Abstract
The present study was undertaken to compare the immunogenicity and reactogenicity of two diphtheria-tetanus-whole cell pertussis (DTwP) vaccines administered to Iranian preschool children. In this randomized, double-blind and multicenter prospective study, 672 children aged 4–6 y were administered with either a local DTwP vaccine (DTwP-Local) (n = 337) or a commercial vaccine (DTwP-Pasteur) (n = 335). All subjects received DTwP vaccine at 4–6 y of age, following the national immunization schedule of Iran. Blood samples were collected before and 2–4 weeks after the vaccination. Immunogenicity of each vaccine was assessed by ELISA using commercial kits. Reactogenicity was assessed by the parents for seven days post-booster using diary cards. The geometric mean titers (GMTs) of the antibodies induced against diphtheria and tetanus by DTwP-Local were 7.7 and 9.4 IU/ml and those of DTwP-Pasteur were 8.2 and 8.6 IU/ml, respectively. There was no significant difference between the immunogenicity of the two vaccines against diphtheria and tetanus. The GMTs of antibodies produced against pertussis were 30.2 EU/ml for DTwP-Local and 47.9 EU/ml for DTwP-Pasteur vaccines (p < 0.001). Pain and fever (axillary temperature > 37.5°C) were the most frequent local and systemic reactions observed after the vaccination. All local and systemic reactions observed after vaccination were significantly higher in subjects immunized with DTwP-Local vaccine. Immunogenicity against diphtheria and tetanus was similar for the two vaccines, but immunogenicity of the local vaccine against pertussis was significantly less efficient than that of DTwP-Pasteur. This difference and the higher side effects of the DTwP-Local vaccine could be due to the bacterial strain or the preparation or formulation protocol of the local pertussis vaccine.
Keywords: Diphtheria-tetanus-pertussis vaccine, vaccination, immunogenicity, reactogenicity, enzyme-linked immunosorbent assay, randomized controlled trial
Introduction
Whole cell pertussis vaccines combined with tetanus and diphtheria toxoid (DTwP) have been in use since 1950s in Iran. The national DTwP vaccination program consists of three primary doses given at 2, 4 and 6 mo of age, with a forth dose given at 18th month and a fifth dose, between 4 to six years of age. For coverage of pertussis vaccination, two different types of pertussis vaccine are currently available, the whole cell (wP) vaccine that was developed in the 1940s and the acellular (aP) vaccine. Both types of vaccine provide protection, though the wP vaccines are thought to induce more efficient immunity.1,2 In spite of the universal vaccination programs against pertussis, Bordetella pertussis resumes to circulate even in populations with high vaccine coverage in infants and children.3 This is evident from the current increase in pertussis incidence in adolescent and young adults,4,5 who serve as an important reservoir of transmission of the pathogen.
In Iran, the registered cases of diphtheria, tetanus and pertussis were 106,14 and 464 cases in 2010 and 132,18 and 650 in 2011, respectively.6 Although the benefit from the DTwP vaccine was established by previous studies,7-9 however, periodic assessment of the vaccine is necessary for national vaccination programs which serves as the basis of systemic immunization policy.10,11 Assessment of local vaccines is performed regularly based on the national regulations of the Food and Drug Administration of the Ministry of Health, Treatment and Medical Education of Iran. According to these regulations a randomized study needs to be performed to evaluate immunogenicity and reactogenicity of such vaccines.
In the present study immunogenicity and reactogenicity of a DTwP vaccine manufactured locally (DTwP-Local) were compared with those induced by a commercial DTwP vaccine (DTwP-Pasteur) in a group of preschool Iranian children.
Results
Demographic data
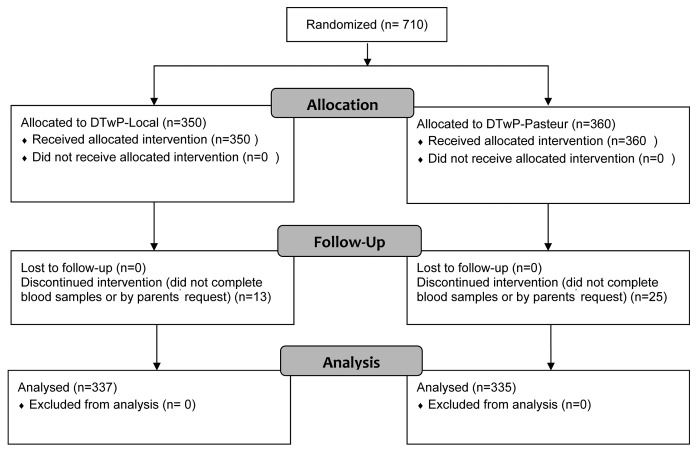
Among a total of 710 children who entered the study, 38 (5%) failed to continue the investigation (Fig. 1). The most common reason for withdrawal was refusal of the parents or the children to give blood samples. None of the participants withdrew due to side reactions to any of the vaccines. Demographic characteristics of the participants were similar between the groups (Table 1).
Figure 1. CONSORT (Consolidated Standards of Reporting Trials) participant flow diagram.
Table 1. Demographic characteristics of the children vaccinated with either DTwP-Local or DTwP-Pasteur vaccine.
| Characteristics | DTwP-Local | DTwP-Pasteur | P-value |
|---|---|---|---|
| Number of subjects | 337 | 335 | NA |
| Gendera (female/male) |
193/144 | 176/159 | 0.22 |
| Weightb (Kg) | 20.57 ± 3.43 | 20.56 ± 3.39 | 0.79 |
| Birth weightb (Kg) | 3.17 ± 0.46 | 3.18 ± 0.47 | 0.99 |
aChi-Square test; b t-test; NA: Not applicable
Immunogenicity
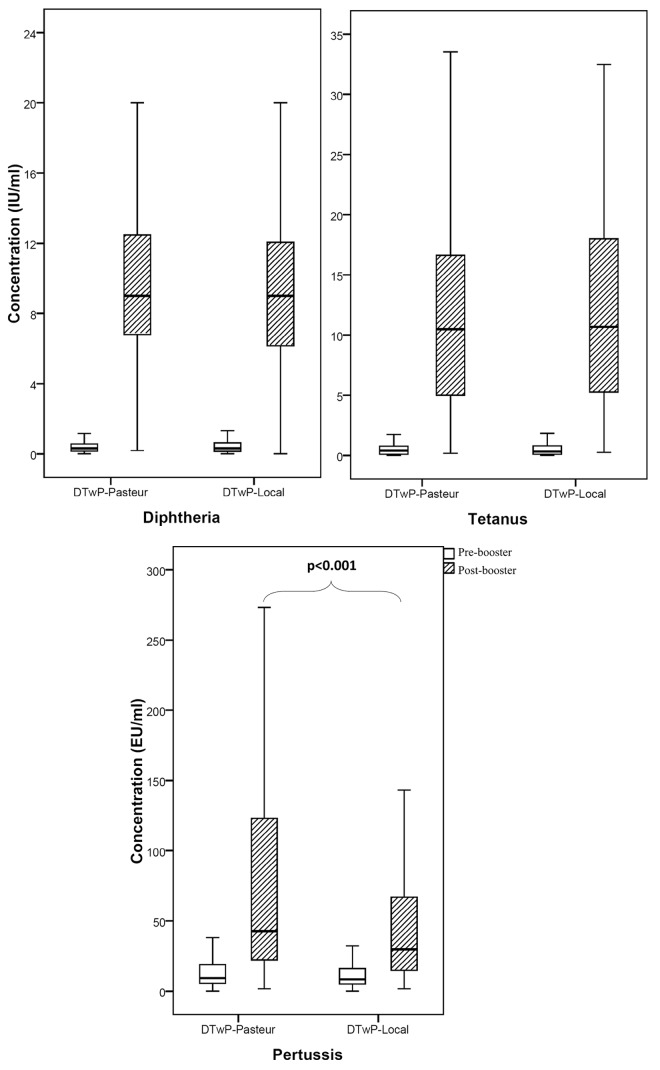
Before vaccination, there was no significant difference in the antibody titers against diphtheria, tetanus and pertussis between the subjects receiving the two vaccines (Table 2). After vaccination, the geometric mean titers (GMTs) of the antibodies induced against diphtheria and tetanus by DTwP-Local were 7.7 and 9.4 IU/ml and those of DTwP-Pasteur were 8.2 and 8.6 IU/ml, respectively (Table 2). There was no significant difference between the immunogenicity of the vaccines against diphtheria (power = 93%) and tetanus (power = 86%). The post-booster GMTs of antibodies produced against pertussis were 30.2 EU/ml for DTwP-Local and 47.9 EU/ml for DTwP-Pasteur vaccines, respectively (p < 0.001). Box plot presentation of antibody concentrations in pre- and post-booster samples is shown in Figure 2. Pre-booster seroprotection rates of pertussis were 25.2% and 31% which were raised to 70.3% and 85.7% upon immunization with DTwP-Local and DTwP-Pasteur vaccines, respectively (Table 2). Using the binary logistic regression analysis, no significant effect of sex, weight and birth weight on post-booster antibody titers of diphtheria, tetanus and pertussis were observed.
Table 2. Comparison of seroprotection rate and geometric mean titer between children immunized with either DTwP-Local or DTwP-Pasteur vaccines.
| Antibody response | Samples | Seroprotection rate | GMT (CI 95%) | post vs. pre | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DTwP-Local n(%) | DTwP-Pasteur n(%) | DTwP-Local | DTwP-Pasteur | p-value1 | Mean ± SD2 | p-value | ||||
| DTwP-Local | DTwP-Pasteur | |||||||||
| Diphtheria (IU/ml) |
Pre-booster | 227(92.2) | 281(83.9) | 0.25(0.22–0.29) | 0.25(0.21–0.29) | 0.76 | 11.68 ± 8 | 10.77 ± 7.3 | 0.122 | |
| Post-booster | 335(99.4) | 335(100) | 7.76(7.03–8.58) | 8.24(7.63–8.90) | 0.36 | |||||
| Tetanus (IU/ml) |
Pre-booster | 234(69.4) | 248(74) | 0.29(0.25–0.33) | 0.30(0.26–0.34) | 0.74 | 9.56 ± 5.17 | 9.47 ± 5.48 | 0.833 | |
| Post-booster | 337(100) | 335(100) | 9.37(8.58–10.24) | 8.66(7.92–9.48) | 0.22 | |||||
| Pertussis (EU/ml) |
Pre-booster | 85(25.2) | 104(31) | 8.41(7.26–9.75) | 9.59(8.12–11.31) | 0.25 | 53.63 ± 28.24 | 72.41 ± 55.66 | < 0.001 | |
| Post-booster | 237(70.3) | 287(85.7) | 30.20(27.01–33.77) | 47.96(42.99–53.51) | < 0.001 | |||||
1 t-test of GMTs;2 Mean (standard deviation) antibody titer differences between pre- and post-booster samples
Figure 2. Box plot presentation of serum antibody titers against diphtheria, tetanus and pertussis in Iranian pre-school children. The box length is the interquartile range. Bars show the range from 10th to 90th percentiles. (▬), median
Safety and reactogenicity
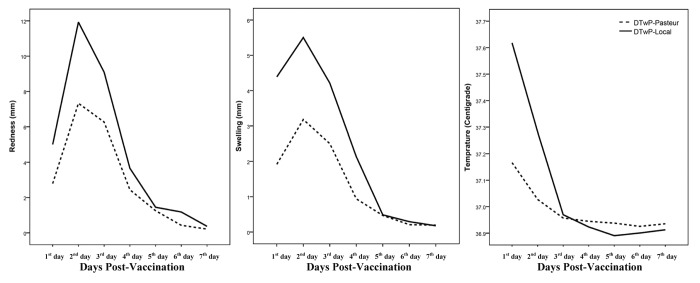
The incidence of local and systemic reactions recorded during the seven days after vaccination is shown in Table 3. The day of vaccine administration was considered as the first day of the study. No serious life-threatening adverse events related to the vaccination were observed. The most frequent local and systemic reactions were pain and auxiliary temperature in both vaccines. All reactions were observed to have significantly reduced during the observation period for both vaccines (p < 0.05). Figure 3 shows the descending trend of redness, swelling and auxiliary temperature during the observation period. The systemic reactions (loss of appetite, gastrointestinal problem, vomiting and eczema) were observed in a small number of children, and disappeared gradually during a week. Significant differences were seen between the two vaccines for pain (1st day until 3rd day, p < 0.01), redness (1st and 2nd days, p < 0.01), swelling (only 1st day, p < 0.01), auxiliary temperature (only 1st day, p < 0.001) and loss of appetite (1st and 2nd days, p ≤ 0.01). The binary logistic regression analysis revealed no significant effect of sex, weight and birth weight of the children on the local and systemic reactions, with the exception of the effect of gender on pain in DTwP-Local vaccine (female, odds ratio = 1.89, 95%, confidence interval = 1.04–3.45, p = 0.037, relative to male) and gender on gastrointestinal problems in DTwP-Local vaccine (female, odds ratio = 4.89, 95%, confidence interval = 1.36–17.64, p = 0.015, relative to male).
Table 3. Frequency of local and systemic reactions reported during the first week of administration of DTwP vaccines in Iranian pre-school children.
| DTwP-Local (n = 337) | DTwP-Pasteur (n = 335) | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st day | 3rd day | 5th day | 7th day | 1st day | 3rd day | 5th day | 7th day | ||
| Local reactions | |||||||||
| Pain | |||||||||
| Any | 86.9 | 56.4 | 21.1 | 10.4 | 77.9 | 48.4 | 18.8 | 13.7 | < 0.001a |
| Grade 3 | 17.8 | 4.2 | 0.3 | 0 | 10.7 | 2.1 | 0 | 0 | |
| Redness | |||||||||
| Any | 39.8 | 35.9 | 8.3 | 2.7 | 31.9 | 25.7 | 7.2 | 2.4 | < 0.001a |
| Grade 3 | 6.8 | 13.6 | 1.8 | 0.6 | 3.3 | 9.9 | 1.2 | 0.3 | |
| Swelling | |||||||||
| Any | 38.6 | 26.7 | 3.3 | 1.2 | 25.4 | 18.5 | 4.8 | 2.4 | < 0.001a |
| Grade 3 | 6.5 | 6.5 | 0.9 | 0 | 2.4 | 3.9 | 0.6 | 0.3 | |
| Systemic reactions | |||||||||
| Auxiliary temperature | |||||||||
| Any | 41.5 | 5.9 | 0.3 | 0.3 | 23 | 5.1 | 1.2 | 0.6 | < 0.001a |
| Grade 3 | 3.6 | 0.3 | 0 | 0 | 0.6 | 0.3 | 0 | 0 | |
| Loss of appetite | 24 | 11.9 | 3 | 1.5 | 16.1 | 6.9 | 1.5 | 0.6 | < 0.001b |
| Gastrointestinal problem | 5.9 | 3.6 | 0.6 | 0.6 | 4.5 | 3.3 | 0.3 | 0 | < 0.001b |
| Vomiting | 8 | 0.9 | 0.6 | 0 | 5.4 | 0 | 0 | 0 | < 0.001b |
| Eczema | 2.7 | 4.2 | 1.2 | 0.6 | 2.1 | 1.5 | 0.3 | 0.3 | < 0.05b |
The results represent percent of cases with or without the specified complications. Pain: Grade 3, crying when limb was moved/spontaneously painful; Tenderness/swelling: Grade 3, > 20 mm. Fever: Grade 3, ≥ 39°C; aFriedman test; bCochran test.
Figure 3. Comparison of reactogenicity of DTwP vaccines in Iranian pre-school children during the first week of vaccination.
Discussion
In the present case-control double-blind study, we investigated immunogenicity and reactogenicity of a local DTwP vaccine in a group of Iranian pre-school children. Previous studies have shown that immunity to all three components of DTwP vaccine declines over time showing an age-related decrease in serum antibody concentrations.15 Waning of the humoral immune response after primary vaccination suggests the need for booster vaccination in pre-school children.16 Thus, administration of the fifth dose of DTwP vaccine at 4–6 y of age has been incorporated in the national EPI vaccination program in many countries, including Iran. Additional vaccination of adults with an extra booster dose of DTaP vaccine has also been recommended to control transmission of pertussis to neonates and children.17
Analysis of the serum antibody levels in pre-booster samples indicates similar seroprotection rates and GMT between the two groups of vaccinees, confirming randomization of the subjects receiving the two types of vaccines. Furthermore, the pre-booster data also indicate that the majority of the subjects were already seroprotected against diphtheria (84–92%) and tetanus (69–74%) components. For pertussis, however, the seroprotection rate was low (25–31%) prior to vaccination, implying lower immunogenicity and waning of the antibody response to pertussis compared with other components of the DTwP vaccine. Although, drop of the serum antibody levels below the protective cut-off does not necessarily indicate complete loss of the specific humoral response due to persistence of a number of the antigen specific circulating memory B cells,18 it proposes the use of booster vaccine doses to keep the adaptive antibody response upregulated in case of exposure to pertussis infection.
Since most recent investigations on DTP immunization have been performed using DTaP vaccine or a combination of DTwP vaccine with other vaccines such as hepatitis and hemophilus influenzae, the results of these studies may not be comparable to our results.15,17,19,20 To be able to compare and contrast the results obtained from this study, we employed a commercial DTwP vaccine (DTwP-Pasteur) which has been extensively used in many countries.13 Analysis of the immunogenicity of the pertussis component of this vaccine had already shown vaccine efficacy and seroprotection rate of DTwP-Pasteur in 84–100% and 92% of subjects, respectively.13
Comparison of GMT and seroprotection rate between DTwP-Local and DTwP-Pasteur vaccines showed similar immunogenicity for diphtheria and tetanus components, but immunogenicity of the pertussis component of DTwP-Local was significantly lower than that of the DTwP- Pasteur. While 25.2% of children were already seroprotected against pertussis prior to booster vaccination with a GMT of 8.41 EU/ml, the seroprotection rate rised to 70.3% and the GMT to 30.20 EU/ml, after administration of a booster dose of DTwP-Local (Table 2). Our results obtained from the commercial DTwP-Pasteur vaccine are also compatible with previous studies in which a DTwP vaccine from another commercial source (Glaxo Smith Kline (GSK) Biologicals, Belgium) was employed as a booster dose.19 The seroprotection rates obtained for all three components of the latter vaccine were similar to those of our control DTwP-Pasteur vaccine.
The age, sex, weight and birth weight of our children were not significantly associated to the immunization outcome, a finding already reported by other investigators.21
Comparison of reactogenicity of the local vaccine with the control vaccine shows higher incidence and intensity of complications in children vaccinated with the local vaccine (Table 3). Assessment of reactogenicity and incidence of serious adverse events of our control vaccine between 1993 to 1997, after administration of 458 million doses, indicates very low incidence of serious adverse events.13 Some of the complications induced by the local vaccine after booster administration to preschool children have already been reported.19
In the present study we compared the incidence of both local and systemic complications induced by both vaccines in a pattern similar to that adapted by other investigators.22-24
Almost all complications were observed at higher frequency in children vaccinated with the local vaccine, particularly in the first day post-booster. Of the children vaccinated with the GSK DTwP vaccine, adverse reactions were reported at a similar frequency as those recorded for our control DTwP-Pasteur vaccinated subjects.19 Approximately 30–70% of children vaccinated with the GSK DTwP vaccine displayed various local and systemic reactions.
The significantly higher reactogenicity and lower immunogenicity of the DTwP-Local vaccine against pertussis may be related to the bacterial strain, the method employed to attenuate the bacteria or the formulation protocol of the vaccine.
Subjects and Methods
Study design
A randomized, double-blind and multicenter prospective study was designed to compare DTwP-Local and DTwP-Pasteur vaccines. The study was performed in four health centers affiliated to Shahid Beheshti University of Medical Sciences in Tehran, Iran from April 2006 to June 2007. Healthy children between 4 to 6 y old who participated in routine EPI vaccination were included in the study. Written informed consent was obtained from the parents of all children before enrollment into the study. Eligibility criteria for inclusion and exclusion in the study followed the Iranian National Immunization Program's Recommendation for routine DTwP vaccination12
Randomization and blinding
Participants were randomized in blocks of size 10, at a 1:1 ratio. All children were randomized to receive either DTwP-Local or DTwP-Pasteur (Fig. 1). The type of vaccine used was blinded to the participants and the study data analyst (double-blind), through assignment of a code to every participant. In the database, the vaccines were designated as codes “0” and “1” to blind the analyst to the type of vaccine. The codes were not identified until finalization of statistical analyses or when requested by a physician due to an adverse event that required inspection. For the analysis (“intent-to-treat”), comparison of GMTs of both groups was disclosed.
Data and sample collection
Reactogenicity was assessed by the parents for seven days post-booster using the prepared questionnaire. A five ml blood sample was collected on the vaccination day and 2–4 weeks after vaccination to measure serum levels of diphtheria, tetanus and pertussis antibodies.
Vaccination
The DTwP vaccines used were (1) DTwP-Local vaccine manufactured by Razi Vaccine and Serum Research Institute of Iran; and (2) DTwP-Pasteur vaccine (D.T.Coq, Aventis Pasteur, Lyon, France) France. Application and handling of the vaccines followed the recommendations from the manufacturers and from the Iranian National Immunization Program.12 Based on the information given in the instruction sheet, DTwP-Local vaccine consisted of 15 Lf (limes flocculation) diphtheria toxoid, 10 Lf tetanus toxoid, 16 International Unit per ml (IU) inactivated B. pertussis bacterial cells, 0.3 to 0.6 mg aluminum phosphate (metal ion) and 0.01% merthiolate (instruction sheet provided by the manufacturer). DTwP-Pasteur vaccine is prepared by heat inactivation of two strains of B. pertussis (IM 1414 and IM 1416, from Massachusetts). The killed B. pertussis bacteria were combined with aluminum hydroxide (0.6–1.25 mg). Each dose of DTwP-Pasteur also contained formaldehyde inactivated diphtheria toxoid (IM1514 from PW8; not less than 30 IU) and formaldehyde inactivated tetanus toxoid (IM1472, From Harvard 49205; not less than 60 IU).13
Ethics
The study protocol was elaborated according to the resolution of Food and Drug Administration of Ministry of Health, Treatment and Medical Education of Iran and approved by the Avicenna Research Institute Ethics Committee. Written informed consent was obtained from all parents of subjects included in the study. The trial was registered in the Iranian Registry of Clinical Trials (www.irct.ir) and assigned a registration number of IRCT138902072471N2.
Laboratory tests
Antibody concentration was determined in serum samples taken immediately before and 2–4 weeks post-booster. All antibody levels were measured by commercial ELISA kits (IBL-Hamburg GmbH, Hamburg, Germany). Optical density was measured at 450 nm by an ELISA reader (Anthos 2020, Anthos Labtec, Salzburg, Austria). The assay cut-offs for protective levels of diphtheria and tetanus antibodies were set at 0.1 IU/ml, based on the position papers of WHO.6,14 Since, there is no defined serological correlate of protection for pertussis, antibody titer was used with an assay cut-off of 16 ELISA units per ml (EU/ml) as defined by the manufacturer. The sensitivity limits of the ELISA kits for diphtheria and tetanus were both 0.004 IU/ml and that of the pertussis ELISA kit was 1 EU/ml.
Assessment of reactogenicity
Subjects were monitored for any immediate reactions for 30 min post vaccination, following the Iranian National Immunization Program’s Recommendation. All participants who received DTwP vaccines were followed for reactogenicity analysis, which evaluates events that may occur from the initiation of vaccination until seven days after vaccination. Parents were trained to record and register all local (pain, injection site redness and swelling) and systemic [fever- auxiliary temperature > 37.5°C, loss of appetite, gastrointestinal problems (diarrhea or constipation), vomiting and eczema] reactions on diary cards daily. The reactogenicity was graded on a 3-point scale (grade 1 = easily tolerated, normal activity, grade 2 = discomfort, interferes with normal activity, and grade 3 = prevents normal activity). Pain was scored as: minor reaction to touch (grade 1); cries/ protests to touch or limb movement (grade 2) or spontaneous pain (grade 3). Tenderness/swelling diameter was graded: < 5 mm (grade 1); 5- 20 mm (grade 2); > 20 mm (grade 3). Fever was graded: 37.5–38°C (grade 1); > 38 and < 39°C (grade 2); ≥ 39°C (grade 3). Other systemic reactions were recorded as yes or no. Parents were also asked to record any additional symptoms that may occur within seven days post vaccination. Serious adverse events were reported immediately during the whole study period. Parents observing a serious adverse event were asked to contact study personnel and bring the subject to the health center for evaluation as soon as possible.
Data analyses
Pre- and post-booster results were compared for both vaccines. The rate of seroprotection or seroconversion, GMT and frequency of complications were calculated. Differences of GMTs between the study groups were analyzed by t-test. The chi-square test was used to evaluate statistical significance of ratios. The data of local and systemic reactions was evaluated by a non-parametric Friedman or Cochran test as appropriate to determine the significance of trend of the decrease of reactogenicity. The effect of age, sex, weight and birth weight on post-booster antibody titers and reactogenicity were assessed using linear regression test with stepwise and backward method. For multiple testing strategies, considering the sample size, all demographic covariates (sex, weight and birth weight) were included in multiple regression models with stepwise and backward method, and type I error for multiple test was 0.05. Confidence intervals of 95% were defined for the estimates. Data was analyzed with the software SPSS version 13.0 for Windows and all probability values of less than 0.05 were considered significant.
Acknowledgments
The authors are grateful to the parents who accepted to allow their infants to enter in the study. We also thank the WHO Regional Office for Middle East Asia, Tehran, for providing the DTwP-Pasteur vaccines. We are indebted to the personnel of Mohammadian, Dawazdah Bahman, Safdari and Salavati Health Centers affiliated to Shahid Beheshti University of Medical Science for their assistance in vaccination and sample collection, especially Dr Afsoon Rahimian, Dr Fatemeh Mostafavi, Mrs Farast Kaveh, Mr Alireza Asgarnejad and Mr Ajatallah Makaremi. This work was supported by a grant from the Food and Drug Administration of the Ministry of Health, Treatment and Medical Education of Iran.
Grant Support
This work was supported by a grant from Food and Drug Administration of the Ministry of Health and Medical Education of Iran
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/24093
References
- 1.Pichichero ME, Green JL, Francis AB, Marsocci SM, Lynd AM, Litteer T. Comparison of a three-component acellular pertussis vaccine with whole cell pertussis vaccine in two-month-old children. Pediatr Infect Dis J. 1994;13:193–6. doi: 10.1097/00006454-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Rieber N, Graf A, Hartl D, Urschel S, Belohradsky BH, Liese J. Acellular pertussis booster in adolescents induces Th1 and memory CD8+ T cell immune response. PLoS One. 2011;6:e17271. doi: 10.1371/journal.pone.0017271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowcroft NS, Pebody RG. Recent developments in pertussis. Lancet. 2006;367:1926–36. doi: 10.1016/S0140-6736(06)68848-X. [DOI] [PubMed] [Google Scholar]
- 4.von König CH, Halperin S, Riffelmann M, Guiso N. Pertussis of adults and infants. Lancet Infect Dis. 2002;2:744–50. doi: 10.1016/S1473-3099(02)00452-8. [DOI] [PubMed] [Google Scholar]
- 5.Campins-Martí M, Cheng HK, Forsyth K, Guiso N, Halperin S, Huang LM, et al. International Consensus Group on Pertussis Immunisation Recommendations are needed for adolescent and adult pertussis immunisation: rationale and strategies for consideration. Vaccine. 2001;20:641–6. doi: 10.1016/S0264-410X(01)00393-0. [DOI] [PubMed] [Google Scholar]
- 6.Tetanus vaccine. Wkly Epidemiol Rec. 2006;81:198–208. [PubMed] [Google Scholar]
- 7.Nazari F. Mass immunity against diphtheria and tetanus in some urban and rural areas in Iran. . Arch Inst Razi. 1973:49–55. [Google Scholar]
- 8.Nazari F, Mirchamsy H, Alé-Agha S, Mahinpour M. A model for developing countries of mass serological survey of children vaccinated against diphtheria and tetanus. J Biol Stand. 1976;4:329–35. doi: 10.1016/S0092-1157(76)80017-0. [DOI] [PubMed] [Google Scholar]
- 9.Zarei S, Jeddi-Tehrani M, Akhondi MM, Zeraati H, Pourheidari F, Ostadkarampour M, et al. Primary immunization with a triple diphtheria-tetanus-whole cell pertussis vaccine in Iranian infants: an analysis of antibody response. Iran J Allergy Asthma Immunol. 2009;8:85–93. [PubMed] [Google Scholar]
- 10.Launay O, Toneatti C, Bernède C, Njamkepo E, Petitprez K, Leblond A, et al. Antibodies to tetanus, diphtheria and pertussis among healthy adults vaccinated according to the French vaccination recommendations. Hum Vaccin. 2009;5:341–6. doi: 10.4161/hv.5.5.7575. [DOI] [PubMed] [Google Scholar]
- 11.Tansel O, Ekuklu G, Eker A, Kunduracilar H, Yuluğkural Z, Yüksel P. Community-based seroepidemiology of diphtheria and tetanus in Edirne, Turkey. Jpn J Infect Dis. 2009;62:275–8. [PubMed] [Google Scholar]
- 12.CDC. Expanded program on immunization (EPI), Islamic republic of Iran, Immunization schedule. Tehran: Ministry of Health, Treatment and Medical Education of Iran, 2010. [Google Scholar]
- 13.Fletcher MA, Saliou P, Ethevenaux C, Plotkin SA. The efficacy of whole cell pertussis immunisation: collected data on a vaccine produced in France. Public Health. 2001;115:119–29. doi: 10.1038/sj/ph/1900745. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. WHO position paper. Diphtheria vaccine. Weekly epidemiological record / Health Section of the Secretariat of the League of Nations. 81. 2006:21–32. [Google Scholar]
- 15.Konda T, Kamachi K, Iwaki M, Matsunaga Y. Distribution of pertussis antibodies among different age groups in Japan. Vaccine. 2002;20:1711–7. doi: 10.1016/S0264-410X(02)00045-2. [DOI] [PubMed] [Google Scholar]
- 16.Forsyth K, Nagai M, Lepetic A, Trindade E. Pertussis immunization in the global pertussis initiative international region: recommended strategies and implementation considerations. Pediatr Infect Dis J. 2005;24(Suppl):S93–7. doi: 10.1097/01.inf.0000160921.74004.12. [DOI] [PubMed] [Google Scholar]
- 17.Van Damme P, Burgess M. Immunogenicity of a combined diphtheria-tetanus-acellular pertussis vaccine in adults. Vaccine. 2004;22:305–8. doi: 10.1016/j.vaccine.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Hendrikx LH, Oztürk K, de Rond LG, Veenhoven RH, Sanders EA, Berbers GA, et al. Identifying long-term memory B-cells in vaccinated children despite waning antibody levels specific for Bordetella pertussis proteins. Vaccine. 2011;29:1431–7. doi: 10.1016/j.vaccine.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Kosuwon P, Warachit B, Hutagalung Y, Borkird T, Kosalaraksa P, Bock HL, et al. Reactogenicity and immunogenicity of reduced antigen content diphtheria-tetanus-acellular pertussis vaccine (dTpa) administered as a booster to 4-6 year-old children primed with four doses of whole-cell pertussis vaccine. Vaccine. 2003;21:4194–200. doi: 10.1016/S0264-410X(03)00496-1. [DOI] [PubMed] [Google Scholar]
- 20.Halperin SA, Scheifele D, Mills E, Guasparini R, Humphreys G, Barreto L, et al. Nature, evolution, and appraisal of adverse events and antibody response associated with the fifth consecutive dose of a five-component acellular pertussis-based combination vaccine. Vaccine. 2003;21:2298–306. doi: 10.1016/S0264-410X(03)00173-7. [DOI] [PubMed] [Google Scholar]
- 21.Christy C, Pichichero ME, Reed GF, Decker MD, Anderson EL, Rennels MB, et al. Effect of gender, race, and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics. 1995;96:584–7. [PubMed] [Google Scholar]
- 22.Blennow M, Granström M, Jäätmaa E, Olin P. Primary immunization of infants with an acellular pertussis vaccine in a double-blind randomized clinical trial. Pediatrics. 1988;82:293–9. [PubMed] [Google Scholar]
- 23.Poland GA, Jacobson RM. Understanding those who do not understand: a brief review of the anti-vaccine movement. Vaccine. 2001;19:2440–5. doi: 10.1016/S0264-410X(00)00469-2. [DOI] [PubMed] [Google Scholar]
- 24.Zarei S, Jeddi-Tehrani M, Zeraati H, Milanifar AR, Ramazankhani A, Shokri F. Short Term Reactogenicity of a Triple Diphtheria-Tetanus-Whole Cell Pertussis Vaccine in Iranian Infants. Iran J Public Health. 2009;38:100–11. [Google Scholar]