Abstract
A range of particulate delivery systems have been considered as vaccine adjuvants. Of these systems, liposomes offer a range of advantages including versatility and flexibility in design format and their ability to incorporate a range of immunomodulators and antigens. Here we briefly outline research, from within our laboratories, which focused on the systematic evaluation of cationic liposomes as vaccines adjuvants. Our aim was to identify physicochemical characteristics that correlate with vaccine efficacy, with particular consideration of the interlink between depot-forming action and immune responses. A variety of parameters were investigated and over a range of studies we have confirmed that cationic liposomes, based on dimethyldioctadecylammonium bromide and trehalose 6,6’-dibehenate formed a depot at the injection site, which stimulates recruitment of antigen presenting cells to the injection site and promotes strong humoral and cell-mediated immune responses. Physicochemical factors which promote a strong vaccine depot include the combination of a high cationic charge and electrostatic binding of the antigen to the liposome system and the use of lipids with high transition temperatures, which form rigid bilayer vesicles. Reduction in vesicle size of cationic vesicles did not promote enhanced drainage from the injection site. However, reducing the cationic nature through substitution of the cationic lipid for a neutral lipid, or by masking of the charge using PEGylation, resulted in a reduced depot formation and reduced Th1-type immune responses, while Th2-type responses were less influenced. These studies confirm that the physicochemical characteristics of particulate-based adjuvants play a key role in the modulation of immune responses.
Keywords: Liposomes, adjuvants, dimethyldioctadecylammonium, formulation design, vaccines, sub-unit antigens, depot formation
Liposomes as Vaccine Adjuvants
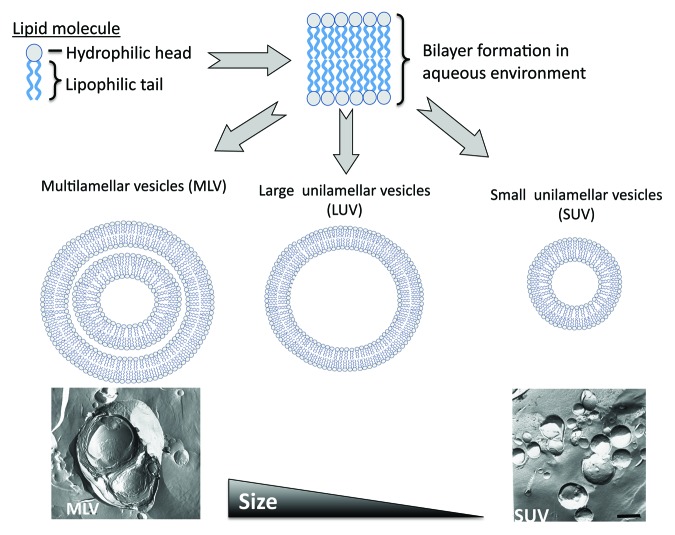
Of the range of delivery systems available, liposomes were the first delivery system to be described as being able to act as immunological adjuvants.1 Liposomes are composed of lipid molecules which, when dispersed into an aqueous phase, form bilayered vesicles (Fig. 1). These lipid building-blocks are composed of three sections (tail, linker and head) and due to the hydrophobic and hydrophilic natures of the tail and head respectively, the water-loving head group is directed outwards, thus forming liposomes. Liposomes can be manufactured in a wide range of morphologies from single to multi-lamellar in structure and ranging in size from ~50 nm up to several microns in size (Fig. 1).
Figure 1. Schematic outline of the formation of liposomes. Lipid molecules, when dispersed in an aqueous phase form bilayers vesicles which can be prepared in a range of sizes from larger multilamellar vesicles, through to small unilamellar vesicles.
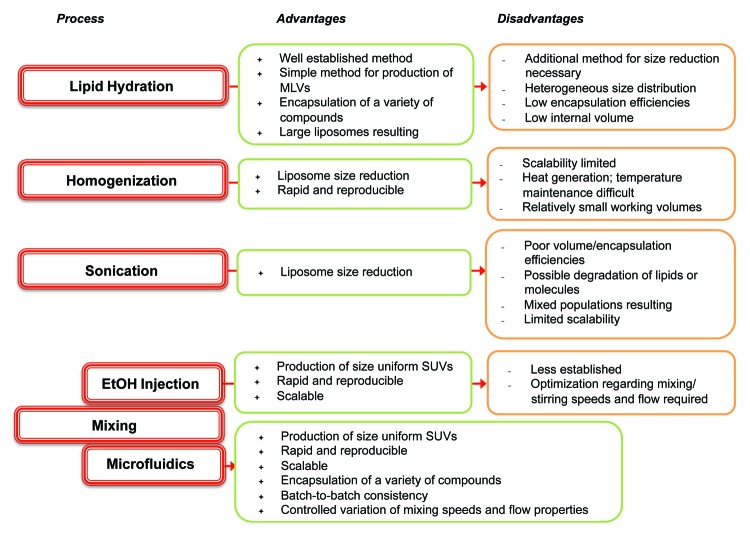
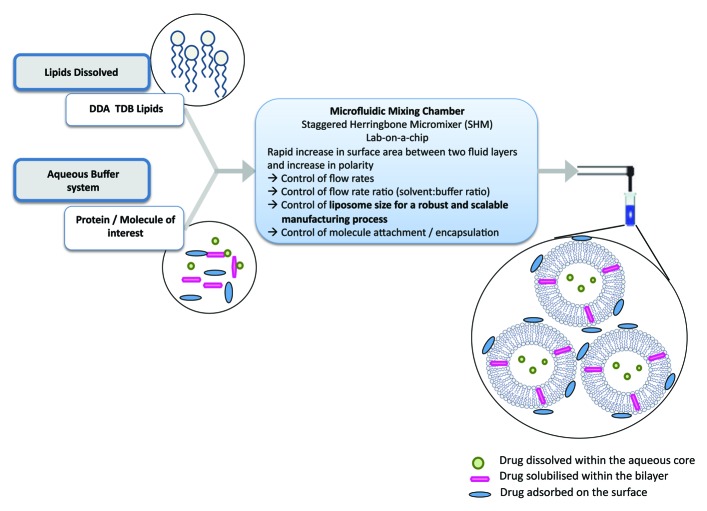
Liposomes are an ideal vaccine delivery system due to their particulate nature, flexibility in formulation, ability to incorporate a range of moieties including immunogenic molecules and antigens; it is these parameters that can be used to promote a range of immune responses.2 The geometry of liposomes is principally determined by their method of manufacturing and their lipid composition. For example, size reduction of vesicles can include sonication, high-shear homogenization or high pressures whereby disruptive energy causes large vesicles to rearrange into smaller ones. An overview of a range of liposome production methods are shown in Figure 2. However, these traditional methods of liposome synthesis raise several difficulties e.g., mechanical stresses, difficultly in up-scaling and methods that rarely lead to size-uniform liposomes.3 To address these issues, the area of microfluidics, and its associated development of novel lab-on-a-chip based devices, has gained increasing attention over recent decades. Besides saving time and money, the use of microfluidics methods reduces space and sample volume (Fig. 3).
Figure 2. Summary of the advantages and disadvantages of some of the common processes used in the manufacture of liposomes.
Figure 3. Schematic outline of the principles involved in the preparation of liposomes using microfluidics.
In terms of developing liposomal adjuvants, a key consideration is their ability to carry and delivery their antigen payload to the appropriate target site. Again, a range of methods have been investigated to promote or improve antigen and liposome association e.g., the dehydration-rehydration.4 A relatively simple and flexible option is to adopt electrostatic interactions to support antigen loading, where the liposomes are designed with the appropriate surface charge to electrostatically adsorb antigen.5 To exploit this method, the overall charge of a peptide or protein must be known; this will be dependent on its amino acids and will change according to the pH of the solution it is suspended in. However, most protein adjuvants have an overall negative charge making cationic liposomes an appropriate design choice.
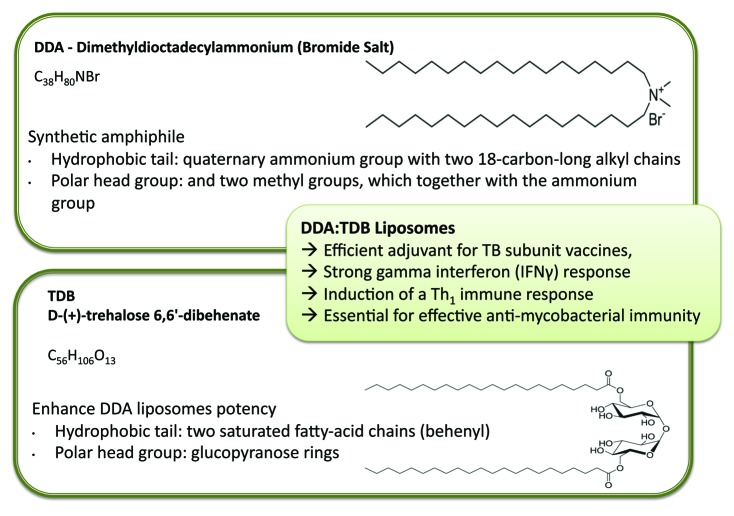
Adopting a cationic change is not just beneficial for enhanced antigen loading; cationic systems can also enhance antigen delivery and immunostimulatory potency. For example, the cationic lipid dimethyldioctadecylammonium (DDA) was discovered as an adjuvant by Gall in the mid-1960s.6 DDA is a synthetic amphiphile, which contains a quaternary ammonium group with two 18-carbon-long alkyl chains forming the hydrophobic moiety and two methyl groups, which together with the ammonium group form the polar head group (Fig. 4). The positively charged head group carries a monovalent counterion, typically bromide or chloride. Due to its amphiphilic character, DDA can form liposomal structures when dispersed in aqueous media at temperatures above its gel-to-liquid phase transition temperature (~47°C).7 DDA is known to induce cell-mediated immunity and delayed-type hypersensitivity8 and, along with its cationic nature and surfactant properties, has been shown to be an effective adjuvant in numerous applications, including mucosal immunization,9 gene delivery10,11 and subunit vaccine delivery.12-15
Figure 4. Liposomal adjuvants can be prepared from a range of lipids, for example the cationic lipid Dimethyldioctadecylammonium bromide and D-(+)- trehalose 6,6’-dibehenate. Combined together, these lipids from liposomes with strong adjuvant properties.
The mechanism of action behind the adjuvant effect of DDA has been attributed to its positive surface charge and its ability to associate antigens.16 This was confirmed and further elaborated by using ovalbumin (OVA) as a model antigen.17 Stimulation of immature bone marrow-derived dendritic cells (BMDCs) with fluorescently labeled OVA showed that adsorption of OVA onto DDA enhanced the cellular acquisition of the antigen. Further, inhibition of active cellular processes by OVA stimulation at 4°C or by the addition of cytochalasin D reduced the cellular uptake, suggesting that active actin-dependent endocytosis is the predominant uptake mechanism.17 DDA-mediated OVA uptake was also associated with a functional enhancement of the APCs. This was shown by measuring the increase in gamma interferon (IFNγ) production and cellular proliferation of purified autologous DO11.10 T-cells transgenic for a T-cell receptor recognizing a major histocompatibility complex (MHC) class II–restricted OVA-epitope (OVA323–339). Both proliferation and IFNγ production was increased upon interaction with either murine BMDCs or purified B-cells, stimulated with OVA adsorbed to DDA.17,18
To further enhance the potency of DDA liposomes, a second component, D-(+)-trehalose 6,6’-dibehenate (TDB) can be added.13 TDB is a synthetic analog of trehalose-6,6-dimycolate, a immunostimulatory component of Mycobacterium tuberculosis. TDB has two saturated fatty-acid chains of 22 carbons (behenyl), each replacing the branched mycobacterial mycolic acids of > 70 carbons (Fig. 4). These two behenyl chains are linked by ester bonds to carbon number 6 of each of the two glucopyranose rings making up the trehalose head group (Fig. 4). TDB has been shown to retain much of the bioactivity of the native form, while showing less toxicity as a result of the shorter fatty acid chains.19,20 Combination with DDA it is an efficient adjuvant for TB subunit vaccines,13 inducing a strong IFNγ response, considered to be the key cytokine for induction of a Th1 immune response and essential for effective anti-mycobacterial immunity.21,22
Interestingly TDB does not only enhance the immunogenicity of the DDA liposomal systems.7 Having shown that DDA:TDB could be effectively prepared in a sterile and stabile format via lyophilization and gamma-sterilization,23,24 the contributing role of TDB to the DDA membranes both in an aqueous25 and freeze-dried format26 was shown to result from the higher attractive forces between the trehalose head group of TDB and water compared with the quaternary ammonium head group of DDA and water.25
Antigen Delivery and Immune-Stimulation
Mycobacteria are well known to exert a number of immunostimulatory effects and are a good source of adjuvants e.g., Freund’s complete adjuvant (an oil emulsion and heat killed mycobacteria). Purified components of mycobacteria such as TDB have also been shown to be effective. Holten-Andersen et al.13 first investigated the combination of DDA:TDB, and studied the ability of seven different immunostimulators, including four mycobacteria-derived immunostimulators, to increase the protective efficacy of DDA, using ESAT-6 as a possible TB antigen. An effective IFNγ response was obtained with the combination of DDA with monophosphoryl lipid A (MPL) and/or TDB. This combination also induced protection in mice similar to that obtained after BCG vaccination. The protective efficacy of DDA:TDB using Ag85B-ESAT-6 was also shown7 and the adjuvant activity of DDA:TDB was compared with aluminum hydroxide. These studies showed that immunization with DDA:TDB leads to high levels of IFNγ secretion and low levels of interleukin-5 (IL-5) secretion by CD4+ T-cells, whereas aluminum hydroxide immunized mice exhibited the opposite pattern, with a negligible IFNγ secretion and higher levels of IL-5 secretion.7 The DDA:TDB-adjuvated vaccine resulted in the same high levels of IgG1 antibody titers, as seen after immunization with the aluminum hydroxide-adjuvated vaccine, whereas the level of IgG2 antibodies were significantly higher after immunization using DDA:TDB as an adjuvant, compared with aluminum hydroxide.7,27 Additionally, studies have shown that DDA:TDB induces a CD4+ T-cell population, predominantly tumor necrosis factor-α (TNF-α)+, IL-2+, IFNγ+, TNF-α+ and IL-2+ multifunctional T-cells, which are long-lived and maintained for at least 1 y in mice.28
In terms of its mechanism of action, the C-type lectin receptor Mincle, expressed in macrophages, is upregulated in the presence of TDM and TDB and triggers the FcRγ-Syk-Card9 pathway. The activation of macrophages by TDB/TDM depends on FcRγ, which represents the Sky-coupling adaptor protein. Deletion of the FcRγ protein resulted in loss of transcriptional responses to TDB/TDM.29 The Syk-Card9 pathway is crucial for antigen-presenting cell activation in vitro and for the adjuvanticity of TDB/TDM in vivo.30,31 The glycolipids TDM and TDB are Mincle-recognized ligands. Molecular mechanisms of TDB/TDM adjuvanticity were identified to be strictly Mincle-dependent and strong IL-17 production post immunization controls the generation of cellular immunity.31,32 Desel et al.32 showed that adjuvanticity of TDM/TDB not only depends on Mincle, but also necessitates MyD88. Whereas in vitro studies identified MyD88 independence, in vivo adjuvanticity was strongly dependent on MyD88 signaling. Immunization experiments included DDA/TDB and H1 subunit vaccine for TB, composed of Ag85B and ESAT-6. Mincle and MyD88 were shown to be indispensable prerequisite for antigen-specific Th1 and Th17 immune responses in vivo supported by IL-1/IL-1R1 signaling.32
Systematically Investigating the Formulation Parameters Controlling the Efficacy of DDA:TDB as a Liposomal Adjuvant
Building on the demonstrated potential of the DDA/TDB system developed by the Staten Serum Institut (e.g., ref. 13), a series of collaborative studies were undertaken to investigate the principles behind the efficacy of this formulation, with one focus being consideration of physicochemical attributes. Our aim was to identify formulation attributes that could be considered to act as correlates for efficacy. These investigations were based on early studies7 which investigated the incorporation of TDB into DDA liposomes and considering the method of liposome preparation and TDB content. These studies demonstrated that incorporation of TDB into DDA liposome bilayers had no impact on antigen loading; however, increasing the TDB concentration (0 to 20 mol%) reduced the transition temperature of the bilayers from 47 to 42°C. The TDB concentration also impacted on immune response. While all the DDA liposome formulations were able to induce high levels of IFNγ, the formulation containing 11% TDB gave the highest responses in terms of IFNγ, IgG1 and IgG2b antigen specific responses in C57B1/6j mice. In comparison to Alum, IFNγ responses from blood lymphocytes isolated from immunized mice were significantly higher for DDA:TDB (11%), while IL-5 levels were significantly lower. In addition to potentiating the immune response of DDA liposomes, incorporation of TDB within the formulation was shown to effectively stabilize the DDA liposomes.7 Given that this DDA:TDB combination was shown to be highly effective, this combination was also incorporated into a range of other particulate based systems including niosomes33 and microspheres.34,35 Incorporation of DDA and TDB within either of these systems did not produce particulates with enhanced adjuvant properties compared with the liposomal DDA:TDB liposome formulation. Therefore, the next stage was to focus on the DDA:TDB liposome formulation and consider the role of its physicochemical characteristics in it performance as an adjuvant, with particular reference to it biodistribution.
The Impact of Lipid Choice—The Controlling Role of Charge
The highly cationic nature of the DDA:TDB liposome formulation offers the advantage of these systems being able to electrostatically bind a range of antigens; however, we also proposed the hypothesis that this cationic charge would influence the biodistribution of the vesicles and their adsorbed antigen. Therefore, we undertook a range of studies applying a relatively simple, effective and reproducible method to follow the biodistribution of the liposomes and their associated antigen using a dual-radiolabeling method developed within our laboratories, where the liposomes are labeled with 3H and the antigen with 125I.36 Using this method we were able to demonstrate that while antigen delivered without liposomes were removed quickly from the body, liposomes based on DDA promoted a depot at the injection site (both after sub-cutaneous or intramuscular injection) and that TDB did not significantly influence this deport effect.37 However, the presence of TDB in the DDA liposomes increased the influx of monocytes to the site of injection, and the subsequent draining of the liposomal adjuvant to the popliteal lymph nodes, in addition to inducing a powerful Th1 response.37
The impact on vesicle charge on the deposition of antigen at the injection site was further considered by comparing cationic DDA:TDB liposomes with a comparable near-neutral liposome formulation composed of Distearoylphosphatidylcholine (DSPC) and TDB (11 mol%).38 This study demonstrates that the cationic nature of the vesicles promotes the retention of the liposomal components at the site of injection, with the DSPC:TDB formulation being more rapidly cleared. Furthermore, the electrostatic adsorption of antigen to the vesicles was demonstrated to be a key requirement for antigen retention. Not only did the neutral liposomes give poor antigen absorption and retention at the injection site, cationic liposomes which were unable to absorb a cationic model antigen (as would be expected) also failed to promote an antigen depot, despite the liposomes being retained at the injection site.38 However, distearoylphosphatidylcholine-based liposomes entrapping antigen within the vesicles were shown to offer a potential alternative to cationic-based systems, especially for the delivery of zwitterionic or cationic molecules.39
Choosing the cationic lipid component
Given that we had demonstrated the controlling role of the cationic lipid in the promotion of a depot at the site of injection, the next consideration addressed whether this was applicable to a range of cationic formulations. Therefore the ability of 3β-[N-(N′,N′-dimethylaminoethane)carbomyl] cholesterol (DC-Chol), 1,2-dioleoyl-3-trimethylammonium propane (DOTAP), or DDA liposomes incorporating immunomodulating trehalose dibehenate (TDB) to form an antigen depot at the site of injection (SOI) and to induce immunological recall responses against co-administered tuberculosis vaccine antigen was investigated.40 Despite all three formulations being cationic in nature, differences in the biodistribution of these vesicles were noted, with the DOTAP formulation draining more rapidly from the injection site compared with the DDA or DC-Chol formulations. This was reflected in a slower drainage to the local lymphoid tissue. This was attributed to the DOTAP liposomes having a lower transition temperature and therefore more fluid in nature, thus promoting easier drainage from the injection site. This was confirmed by comparing liposomes prepared using DDA with liposomes prepared with its unsaturated analog dimethyldioleoylammonium bromide (DODA), which contained one unsaturated C = C bound in each of the lipophilic acyl chains. By comparing the delivery properties of liposomes prepared using these two lipids, liposomes which were rigid (DDA:TDB liposomes) or fluid (DODA:TDB liposomes) in nature at physiological temperatures could be considered.41 Through a series of studies it was shown that these two different formulations showed major differences in their ability to drive a Th1 immune response. The rigid DDA-based liposomes retained higher levels of antigen at the injection site, resulting in a continuous attraction of antigen-presenting cells that expressed elevated levels of the co-stimulatory molecules CD40 and CD86.41 Overall the rigid, DDA-liposomes induced 100-fold higher Th1 responses than the fluid DODA liposome counterparts, confirming that a range of physicochemical properties have a major influence on the efficacy of liposomal adjuvants.
Enhancing delivery of DDA:TDB to the target site
While all the studies undertaken with the DDA:TDB had proved it gave strong Th1 responses and formed a strong depot at the site of injection, this did not necessarily demonstrate a direct correlation between the these two attributes, and enhancing the delivery of DDA:TDB+ antigen to APC should further boost its potency. This was nicely demonstrated by a study led by Johansen’s group,42 where the immune responses achieved by DDA:TDB when administered via the sub-cutaneous, intradermal, intramuscular or intra-lymphatic routes. This study showed that the route of administration promoted no notable differences in IgG1. However, the administration route had a major influence on Th1 responses; intra-lymphatic injection gave strong early IgG2a responses and significantly higher IFNγ secretion from splenocytes collected from immunized mice via this route compared with the other routes.42 This suggested that while the highly effective DDA:TDB liposome formulation formed a strong depot effect at the injection site, trafficking of this formulation to the draining lymphatics would enhance vaccine efficacy.
The role of particulate size in the biodistribution of DDA:TDB liposomal adjuvants
When considering the biodistribution of liposomes, size is often shown to have a significant impact. Hence, liposome size was also an important attribute to consider. In the above studies the DDA:TDB liposomes studied were around 500 - 600 nm in size, therefore consideration of the impact of the size of the DDA:TDB vesicles on their role as adjuvants was also investigated.43 DDA:TDB liposomes were prepared in the following size ranges: small (< 200 nm), medium (500–600 nm), and large (~1,500 nm). No significant difference in the drainage of the liposomes or their adsorbed antigen from the site of injection was seen between the different sized liposomes. However, significantly higher levels (but still relatively low amounts of the total dose) of the smaller liposomes were noted at the popliteal lymph node, 6 h after injection.43 This was shown to be independent to cellular phagocytosis, as macrophage uptake of these various liposomes was not shown to be size-dependent.43 This would suggest that due to their cationic nature, and independent of their size, the vesicles aggregate after administration, due to interaction with interstitial proteins which are generally anionic in nature, thus prohibiting their clearance from the site of injection.
Retaining the DDA component but masking its cationic nature—pegylated DDA:TDB
Given that the depot effect had been shown to be primarily due to the cationic nature of the vesicles, which results in electrostatic adsorption of the antigen and aggregation of the vesicles at the site of injection, a second method to promote drainage (yet retain the DDA content of the DDA:TDB:antigen formulation) to the lymphatics was considered. A series of studies were undertaken where the cationic DDA component of the liposomes was retained, yet the cationic nature masked with polyethylene glycol. Polyethylene glycol (PEG) is the most widely used hydrophilic polymer for the steric stabilization of liposome drug delivery systems. It is a linear polyether diol with many useful properties, including the ability to be conjugated to a liposomal surface to create a steric, hydrophilic barrier which can enhance increasing half-life of the liposomes, through steric stabilization.44 Coating of liposomes with PEG (often referred to as PEGylation), is exploited in therapeutic products such as Caelyx, which is a PEGylated liposomal delivery system for doxorubicin. PEG can be incorporated onto the surface of liposomes in different ways, but the most widely used method at present is to anchor the polymer in the liposomal membrane via a cross-linked lipid (i.e., PEG-distearoylphosphatidylethanolamine [DSPE]).
DDA:TDB liposomes were prepared with increasing PEG concentrations (0 to 25 mol%). This resulted in the cationic zeta potential of the vesicles dropping from ~55 mV for DDA:TDB down to 39 mV with the addition of 10% PEG, and to 3 mV when 25% PEG was added.45 This drop in zeta potential results in a reduction in both the liposome and antigen depot at the injection site, and reduced monocyte influx to the injection site. However, while PEGylation of DDA:TDB tended to promote an increased drainage of liposomes to the local lymph node, this did not translate to an increased antigen delivery to the draining lymph nodes.45 In terms of immune responses, while increasing PEG concentrations in the DDA:TDB liposomal adjuvant made no significant difference in IgG1 responses, both IgG2b and IFNγ responses reduced with increasing PEG concentrations.45 This suggested that PEGylation was able to block the formation at the site of injection, yet the PEGylated liposomes were less able to carry the antigen with them to the draining lymph nodes.
In an attempt to address this and improve co-delivery of the liposomes and antigen to the draining lymph nodes, a second series of PEGylated liposomes were prepared where the antigen was incorporated within the liposomes rather than surface adsorbed.45 However, this did not significantly enhance antigen delivery to the PLN, nor change the immune-responses compared with the DDA:TDB formulations with the surface-adsorbed antigen.45 To consider if a combination of size reduction and PEGylation of the vesicles could further modify the clearance kinetics of these liposomal adjuvants, DDA:TDB liposomes with and without PEGylation were also prepared as small unilamellar vesicles (SUV).46 By using DDA:TDB:10% PEG vesicles which were ~150 nm in size, both liposome and antigen dose at the popliteal lymph node 4 d after injection was increased and earlier antibody responses noted.46
The ability of PEGylation of small cationic liposomes to enhance drainage was also demonstrated with DNA vaccine carriers. Liposomes composed of phosphtidylcholine, dioleoylphosphatidylethanolamine and the cationic lipid DOTAP were prepared with entrapped OVA-encoding plasmid DNA; the introduction of PEG onto the surface of these small cationic vesicles resulted in enhanced lymphatic drainage after sub-cutaneous injection, but the immune responses measured were not improved when compared with non-PEGylated liposomes.47
The controlling role of the adjuvant depot on Th1 but not Th2 responses
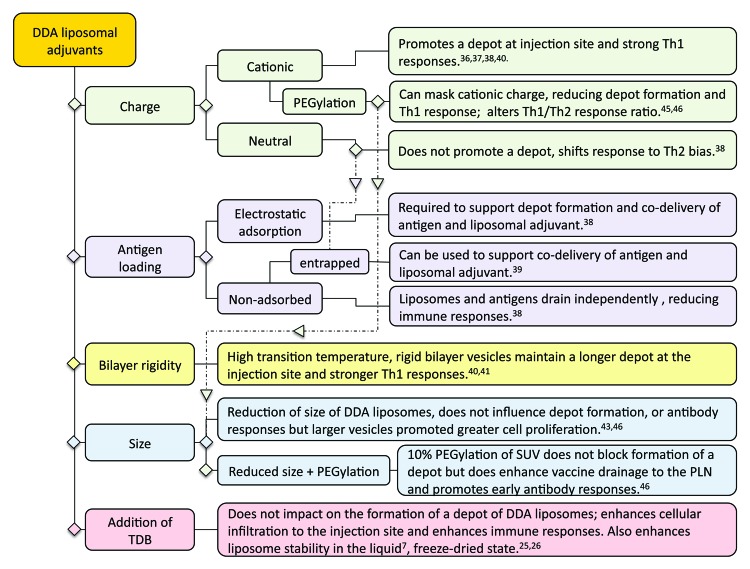
A particular reference point in the above outlined studies was the ability of the liposomal formulation to form a depot at the site of injection and the overall design factors identified to control the formation of a depot at the site of injection for liposomal adjuvants are summarized in Figure 5. Until recently, it had been widely accepted that the activity of alum-based adjuvants was attributed to its ability to retain the antigen at the site of injection.48 This theory was recently put into question through various studies which demonstrated that alum with non-adsorbed or adsorbed antigen gave rise to similar antibody responses49,50 and indeed removal of the alum injection site did not influence the subsequent immune response.51
Figure 5. Formulation parameters shown to impact on the formation of a DDA:TDB liposome depot at the injection site and a summary of the impact this has on immune responses.
Through the above series of studies, we have systematically considered the correlation between the induction of immune responses and the ability of the liposomal formulation to promote depot formation. These studies demonstrate that (1) liposomes that promote a strong depot effect also potentiate a strong Th1 response, and (2) Th2 responses generated by these liposomal adjuvants were not influenced by/reliant upon the depot formation. These findings are in line with the more recent understanding of Alum’s adjuvant action, which predominately drives a Th2 response and appears to act independent of a depot.49-51
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by NewTBVAC and made possible by the European Commission (contract no. LSHP-CT-2003-503367 and FP7-HEALTH-F3-2009-241745).
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/24694
References
- 1.Allison AG, Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974;252:252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- 2.Foged C, Arigita C, Sundblad A, Jiskoot W, Storm G, Frokjaer S. Interaction of dendritic cells with antigen-containing liposomes: effect of bilayer composition. Vaccine. 2004;22:1903–13. doi: 10.1016/j.vaccine.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Mozafari MR. Liposomes: an overview of manufacturing techniques. Cell Mol Biol Lett. 2005;10:711–9. [PubMed] [Google Scholar]
- 4.Kirby C, Gregoriadis G. Dehydration-rehydration vesicles (DRV): A new method for high yield drug entrapment in liposomes. Biotechnology. 1984;2:979–84. doi: 10.1038/nbt1184-979. [DOI] [Google Scholar]
- 5.Gammon B, Virden J, Berg J. The aggregation kinetics of an electrostatically stabilized dipalmitoyl phosphatidylcholine system. J Colloid Interface Sci. 1989;132:125–38. doi: 10.1016/0021-9797(89)90223-3. [DOI] [Google Scholar]
- 6.Gall D. The adjuvant activity of aliphatic nitrogenous bases. Immunology. 1966;11:369–86. [PMC free article] [PubMed] [Google Scholar]
- 7.Davidsen J, Rosenkrands I, Christensen D, Vangala A, Kirby D, Perrie Y, et al. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)-a novel adjuvant inducing both strong CMI and antibody responses. Biochim Biophys Acta. 2005;1718:22–31. doi: 10.1016/j.bbamem.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Snippe H, de Reuver MJ, Beunder JW, van der Meer JB, van Wichen DF, Willers JM. Delayed-type hypersensitivity in rabbits. Comparison of the adjuvants dimethyl dioctadecyl ammonium bromide and Freund’s complete adjuvant. Int Arch Allergy Appl Immunol. 1982;67:139–44. doi: 10.1159/000233004. [DOI] [PubMed] [Google Scholar]
- 9.Klinguer C, Beck A, De-Lys P, Bussat MC, Blaecke A, Derouet F, et al. Lipophilic quaternary ammonium salt acts as a mucosal adjuvant when co-administered by the nasal route with vaccine antigens. Vaccine. 2001;19:4236–44. doi: 10.1016/S0264-410X(01)00156-6. [DOI] [PubMed] [Google Scholar]
- 10.Esposito E, Sebben S, Cortesi R, Menegatti E, Nastruzzi C. Preparation and characterization of cationic microspheres for gene delivery. Int J Pharm. 1999;189:29–41. doi: 10.1016/S0378-5173(99)00231-8. [DOI] [PubMed] [Google Scholar]
- 11.Vangala A, Bramwell VW, McNeil S, Christensen D, Agger E-M, Perrie Y. Comparison of vesicle based antigen delivery systems for delivery of hepatitis B surface antigen. J Control Release. 2007;119:102–10. doi: 10.1016/j.jconrel.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Lindblad EB, Elhay MJ, Silva R, Appelberg R, Andersen P. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect Immun. 1997;65:623–9. doi: 10.1128/iai.65.2.623-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt L, Elhay M, Rosenkrands I, Lindblad EB, Andersen P. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect Immun. 2000;68:791–5. doi: 10.1128/IAI.68.2.791-795.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holten-Andersen L, Doherty TM, Korsholm KS, Andersen P. Combination of the cationic surfactant dimethyl dioctadecyl ammonium bromide and synthetic mycobacterial cord factor as an efficient adjuvant for tuberculosis subunit vaccines. Infect Immun. 2004;72:1608–17. doi: 10.1128/IAI.72.3.1608-1617.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenkrands I, Agger EM, Olsen AW, Korsholm KS, Andersen CS, Jensen KT, et al. Cationic liposomes containing mycobacterial lipids: a new powerful Th1 adjuvant system. Infect Immun. 2005;73:5817–26. doi: 10.1128/IAI.73.9.5817-5826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilgers LA, Snippe H, Jansze M, Willers JM. Combinations of two synthetic adjuvants: synergistic effects of a surfactant and a polyanion on the humoral immune response. Cell Immunol. 1985;92:203–9. doi: 10.1016/0008-8749(85)90001-2. [DOI] [PubMed] [Google Scholar]
- 17.Korsholm KS, Agger EM, Foged C, Christensen D, Dietrich J, Andersen CS, et al. The adjuvant mechanism of cationic dimethyldioctadecylammonium liposomes. Immunology. 2007;121:216–26. doi: 10.1111/j.1365-2567.2007.02560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen D, Agger EM, Andreasen LV, Kirby D, Andersen P, Perrie Y. Liposome-based cationic adjuvant formulations (CAF): past, present, and future. J Liposome Res. 2009;19:2–11. doi: 10.1080/08982100902726820. [DOI] [PubMed] [Google Scholar]
- 19.Olds GR, Chedid L, Lederer E, Mahmoud AA. Induction of resistance to Schistosoma mansoni by natural cord factor and synthetic lower homologues. J Infect Dis. 1980;141:473–8. doi: 10.1093/infdis/141.4.473. [DOI] [PubMed] [Google Scholar]
- 20.Pimm MV, Baldwin RW, Polonsky J, Lederer E. Immunotherapy of an ascitic rat hepatoma with cord factor (trehalose-6, 6′-dimycolate) and synthetic analogues. Int J Cancer. 1979;24:780–5. doi: 10.1002/ijc.2910240614. [DOI] [PubMed] [Google Scholar]
- 21.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammed AR, Bramwell VW, Coombes AGA, Perrie Y. Lyophilisation and sterilisation of liposomal vaccines to produce stable and sterile products. Methods. 2006;40:30–8. doi: 10.1016/j.ymeth.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Mohammed AR, Bramwell VW, Kirby DJ, McNeil SE, Perrie Y. Increased potential of a cationic liposome-based delivery system: enhancing stability and sustained immunological activity in pre-clinical development. Eur J Pharm Biopharm. 2010;76:404–12. doi: 10.1016/j.ejpb.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Christensen D, Foged C, Rosenkrands I, Nielsen HM, Andersen P, Agger EM. Trehalose preserves DDA/TDB liposomes and their adjuvant effect during freeze-drying. Biochim Biophys Acta. 2007;1768:2120–9. doi: 10.1016/j.bbamem.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Christensen D, Kirby D, Foged C, Agger EM, Andersen P, Perrie Y, et al. alpha,alpha’-trehalose 6,6′-dibehenate in non-phospholipid-based liposomes enables direct interaction with trehalose, offering stability during freeze-drying. Biochim Biophys Acta. 2008;1778:1365–73. doi: 10.1016/j.bbamem.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Agger EM, Rosenkrands I, Hansen J, Brahimi K, Vandahl BS, Aagaard C, et al. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLoS One. 2008;3:e3116. doi: 10.1371/journal.pone.0003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindenstrøm T, Agger EM, Korsholm KS, Darrah PA, Aagaard C, Seder RA, et al. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol. 2009;182:8047–55. doi: 10.4049/jimmunol.0801592. [DOI] [PubMed] [Google Scholar]
- 29.Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol. 2010;184:2756–60. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang R, Schoenen H, Desel C. Targeting Syk-Card9-activating C-type lectin receptors by vaccine adjuvants: findings, implications and open questions. Immunobiology. 2011;216:1184–91. doi: 10.1016/j.imbio.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Werninghaus K, Babiak A, Gross O, Hölscher C, Dietrich H, Agger EM, et al. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation. J Exp Med. 2009;206:89–97. doi: 10.1084/jem.20081445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desel C, Werninghaus K, Ritter M, Jozefowski K, Wenzel J, Russkamp N, et al. The Mincle-activating adjuvant TDB induces MyD88-dependent Th1 and Th17 responses through IL-1R signaling. PLoS One. 2013;8:e53531. doi: 10.1371/journal.pone.0053531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vangala AK, Kirby D, Rosenkrands I, Agger E-M, Andersen P, Perrie Y. A comparative study of cationic liposome and niosome-based adjuvant systems for protein subunit vaccines: characterisation, environmental scanning electron microscopy and immunisation studies in mice. J Pharm Pharmacol. 2006;58:787–99. doi: 10.1211/jpp.58.6.0009. [DOI] [PubMed] [Google Scholar]
- 34.Kirby DJ, Rosenkrands I, Agger E-M, Andersen P, Coombes AGA, Perrie Y. PLGA microspheres for the delivery of a novel subunit TB vaccine. J Drug Target. 2008;16:282–93. doi: 10.1080/10611860801900462. [DOI] [PubMed] [Google Scholar]
- 35.Kirby DJ, Rosenkrands I, Agger E-M, Andersen P, Coombes AGA, Perrie Y. Liposomes act as stronger sub-unit vaccine adjuvants when compared to microspheres. J Drug Target. 2008;16:543–54. doi: 10.1080/10611860802228558. [DOI] [PubMed] [Google Scholar]
- 36.Henriksen-Lacey M, Bramwell VW, Perrie Y. Radiolabelling of Antigen and Liposomes for Vaccine Biodistribution Studies. Pharmaceutics. 2010;2:91–104. doi: 10.3390/pharmaceutics2020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henriksen-Lacey M, Bramwell VW, Christensen D, Agger E-M, Andersen P, Perrie Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J Control Release. 2010;142:180–6. doi: 10.1016/j.jconrel.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 38.Henriksen-Lacey M, Christensen D, Bramwell VW, Lindenstrøm T, Agger E-M, Andersen P, et al. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and immunogenicity of the vaccine. J Control Release. 2010;145:102–8. doi: 10.1016/j.jconrel.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 39.McNeil SE, Rosenkrands I, Agger EM, Andersen P, Perrie Y. Subunit vaccines: distearoylphosphatidylcholine-based liposomes entrapping antigen offer a neutral alternative to dimethyldioctadecylammonium-based cationic liposomes as an adjuvant delivery system. J Pharm Sci. 2011;100:1856–65. doi: 10.1002/jps.22427. [DOI] [PubMed] [Google Scholar]
- 40.Henriksen-Lacey M, Christensen D, Bramwell VW, Lindenstrøm T, Agger E-M, Andersen P, et al. Comparison of the depot effect and immunogenicity of liposomes based on DDA, DC-Chol and DOTAP: Prolonged liposome retention mediates stronger Th1 responses. Molecular Pharmaceutics. Mol Pharm. 2011;8:153–61. doi: 10.1021/mp100208f. [DOI] [PubMed] [Google Scholar]
- 41.Christensen D, Henriksen-Lacey M, Kamath AT, Lindenstrøm T, Korsholm KS, Christensen JP, et al. Vaccine adjuvants based on saturated quaternary ammonium lipids have different in vivo distribution kinetics and display distinct T cell-inducing capacity compared to their unsaturated analogues. J Control Release. J Control Release. 2012;160:468–76. doi: 10.1016/j.jconrel.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 42.Mohanan D, Slütter B, Henriksen-Lacey M, Jiskoot W, Bouwstra JA, Perrie Y, et al. Administration routes affect the quality of immune responses: A cross-sectional evaluation of particulate antigen-delivery systems. J Control Release. 2010;147:342–9. doi: 10.1016/j.jconrel.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Henriksen-Lacey M, Devitt A, Perrie Y. The vesicle size of DDA:TDB liposomal adjuvants plays a role in the cell-mediated immune response but has no significant effect on antibody production. J Control Release. 2011;154:131–7. doi: 10.1016/j.jconrel.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 44.Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim Biophys Acta. 1991;1066:29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- 45.Kaur R, Bramwell VW, Kirby DJ, Perrie Y. Pegylation of DDA:TDB liposomal adjuvants reduces the vaccine depot effect and alters the Th1/Th2 immune responses. J Control Release. 2012;158:72–7. doi: 10.1016/j.jconrel.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Kaur R, Bramwell VW, Kirby DJ, Perrie Y. Blocking the depot-formation of cationic liposomal adjuvants at the injection site, by reducing their size and pegylating their surface, promotes rapid antibody production but reduced Th1 responses. J Control Release. 2012;164:331–7. doi: 10.1016/j.jconrel.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 47.Carstens MG, Camps MGM, Henriksen-Lacey M, Franken K, Ottenhoff THM, Perrie Y, et al. Effect of vesicle size on tissue localization and immunogenicity of liposomal DNA vaccines. Vaccine. 2011;29:4761–70. doi: 10.1016/j.vaccine.2011.04.081. [DOI] [PubMed] [Google Scholar]
- 48.Glenny A, Pope C, Waddington H, Wallace U. The antigenic value of toxoid precipitated by potassium alum. J Pathol Bacteriol. 1926;29:38–45. [Google Scholar]
- 49.Noe SM, Green MA, HogenEsch H, Hem SL. Mechanism of immunopotentiation by aluminum-containing adjuvants elucidated by the relationship between antigen retention at the inoculation site and the immune response. Vaccine. 2010;28:3588–94. doi: 10.1016/j.vaccine.2010.02.085. [DOI] [PubMed] [Google Scholar]
- 50.Romero Méndez IZ, Shi Y, HogenEsch H, Hem SL. Potentiation of the immune response to non-adsorbed antigens by aluminum-containing adjuvants. Vaccine. 2007;25:825–33. doi: 10.1016/j.vaccine.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 51.Hutchison S, Benson RA, Gibson VB, Pollock AH, Garside P, Brewer JM. Antigen depot is not required for alum adjuvanticity. FASEB J. 2012;26:1272–9. doi: 10.1096/fj.11-184556. [DOI] [PMC free article] [PubMed] [Google Scholar]