107. [DOI] [PubMed] [Google Scholar]
- 39.Zollinger WD, Boslego J, Frøholm LO, Ray JS, Moran EE, Brandt BL. Human bactericidal antibody response to meningococcal outer membrane protein vaccines. Antonie Van Leeuwenhoek. 1987;53:403–11. doi: 10.1007/BF00415494. [DOI] [PubMed] [Google Scholar]
- 40.Zollinger WD, Mandrell RE, Altieri P, Berman S, Lowenthal J, Artenstein MS. Safety and immunogenicity of a Neisseria meningitidis type 2 protein vaccine in animals and humans. J Infect Dis. 1978;137:728–39. doi: 10.1093/infdis/137.6.728. [DOI] [PubMed] [Google Scholar]
- 41.Frasch CE, Robbins JD. Protection against group B meningococcal disease. III. Immunogenicity of serotype 2 vaccines and specificity of protection in a guinea pig model. J Exp Med. 1978;147:629–44. doi: 10.1084/jem.147.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sierra GV, Campa HC, Varcacel NM, Garcia IL, Izquierdo PL, Sotolongo PF, et al. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14:195–207, discussion 208-10. [PubMed] [Google Scholar]
- 43.Bjune G, Høiby EA, Grønnesby JK, Arnesen O, Fredriksen JH, Halstensen A, et al. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338:1093–6. doi: 10.1016/0140-6736(91)91961-S. [DOI] [PubMed] [Google Scholar]
- 44.Holst J, Feiring B, Fuglesang JE, Høiby EA, Nøkleby H, Aaberge IS, et al. Serum bactericidal activity correlates with the vaccine efficacy of outer membrane vesicle vaccines against Neisseria meningitidis serogroup B disease. Vaccine. 2003;21:734–7. doi: 10.1016/S0264-410X(02)00591-1. [DOI] [PubMed] [Google Scholar]
- 45.Rosenqvist E, Høiby EA, Wedege E, Bryn K, Kolberg J, Klem A, et al. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect Immun. 1995;63:4642–52. doi: 10.1128/iai.63.12.4642-4652.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feiring B, Fuglesang J, Oster P, Naess LM, Helland OS, Tilman S, et al. Persisting immune responses indicating long-term protection after booster dose with meningococcal group B outer membrane vesicle vaccine. Clin Vaccine Immunol. 2006;13:790–6. doi: 10.1128/CVI.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perkins BA, Jonsdottir K, Briem H, Griffiths E, Plikaytis BD, Høiby EA, et al. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J Infect Dis. 1998;177:683–91. doi: 10.1086/514232. [DOI] [PubMed] [Google Scholar]
- 48.Tappero JW, Lagos R, Ballesteros AM, Plikaytis B, Williams D, Dykes J, et al. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA. 1999;281:1520–7. doi: 10.1001/jama.281.16.1520. [DOI] [PubMed] [Google Scholar]
- 49.Wenger JD. Serogroup B meningococcal disease: new outbreaks, new strategies. JAMA. 1999;281:1541–3. doi: 10.1001/jama.281.16.1541. [DOI] [PubMed] [Google Scholar]
- 50.Martin DR, Walker SJ, Baker MG, Lennon DR. New Zealand epidemic of meningococcal disease identified by a strain with phenotype B:4:P1.4. J Infect Dis. 1998;177:497–500. doi: 10.1086/517385. [DOI] [PubMed] [Google Scholar]
- 51.O’Hallahan J, Lennon D, Oster P, Lane R, Reid S, Mulholland K, et al. From secondary prevention to primary prevention: a unique strategy that gives hope to a country ravaged by meningococcal disease. Vaccine. 2005;23:2197–201. doi: 10.1016/j.vaccine.2005.01.061. [DOI] [PubMed] [Google Scholar]
- 52.O'Hallahan J, Martin D, Oster P. An epidemic of group B meningococcal of disease controlled by a vaccine - the final chapter. 15th International Pathogenic Neisseria Conference; 2006 September 10-15, 2006; Cairns, Australia; 2006. p. 42. [Google Scholar]
- 53.Loring BJ, Turner N, Petousis-Harris H. MeNZB vaccine and epidemic control: when do you stop vaccinating? Vaccine. 2008;26:5899–904. doi: 10.1016/j.vaccine.2008.08.062. [DOI] [PubMed] [Google Scholar]
- 54.Holst J, Aaberge IS, Oster P, Lennon D, Martin D, O'Hallahan J, et al. A “tailor made” vaccine trialled as part of public health response to group B meningococcal epidemic in New Zealand. Euro Surveill. 2003;7:pii=2262. [Google Scholar]
- 55.Martin DR, Ruijne N, McCallum L, O’Hallahan J, Oster P. The VR2 epitope on the PorA P1.7-2,4 protein is the major target for the immune response elicited by the strain-specific group B meningococcal vaccine MeNZB. Clin Vaccine Immunol. 2006;13:486–91. doi: 10.1128/CVI.13.4.486-491.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thornton V, Lennon D, Rasanathan K, O’Hallahan J, Oster P, Stewart J, et al. Safety and immunogenicity of New Zealand strain meningococcal serogroup B OMV vaccine in healthy adults: beginning of epidemic control. Vaccine. 2006;24:1395–400. doi: 10.1016/j.vaccine.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 57.Galloway Y, Stehr-Green P, McNicholas A, O’Hallahan J. Use of an observational cohort study to estimate the effectiveness of the New Zealand group B meningococcal vaccine in children aged under 5 years. Int J Epidemiol. 2009;38:413–8. doi: 10.1093/ije/dyn228. [DOI] [PubMed] [Google Scholar]
- 58.Jackson C, Lennon DR, Sotutu VT, Yan J, Stewart JM, Reid S, et al. Phase II meningococcal B vesicle vaccine trial in New Zealand infants. Arch Dis Child. 2009;94:745–51. doi: 10.1136/adc.2007.132571. [DOI] [PubMed] [Google Scholar]
- 59.Giuliani MM, Adu-Bobie J, Comanducci M, Aricò B, Savino S, Santini L, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A. 2006;103:10834–9. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bai X, Findlow J, Borrow R. Recombinant protein meningococcal serogroup B vaccine combined with outer membrane vesicles. Expert Opin Biol Ther. 2011;11:969–85. doi: 10.1517/14712598.2011.585965. [DOI] [PubMed] [Google Scholar]
- 61.Su EL, Snape MD. A combination recombinant protein and outer membrane vesicle vaccine against serogroup B meningococcal disease. Expert Rev Vaccines. 2011;10:575–88. doi: 10.1586/erv.11.32. [DOI] [PubMed] [Google Scholar]
- 62.Pizza M, DeTora L, Wassil J. Advances in meningococcal vaccines. Clin Pract. 2012;9:101–17. doi: 10.2217/cpr.11.128. [DOI] [Google Scholar]
- 63.Bai XF, Findlow J, Borrow R. Recombinant protein meningococcal serogroup B vaccine combined with outer membrane vesicles. Expert Opin Biol Ther. 2011;11:969–85. doi: 10.1517/14712598.2011.585965. [DOI] [PubMed] [Google Scholar]
- 64.Sow SO, Okoko BJ, Diallo A, Viviani S, Borrow R, Carlone G, et al. Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans. N Engl J Med. 2011;364:2293–304. doi: 10.1056/NEJMoa1003812. [DOI] [PubMed] [Google Scholar]
- 65.Bambini S, Muzzi A, Olcen P, Rappuoli R, Pizza M, Comanducci M. Distribution and genetic variability of three vaccine components in a panel of strains representative of the diversity of serogroup B meningococcus. Vaccine. 2009;27:2794–803. doi: 10.1016/j.vaccine.2009.02.098. [DOI] [PubMed] [Google Scholar]
- 66.Jansen KU, McNeil LK, Dragalin V, Anderson AS, Hoiseth SK, Arora A, et al. Bivalent recombinant LP2086 vaccine to provide broad protection against Neisseria meningitidis B disease: Immunological correlates of protection and how to assess coverage against invasive MnB strains. In: van Alphen L, van Ley P, van den Dobbelsteen G, editors. 16th International Pathogenic Neisseria Conference. Rotterdam, The Netherlands, 2008: abstract 064. [Google Scholar]
- 67.Murphy E, Andrew L, Lee KL, Dilts DA, Nunez L, Fink PS, et al. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J Infect Dis. 2009;200:379–89. doi: 10.1086/600141. [DOI] [PubMed] [Google Scholar]
- 68.Jiang HQ, Hoiseth SK, Harris SL, McNeil LK, Zhu D, Tan C, et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine. 2010;28:6086–93. doi: 10.1016/j.vaccine.2010.06.083. [DOI] [PubMed] [Google Scholar]
- 69.Tyson J, Norman R. Fighting a Fearful Disease; Controlling New Zealand's meningococcal B epidemic. Institute of Policy Studies, Victoria University of Wellington, The Australian and New Zealand School of Government 2007. [Google Scholar]
- 70.Claassen I, Meylis J, van der Ley P, Peeters C, Brons H, Robert J, et al. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine. 1996;14:1001–8. doi: 10.1016/0264-410X(96)00020-5. [DOI] [PubMed] [Google Scholar]
- 71.Cartwright K, Morris R, Rümke H, Fox A, Borrow R, Begg N, et al. Immunogenicity and reactogenicity in UK infants of a novel meningococcal vesicle vaccine containing multiple class 1 (PorA) outer membrane proteins. Vaccine. 1999;17:2612–9. doi: 10.1016/S0264-410X(99)00044-4. [DOI] [PubMed] [Google Scholar]
- 72.Holst J. Strategies for development of universal vaccines against meningococcal serogroup B disease: the most promising options and the challenges evaluating them. Hum Vaccin. 2007;3:290–4. doi: 10.4161/hv.4513. [DOI] [PubMed] [Google Scholar]
- 73.Fredriksen JH, Rosenqvist E, Wedege E, Bryn K, Bjune G, Frøholm LO, et al. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1991;14:67–79, discussion 79-80. [PubMed] [Google Scholar]
- 74.Bjaering Hansen N, Høiby EA, Caugant DA, Frøholm LO, Holst J, Tangen T, et al. Development and evaluation of experimental meningococcal B:4:P1.(7),4 outer membrane vaccines. In: Nassif X, Quentin-Millet M-J, Taha M-K, editors. 11th International Pathogenic Neisseria Conference; 1998 November 1-6, 1998; Nice, France: Editions E.D.K.; 1998. p. 169. [Google Scholar]
- 75.Rosenqvist E, Bryn K, Harbak K, Holst J, Høiby EA, Kristiansen P, et al. Development of a tailor-made outer membrane vesicle vaccine against the group B meningococcal epidemic in New Zealand. In: Caugant, DA Wedege, E editors 13th International Pathogenic Neisseria Conference; September 1-6, 2002 Oslo: Nordberg Aksidenstrykkeri AS; 2002 p64 2002. [Google Scholar]
- 76.Lennon D, Jackson C, Wong S, Horsfall M, Stewart J, Reid S. Fast tracking the vaccine licensure process to control an epidemic of serogroup B meningococcal disease in New Zealand. Clin Infect Dis. 2009;49:597–605. doi: 10.1086/603552. [DOI] [PubMed] [Google Scholar]
- 77.Oster P, Lennon D, O’Hallahan J, Mulholland K, Reid S, Martin D. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine. 2005;23:2191–6. doi: 10.1016/j.vaccine.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 78.Oster P, O’Hallahan J, Aaberge I, Tilman S, Ypma E, Martin D. Immunogenicity and safety of a strain-specific MenB OMV vaccine delivered to under 5-year olds in New Zealand. Vaccine. 2007;25:3075–9. doi: 10.1016/j.vaccine.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 79.Wong S, Lennon D, Jackson C, Stewart J, Reid S, Crengle S, et al. New zealand epidemic strain meningococcal B outer membrane vesicle vaccine in children aged 16-24 months. Pediatr Infect Dis J. 2007;26:345–50. doi: 10.1097/01.inf.0000258697.05341.2c. [DOI] [PubMed] [Google Scholar]
- 80.Balmer P, Borrow R. Serologic correlates of protection for evaluating the response to meningococcal vaccines. Expert Rev Vaccines. 2004;3:77–87. doi: 10.1586/14760584.3.1.77. [DOI] [PubMed] [Google Scholar]
- 81.Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection--serum bactericidal antibody activity. Vaccine. 2005;23:2222–7. doi: 10.1016/j.vaccine.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 82.Borrow R, Carlone GM, Rosenstein N, Blake M, Feavers I, Martin D, et al. Neisseria meningitidis group B correlates of protection and assay standardization--international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine. 2006;24:5093–107. doi: 10.1016/j.vaccine.2006.03.091. [DOI] [PubMed] [Google Scholar]
- 83.Martin D, McCallum L, Glennie A, Ruijne N, Blatchford P, O’Hallahan J, et al. Validation of the serum bactericidal assay for measurement of functional antibodies against group B meningococci associated with vaccine trials. Vaccine. 2005;23:2218–21. doi: 10.1016/j.vaccine.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 84.Borrow R, Aaberge IS, Santos GF, Eudey TL, Oster P, Glennie A, et al. Interlaboratory standardization of the measurement of serum bactericidal activity by using human complement against meningococcal serogroup b, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin Diagn Lab Immunol. 2005;12:970–6. doi: 10.1128/CDLI.12.8.970-976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kelly C, Arnold R, Galloway Y, O’Hallahan J. A prospective study of the effectiveness of the New Zealand meningococcal B vaccine. Am J Epidemiol. 2007;166:817–23. doi: 10.1093/aje/kwm147. [DOI] [PubMed] [Google Scholar]
- 86.Arnold R, Galloway Y, McNicholas A, O’Hallahan J. Effectiveness of a vaccination programme for an epidemic of meningococcal B in New Zealand. Vaccine. 2011;29:7100–6. doi: 10.1016/j.vaccine.2011.06.120. [DOI] [PubMed] [Google Scholar]
- 87.Wong SH, Lennon DR, Jackson CM, Stewart JM, Reid S, Ypma E, et al. Immunogenicity and tolerability in infants of a New Zealand epidemic strain meningococcal B outer membrane vesicle vaccine. Pediatr Infect Dis J. 2009;28:385–90. doi: 10.1097/INF.0b013e318195205e. [DOI] [PubMed] [Google Scholar]
- 88.O’Hallahan J, Lennon D, Oster P. The strategy to control New Zealand’s epidemic of group B meningococcal disease. Pediatr Infect Dis J. 2004;23(Suppl):S293–8. [PubMed] [Google Scholar]
- 89.Tatley MV, Kunac DL, McNicholas A, Zhou L, Ballantyne S, Ashton J, et al. The Intensive Vaccines Monitoring Programme (IVMP): an electronic system to monitor vaccine safety in New Zealand. Vaccine. 2008;26:2746–52. doi: 10.1016/j.vaccine.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 90.McNicholas A, Galloway Y, Stehr-Green P, Reid S, Radke S, Sexton K, et al. Post-marketing safety monitoring of a new group B meningococcal vaccine in New Zealand, 2004-2006. Hum Vaccin. 2007;3:196–204. doi: 10.4161/hv.3.5.4458. [DOI] [PubMed] [Google Scholar]
- 91.Holst J, Nøkleby H, Bettinger JA. Considerations for controlling invasive meningococcal disease in high income countries. Vaccine. 2012;30(Suppl 2):B57–62. doi: 10.1016/j.vaccine.2011.12.093. [DOI] [PubMed] [Google Scholar]
- 92.O’Hallahan J, McNicholas A, Galloway Y, O’Leary E, Roseveare C. Delivering a safe and effective strain-specific vaccine to control an epidemic of group B meningococcal disease. N Z Med J. 2009;122:48–59. [PubMed] [Google Scholar]
- 93.Sexton K, McNicholas A, Galloway Y, Radke S, Kieft C, Stehr-Green P, et al. Henoch-Schönlein purpura and meningococcal B vaccination. Arch Dis Child. 2009;94:224–6. doi: 10.1136/adc.2007.125195. [DOI] [PubMed] [Google Scholar]
- 94.Stehr-Green P, Radke S, Kieft C, Galloway Y, McNicholas A, Reid S. The risk of simple febrile seizures after immunisation with a new group B meningococcal vaccine, New Zealand. Vaccine. 2008;26:739–42. doi: 10.1016/j.vaccine.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 95.Stehr-Green P, Galloway Y, Kieft C, McNicholas A. The risk of bronchiolitis hospitalisation following administration of a group B meningococcal vaccine in New Zealand. N Z Med J. 2007;120:U2746. [PubMed] [Google Scholar]
- 96.Nøkleby H, Aavitsland P, O’Hallahan J, Feiring B, Tilman S, Oster P. Safety review: two outer membrane vesicle (OMV) vaccines against systemic Neisseria meningitidis serogroup B disease. Vaccine. 2007;25:3080–4. doi: 10.1016/j.vaccine.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 97.Sexton K, Lennon D, Oster P, Crengle S, Martin D, Mulholland K, et al. The New Zealand Meningococcal Vaccine Strategy: a tailor-made vaccine to combat a devastating epidemic. N Z Med J. 2004;117:U1015. [PubMed] [Google Scholar]
- 98.McNicholas A, Galloway Y, Martin D, Sexton K, O’Hallahan J. Surveillance of vaccine breakthrough cases following MeNZB vaccination. N Z Med J. 2008;121:38–46. [PubMed] [Google Scholar]
- 99.Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci U S A. 2010;107:19490–5. doi: 10.1073/pnas.1013758107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wedege E, Høiby EA, Rosenqvist E, Bjune G. Immune responses against major outer membrane antigens of Neisseria meningitidis in vaccinees and controls who contracted meningococcal disease during the Norwegian serogroup B protection trial. Infect Immun. 1998;66:3223–31. doi: 10.1128/iai.66.7.3223-3231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martin D, Lopez L, Sexton K. The epidemiology of meningococcal disease in New Zealand in 2006. Report from Institute of Environmental Science and Research Ltd, Wellington 2007. [Google Scholar]
- 102.Sotolongo FP, Campa C, Casanueva VG, Fajardo EM, Cuevas IE, González N. Cuban meningococcal BC Vaccine: experiences and contributions from 20 years of application. MEDICC Rev. 2007;9:16–22. doi: 10.37757/MR2007V9.N1.6. [DOI] [PubMed] [Google Scholar]
- 103.Lennon D, Reid S, Stewart J, Jackson C, Crengle S, Percival T. Reducing inequalities with vaccines: New Zealand’s MeNZB vaccine initiative to control an epidemic. J Paediatr Child Health. 2012;48:193–201. doi: 10.1111/j.1440-1754.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- 104.Caron F, du Châtelet IP, Leroy JP, Ruckly C, Blanchard M, Bohic N, et al. From tailor-made to ready-to-wear meningococcal B vaccines: longitudinal study of a clonal meningococcal B outbreak. Lancet Infect Dis. 2011;11:455–63. doi: 10.1016/S1473-3099(11)70027-5. [DOI] [PubMed] [Google Scholar]
- 105.Caron F, Delbos V, Houivet E, Deghmane AE, Leroy JP, Hong E, et al. Evolution of immune response against Neisseria meningitidis B:14:P1.7,16 before and after the outer membrane vesicle vaccine MenBvac. Vaccine. 2012;30:5059–62. doi: 10.1016/j.vaccine.2012.05.051. [DOI] [PubMed] [Google Scholar]
- 106.Koeberling O, Seubert A, Granoff DM. Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor H-binding protein and genetically attenuated endotoxin. J Infect Dis. 2008;198:262–70. doi: 10.1086/589308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pajon R, Fergus AM, Koeberling O, Caugant DA, Granoff DM. Meningococcal factor H binding proteins in epidemic strains from Africa: implications for vaccine development. PLoS Negl Trop Dis. 2011;5:e1302. doi: 10.1371/journal.pntd.0001302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fisseha M, Chen P, Brandt B, Kijek T, Moran E, Zollinger W. Characterization of native outer membrane vesicles from lpxL mutant strains of Neisseria meningitidis for use in parenteral vaccination. Infect Immun. 2005;73:4070–80. doi: 10.1128/IAI.73.7.4070-4080.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Keiser PB, Biggs-Cicatelli S, Moran EE, Schmiel DH, Pinto VB, Burden RE, et al. A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine. 2011;29:1413–20. doi: 10.1016/j.vaccine.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 110.Zollinger WD, Babcock JG, Moran EE, Brandt BL, Matyas GR, Wassef NM, et al. Phase I study of a Neisseria meningitidis liposomal vaccine containing purified outer membrane proteins and detoxified lipooligosaccharide. Vaccine. 2012;30:712–21. doi: 10.1016/j.vaccine.2011.11.084. [DOI] [PubMed] [Google Scholar]
- 111.Moran EE, Burden R, Labrie JE, 3rd, Wen Z, Wang XM, Zollinger WD, et al. Analysis of the bactericidal response to an experimental Neisseria meningitidis vesicle vaccine. Clin Vaccine Immunol. 2012;19:659–65. doi: 10.1128/CVI.00070-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van der Ley P, van den Dobbelsteen G. Next-generation outer membrane vesicle vaccines against Neisseria meningitidis based on nontoxic LPS mutants. Hum Vaccin. 2011;7:886–90. doi: 10.4161/hv.7.8.16086. [DOI] [PubMed] [Google Scholar]