Abstract
Immunization information systems (IIS) have been useful for consolidating immunization data and increasing coverage, and have the potential to be a valuable resource for immunization research, but the extent which IIS data are used for research purposes has not been evaluated. We reviewed studies conducted using data from federally supported state and city immunization program IIS, and categorized research type based on study objectives to evaluate patterns in the types of research conducted. Research papers using IIS data published between 1999 and July 3, 2012 were identified by searching the CDC IIS publication database and PubMed. These searches produced 304 and 884 papers, respectively, 44 of which were eligible to be included in this evaluation. The most common research category was evaluation of factors associated with vaccine coverage and vaccine coverage estimates (n = 20). This study shows that IIS may not be used to their full potential with regards to research. Further research is needed to determine barriers to using IIS data for research purposes.
Keywords: immunization information systems, research, review, registry, functionality
Introduction
The Centers for Disease Control and Prevention (CDC) defines immunization information systems (IIS) as “confidential, population-based, computerized databases that record all immunization doses administered by participating providers to persons residing within a given geopolitical area”.1 Development of IIS largely began in the 1980s but was primarily focused in managed care organizations. Starting in 1993 federal funding was provided for the creation of population-based IIS.2 By 1999, 61 of the 64 state and local immunization programs were using federal funds to implement these systems, and 84% of all children in the United States under the age of 6 had two or more vaccinations documented in an IIS in 2011.3
The use of IIS has been suggested as a way to address the fact that children with multiple healthcare providers are less likely to be up to date with their immunizations.4,5 In addition to improved vaccination coverage, there are other important outcomes of IIS use, such as cost savings, generating vaccination recall notices, vaccination reminders and providing official vaccination history forms for use in meeting school entry immunization requirements.6
IIS are especially useful because they provide population-based data and thus are less prone to bias introduced by only including people who are able to seek out medical services,7 though the extent to which this bias is reduced depends on the completeness of provider participation in the IIS. Such reduction of bias makes IIS data a valuable research tool for creating new immunization schedule recommendations,2 or monitoring the impact of vaccine shortages.8 Compiling such comprehensive data opens novel avenues for research including analyzing immunization accessibility, quality and disparities.7 Research into these areas with the accuracy that IIS can provide has the potential to increase vaccination coverage and lower the rate of vaccine preventable diseases, especially in vulnerable populations. In fact, South Carolina law states that the use of IIS “will enable research on the causes, distribution and prevention of vaccine preventable diseases,” and New York law states that IIS data may be used “for the purposes of outreach, quality improvement and vaccine accountability, research, epidemiological studies and disease control.”9,10 In order to maximize the potential of this powerful tool, it important to determine what type of research is being done with data produced by IIS.
In 2010, the Guide to Community Preventive Services conducted a systematic review of papers published using IIS data. Using the 71 published papers and 123 conference abstracts they found, they concluded that IIS are useful for surveillance and investigation of vaccination rates, provider assessment and feedback, providing vaccine reminders and recalls, assisting during outbreaks of vaccine-preventable diseases, facilitating management of vaccines and identifying missed opportunities, invalid dose administration and disparities in coverage.11 This review included conference abstracts, papers that included data from multiple sites (such as Sentinel Site data) and was not restricted to one country. In addition, this review did not examine the use of IIS data specifically for research purposes. Therefore, though findings from this review are important for understanding the utility of IIS, it does not examine the use of individual IIS associated with the 64immunization programs in the United States for research purposes, and it is possible that the full potential of this powerful tool is not being realized. We examined patterns in the use of individual IIS data for research by reviewing all papers published since 1999 that used IIS data.
Results
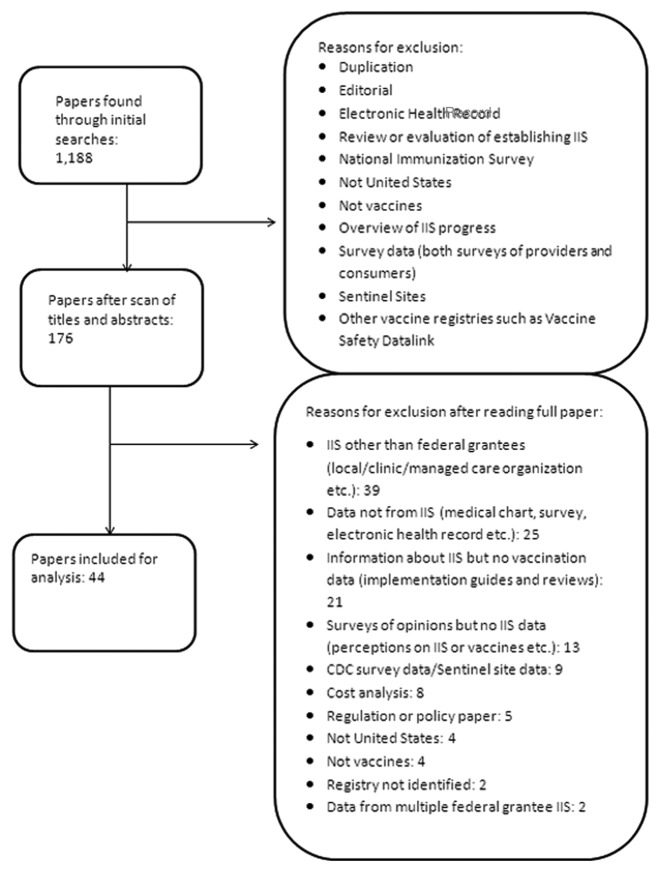
We identified 304 and 884 papers from the CDC IIS publication database and PubMed search results, respectively. No additional new manuscripts were found through reference list review. Following removal of duplicates and applying inclusion and exclusion criteria, 44 papers were available for analysis (Fig. 1).12-55 These 44 manuscripts were produced through research at 18 IIS locations. The most number of manuscripts were affiliated with a university (n = 16),12,16,17,19,20,24,25,27,32-34,36,40,41,48,55 followed by affiliations with a health department (n = 9).14,20,22,30,31,38,46,47,49 Other affiliations included the CDC (n = 8),18,28,29,37,39,42,44,45 a hospital (n = 8),12,15,26,35,41,53 an HMO (n = 1),23 a consulting service (n = 1)54 and an independent research group (n = 1). Data from the Michigan IIS (Michigan Care Improvement Registry) were used to generate 9 manuscripts12,14,15,30,39-41,43,48 the most from any IIS, followed by Philadelphia (8)27,28,33,35,37,44,45,55 and New York City (6)17,18,32,46,49,51 (Table 1). Other than these immunization programs, no IIS produced more than three manuscripts. The number of IIS manuscripts published each year followed an increasing trajectory between 1999 and 2011, with the majority of publications data in the year 2008 or later (Fig. 2).
Figure 1. Research papers published between 1999 and July 3, 2012 utilizing data from immunization information systems, identified through a review of a CDC database [54] and PubMed, with searches conducted between February 13, 2012 and July 3, 2012.
Table 1. Number of papers published using IIS data from individual Immunization Programs and the number of minimum functional standards met by those Immunization Programs as of 2009.
| Immunization Program | Number of Papers |
|---|---|
| Michigan | 9 |
| Philadelphia | 8 |
| New York City | 6 |
| Arizona | 3 |
| North Carolina | 2 |
| Oregon | 2 |
| Houston | 2 |
| Connecticut | 2 |
| Wisconsin | 1 |
| District of Columbia | 1 |
| Minnesota | 1 |
| Utah | 1 |
| Washington State | 1 |
| San Antonio | 1 |
| Chicago | 1 |
| Colorado | 1 |
| Delaware | 1 |
| North Dakota | 1 |
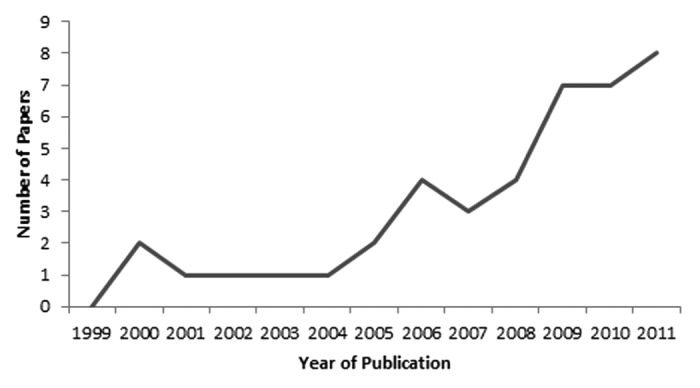
Figure 2. Number of published research papers using data from an immunization information system, by year of publication, 1999–2011. Note that only January–July was included for 2012, so the three publications from 2012 are not included in this figure
The largest group, was coverage associations and estimate evaluations (n = 22 papers), including 11 describing associations with coverage,12,17,21,27,38-40,51,54,55 2 estimating coverage rates,21,22 2 evaluating completion vaccine series completion,23,24 3 evaluating the accuracy of coverage estimates from parents25-27 and 4 describing the completeness of data in the IIS.28,29,43,44 The Policy implementation/change category included 11 papers, including one that described the coverage of a new vaccine compared with an older vaccine,30 one that examined recommended ages,35 two that examined the impact of a policy change,36,37 and 7 that evaluated an intervention.31-34,45-47 Two manuscripts described a response to short-term vaccine supply issues, including the impact of an outbreak38 and a shortage.39 There were six papers in the reminder/recall group, with three papers evaluating the use of an IIS in a vaccine recall40,48,49 and three evaluating the use of IIS in vaccination reminders.41,50,51 Three papers evaluated vaccine effectiveness,42,52,53 all of which focused on the rotavirus vaccine.
Discussion and Conclusion
We conducted the first assessment, to our knowledge, on the extent of use of publicly funded IIS in the US for research purposes. While IIS have been in place for many immunization programs for over a decade, there have been relatively few research reports utilizing these data. Additionally, the IIS locations conducting these research studies are limited, with more than half of the published research papers coming from three immunization programs. While IIS have served many purposes to aid public health practice (e.g., reminder/recall systems, generation of immunization reports for school entry and surveillance for immunization coverage), it appears that we are not currently using IIS to their full potential. On the other hand only 2 manuscripts used data collected after 2009,12,25 indicating that there is a lag between when data are collected and when research is published. Therefore, it is possible that now that IIS have matured and include more data they are more useful to researchers and manuscripts using current data will be published in the near future.
The largest groups of studies dealt with factors associated with coverage and evaluation of an intervention. Using IIS to answer these questions not only takes advantage of the reduced bias in the population-based data available with IIS, but also helps with one of the main purposes of IIS; increased coverage. Though IIS are useful for researching factors associated with vaccine coverage, they can also be used for other vaccine-related research, such as vaccine effectiveness, or adverse events. Only one study examined recommended ages for vaccination, and all three of the studies examining vaccine effectiveness focused on rotavirus vaccine (though a study published after the time period covered by this review used IIS data to research influenza vaccine effectiveness, showing that these data are being utilized to study multiple vaccine preventable diseases).56 There were no studies on adverse events. On the other hand, the systematic review conducted by the Guide to Community Preventive Services found more papers and abstracts than included in this review, so it is possible that research into these areas is being conducted, but did not fit our inclusion criteria (e.g., multi-site research or research done with HMO databases).
There are many barriers to research, including possible issues with data sharing and confidentiality, as well as staffing limitations due to recent cuts in the public health workforce.57 In addition, it is possible that immunization program staff have other priorities regarding IIS (such as generating vaccination reports or managing vaccines), and are under time constraints. Though IIS have been shown to be useful in immunization research, such barriers may prevent them from being used in this manner. Our results imply that partnerships with academic institutions may be one way to overcome these barriers and use the data from IIS most effectively. Future research is needed to understand these issues. In response to this need we have conducted a survey of Immunization Program Managers and future direction of work includes analysis of possible barriers to research with IIS research and data sharing and usage.
This study has some limitations. It was assumed that regional registries that covered an area different from a federally funded state or local registry. Since regional registries have been known to combine to form what we now consider state or local registries, it is possible that there were some studies used data from regional registries that later joined to become what are now known as the 64 federally funded state and local registries58. However, as we were interested in how those immunization programs in particular were using their IIS data, the resulting bias is likely minimal. Only published studies included in the CDC website and PubMed were reviewed. Therefore, it is possible that gray literature or studies that have been completed but not published were missed. On the other hand, most high quality research is published in peer reviewed databases. Our study shows that IIS are not being used to their full potential with regard to research. Since the largest number of studies were affiliated with a university, it is possible that lack of a relationship with a university could be seen as a barrier to research, and immunization programs that want to use the IIS data for research purposes could be advised to seek such a relationship. There are other possible barriers to the use of IIS data, including concerns regarding confidentiality, data quality and budget constrictions, but further research is needed in this area. Our study highlights the need for future research, both with IIS data itself and barriers to such research.
Methods
Literature search
We identified published IIS research manuscripts using two systems. First, we searched the CDC IIS publication database59 for papers published from 1999- July 3, 2012. Next, We searched Pubmed during the period April 9, 2012 to July 3, 2012 using the search terms “(immunization OR vaccination) AND [(information system*) OR registry]” with results limited to papers written in English and published after January 1, 1999. Titles and abstracts were reviewed for possible IIS data usage for research purposes, and the full article was reviewed for those studies that reported research from an IIS. Papers were included if they described using data from an IIS affiliated with an immunization program registry or a “regional registry” that covered the same geographic area as an IIS affiliated with an immunization program registry.60 For example, a regional registry that covered Philadelphia was assumed to be the Philadelphia citywide IIS, and was included. However, if a “regional registry” was referred to and the study area differed from that of a federal registry, for example a regional registry that covered Boston, MA, the paper was excluded.61 Papers were also excluded if they were not from the United States, used a managed care organization, hospital or other IIS. In addition, to see how individual immunization program registries were using their data rather than how it was being used as part of a research consortium, we only included studies that covered the area of one IIS, thereby further excluding analyses reported using National Immunization Survey, the Vaccine Safety Datalink, the CDC Sentinel sites, and other studies that used data from multiple IIS. Our focus for this evaluation was on research activities using IIS data, as defined by an activity that “contribute[s] to generalizable knowledge to improve public health practice,” the results of which can be used to benefit a population beyond the scope of the study;62 therefore papers specifically addressing IIS implementation, methodology, or cost issues were excluded. In accordance with this objective, we excluded gray literature, such as information posted on websites or reports, and only included papers published in journals. The reference lists of the IIS papers we had included were then searched for any additional research manuscripts.
Analysis
For each research manuscript, information on publication date, IIS location, study objective and author affiliation (e.g., university, health department etc.) was extracted. For papers with more than one author affiliation reported, only the affiliation of the corresponding author was included. The number of times each IIS was used was totaled, as was the total number of publications in each year. Categories for qualitative grouping were created based on study objectives to assess patterns in the type of research being conducted through IIS. The manuscripts were grouped into five main categories: coverage associations and estimate evaluations, policy implementation/change, response to short-term vaccine supply issues, reminder/recall and vaccine effectiveness.
IRB
Since this was a review and used no human subjects, no IRB approval or informed consent was needed.
Acknowledgments
The authors would like to acknowledge the Emory Preparedness and Emergency Response Research Center for their assistance with submitting this paper.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/24033
References
- 1.Centers For Disease Control And Prevention. Immunization Information Systems (IIS) May 15, 2012 [cited 2012 July 10].
- 2.Freeman VA, DeFriese GH. The challenge and potential of childhood immunization registries. Annu Rev Public Health. 2003;24:227–46. doi: 10.1146/annurev.publhealth.24.100901.140831. [DOI] [PubMed] [Google Scholar]
- 3.Fath J, Ng TW, Pabst LJ, Centers for Disease Control and Prevention (CDC) Progress in immunization information systems - United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:464–7. [PubMed] [Google Scholar]
- 4.Allred NJ, Wooten KG, Kong Y. The association of health insurance and continuous primary care in the medical home on vaccination coverage for 19- to 35-month-old children. Pediatrics. 2007;119(Suppl 1):S4–11. doi: 10.1542/peds.2006-2089C. [DOI] [PubMed] [Google Scholar]
- 5.Hinman AR, Eichwald J, Linzer D, Saarlas KN. Integrating child health information systems. Am J Public Health. 2005;95:1923–7. doi: 10.2105/AJPH.2004.051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett DL, Washington ML, Bryant A, Thurston N, Perfili CA. Cost savings associated with using immunization information systems for Vaccines for Children administrative tasks. J Public Health Manag Pract. 2007;13:559–66. doi: 10.1097/01.PHH.0000296130.39519.f0. [DOI] [PubMed] [Google Scholar]
- 7.Hinman AR, Ross DA. Immunization registries can be building blocks for national health information systems. Health Aff (Millwood) 2010;29:676–82. doi: 10.1377/hlthaff.2007.0594. [DOI] [PubMed] [Google Scholar]
- 8.Hinman AR, Urquhart GA, Strikas RA, National Vaccine Advisory Committee Immunization information systems: National Vaccine Advisory Committee progress report, 2007. J Public Health Manag Pract. 2007;13:553–8. doi: 10.1097/01.PHH.0000296129.31896.5a. [DOI] [PubMed] [Google Scholar]
- 9.S. C. Code § 44-29-40 (2011)
- 10.N. Y. Code § 66-1.2 (2008)
- 11.The Guide to Community Preventive Services. Universally recommended vaccinations: Immunization Information Systems July, 2010 [cited 2013 Jan 7]; Available from; www.thecommunityguide.org/vaccines/universally/imminfosystems.html
- 12.Boulton ML, Grossman AM, Potter R, Vranesich PA, Clayton J. Assessing the relationship between seasonal and H1N1 influenza vaccination status in Michigan children, 2009-2010. Public Health Rep. 2011;126(Suppl 2):70–7. doi: 10.1177/00333549111260S209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ou H, Cleary P, Sie K. Assessing the immunization status of pediatric cochlear implant recipients using a state-maintained immunization registry. Otolaryngol Head Neck Surg. 2010;143:487–91. doi: 10.1016/j.otohns.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Weston AL, Enger KS. Factors associated with hepatitis A vaccination receipt in one-year-olds in the state of Michigan. J Biomed Biotechnol. 2010;2010:360652. doi: 10.1155/2010/360652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu LY, Cowan N, McLaren R, Engstrom R, Teach SJ. Spatial accessibility to providers and vaccination compliance among children with medicaid. Pediatrics. 2009;124:1579–86. doi: 10.1542/peds.2009-0233. [DOI] [PubMed] [Google Scholar]
- 16.Omer SB, Enger KS, Moulton LH, Halsey NA, Stokley S, Salmon DA. Geographic clustering of nonmedical exemptions to school immunization requirements and associations with geographic clustering of pertussis. Am J Epidemiol. 2008;168:1389–96. doi: 10.1093/aje/kwn263. [DOI] [PubMed] [Google Scholar]
- 17.Verani JR, Irigoyen M, Chen S, Chimkin F. Influenza vaccine coverage and missed opportunities among inner-city children aged 6 to 23 months: 2000-2005. Pediatrics. 2007;119:e580–6. doi: 10.1542/peds.2006-1580. [DOI] [PubMed] [Google Scholar]
- 18.Watson JT, Ramirez E, Evens A, Bellini WJ, Johnson H, Morita J. Measles immunization coverage determined by serology and immunization record from children in two Chicago communities. Public Health Rep. 2006;121:262–9. doi: 10.1177/003335490612100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irigoyen M, Findley SE, Chen S, Vaughan R, Sternfels P, Caesar A, et al. Early continuity of care and immunization coverage. Ambul Pediatr. 2004;4:199–203. doi: 10.1367/A03-138R1.1. [DOI] [PubMed] [Google Scholar]
- 20.Ortega AN, Stewart DC, Dowshen SA, Katz SH. The impact of a pediatric medical home on immunization coverage. Clin Pediatr (Phila) 2000;39:89–96. doi: 10.1177/000992280003900203. [DOI] [PubMed] [Google Scholar]
- 21.LoMurray K, Sander M. Using the North Dakota Immunization Information System to determine adolescent vaccination rates and uptake. Public Health Rep. 2011;126(Suppl 2):78–86. doi: 10.1177/00333549111260S210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robison SG, Kurosky SK, Young CM, Gallia CA, Arbor SA. Immunization milestones: a more comprehensive picture of age-appropriate vaccination. J Biomed Biotechnol. 2010;2010:916525. doi: 10.1155/2010/916525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gold R, Naleway AL, Jenkins LL, Riedlinger KK, Kurosky SK, Nystrom RJ, et al. Completion and timing of the three-dose human papillomavirus vaccine series among adolescents attending school-based health centers in Oregon. Prev Med. 2011;52:456–8. doi: 10.1016/j.ypmed.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Tan W, Viera AJ, Rowe-West B, Grimshaw A, Quinn B, Walter EB. The HPV vaccine: are dosing recommendations being followed? Vaccine. 2011;29:2548–54. doi: 10.1016/j.vaccine.2011.01.066. [DOI] [PubMed] [Google Scholar]
- 25.Poehling KA, Vannoy L, Light LS, Suerken CK, Snively BM, Guitierrez A, et al. Assessment of parental report for 2009-2010 seasonal and monovalent H1N1 influenza vaccines among children in the emergency department or hospital. Acad Pediatr. 2012;12:36–42. doi: 10.1016/j.acap.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stecher DS, Adelman R, Brinkman T, Bulloch B. Accuracy of a state immunization registry in the pediatric emergency department. Pediatr Emerg Care. 2008;24:71–4. doi: 10.1097/PEC.0b013e318163db4d. [DOI] [PubMed] [Google Scholar]
- 27.Czaja C, Crossette L, Metlay JP. Accuracy of adult reported pneumococcal vaccination status of children. Ann Epidemiol. 2005;15:253–6. doi: 10.1016/j.annepidem.2004.07.091. [DOI] [PubMed] [Google Scholar]
- 28.Kolasa MS, Cherry JE, Chilkatowsky AP, Reyes DP, Lutz JP. Practice-based electronic billing systems and their impact on immunization registries. J Public Health Manag Pract. 2005;11:493–9. doi: 10.1097/00124784-200511000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Boyd TD, Linkins RW, Mason K, Bulim I, Lemke B. Assessing immunization registry data completeness in Bexar County, Texas. Am J Prev Med. 2002;22:184–7. doi: 10.1016/S0749-3797(01)00427-5. [DOI] [PubMed] [Google Scholar]
- 30.Enger KS, Stokley S. Meningococcal conjugate vaccine uptake, measured by Michigan’s immunization registry. J Adolesc Health. 2007;40:398–404. doi: 10.1016/j.jadohealth.2006.11.141. [DOI] [PubMed] [Google Scholar]
- 31.Schauer SL, Maerz TR, Hurie MB, Gabor GW, Flynn JM, Davis JP. The use of an immunization information system to establish baseline childhood immunization rates and measure contract objectives. J Public Health Manag Pract. 2009;15:E6–12. doi: 10.1097/PHH.0b013e3181a391ba. [DOI] [PubMed] [Google Scholar]
- 32.Findley SE, Irigoyen M, Sanchez M, Stockwell MS, Mejia M, Guzman L, et al. Effectiveness of a community coalition for improving child vaccination rates in New York City. Am J Public Health. 2008;98:1959–62. doi: 10.2105/AJPH.2007.121046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilcox SA, Koepke CP, Levenson R, Thalheimer JC. Registry-driven, community-based immunization outreach: a randomized controlled trial. Am J Public Health. 2001;91:1507–11. doi: 10.2105/AJPH.91.9.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stille CJ, Christison-Lagay J. Determining immunization rates for inner-city infants: statewide registry data vs medical record review. Am J Public Health. 2000;90:1613–5. doi: 10.2105/AJPH.90.10.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daskalaki I, Spain CV, Long SS, Watson B. Implementation of rotavirus immunization in Philadelphia, Pennsylvania: high levels of vaccine ineligibility and off-label use. Pediatrics. 2008;122:e33–8. doi: 10.1542/peds.2007-2464. [DOI] [PubMed] [Google Scholar]
- 36.Ernst KC, Pogreba-Brown K, Rasmussen L, Erhart LM. The effect of policy changes on Hepatits A vaccine uptake in arizone children, 1995-2008. Public Health Rep. 2011;•••:126. doi: 10.1177/00333549111260S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolasa MS, Chilkatowsky AP, Stevenson JM, Lutz JP, Watson BM, Levenson R, et al. Do laws bring children in child care centers up to date for immunizations? Ambul Pediatr. 2003;3:154–7. doi: 10.1367/1539-4409(2003)003<0154:DLBCIC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 38.Bronson-Lowe D, Anderson SM. Effects of a minimum interval immunization schedule for diphtheria and tetanus toxoids and acellular pertussis vaccination during a pertussis outbreak. Arch Pediatr Adolesc Med. 2009;163:417–21. doi: 10.1001/archpediatrics.2009.53. [DOI] [PubMed] [Google Scholar]
- 39.Allred NJ, Stevenson JM, Kolasa M, Bartlett DL, Schieber R, Enger KS, et al. Using registry data to evaluate the 2004 pneumococcal conjugate vaccine shortage. Am J Prev Med. 2006;30:347–50. doi: 10.1016/j.amepre.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Dombkowski KJ, Reeves SL, Dong S, Stevenson J, Clark SJ. Assessing the burden of undeliverable immunization reminder and recall notifications. Prev Med. 2011;53:424–6. doi: 10.1016/j.ypmed.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Dombkowski KJ, Harrington LB, Dong S, Clark SJ. Seasonal influenza vaccination reminders for children with high-risk conditions: a registry-based randomized trial. Am J Prev Med. 2012;42:71–5. doi: 10.1016/j.amepre.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 42.Guh AY, Hadler JL. Use of the state immunization information system to assess rotavirus vaccine effectiveness in Connecticut, 2006-2008. Vaccine. 2011;29:6155–8. doi: 10.1016/j.vaccine.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 43.O’Connor AC, Layton CM, Osbeck TJ, Hoyle TM, Rasulnia B. Health plan use of immunization information systems for quality measurement. Am J Manag Care. 2010;16:217–24. [PubMed] [Google Scholar]
- 44.Kolasa MS, Chilkatowsky AP, Clarke KR, Lutz JP. How complete are immunization registries? The Philadelphia story. Ambul Pediatr. 2006;6:21–4. doi: 10.1016/j.ambp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Kolasa MS, Lutz JP, Cofsky A, Jones T. Provider chart audits and outreach to parents: impact in improving childhood immunization coverage and immunization information system completeness. J Public Health Manag Pract. 2009;15:459–63. doi: 10.1097/PHH.0b013e3181abbee6. [DOI] [PubMed] [Google Scholar]
- 46.Metroka AE, Hansen MA, Papadouka V, Zucker JR. Using an immunization information system to improve accountability for vaccines distributed through the Vaccines for Children program in New York City, 2005-2008. J Public Health Manag Pract. 2009;15:E13–21. doi: 10.1097/PHH.0b013e3181a8c31f. [DOI] [PubMed] [Google Scholar]
- 47.White KE, Anderson J, Stanley M, Ehresmann K. Evaluating hepatitis B universal birth dose vaccination at Minnesota birthing hospitals by utilizing immunization information systems, birth certificates, and chart reviews, 2007-2008. J Public Health Manag Pract. 2009;15:464–70. doi: 10.1097/PHH.0b013e3181aab5e0. [DOI] [PubMed] [Google Scholar]
- 48.Dombkowski KJ, Cowan AE, Harrington LB, Allred NJ, Hudson E, Clark SJ. Feasibility of initiating and sustaining registry-based immunization recall in private practices. Acad Pediatr. 2012;12:104–9. doi: 10.1016/j.acap.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Papadouka V, Metroka A, Zucker JR. Using an immunization information system to facilitate a vaccine recall in New York City, 2007. J Public Health Manag Pract. 2011;17:565–8. doi: 10.1097/PHH.0b013e3182214746. [DOI] [PubMed] [Google Scholar]
- 50.Saville AW, Albright K, Nowels C, Barnard J, Daley MF, Stokley S, et al. Getting under the hood: exploring issues that affect provider-based recall using an immunization information system. Acad Pediatr. 2011;11:44–9. doi: 10.1016/j.acap.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Irigoyen MM, Findley S, Wang D, Chen S, Chimkin F, Pena O, et al. Challenges and successes of immunization registry reminders at inner-city practices. Ambul Pediatr. 2006;6:100–4. doi: 10.1016/j.ambp.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Boom JA, Tate JE, Sahni LC, Rench MA, Hull JJ, Gentsch JR, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics. 2010;125:e199–207. doi: 10.1542/peds.2009-1021. [DOI] [PubMed] [Google Scholar]
- 53.Sahni LC, Boom JA, Patel MM, Baker CJ, Rench MA, Parashar UD, et al. Use of an immunization information system to assess the effectiveness of pentavalent rotavirus vaccine in US children. Vaccine. 2010;28:6314–7. doi: 10.1016/j.vaccine.2010.06.109. [DOI] [PubMed] [Google Scholar]
- 54.Happe LE, Lunacsek OE, Marshall GS, Lewis T, Spencer S. Combination vaccine use and vaccination quality in a managed care population. Am J Manag Care. 2007;13:506–12. [PubMed] [Google Scholar]
- 55.Feemster KA, Spain CV, Eberhart M, Pati S, Watson B. Identifying infants at increased risk for late initiation of immunizations: maternal and provider characteristics. Public Health Rep. 2009;124:42–53. doi: 10.1177/003335490912400108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hadler JL, Baker TN, Papadouka V, France AM, Zimmerman C, Livingston KA, et al. Effectiveness of 1 dose of 2009 influenza A (H1N1) vaccine at preventing hospitalization with pandemic H1N1 influenza in children aged 7 months-9 years. J Infect Dis. 2012;206:49–55. doi: 10.1093/infdis/jis306. [DOI] [PubMed] [Google Scholar]
- 57.National Association of County and City and Health Officials. Local health department job losses and program cuts: Findings from January 2012 survey. 2012.
- 58.California Department of Public Health. Statewide Immunization Information System (SIIS) project: feasibility study report version 1.1. July 23, 2008 [cited 2013 Feb 4]; Available from: http://www.itsp.ca.gov/pdf/0530-4265-FSR-4265-11.pdf
- 59.Centers For Disease Control And Prevention. IIS Publications Searchable Database May 15, 2012 [cited 2012 July 3]; Available from: http://www2a.cdc.gov/vaccines/IIS/IISPubs/IISPubsMain.asp
- 60.Centers For Disease Control And Prevention. IIS State/Territory/City Registry Staff- Main & Technical Contacts July 11, 2012 [cited 2012 July 17]; Available from: http://www.cdc.gov/vaccines/programs/iis/contacts-registry-staff.html
- 61.Mahon BE, Shea KM, Dougherty NN, Loughlin AM. Implications for registry-based vaccine effectiveness studies from an evaluation of an immunization registry: a cross-sectional study. BMC Public Health. 2008;8:160. doi: 10.1186/1471-2458-8-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention. Distinguishing public health research and public health nonresearch July 29, 2010 [cited 2013 Jan 7]; Available from: http://www.cdc.gov/od/science/integrity/docs/cdc-policy-distinguishing-public-health-research-nonresearch.pdf