Abstract
With a falling birth rate and increasing life expectancy we are experiencing dramatic demographic changes that result in an ever ageing population. Aging is accompanied by increased susceptibility to bacterial and viral infections indicative of a decline in immunity, termed immunesenescence. Immunesenescence also has implications for vaccination programmes and there is clear evidence that the decline in adaptive immunity results in dramatically reduced vaccine responses and vaccine longevity in older adults. This review summarises some of the key changes in the adaptive immune response that change with age and suggests approaches that could be used to try and improve vaccine responses in older adults.
Keywords: Immunesenescence, aging, vaccine efficacy
The Aging Population
UK statistics report that in the period 1984–2009 the number of individuals aged ≥ 65 y increased by 1.7 million and the number of individuals aged ≥ 85 y more than doubled to 1.4 million. It is predicted that by 2034 23% of the population will be aged ≥ 65 y and 5% aged ≥ 85 y.1 Despite lengthening life expectancy there is evidence to suggest that healthy life expectancy (HLE) is growing more slowly than life expectancy alone. This corresponds to an increase in what might be termed “unhealthy life expectancy.”2 Understanding the contribution made by a decline in immunity with age to ill health in old age and how this can be ameliorated, will allow us to develop interventions to optimize immune function in older adults.
What is the Evidence that Immune Function Declines with Age?
There is much evidence for the decline in function of the immune system with age, termed immunesenescence. The role of the immune system is 3-fold: to protect the host from pathogens; to recognize “altered cells” such as cancers; and to do both of these things while not reacting to self-antigens. With aging each of these arms of the immune system is compromised. In relation to protection from infection, the incidence of bacterial infections such as the hospital superbugs Clostridum difficile and Methicillin-resistant staphylococcus aureus (MRSA) is highest in people aged over 75 y and bacterial infections such as pneumonia are not only more frequent in the over 65s but the patients have much higher mortality.3 Detection and elimination of tumors is also reduced as cancer incidence increases with age for most cancers. The immune system is also less tolerant of self and several autoimmune conditions such as Rheumatoid arthritis have a peak age of onset in late middle age.
Another key element of the immune system is the ability to generate immune memory, allowing the host to produce a more rapid and specific response to the pathogen when encountered for a second time. This aspect of immunity is of course also the basis of vaccinations. Vaccination is an important prophylactic measure against infections but the response to vaccination in terms of the titer, efficacy and affinity of antibody produced has also been shown to diminish with aging. One study in rhesus monkeys demonstrated immunesenescence in old and very old monkeys, for example reduced antibody response to influenza vaccination was noted.4 In another study, the role of humoral and cell-mediated immunity in protection from influenza after immunization was examined in healthy older people. It was found that the percentages of both the young and old demonstrating protective titers (i.e., HI ≥ 40) increased post-immunization each year, but were consistently higher in the young compared with the old subjects. It was also found that the risk of developing influenza after immunization was highest among the old subjects who demonstrated neither antibody nor cell-mediated responses.5 In a study to assess vaccination-induced changes in the human B-cell repertoire and pneumococcal IgM and IgA antibody at different ages, it was found that IgA and IgM responses were significantly impaired in the old subjects.6
Importantly, it is not only the initial antibody response to vaccination that has been shown to diminish with age, it has also been shown that older people have reduced vaccination longevity. Antibodies specific for tetanus or tick-borne encephalitis (TBE) virus were measured in 734 adults (aged 18–93 y) and data were evaluated in connection with the time point of the last vaccination against tetanus or TBE and age. This analysis revealed that the time of the last vaccination as well as age had highly significant effects on tetanus and TBE titers (p < 0.001). These results showed a strong decline in post-vaccination antibody concentrations with age, which set in at the age of 40 in the case of tetanus, and was observed right throughout adult life in the case of TBE. Results thus suggest that people > 60 y of age frequently do not have protective antibody concentrations.7
Processes Underlying Immunesenescence
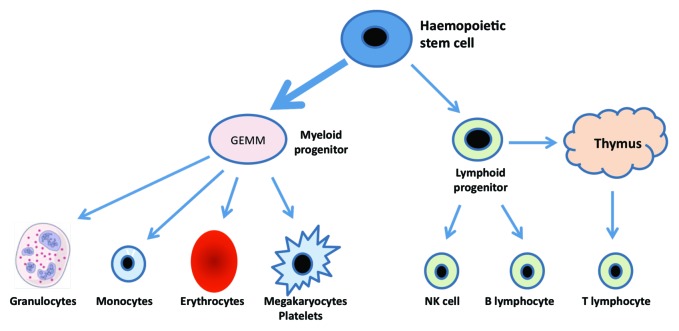
As shown in Figure 1, aging results in a shift toward the myeloid lineage with expansion of the myeloid progenitor cell population at the expense of lymphoid progenitors. In addition from late childhood in humans the thymus atrophies leading to a 3% decline in thymic output of naive T lymphocytes per year. As the peripheral lymphocyte pool remains constant the homeostatic balance is maintained by expansion of the peripheral pool, resulting in an increase in the ratio of memory to naive T cells with age.
Figure 1. Blood cell generation from the pluripotent stem cell showing predisposition toward the myeloid lineage with aging.
The increase in memory T cells with age is potentially advantageous as the host essentially develops a memory cell repertoire against the pathogens encountered on a regular basis. However, lymphocytes cannot proliferate indefinitely and lymphocyte proliferative senescence is another factor contributing to immunesenescence, leading to re-emergence of infections that the person was previously immune to. An example of the latter includes Varicella zoster acquired in childhood giving chicken pox and emerging as shingles in old age. The loss of proliferative capacity is a result of the shortening of telomeres. Telomeres located at the tip of chromosomes range from 4 to 12 Kb in humans and protect coding-DNA from degradation and confer stability to the chromosome. Telomere length decreases with each round of cell division (50–100 bp per division). In the absence of compensatory mechanisms, telomeres eventually reach a critical length which leads to growth arrest and diminished lymphocyte proliferation. Telomerase has a role as this enzyme is expressed in lymphocytes and is a reverse transcriptase that synthesizes telomeric repeats and allows these cells to proliferate much more than other cells in the body. A study from Arne Akbar’s group demonstrated substantial telomere erosion and loss of replicative capacity of CD8+ T cells during aging.8
As summarized in Figure 2, in healthy individuals, the generation of new T cells progressively declines due to thymic atrophy. This decline is compensated for by the homeostatic proliferation of mature T cells in the periphery. Eventually, the continually replicating mature T cells become exhausted due to telomere shortening and take on a senescent phenotype characterized by blunted replicative potential, contracted T-cell repertoire, loss of CD28 co-stimulatory receptor expression, and de novo expression of stimulatory receptors such as killer-like immunoglobulin receptors (KIR).9
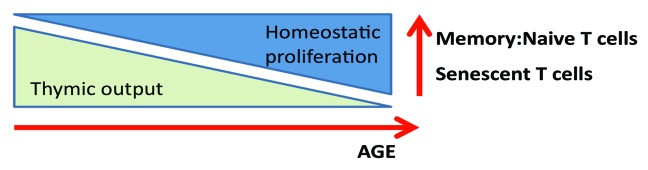
Figure 2. The effects of aging on T cell proliferative capacity and phenotype.
It has been reported that persistent CMV infection is a major factor in T cell immunesenescence, driving T cells toward inexorable end-stage differentiation, shortening telomeres which may eventually lead to the loss of functional CMV-specific memory T cells in older subjects and reduced vaccination responses.10 In addition CMV specific T cells dominate the peripheral pool, with levels in excess of 10% seen in some older subjects,8 leading to the immunological space being occupied by large numbers of cells with poor proliferative capacity. The T cell receptor Vβ repertoire of CMV-specific CD4 T cells like the CMV-specific CD8 T cell population is highly restricted in both young and old subjects. Evidence suggests that CMV-specific CD4 T cells may have reduced capacity to induce telomerase and that this may be one reason for their short telomeres. It has also been suggested that CMV-specific CD4 T cell populations are susceptible to replicative senescence upon repeated stimulation since they have short telomeres and have lost their ability to induce telomerase after activation.11 Thus, CMV-specific CD4 T cell expansion limits immune responses due to high frequency, highly differentiated surface phenotype, having significantly shorter telomeres, low telomerase activity and reduced replicative capacity.
Improving Vaccination Responses
There are a number of strategies that could be tried to improve response to vaccination in older adults. These include:
Boosting
Time of day of vaccine administration
Mitigating the effects of stress
Improved adjuvants
Exercise
Boosting
In an in vivo study of rhesus monkeys it was shown that antibody responses were significantly reduced to primary immunizations in old monkeys, but by administering a second vaccine at 1 mo, it was possible to boost antibody titers up to the level found in young adults during their primary phase.4 Currently the annual vaccination to influenza and the Streptococcus pneumoniae vaccines are given only once and it would be worth investigating whether a second vaccination would improve the current very low response to the vaccines in older adults.
Time of day of vaccine administration
One extremely cost effective change to current vaccine practice may be to specify the time of day of administration. Data from a recent study which examined morning vs. afternoon influenza vaccine administration in older community-based adults showed that men, but not women, vaccinated in the morning mounted a better peak antibody response to the A/Panama influenza strain. These results indicate that hormonal and cytokine diurnal rhythms may influence the magnitude of response to some vaccines.12
Effect of stress
Several studies have compared antibody responses to vaccination in caregivers, including parents of children with extreme special needs and older adults caring for partners with dementia or other chronic conditions. Results show that relative to non-carers, caregivers mount a poorer antibody response than controls to the influenza vaccine.13,14 The emotional stress of bereavement also has a negative impact on influenza vaccine responses in older adults.15
Improved adjuvants
The TLR5 ligand flagellin is a potent activator of a broad range of cell types involved in innate and adaptive immunity. Flagellin administration can induce prominent local and systemic immune/inflammatory responses in vivo. The incorporation of flagellin into vaccines has been shown to enhance the immune response in influenza vaccines and cancer immunotherapeutic studies. Findings of a recent study on age-associated elevation in TLR5-mediated immune responses offer novel opportunities for flagellin-related therapeutic uses and vaccines.16 This may offer a potentially powerful strategy to harness the innate immune system to address the increased susceptibility to infections and decreased response to vaccines in the aged population.
Exercise
There have been many studies looking at the effect of physical activity on vaccination responses, with most showing an enhancing effect of exercise. One study from the Kohut group asked subjects to exercise (three aerobics classes per week) for 12 weeks prior to an influenza vaccination. The data showed that the exercise intervention gave a significant benefit with respect to antibody titer suggesting that exercise can improve immune responses and specifically vaccination in the aged.17
Conclusion
In conclusion, the age-related decline in immunity results not only in increased susceptibility to infection but also reduces the prophylactic efficacy of vaccinations. The two combined lead to increased risk of infections and mortality in older adults. Current research is indicating routes to improved vaccination regimes in older adults, such as adjuvants focused upon aspects of the immune response that remain intact, or simple interventions such as the time of day the vaccine is given or the use of booster vaccinations in old subjects. Further research is, however, needed to optimize vaccination protocols for older adults and provide the evidence based for altered health policy in this area.
Disclosure of Potential Conflicts of Interest
The author has no conflicts of interest to declare.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/24696
References
- 1.Office for National Statistics online, 2012. Available from URL: http://www.ons.gov.uk/ons/rel/mortality-ageing/mortality-in-england-and-wales/average-life-span/rpt-average-life-span.html (Accessed 23.03.13).
- 2.House of Lords Report: Ageing: Scientific Aspects. July 2005. Available from URL: http://www.publications.parliament.uk/pa/ld200506/ldselect/ldsctech/20/20i.pdf
- 3.Fleming DM, Elliot AJ. The impact of influenza on the health and health care utilisation of elderly people. Vaccine. 2005;23(Suppl 1):S1–9. doi: 10.1016/j.vaccine.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Coe CL, Lubach GR, Kinnard J. Immune senescence in old and very old rhesus monkeys: reduced antibody response to influenza vaccination. Age (Dordr) 2012;34:1169–77. doi: 10.1007/s11357-011-9356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murasko DM, Bernstein ED, Gardner EM, Gross P, Munk G, Dran S, et al. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002;37:427–39. doi: 10.1016/S0531-5565(01)00210-8. [DOI] [PubMed] [Google Scholar]
- 6.Ademokun A, Wu YC, Martin V, Mitra R, Sack U, Baxendale H, et al. Vaccination-induced changes in human B-cell repertoire and pneumococcal IgM and IgA antibody at different ages. Aging Cell. 2011;10:922–30. doi: 10.1111/j.1474-9726.2011.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hainz U, Jenewein B, Asch E, Pfeiffer KP, Berger P, Grubeck-Loebenstein B. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine. 2005;23:3232–5. doi: 10.1016/j.vaccine.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, et al. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol. 2005;175:8218–25. doi: 10.4049/jimmunol.175.12.8218. [DOI] [PubMed] [Google Scholar]
- 9.Lindstrom TM, Robinson WH. Rheumatoid arthritis: a role for immunosenescence? J Am Geriatr Soc. 2010;58:1565–75. doi: 10.1111/j.1532-5415.2010.02965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derhovanessian E, Theeten H, Hähnel K, Van Damme P, Cools N, Pawelec G. Cytomegalovirus-associated accumulation of late-differentiated CD4 T-cells correlates with poor humoral response to influenza vaccination. Vaccine. 2013;31:685–90. doi: 10.1016/j.vaccine.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 11.Moro-Garcia MA, Alonso-Arias R, Lopez-Larrea C. Molecular Mechanisms Involved in the Aging of the T-cell Immune Response. Curr Genomics. 2012;13:589–602. doi: 10.2174/138920212803759749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips AC, Gallagher S, Carroll D, Drayson M. Preliminary evidence that morning vaccination is associated with an enhanced antibody response in men. Psychophysiology. 2008;45:663–6. doi: 10.1111/j.1469-8986.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher S, Phillips AC, Drayson MT, Carroll D. Caregiving for children with developmental disabilities is associated with a poor antibody response to influenza vaccination. Psychosom Med. 2009;71:341–4. doi: 10.1097/PSY.0b013e31819d1910. [DOI] [PubMed] [Google Scholar]
- 14.Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93:3043–7. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips AC, Carroll D, Burns VE, Ring C, Macleod J, Drayson M. Bereavement and marriage are associated with antibody response to influenza vaccination in the elderly. Brain Behav Immun. 2006;20:279–89. doi: 10.1016/j.bbi.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Qian F, Wang X, Zhang L, Chen S, Piecychna M, Allore H, et al. Age-associated elevation in TLR5 leads to increased inflammatory responses in the elderly. Aging Cell. 2012;11:104–10. doi: 10.1111/j.1474-9726.2011.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohut ML, Lee W, Martin A, Arnston B, Russell DW, Ekkekakis P, et al. The exercise-induced enhancement of influenza immunity is mediated in part by improvements in psychosocial factors in older adults. Brain Behav Immun. 2005;19:357–66. doi: 10.1016/j.bbi.2004.12.002. [DOI] [PubMed] [Google Scholar]