Introduction
Diabetes mellitus (DM) is caused by inadequate insulin secretion or an inability to respond appropriately to secreted insulin, which leads to chronic hyperglycemia. An estimated 171 million people worldwide have DM, and the prevalence of DM will more than double over the next two decades.1 Patients with diabetes have a two- to four-fold increased risk of coronary artery disease (CAD) over non-diabetic patients,2 and 75% of diabetic patients die from a cardiovascular cause.3 Diabetic patients commonly undergo percutaneous revascularization procedures, as 25-30% of all percutaneous coronary interventions (PCIs) are performed in patients with DM.4 A diagnosis of DM is also considered equivalent to having CAD, since diabetic patients without a history of CAD have a 5-year cardiovascular mortality that is similar to that of non-diabetic patients who have a history of myocardial infarction (MI).5 By current ACC/AHA guidelines, patients with DM are therefore treated as having a coronary artery disease equivalent.6, 7 Previous review articles have summarized specific medical therapies for patients with diabetes.8, 9 This review focuses on mechanisms of accelerated atherosclerosis, percutaneous and surgical revascularization strategies, and outcomes among patients with DM and CAD.
Mechanisms Linking Diabetes, Atherosclerosis, and Outcomes After Coronary Revascularization
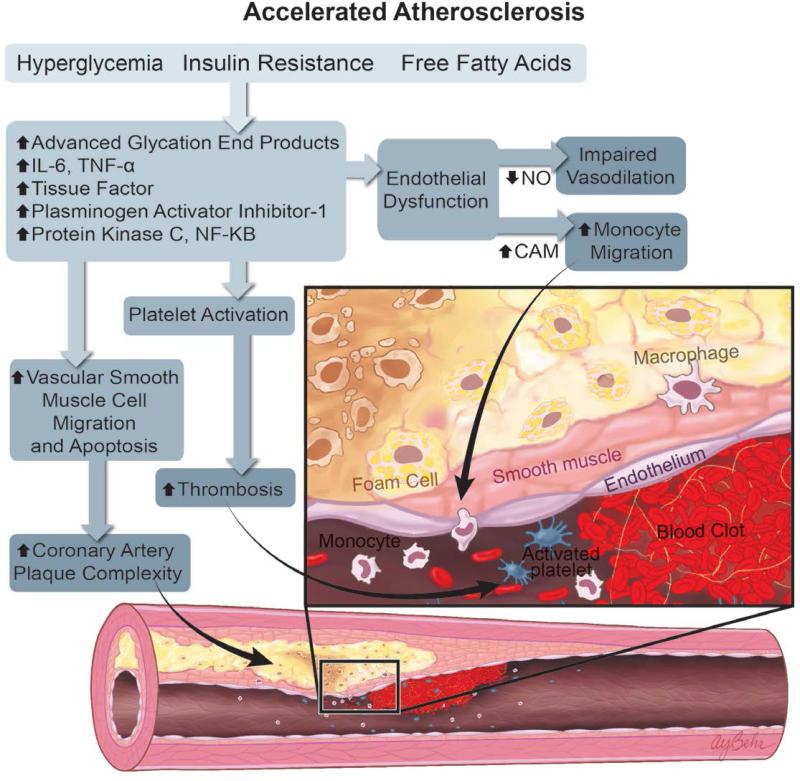
Diabetic patients have increased rates, extent, and complexity of atherosclerotic CAD.8 After coronary revascularization, diabetics have an increased risk of target vessel failure and need for repeat interventions.10 Altered inflammatory pathways stemming from the effects of hyperglycemia, insulin resistance, and altered free fatty acid metabolism predispose diabetic patients to endothelial dysfunction, thrombogenesis, monocyte activation, foam cell transformation, and altered smooth muscle cell migration.11-13 These mechanisms converge to create increased coronary artery plaque burden and more complex coronary artery disease (Figure 1A).
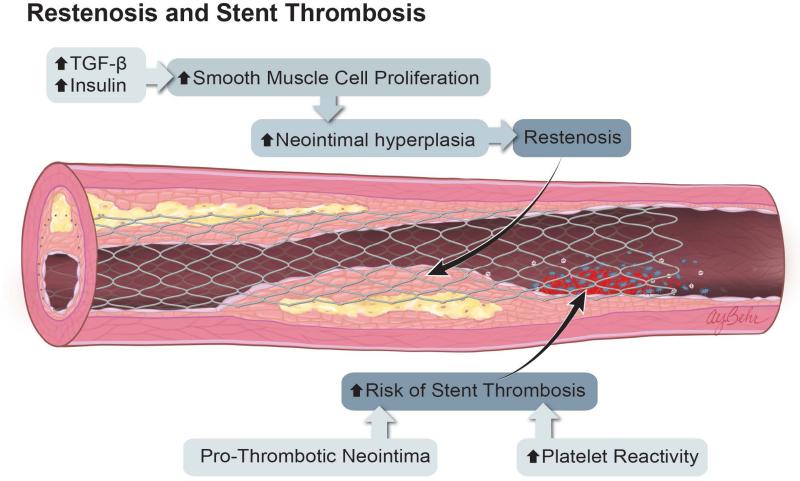
Figure 1.
Mechanisms of Atherosclerosis and Restenosis in Diabetes. A. The combination of hyperglycemia, insulin resistance, and increased circulating free fatty acids activate multiple inflammatory pathways, leading to endothelial dysfunction, increased monocyte activation and localization to sites of nascent plaque, increased vascular smooth muscle cell migration, and apoptosis. These inflammatory pathways also increase platelet activation, leading to an increased risk of atherothrombosis and coronary artery plaque complexity. B. After percutaneous coronary intervention, elevated levels of insulin and TGF-β promote greater smooth muscle cell proliferation, neointimal hyperplasia, and restenosis. Patients with DM may also have prothrombotic neointima, as well as increased platelet reactivity. The sum of these effects results in an increased risk of stent thrombosis.
Endothelial Dysfunction and Immune Cell Migration
The endothelium plays a pivotal role in the maintenance of vessel tone and blood flow.14 Disruption of endothelial cell homeostasis can increase smooth muscle cell, leukocyte and platelet activity.15 The role of hyperglycemia and insulin resistance in endothelial cell dysfunction is multifactorial. Endothelial cells control vessel tone by the regulated production of nitric oxide (NO) via phosphoinositol-3 kinase (PI3K)-dependent activation of endothelial nitric oxide synthase (eNOS). NO promotes vasodilation but also possesses antiplatelet, antiproliferative, and antioxidant properties.16 In healthy individuals, insulin induces PI3K signaling, leading to the production of NO and increased NO bioavailability. However, in patients with type 2 DM, the production of NO is impaired leading to a decrease in vasodilation.17
DM is also associated with increased production of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) that bind to endothelial surface receptors and activate nuclear factor-κB (NF-κB) to induce transcription of endothelial cell adhesion molecules.18 The increase in adhesion molecule expression enhances binding of leukocytes and platelets to the surface of the endothelium, leading to increased thrombogenesis. Increased leukocyte migration to sites of coronary plaque may also promote local plaque inflammation and plaque instability.
Platelet Activation
Platelet activity is enhanced in patients with DM, with increased expression of P-selectin on the platelet surface and glycation of platelet surface receptors leading to increased platelet adhesion.19 DM is also associated with an increase in advanced glycation end products (AGE), which result from the attachment of reducing sugars such as glucose to free amino groups via the Maillard reaction.20 AGE induce endothelial cell signaling via receptors for AGE (RAGE).21 Through their binding to RAGE, AGE can also induce the synthesis of proinflammatory cytokines and growth factors to increase tissue proliferation, induce modification of the endothelial cell extracellular matrix, and disrupt NO production. Production of AGE are known to be enhanced in vivo in the setting of hyperglycemia and are thought to mediate many of the complications of DM, including vascular dysfunction.22
Restenosis and Stent Thrombosis
The above mechanisms are associated with the increased development of clinically significant CAD among patients with DM. Patients with DM also have higher rates of adverse events after PCI, due to both increased neointimal hyperplasia and an increased propensity for thrombosis (Figure 1B). Accelerated rates of neointimal hyperplasia have been demonstrated in both rat23 and human studies following angioplasty in type 2 DM.24, 25 Increased neointimal hyperplasia in the diabetic artery following coronary intervention may result partly from increased production of transforming growth factor-β (TGF-β) and smooth muscle cell migration and proliferation caused by the hyperglycemic state.24 Animal models of endovascular stent placement have also shown that diabetes is associated with increased extracellular signal-related kinase (ERK) activation but a reduction in Akt signaling.26 Sirolimus, but not paclitaxel, activates Akt signaling, leading to increased smooth muscle cell proliferation in the setting of hyperglycemia.27 These drug-specific signaling effects of antiproliferative agents may in part explain the differential efficacy of sirolimus-eluting stents in patients with diabetes (see below).
The neointima of patients with diabetes may also have biologic alterations that predispose to stent thrombosis: when visualized by optical coherence tomography, the neointima in diabetic patients has a low signal pattern that may be associated with increased proteoglycan content and organized thrombi.28 Platelets from diabetic patients are more reactive than those of non-diabetic patients, further increasing the risk of thrombosis.29 Recent advances in antiplatelet therapies have been shown to be beneficial to both diabetic and non-diabetic patients in prevention of atherothrombosis,30 and in certain studies antiplatelet agents have decreased the gap in thrombosis risk between diabetics and non-diabetics for endpoints such as stent thrombosis (see “Pharmacotherapy After Revascularization,” below).31
These findings emphasize that the choice of antiplatelet therapies, lipid-lowering therapies, method of glycemic control, and device choice for PCI must be considered as a whole when treating patients with diabetes. Due to the multiplicity and redundancy of pathophysiologic mechanisms in diabetics, no single therapy will be effective in all patients. Therapies that affect multiple pathophysiologic mechanisms, such as weight loss and exercise, are likely to be the most effective treatments in the long term.
Appropriateness and Timing of Revascularization in Patients With Diabetes
Patients with DM and CAD are at high risk of subsequent cardiovascular events, regardless of symptoms.32 Whether such patients with stable CAD should undergo prompt revascularization is an important clinical question with broad implications for risk stratification and treatment. The prospective randomized BARI 2D Trial compared prompt revascularization (either CABG or PCI) of patients with DM and stable CAD with concurrent aggressive medical treatment to aggressive medical treatment alone, as well as glycemic control strategies.33 A total of 2,368 type 2 diabetic patients were enrolled and followed for 5 years. The primary endpoint of the trial was 5-year mortality, which demonstrated no difference between the revascularization plus medical treatment group vs. the initial medical treatment alone group. There was also no difference in outcomes between the two glycemic control strategy groups at 5 years.34
While the BARI 2D Trial was not designed to compare CABG vs. PCI, there was a significant decrease in the rate of composite cardiovascular events when CABG revascularization was compared to the medical therapy alone group that was not seen in the PCI group. This suggested that there was a benefit to prompt revascularization in diabetic patients in whom CABG was the preferred revascularization treatment, but that this benefit was not seen in those in whom PCI was the preferred treatment.34 Of note, this study was carried out during the first clinical use of DES. Approximately 35% of diabetic patients undergoing PCI as part of the BARI 2D Trial received DES, while the remainder received either a BMS (56%) or no stent (9%).
The results of the BARI 2D trial suggest that an initial strategy of medical therapy is reasonable in patients with DM and stable CAD, with the recognition that a large percentage of such patients (42% at 5 year follow up in the BARI 2D trial) may eventually require revascularization. The initial 2009 Appropriate Use Criteria (AUC) document for coronary revascularization included diabetes as a clinical decision point for the type of revascularization (e.g., CABG vs. PCI), but the presence of diabetes did not alter the appropriateness of a given method of revascularization.35 The 2012 AUC update does not include diabetes as a variable for the appropriateness of revascularization or method of revascularization, but instead uses the SYNTAX score to stratify decision-making.36 Current AUC for revascularization therefore remain primarily based on patient symptoms, documentation of ischemia, and anatomic extent of disease. The presence of diabetes in a given patient, however, may be an important clinical factor to take into account for scenarios in the “uncertain” range, where the presence of diabetes may identify a patient as having a higher risk profile who may therefore benefit from closer clinical monitoring and possible revascularization.
Clinical Trials Comparing Surgical Revascularization to Percutaneous Coronary Intervention in Patients with Diabetes
A number of large-scale trials have compared CABG to PCI (Table 1). These trials have been conducted in parallel with development of new PCI technologies and refinement in surgical techniques, including angioplasty (BARI trial), bare metal stents (ARTS-I), and most recently, first generation drug-eluting stents (SYNTAX trial). Each of these trials included a large percentage of patients with DM. More recently, the FREEDOM trial specifically randomized patients with DM to CABG or PCI.
Table 1.
Major Recent Studies Comparing PCI with CABG Among Patients with Diabetes.
| Trial Name | Study Period | Type of PCI | # Patients per Arm | # With DM (%) | Follow-up | Primary Endpoint in DM | Outcome in DM |
|---|---|---|---|---|---|---|---|
| BMS or DES | |||||||
| ARTS-I | 1997-1998 | BMS | 600 BMS 605 CABG |
208 (17.3) | 5 yr | Composite MACCE | CABG<BMS |
| ARTS-II | 2003-2003 | Cypher SES | 607 SES 605 CABG (from ARTS-I) |
255 (21.0) | 5 yr | Composite MACCE | CABG<SES<BMS |
| BARI 2D | 2000-2008 | PTCA/BMS/DES | 1605 PCI 763 CABG |
2368 (100) | 5 yr | All-cause mortality | Similar outcomes for medical therapy or revascularization |
| CARDia | 2002-2007 | BMS or Cypher SES | 256 PCI 254 CABG |
510 (100) | 5 yr | Composite of all-cause death, non-fatal MI, non-fatal stroke | No difference between CABG and PCI |
| DES | |||||||
| SYNTAX | 2005-2007 | Taxus PES | 903 PES 897 CABG |
452 (25.1) | 3 yr | Composite MACCE | Increased MACCE in PCI |
| FREEDOM | 2005-2010 | Taxus PES or Cypher SES | 953 PCI 947 CABG |
1900 (100) | 5 yr | Composite all-cause mortality, MI and stroke | CABG better for DM in all outcomes |
| PRECOMBAT | 2004-2009 | Cypher SES | 300 SES 300 CABG |
192 | 1 yr | Composite MACCE | No difference between PCI and CABG |
| EXCEL | 2010-current | Xience V EES |
2600 (estimated) | Ongoing | 3 yr | Composite all cause mortality, MI, stroke | Ongoing |
CABG=Coronary artery bypass graft; BMS=Bare metal stent; DES= drug-eluting stent; SES=Sirolimus-eluting stent; EES=Everolimus-eluting stent; PES=Paclitaxel-eluting stent; E-ZES=Endeavor zotarolimus-eluting stent; R-ZES=Resolute zotarolimus-eluting stent
The Bypass Angioplasty Revascularization Investigation (BARI) Trial compared the safety and efficacy of coronary artery bypass grafting (CABG) and percutaneous transluminal coronary angioplasty (PTCA) in a randomized population of patients with multivessel disease. This trial, which enrolled 1,829 patients, showed that diabetic patients who underwent CABG had increased rates of 10-year survival and decreased rates of MI compared to those who underwent PTCA.37 Contemporaneous smaller trials yielded conflicting results, with some finding increased survival of diabetic patients undergoing CABG vs. PTCA,38-40 and others finding no difference in survival of diabetic patients treated with CABG vs. PCI.41-44
Application of BMS or early generation DES led to improved outcomes of PCI among diabetic patients, thereby narrowing the outcomes gap with CABG. The Arterial Revascularization Therapies Study (ARTS-I and -II) compared the safety and efficacy of CABG vs. BMS (ARTS-I) and CABG vs. SES (ARTS-II) in patients with and without DM. Among patients with DM, there was no difference in 3-year MACCE between CABG, BMS and SES, but CABG and SES each showed decreased rates of death and MI when compared to BMS historical comparisons.45 At 5-year follow-up, SES-treated patients had lower rates of MACCE than those previously randomized to BMS, but CABG remained superior to both PCI strategies. SES was also associated with an increased risk of repeat revascularization at 5 years when compared to CABG.46 Similarly, in the Coronary Artery Revascularization in Diabetes (CARDia) Trial, PCI (either BMS or DES) was compared to CABG in diabetic patients with multivessel disease and demonstrated that there were no differences in death, MI, or stroke when comparing PCI and CABG. However, treatment with PCI in diabetic patients was associated with an increased incidence of late MI and the need for repeat revascularization at 1 year.47
The Synergy Between PCI With TAXUS and Cardiac Surgery (SYNTAX) study examined the use of the TAXUS PES vs. CABG for treatment of diabetic and non-diabetic patients with multivessel disease. In agreement with many other studies, this study found that diabetic patients had increased rates of major adverse cardiac and cerebrovascular events (MACCE) and revascularization when compared to non-diabetic patients.48 Furthermore, both diabetic and non-diabetic patients treated with PES demonstrated increased rates of MACCE and repeat revascularization when compared to those treated with CABG out to final five year follow up.49 These results suggested that in patients with complex disease (as determined by the SYNTAX score), CABG remains the preferred method of revascularization over PES. However, for patients with lower disease complexity (SYNTAX Score ≤ 22), PCI was non-inferior to CABG in terms of all MACCE endpoints. Therefore, PCI may be an acceptable alternative to CABG in diabetic patients with lower disease complexity.50
The Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) Trial, a randomized trial of 1900 patients with multivessel disease and DM, examined the use of PCI (primarily first-generation PES or SES) vs. CABG.51 Patients with DM who underwent CABG had a decreased incidence of MI (6.0% vs. 13.9%) and all-cause mortality (10.9% vs. 16.3%) at 5 years compared to those who underwent PCI. However, patients randomized to CABG did have increased rates of stroke (5.2% vs. 2.4%). Of note, there was no interaction between SYNTAX score and outcomes among the overall population, suggesting the increased event rates among patients randomized to PCI was not related to the anatomic complexity of disease at the time of revascularization. The FREEDOM trial enrolled lower surgical risk patients with preserved ejection fractions, and the conclusions of the trial may therefore not be applicable to patients at higher risk for surgery with comorbidities such as left ventricular dysfunction, stroke, renal insufficiency, neuropathy, peripheral arterial disease, and frailty. Because high surgical risk patients have not been studied in any of the trials reviewed here, it is reasonable that a multidisciplinary heart team evaluate high surgical risk patients for the best revascularization strategy. In many of these cases, PCI may remain the preferred strategy due to the less invasive nature of this approach.
Upcoming randomized studies of second-generation stents will continue to address important questions regarding revascularization strategies in patients with diabetes. The Evaluation of Xience Prime™ or Xience V™ stents versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) trial will enroll approximately 2600 patients with left main disease and a SYNTAX score of ≤ 32 to compare the Xience Prime EES vs. CABG.52 Patients with DM will be a pre-specified subgroup of this study. Additionally, the Bypass Surgery versus Everolimus-Eluting Stent Implantation for Multivessel Disease (BEST) trial (NCT00997828) will examine the use of the Xience V EES vs. CABG in patients with multivessel disease. Analysis of the diabetic subgroups of the FREEDOM, EXCEL and BEST trials will shed further light on the safety and efficacy of second generation DES in patients with DM.
Drug Eluting Stents in Patients with Diabetes
DES are associated with a decreased rate of restenosis compared to BMS in both diabetic and non-diabetic patients.53-56,57, 58 Pooled analysis of these studies has raised some controversy regarding the relative efficacy of different DES types in DM. A recent mixed-treatment comparison meta-analysis of 42 randomized trials that included 10,714 patients with DM found that DES as a whole were associated with a 37-69% reduction in target vessel revascularization (TVR) compared to BMS, but the magnitude of this reduction varied with stent type.59 In the following discussion, we review recent data on the efficacy of first, second, and newer generation DES platforms among patients with DM. In each case, we highlight the available data comparing stents types among patients with DM (Table 2).
Table 2.
Major Studies Comparing DES Types Among Patients with Diabetes.
| Trial Name | Study Period | Type of PCI | # Patients per Arm | # with DM (%) | Follow-up | Primary Endpoint in DM | Outcome in DM |
|---|---|---|---|---|---|---|---|
| SPIRIT V | 2006-2007 | Xience EES vs. Taxus PES | 218 EES 106 PES |
324 | 1 yr | In-stent late loss | PES increased late loss |
| ESSENCE-DIABETES | 2008-2009 | Xience EES vs. Cypher SES | 149 EES 151 SES |
300 | 1 yr | Angiographic in-segment late loss | No difference between EES and SES |
| PROTECT | 2007-2008 | Endeavor ZES vs. Cypher SES | 4357 ZES 4352 SES |
2410 | 3 yr | Stent thrombosis | ZES better in all pts, no difference between DM and non- DM |
| NAPLES-DIABETES | 2005-2007 | Endeavor ZES vs. Taxus PES vs. Cypher SES | 75 ZES 75 PES 76 SES |
226 | 3 yr | Composite MACE | Increased MACE in ZES vs. PES and SES |
| SCAAR | 2003-2006 | Endeavor ZES vs. Taxus PES vs. Cypher SES | 333 ZES 2852 PES 1569 SES |
9710 | 4 yr | Death and MI | No difference between stent types or DES vs. BMS |
| RESOLUTE US | 2008-2009 | Resolute ZES vs. Endeavor ZES | 1402 R-ZES 2270 E-ZES (historical) |
374 | 1 yr | TLF and Composite TVF | No difference between E-ZES and R-ZES in DM or non-DM |
| TWENTE | 2008-2010 | Endeavor ZES vs. Xience V EES | 697 ZES 694 EES |
301 | 1 yr | TVF | No difference between ZES and EES |
SES=Sirolimus-eluting stent; EES=Everolimus-eluting stent; PES=Paclitaxel-eluting stent; E-ZES=Endeavor zotarolimus-eluting stent; R-ZES=Resolute zotarolimus-eluting stent
First Generation DES
Paclitaxel Eluting Stents (PES)
A large meta-analysis examined the outcomes of BMS vs. the first generation TAXUS PES in five prospective, randomized trials enrolling 2,797 patients (TAXUS Clinical Program). The authors demonstrated similar 5-year safety and efficacy between PES and BMS in diabetic patients.54, 60 In the TAXUS IV study, PES decreased the overall rates of target vessel failure (TVF), target lesion revascularization (TLR) and major adverse cardiovascular events (MACE) in diabetic and non-diabetic patients.61 Additional studies demonstrated no difference in rates of stent thrombosis (ST), MI, death or neointimal proliferation in diabetic and non-diabetic patients treated with PES.62 Importantly, patients with type 2 DM who required insulin therapy were at increased risk for MACE, TVF and TVR when compared to those with Type 2 DM who were treated with oral medications.
Sirolimus Eluting Stents (SES)
The German Multicenter Randomized Single Blind Study of the CYPHER Sirolimus-Eluting Stent in the Treatment of Diabetic Patients with De Novo Native Coronary Artery Lesions (SCORPIUS) Trial examined the safety and efficacy of SES vs. BMS in a small group of diabetic patients. Treatment with SES led to a reduction in 5-year overall MACE, mostly attributable to a decrease in 5-year TLR. Safety endpoints of all cause mortality, cardiac death, MI and ST were similar between SES and BMS in diabetic patients.56 A combined analysis of four randomized trials comparing SES to BMS with five years of follow up found no difference in overall rates of MACE among the overall study population. However, patients with DM treated with SES had significantly higher rates of death due to cardiac causes than patients treated with BMS (15.9% vs. 9.0%).63 This finding has raised the concern of possible increased stent thrombosis rates among patients with DM treated with SES, although other studies have suggested decreased rates of mortality among diabetic patients treated with first generation DES.64
Comparisons of PES and SES Among Patients with Diabetes
A mixed comparison meta-analysis comparing SES, PES and BMS in 3,852 diabetic patients found that the two DES types were associated with lower mortality in diabetic patients than BMS, but, as suggested by other studies, mortality in diabetic patients remained higher than in non-diabetic patients.55 SES also showed an advantage over PES for ST and longer event-free follow-up in diabetic patients at 1 year. When these results were followed to 5 years the early advantage of SES was lost in the general population, but SES remained advantageous for diabetic patients.65, 66 Further stratification of diabetic patients into those requiring insulin has shown that patients with DM who require insulin treatment have the highest rates of restenosis regardless of stent type. The above-mentioned mixed treatment comparison also favored SES over PES in a head to head comparison of the outcome of TVR for treatment of diabetic patients.59
Second-Generation DES
Second-generation DES have optimized drug deliverability while seeking to minimize TLR as well as the risk of ST. Numerous studies have examined the relative efficacy of second-generation DES among patients with DM. Overall, these studies have found that event rates among diabetic patients remain higher than for the general population, but that most of this effect on outcomes is driven by the subset of patients with DM who require insulin therapy.
Everolimus Eluting Stents (EES)
Initial studies with follow-up angiography demonstrated decreased rates of angiographic restenosis among diabetic patients treated with EES as compared to PES. A pooled study comparing the use of the Xience V EES and TAXUS LIBERTÉ™ PES (SPIRIT V diabetic study) determined that the rate of angiographic lumen loss, which reflects the degree of neointimal hyperplasia, was reduced in diabetic patients treated with EES as compared to PES, without any effect on safety outcomes.58 Further studies demonstrated that EES were associated with decreased neointima formation, lumen loss and vessel narrowing in diabetic patients, as measured by intravascular ultrasound, when compared to PES.67
These findings concur with recent studies in which EES decreased rates of ST up to one year following treatment in diabetic patients when compared to PES, with similar composite TLR-MACE outcomes between the stent types.68 In the ESSENCE-DIABETES Trial comparing EES and SES in diabetic patients, EES was associated with decreased in-segment lumen loss, restenosis rates, and ST in diabetic patients, while maintaining similar safety outcomes to SES.69
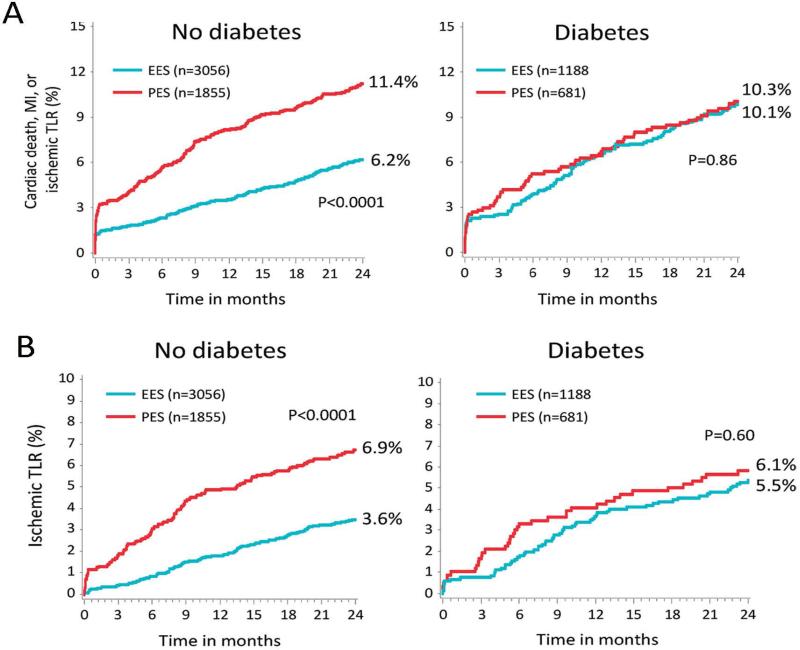
Other studies have suggested that EES may have less relative benefit among patients with DM. A pooled analysis of the SPIRIT II, SPIRIT III, SPIRIT IV and comparison of the everolimus eluting XIENCE-V stent with the paclitaxel eluting TAXUS LIBERTÉ stent in all-comers (COMPARE) trials evaluated the second-generation EES system, Xience V/PROMUS vs. the first-generation TAXUS LIBERTÉ PES. Strikingly, non-diabetic patients receiving EES had decreased mortality, MI, ST, and TLR as compared to PES in the overall population, whereas there were no differences in efficacy or safety outcomes between the two stent platforms among patients with diabetes (Figure 2).70 These unexpected differential effects in patients with diabetes versus those without diabetes highlight the as yet uncertain mechanistic links between stent drug-elution and adverse events after PCI. Similarly, the SPIRIT IV Trial demonstrated no difference in efficacy between EES and PES in diabetic patients with 3 or fewer de novo lesions, even though marked increases in efficacy were shown in the non-diabetic population.71 Consistent with other studies, patients with DM who required insulin had higher rates of these adverse outcomes than patients with DM treated with oral agents.
Figure 2.
Differential Outcomes After PCI Among Patients with and without Diabetes in the SPIRIT and COMPARE Trials. Pooled analysis of the SPIRIT and COMPARE trials demonstrate a significant interaction effect of diabetes on the outcomes of everolimus-eluting stents (EES) vs. paclitaxel-eluting stents (PES). Top panel: Among patients without diabetes, the rates of cardiac death, MI, or ischemic TLR were significantly lower for EES compared to PES. In comparison, there was no difference in outcomes of EES vs. PES among patients with DM. Bottom panel: Among patients without diabetes, EES were associated with significantly decreased rates of ischemic TLR. There was no difference in rates of ischemic TLR among patients with diabetes. (Reproduced, with permission, from Stone et al, Circulation 2011;124:893-900.)
Zotarolimus Eluting Stents (ZES)
The ENDEAVOR IV trial examined the second-generation Endeavor™ zotarolimus-eluting stent (E-ZES) in patients with a single de novo lesion and demonstrated non-inferiority of E-ZES to the TAXUS PES in both diabetic and non-diabetic patient populations in terms of safety, efficacy and clinical outcomes.72, 73 However, the percentage of in-stent restenosis was increased in diabetic patients treated with E-ZES compared to non-diabetic patients treated with E-ZES. Further stratification revealed that this effect was independent of whether insulin was necessary to treat a patient's diabetes. Additional comparisons between diabetic and non-diabetic patients treated with E-ZES in the E-FIVE Registry demonstrated that diabetic patients had significantly higher rates of MACE, TLR, TVF, and early ST than non-diabetic patients at one year, and that insulin dependence led to increased MACE and TVF when compared to non-insulin dependent diabetic patients.74
Comparisons of PES, SES and ZES
Direct comparison of E-ZES with SES in the SORT OUT III Trial demonstrated that treatment with E-ZES was associated with increased rates of MACE, as well as TVR and TLR in both diabetic and non-diabetic patients at 18 months, but these increases were much greater in the diabetic population.75 In comparison, the PROTECT study showed comparable levels of definite ST at 3 years in both diabetic and non-diabetic populations treated with E-ZES and SES,76 suggesting similar long-term safety outcomes between the two stents.
A direct comparison of SES, PES and E-ZES in type 2 diabetes patients was undertaken in the Novel Approaches for Preventing or Limiting Events in Diabetic Patients (NAPLES-Diabetes) Trial. Results from this trial indicated that treatment of diabetic patients with E-ZES, as compared to either PES or SES, led to increased 3-year rates of MACE, due largely to a higher rate of TLR.57 Similar results were found in the Swedish Coronary Angiography and Angioplasty Register (SCAAR) study.77
Resolute Zotarolimus-Eluting Stent (R-ZES)
Recently, the latest-generation Resolute™ ZES (R-ZES) became the first DES to gain an FDA labeling indication for patients with DM. The R-ZES sought to improve on E-ZES with controlled drug release over a longer time period, while maintaining the safety outcomes observed with E-ZES.78 FDA approval was based on a pre-specified performance goal in diabetic patients.79 The study population included 878 diabetic and matched control subjects from the Global Resolute Clinical Trial Program. A pre-specified performance goal of 14.5% TVF, which included cardiac death, MI not attributable to other vessels, and TVR, was implemented based on a meta-analysis of published studies in diabetic patients treated with first-generation SES and PES stents and data from pooled Endeavor studies.
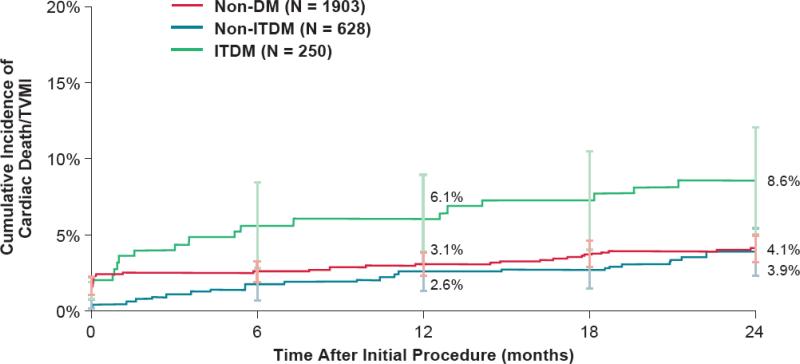
At 1-year follow-up, the R-ZES TVF rate in diabetic patients was superior (7.8%) to the pre-specified performance goal of 14.5% (p<0.001). In results from the 2-year follow-up to this pooled study, R-ZES continued to perform similarly in both diabetic and non-diabetic patient populations and, importantly, the rates of ST were not significantly different between diabetic patients and non-diabetic patients. Further stratification of the diabetic population into patients requiring treatment with insulin demonstrated that TLF rates in the non-insulin treated population remained similar to those in the non-diabetic population, while the rate of TLF was increased in the insulin treated population (Figure 3). These findings emphasize that insulin-dependence plays an important role in determining the safety and efficacy of DES in diabetic patients.
Figure 3.
Outcomes After PCI Among Patients with Diabetes Treated with Resolute Zotarolimus-Eluting Stents. During two-year follow-up, the rates of cardiac death or target vessel MI were similar between patients without diabetes and those with diabetes who did not require treatment with insulin (non-ITDM). In comparison, patients with diabetes who required treatment with insulin (ITDM) had significantly higher rates of cardiac death or target vessel MI at two years of follow-up. (Reproduced, with permission, from Silber et al J. Am. Coll. Cardiol. Intv, 2013; 6:357-368.)
Recent trials have also compared R-ZES to other DES types and found no significant differences in clinically driven outcomes. In the TWENTE Trial, the safety and efficacy of R-ZES was compared to that of the Xience V EES in an all-comers population. This trial demonstrated the overall non-inferiority of R-ZES as compared to EES, and there were no significant differences in the primary endpoint of TVF between R-ZES and EES in the subset of diabetic patients.80
These initial trials with the R-ZES provide encouraging results for patients with diabetes undergoing PCI. The pre-specified analysis of outcomes for patients with DM treated with RZES did not include the higher risk cohorts of patients treated in the RESOLUTE All Comers or RESOLUTE International trials.81, 82 Real-world outcomes among patients with diabetes and more complex lesions may therefore be associated with higher target lesion event rates during long-term follow-up. However, current data support improved outcomes of R-ZES in patients with DM compared to first-generation DES.
Pharmacotherapy After Revascularization in Patients with Diabetes
Although patients with diabetes are at high risk for recurrent cardiovascular events after revascularization, a number of studies have shown that these patients are not adequately managed for modifiable risk factors.83 Close attention must be paid to secondary risk reduction after both CABG and PCI with a goal of meeting current guideline directed therapies for control of hypertension, cholesterol, smoking cessation, and hemoglobin A1C. Current guidelines for diabetic patients recommend a target blood pressure of <130/80 mm Hg, LDL <100 mg/dl for established CAD and <70 mg/dL in the highest risk patients, immediate smoking cessation, and strong consideration of aspirin therapy.84 Although strict glucose control for reduction of cardiovascular events has met with mixed results in randomized trials, a goal hemoglobin A1C of <7% is a reasonable target for patients with a life expectancy exceeding five years.85 It remains uncertain whether specific medications are favored for control of glucose in patients with diabetes and CAD. Although DM requiring insulin is associated with increased cardiovascular event rates after PCI, it is uncertain whether the increased event rates are due to insulin use or confounded by the presence of more severe diabetes. Recent research has also suggested that metformin may be associated with impaired re-endothelialization after PCI, although no clinical studies have yet investigated whether metformin increases rates of restenosis or target lesion failure after PCI.86
Diabetic patients who have undergone PCI may also benefit from more intensive antiplatelet therapy. The TRITON-TIMI 38 and PLATO trials both found an overall improvement in net clinical outcomes for prasugrel or ticagrelor compared to clopidogrel after PCI.87, 88 Subgroup analyses of patients from these trials with DM reported that diabetics have equal or greater relative reduction in MACE compared to patients without diabetes. In the TRITON TIMI-38 trial, patients with diabetes treated with prasugrel had a greater net clinical benefit than the overall population, with an observed improvement in outcomes for both insulin requiring and non-insulin requiring diabetic patients. Similar trends were observed among the cohort of patients with diabetes treated with ticagrelor in the PLATO trial, although these results were not statistically significant. Treatment of diabetic patients with prasugrel in the TIMI-38 trial was also associated with a significant reduction in the risk of stent thrombosis (2.0% vs. 3.6%, p<0.001) among this high-risk cohort.87 Strong consideration should therefore be given towards administering prasugrel or ticagrelor as part of a dual antiplatelet strategy after PCI in diabetic patients, while weighing the possible increased risk of bleeding.
Future Directions
Although modern revascularization strategies have greatly improved the outcomes of patients with DM and CAD, much work remains to better understand the underlying mechanisms of CAD in the setting of diabetes and to improve clinical outcomes in this challenging patient population. Further characterization of the signaling mechanisms that link diabetes to restenosis after percutaneous intervention could lead to development of novel anti-restenotic agents specific to diabetic patients. While CABG remains superior to PCI among patients with diabetes who are candidates for surgical revascularization (and particularly for those with higher angiographic disease complexity), the gap between CABG and PCI has narrowed over time. Advances in stent technology including bioresorbable stents may further minimize the risk of target lesion failure and the long-term risk of stent thrombosis.89 Additionally, invasive assessment of lesion significance with fractional flow reserve will help with identifying hemodynamically important lesions that benefit most from revascularization. Such a strategy may have important prognostic utility in reclassifying patients with apparent three-vessel disease into functional one- or two-vessel CAD.90, 91 As the prevalence of diabetes continues to rise, development of new treatment strategies and increased recognition of the association between diabetes and outcomes after revascularization will help identify novel treatments for this high-risk cohort of patients.
Supplementary Material
Acknowledgments
The authors thank Chantelle Rein-Smith, PhD and Robin Whitsell, BA, BPh for their assistance in the preparation of this manuscript and Amanda Behr, MA, CMI for assistance in the preparation of Figure 1.
Funding Sources: Ehrin Armstrong was supported by American Heart Association grant 11CRP7260031. John Rutledge was supported by the NIH-NIA AG039094 and the Richard A. Harrison Endowed Chair in Diabetes Research.
Footnotes
Conflict of Interest Disclosures: Dr. Rogers is a consultant for Medtronic and Boston Scientific. Drs. Armstrong and Rutledge have no relevant disclosures.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention. Atlanta G. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the us 2011. US Department of Health and Human Services; 2011. [Google Scholar]
- 3.Hammoud T, Tanguay JF, Bourassa MG. Management of coronary artery disease: Therapeutic options in patients with diabetes. J Am Coll Cardiol. 2000;36:355–365. doi: 10.1016/s0735-1097(00)00732-4. [DOI] [PubMed] [Google Scholar]
- 4.Aronson D, Edelman ER. Revascularization for coronary artery disease in diabetes mellitus: Angioplasty, stents and coronary artery bypass grafting. Reviews in Endocrine & Metabolic Disorders. 2010;11:75–86. doi: 10.1007/s11154-010-9135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schramm TK, Gislason GH, Kober L, Rasmussen S, Rasmussen JN, Abildstrom SZ, Hansen ML, Folke F, Buch P, Madsen M, Vaag A, Torp-Pedersen C. Diabetes patients requiring glucose-lowering therapy and nondiabeticsf with a prior myocardial infarction carry the same cardiovascular risk: A population study of 3.3 million people. Circulation. 2008;117:1945–1954. doi: 10.1161/CIRCULATIONAHA.107.720847. [DOI] [PubMed] [Google Scholar]
- 6.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. American College of Cardiology F, American Heart Association Task Force on Practice G, Society for Cardiovascular A, Interventions. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF, Hutter AM, Jr., Jessen ME, Keeley EC, Lahey SJ, Lange RA, London MJ, Mack MJ, Patel MR, Puskas JD, Sabik JF, Selnes O, Shahian DM, Trost JC, Winniford MD. 2011 accf/aha guideline for coronary artery bypass graft surgery. A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e123–210. doi: 10.1016/j.jacc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 9.Berry C, Tardif JC, Bourassa MG. Coronary heart disease in patients with diabetes: Part I: Recent advances in prevention and noninvasive management. J Am Coll Cardiol. 2007;49:631–642. doi: 10.1016/j.jacc.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 10.Bangalore S, Kumar S, Fusaro M, Amoroso N, Kirtane AJ, Byrne RA, Williams DO, Slater J, Cutlip DE, Feit F. Outcomes with various drug eluting or bare metal stents in patients with diabetes mellitus: Mixed treatment comparison analysis of 22,844 patient years of follow-up from randomised trials. BMJ. 2012;345:e5170. doi: 10.1136/bmj.e5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88:2510–2516. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997;100:1230–1239. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Woo V, Bose R. Platelet hyperactivity and abnormal ca(2+) homeostasis in diabetes mellitus. Am J Physiol Heart Circ Physiol. 2001;280:H1480–1489. doi: 10.1152/ajpheart.2001.280.4.H1480. [DOI] [PubMed] [Google Scholar]
- 14.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 15.Zeiher AM, Fisslthaler B, Schray-Utz B, Busse R. Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells. Circ Res. 1995;76:980–986. doi: 10.1161/01.res.76.6.980. [DOI] [PubMed] [Google Scholar]
- 16.Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:998–1005. doi: 10.1161/01.ATV.0000125114.88079.96. [DOI] [PubMed] [Google Scholar]
- 17.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartge MM, Unger T, Kintscher U. The endothelium and vascular inflammation in diabetes. Diabetes & Vascular Disease Research. 2007;4:84–88. doi: 10.3132/dvdr.2007.025. [DOI] [PubMed] [Google Scholar]
- 19.Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL. Platelet dysfunction in type 2 diabetes. Diabetes Care. 2001;24:1476–1485. doi: 10.2337/diacare.24.8.1476. [DOI] [PubMed] [Google Scholar]
- 20.Bierhaus A, Hofmann MA, Ziegler R, Nawroth PP. AGEs and their interaction with age receptors in vascular disease and diabetes mellitus. I. The age concept. Cardiovasc Res. 1998;37:586–600. doi: 10.1016/s0008-6363(97)00233-2. [DOI] [PubMed] [Google Scholar]
- 21.Stern DM, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev. 2002;1:1–15. doi: 10.1016/s0047-6374(01)00366-9. [DOI] [PubMed] [Google Scholar]
- 22.Bierhaus A, Nawroth PP. Multiple levels of regulation determine the role of the receptor for age (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia. 2009;52:2251–2263. doi: 10.1007/s00125-009-1458-9. [DOI] [PubMed] [Google Scholar]
- 23.Park SH, Marso SP, Zhou Z, Foroudi F, Topol EJ, Lincoff AM. Neointimal hyperplasia after arterial injury is increased in a rat model of non-insulin-dependent diabetes mellitus. Circulation. 2001;104:815–819. doi: 10.1161/hc3301.092789. [DOI] [PubMed] [Google Scholar]
- 24.Kornowski R, Mintz GS, Kent KM, Pichard AD, Satler LF, Bucher TA, Hong MK, Popma JJ, Leon MB. Increased restenosis in diabetes mellitus after coronary interventions is due to exaggerated intimal hyperplasia. A serial intravascular ultrasound study. Circulation. 1997;95:1366–1369. doi: 10.1161/01.cir.95.6.1366. [DOI] [PubMed] [Google Scholar]
- 25.Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part II. Circulation. 2003;108:1655–1661. doi: 10.1161/01.CIR.0000089189.70578.E2. [DOI] [PubMed] [Google Scholar]
- 26.Jonas M, Edelman ER, Groothuis A, Baker AB, Seifert P, Rogers C. Vascular neointimal formation and signaling pathway activation in response to stent injury in insulin-resistant and diabetic animals. Circ Res. 2005;97:725–733. doi: 10.1161/01.RES.0000183730.52908.C6. [DOI] [PubMed] [Google Scholar]
- 27.Patterson C, Mapera S, Li HH, Madamanchi N, Hilliard E, Lineberger R, Herrmann R, Charles P. Comparative effects of paclitaxel and rapamycin on smooth muscle migration and survival: Role of akt-dependent signaling. Arterioscler Thromb Vasc Biol. 2006;26:1473–1480. doi: 10.1161/01.ATV.0000223866.42883.3b. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka N, Terashima M, Rathore S, Itoh T, Habara M, Nasu K, Kimura M, Itoh T, Kinoshita Y, Ehara M, Tsuchikane E, Asakura K, Asakura Y, Katoh O, Suzuki T. Different patterns of vascular response between patients with or without diabetes mellitus after drug-eluting stent implantation: Optical coherence tomographic analysis. JACC Cardiovasc Interv. 2010;3:1074–1079. doi: 10.1016/j.jcin.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Colwell JA, Nesto RW. The platelet in diabetes: Focus on prevention of ischemic events. Diabetes Care. 2003;26:2181–2188. doi: 10.2337/diacare.26.7.2181. [DOI] [PubMed] [Google Scholar]
- 30.Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J. 2011;32:2748–2757. doi: 10.1093/eurheartj/ehr305. [DOI] [PubMed] [Google Scholar]
- 31.Wiviott SD, Braunwald E, McCabe CH, Horvath I, Keltai M, Herrman JP, Van de Werf F, Downey WE, Scirica BM, Murphy SA, Antman EM, Investigators T-T. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the Triton-TIMI 38 trial: A subanalysis of a randomised trial. Lancet. 2008;371:1353–1363. doi: 10.1016/S0140-6736(08)60422-5. [DOI] [PubMed] [Google Scholar]
- 32.Dagenais GR, Lu J, Faxon DP, Bogaty P, Adler D, Fuentes F, Escobedo J, Krishnaswami A, Slater J, Frye RL. Prognostic impact of the presence and absence of angina on mortality and cardiovascular outcomes in patients with type 2 diabetes and stable coronary artery disease: Results from the BARI 2D (bypass angioplasty revascularization investigation 2 diabetes) trial. J Am Coll Cardiol. 2013;61:702–711. doi: 10.1016/j.jacc.2012.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooks MM, Frye RL, Genuth S, Detre KM, Nesto R, Sobel BE, Kelsey SF, Orchard TJ. Hypotheses, design, and methods for the bypass angioplasty revascularization investigation 2 diabetes (BARI 2D) trial. Am J Cardiol. 2006;97:9G–19G. doi: 10.1016/j.amjcard.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 34.Group TBDS A randomized trial of therapies for type 2 diabetes and coronary artery disease. New England Journal of Medicine. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 appropriateness criteria for coronary revascularization: A report of the american college of cardiology foundation appropriateness criteria task force, society for cardiovascular angiography and interventions, society of thoracic surgeons, american association for thoracic surgery, american heart association, and the american society of nuclear cardiology: Endorsed by the american society of echocardiography, the heart failure society of america, and the society of cardiovascular computed tomography. Circulation. 2009;119:1330–1352. doi: 10.1161/CIRCULATIONAHA.108.191768. [DOI] [PubMed] [Google Scholar]
- 36.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update: A report of the american college of cardiology foundation appropriate use criteria task force, society for cardiovascular angiography and interventions, society of thoracic surgeons, american association for thoracic surgery, american heart association, american society of nuclear cardiology, and the society of cardiovascular computed tomography. J Am Coll Cardiol. 2012;59:857–881. doi: 10.1016/j.jacc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 37.The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol. 2007;49:1600–1606. doi: 10.1016/j.jacc.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 38.Gum PA, O'Keefe JH, Jr., Borkon AM, Spertus JA, Bateman TM, McGraw JP, Sherwani K, Vacek J, McCallister BD. Bypass surgery versus coronary angioplasty for revascularization of treated diabetic patients. Circulation. 1997;96:II–7-10. [PubMed] [Google Scholar]
- 39.King SB, 3rd, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eight-year mortality in the emory angioplasty versus surgery trial (east). J Am Coll Cardiol. 2000;35:1116–1121. doi: 10.1016/s0735-1097(00)00546-5. [DOI] [PubMed] [Google Scholar]
- 40.Niles NW, McGrath PD, Malenka D, Quinton H, Wennberg D, Shubrooks SJ, Tryzelaar JF, Clough R, Hearne MJ, Hernandez F, Jr., Watkins MW, O'Connor GT. Survival of patients with diabetes and multivessel coronary artery disease after surgical or percutaneous coronary revascularization: Results of a large regional prospective study. Northern new england cardiovascular disease study group. J Am Coll Cardiol. 2001;37:1008–1015. doi: 10.1016/s0735-1097(00)01205-5. [DOI] [PubMed] [Google Scholar]
- 41.Barsness GW, Peterson ED, Ohman EM, Nelson CL, DeLong ER, Reves JG, Smith PK, Anderson RD, Jones RH, Mark DB, Califf RM. Relationship between diabetes mellitus and long-term survival after coronary bypass and angioplasty. Circulation. 1997;96:2551–2556. doi: 10.1161/01.cir.96.8.2551. [DOI] [PubMed] [Google Scholar]
- 42.Weintraub WS, Stein B, Kosinski A, Douglas JS, Jr., Ghazzal ZM, Jones EL, Morris DC, Guyton RA, Craver JM, King SB., 3rd. Outcome of coronary bypass surgery versus coronary angioplasty in diabetic patients with multivessel coronary artery disease. J Am Coll Cardiol. 1998;31:10–19. doi: 10.1016/s0735-1097(97)00441-5. [DOI] [PubMed] [Google Scholar]
- 43.Kurbaan AS, Bowker TJ, Ilsley CD, Sigwart U, Rickards AF, Investigators C. Difference in the mortality of the cabri diabetic and nondiabetic populations and its relation to coronary artery disease and the revascularization mode. Am J of Cardiol. 2001;87:947–950. A943. doi: 10.1016/s0002-9149(01)01426-6. [DOI] [PubMed] [Google Scholar]
- 44.Park DW, Kim YH, Song HG, Ahn JM, Kim WJ, Lee JY, Kang SJ, Lee SW, Lee CW, Park SW, Yun SC, Her SH, Hur SH, Park JS, Kim MK, Choi YS, Kim HS, Cho JH, Lee SG, Park YW, Jeong MH, Lee BK, Lee NH, Lim DS, Yoon J, Seung KB, Shin WY, Rha SW, Kim KS, Tahk SJ, Park BE, Ahn T, Yang JY, Jeong YS, Rhew JH, Park SJ, Investigators I-D. Outcomes after unrestricted use of everolimus-eluting and sirolimus eluting stents in routine clinical practice: A multicenter, prospective cohort study. Circ: Cardiovasc Interv. 2012;5:365–371. doi: 10.1161/CIRCINTERVENTIONS.111.966549. [DOI] [PubMed] [Google Scholar]
- 45.Daemen J, Kuck KH, Macaya C, LeGrand V, Vrolix M, Carrie D, Sheiban I, Suttorp MJ, Vranckx P, Rademaker T, Goedhart D, Schuijer M, Wittebols K, Macours N, Stoll HP, Serruys PW, Investigators A-I. Multivessel coronary revascularization in patients with and without diabetes mellitus: 3-year follow-up of the arts-II (arterial revascularization therapies study-part II) trial. J Am Coll Cardiol. 2008;52:1957–1967. doi: 10.1016/j.jacc.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Onuma Y, Wykrzykowska JJ, Garg S, Vranckx P, Serruys PW, Arts I, Investigators II. 5-year follow-up of coronary revascularization in diabetic patients with multivessel coronary artery disease: Insights from ARTS (arterial revascularization therapy study)-II and ARTS-I trials. JACC: Cardiovasc Interv. 2011;4:317–323. doi: 10.1016/j.jcin.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Kapur A, Hall RJ, Malik IS, Qureshi AC, Butts J, de Belder M, Baumbach A, Angelini G, de Belder A, Oldroyd KG, Flather M, Roughton M, Nihoyannopoulos P, Bagger JP, Morgan K, Beatt KJ. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDiA (coronary artery revascularization in diabetes) trial. J Am Coll Cardiol. 2010;55:432–440. doi: 10.1016/j.jacc.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Kappetein AP, Head SJ, Morice MC, Banning AP, Serruys PW, Mohr FW, Dawkins KD, Mack MJ. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the syntax trial. Eur J Cardiothorac Surg. 2013;43:1006–1013. doi: 10.1093/ejcts/ezt017. [DOI] [PubMed] [Google Scholar]
- 49.Mohr FW, Morice MC, Kappetein AP, Feldman TE, Stahle E, Colombo A, Mack MJ, Holmes DR, Jr., Morel MA, Van Dyck N, Houle VM, Dawkins KD, Serruys PW. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–638. doi: 10.1016/S0140-6736(13)60141-5. [DOI] [PubMed] [Google Scholar]
- 50.Mack MJ, Banning AP, Serruys PW, Morice MC, Taeymans Y, Van Nooten G, Possati G, Crea F, Hood KL, Leadley K, Dawkins KD, Kappetein AP. Bypass versus drug-eluting stents at three years in syntax patients with diabetes mellitus or metabolic syndrome. Ann Thorac Surg. 2011;92:2140–2146. doi: 10.1016/j.athoracsur.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 51.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S, 3rd, Bertrand M, Fuster V, Investigators FT Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 52.Capodanno D, Caggegi A, Capranzano P, Cincotta G, Miano M, Barrano G, Monaco S, Calvo F, Tamburino C. Validating the excel hypothesis: A propensity score matched 3-year comparison of percutaneous coronary intervention versus coronary artery bypass graft in left main patients with syntax score </=32. Catheter Cardiovasc Interv. 2011;77:936–943. doi: 10.1002/ccd.22992. [DOI] [PubMed] [Google Scholar]
- 53.Moussa I, Leon MB, Baim DS, O'Neill WW, Popma JJ, Buchbinder M, Midwall J, Simonton CA, Keim E, Wang P, Kuntz RE, Moses JW. Impact of sirolimus-eluting stents on outcome in diabetic patients: A SIRIUS (sirolimus-coated bx velocity balloon- expandable stent in the treatment of patients with de novo coronary artery lesions) substudy. Circulation. 2004;109:2273–2278. doi: 10.1161/01.CIR.0000129767.45513.71. [DOI] [PubMed] [Google Scholar]
- 54.Kirtane AJ, Ellis SG, Dawkins KD, Colombo A, Grube E, Popma JJ, Fahy M, Leon MB, Moses JW, Mehran R, Stone GW. Paclitaxel-eluting coronary stents in patients with diabetes mellitus: Pooled analysis from 5 randomized trials. J Am Coll Cardiol. 2008;51:708–715. doi: 10.1016/j.jacc.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 55.Stettler C, Allemann S, Wandel S, Kastrati A, Morice MC, Schomig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, Goy JJ, Park SJ, Sabate M, Suttorp MJ, Kelbaek H, Spaulding C, Menichelli M, Vermeersch P, Dirksen MT, Cervinka P, De Carlo M, Erglis A, Chechi T, Ortolani P, Schalij MJ, Diem P, Meier B, Windecker S, Juni P. Drug eluting and bare metal stents in people with and without diabetes: Collaborative network meta-analysis. BMJ. 2008;337:a1331. doi: 10.1136/bmj.a1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinning JM, Baumgart D, Werner N, Klauss V, Baer FM, Hartmann F, Drexler H, Motz W, Klues H, Voelker W, Pfannebecker T, Stoll HP, Nickenig G, Study S. Five-year results of the multicenter randomized controlled open-label study of the cypher sirolimus eluting stent in the treatment of diabetic patients with de novo native coronary artery lesions (SCORPIUS) study: A german multicenter investigation on the effectiveness of sirolimus-eluting stents in diabetic patients. Am Heart J. 2012;163:446–453. 453, e441. doi: 10.1016/j.ahj.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 57.Briguori C, Airoldi F, Visconti G, Focaccio A, Caiazzo G, Golia B, Biondi-Zoccai G, Ricciardelli B, Condorelli G. Novel approaches for preventing or limiting events in diabetic patients (naples-diabetes) trial: A randomized comparison of 3 drug-eluting stents in diabetic patients. Circ Cardiovasc Interv. 2011;4:121–129. doi: 10.1161/CIRCINTERVENTIONS.110.959924. [DOI] [PubMed] [Google Scholar]
- 58.Grube E, Chevalier B, Guagliumi G, Smits PC, Stuteville M, Dorange C, Papeleu P, Kaul U, Dzavik V. The spirit v diabetic study: A randomized clinical evaluation of the xience v everolimus-eluting stent vs the taxus liberte paclitaxel-eluting stent in diabetic patients with de novo coronary artery lesions. Am Heart J. 2012;163:867–875. e861. doi: 10.1016/j.ahj.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Bangalore S, Kumar S, Fusaro M, Amoroso N, Kirtane AJ, Byrne RA, Williams DO, Slater J, Cutlip DE, Feit F. Outcomes with various drug eluting or bare metal stents in patients with diabetes mellitus: Mixed treatment comparison analysis of 22 844 patient years of follow-up from randomised trials. BMJ. 2012;345:e5170–e5170. doi: 10.1136/bmj.e5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stone GW, Ellis SG, Colombo A, Grube E, Popma JJ, Uchida T, Bleuit JS, Dawkins KD, Russell ME. Long-term safety and efficacy of paclitaxel-eluting stents final 5-year analysis from the taxus clinical trial program. JACC Cardiovasc Interv. 2011;4:530–542. doi: 10.1016/j.jcin.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Hermiller JB, Raizner A, Cannon L, Gurbel PA, Kutcher MA, Wong SC, Russell ME, Ellis SG, Mehran R, Stone GW, Investigators T-I. Outcomes with the polymer-based paclitaxel-eluting taxus stent in patients with diabetes mellitus: The taxus-iv trial. J Am Coll Cardiol. 2005;45:1172–1179. doi: 10.1016/j.jacc.2004.10.075. [DOI] [PubMed] [Google Scholar]
- 62.Mahmud E, Ormiston JA, Turco MA, Popma JJ, Weissman NJ, O'Shaughnessy CD, Mann T, Hall JJ, McGarry TF, Cannon LA, Webster MW, Mandinov L, Baim DS. Taxus liberte attenuates the risk of restenosis in patients with medically treated diabetes mellitus: Results from the taxus atlas program. JACC Cardiovasc Interv. 2009;2:240–252. doi: 10.1016/j.jcin.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 63.Caixeta A, Leon MB, Lansky AJ, Nikolsky E, Aoki J, Moses JW, Schofer J, Morice MC, Schampaert E, Kirtane AJ, Popma JJ, Parise H, Fahy M, Mehran R. 5-year clinical outcomes after sirolimus-eluting stent implantation insights from a patient-level pooled analysis of 4 randomized trials comparing sirolimus-eluting stents with bare-metal stents. J Am Coll Cardiol. 2009;54:894–902. doi: 10.1016/j.jacc.2009.04.077. [DOI] [PubMed] [Google Scholar]
- 64.Garg P, Normand SL, Silbaugh TS, Wolf RE, Zelevinsky K, Lovett A, Varma MR, Zhou Z, Mauri L. Drug-eluting or bare-metal stenting in patients with diabetes mellitus: Results from the Massachusetts data analysis center registry. Circulation. 2008;118:2277–2285. doi: 10.1161/CIRCULATIONAHA.108.820159. 2277p following 2285. [DOI] [PubMed] [Google Scholar]
- 65.Raber L, Wohlwend L, Wigger M, Togni M, Wandel S, Wenaweser P, Cook S, Moschovitis A, Vogel R, Kalesan B, Seiler C, Eberli F, Luscher TF, Meier B, Juni P, Windecker S. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: Results of the sirolimus-eluting versus paclitaxel-eluting stents for coronary revascularization late trial. Circulation. 2011;123:2819–2828. doi: 10.1161/CIRCULATIONAHA.110.004762. [DOI] [PubMed] [Google Scholar]
- 66.Billinger M, Raber L, Hitz S, Stefanini GG, Pilgrim T, Stettler C, Zanchin T, Pulver C, Pfaffli N, Eberli F, Meier B, Kalesan B, Juni P, Windecker S. Long-term clinical and angiographic outcomes of diabetic patients after revascularization with early generation drug-eluting stents. Am Heart J. 2012;163:876–886. doi: 10.1016/j.ahj.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 67.Otake H, Ako J, Yamasaki M, Tsujino I, Shimohama T, Hasegawa T, Sakurai R, Waseda K, Honda Y, Sood P, Sudhir K, Stone GW, Fitzgerald PJ. Comparison of everolimus-versus paclitaxel-eluting stents implanted in patients with diabetes mellitus as evaluated by three-dimensional intravascular ultrasound analysis. Am J Cardiol. 2010;106:492–497. doi: 10.1016/j.amjcard.2010.03.059. [DOI] [PubMed] [Google Scholar]
- 68.Laynez A, Sardi G, Hauville C, Barbash IM, Pakala R, Torguson R, Xue Z, Satler LF, Pichard AD, Waksman R. Safety and efficacy of everolimus-eluting stents versus paclitaxel-eluting stents in a diabetic population. Catheter Cardiovasc Intv. 2013;81:759–765. doi: 10.1002/ccd.24438. [DOI] [PubMed] [Google Scholar]
- 69.Kim WJ, Lee SW, Park SW, Kim YH, Yun SC, Lee JY, Park DW, Kang SJ, Lee CW, Lee JH, Choi SW, Seong IW, Lee BK, Lee NH, Cho YH, Shin WY, Lee SJ, Lee SW, Hyon MS, Bang DW, Park WJ, Kim HS, Chae JK, Lee K, Park HK, Park CB, Lee SG, Kim MK, Park KH, Choi YJ, Cheong SS, Yang TH, Jang JS, Her SH, Park SJ, Investigators E-DS. Randomized comparison of everolimus-eluting stent versus sirolimus-eluting stent implantation for de novo coronary artery disease in patients with diabetes mellitus (essence-diabetes): Results from the essence-diabetes trial. Circulation. 2011;124:886–892. doi: 10.1161/CIRCULATIONAHA.110.015453. [DOI] [PubMed] [Google Scholar]
- 70.Stone GW, Kedhi E, Kereiakes DJ, Parise H, Fahy M, Serruys PW, Smits PC. Differential clinical responses to everolimus-eluting and paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation. 2011;124:893–900. doi: 10.1161/CIRCULATIONAHA.111.031070. [DOI] [PubMed] [Google Scholar]
- 71.Kereiakes DJ, Cutlip DE, Applegate RJ, Wang J, Yaqub M, Sood P, Su X, Su G, Farhat N, Rizvi A, Simonton CA, Sudhir K, Stone GW. Outcomes in diabetic and nondiabetic patients treated with everolimus- or paclitaxel-eluting stents: Results from the SPIRIT IV clinical trial (clinical evaluation of the xience v everolimus eluting coronary stent system). J Am Coll Cardiol. 2010;56:2084–2089. doi: 10.1016/j.jacc.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 72.Kirtane AJ, Patel R, O'Shaughnessy C, Overlie P, McLaurin B, Solomon S, Mauri L, Fitzgerald P, Popma JJ, Kandzari DE, Leon MB. Clinical and angiographic outcomes in diabetics from the ENDEAVOR IV trial: Randomized comparison of zotarolimus- and paclitaxel-eluting stents in patients with coronary artery disease. JACC Cardiovasc Interv. 2009;2:967–976. doi: 10.1016/j.jcin.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Leon MB, Mauri L, Popma JJ, Cutlip DE, Nikolsky E, O'Shaughnessy C, Overlie PA, McLaurin BT, Solomon SL, Douglas JS, Jr., Ball MW, Caputo RP, Jain A, Tolleson TR, Reen BM, 3rd, Kirtane AJ, Fitzgerald PJ, Thompson K, Kandzari DE, Investigators EI A randomized comparison of the endeavor zotarolimus-eluting stent versus the taxus paclitaxel-eluting stent in de novo native coronary lesions 12-month outcomes from the ENDEAVOR IV trial. J Am Coll Cardiol. 2010;55:543–554. doi: 10.1016/j.jacc.2009.08.067. [DOI] [PubMed] [Google Scholar]
- 74.Jain AK, Lotan C, Meredith IT, Feres F, Zambahari R, Sinha N, Rothman MT, Investigators EFR. Twelve-month outcomes in patients with diabetes implanted with a zotarolimus-eluting stent: Results from the E-FIVE registry. Heart. 2010;96:848–853. doi: 10.1136/hrt.2009.184150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maeng M, Jensen LO, Tilsted HH, Kaltoft A, Kelbaek H, Abildgaard U, Villadsen A, Aaroe J, Thayssen P, Krusell LR, Christiansen EH, Botker HE, Kristensen SD, Ravkilde J, Madsen M, Sorensen HT, Rasmussen K, Thuesen L, Lassen JF. Outcome of sirolimus eluting versus zotarolimus-eluting coronary stent implantation in patients with and without diabetes mellitus (a SORT-OUT III substudy). Am J Cardiol. 2011;108:1232–1237. doi: 10.1016/j.amjcard.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 76.Camenzind E, Wijns W, Mauri L, Kurowski V, Parikh K, Gao R, Bode C, Greenwood JP, Boersma E, Vranckx P, McFadden E, Serruys PW, O'Neil WW, Jorissen B, Van Leeuwen F, Steg PG. Stent thrombosis and major clinical events at 3 years after zotarolimus-eluting or sirolimus-eluting coronary stent implantation: A randomised, multicentre, open-label, controlled trial. The Lancet. 2012;380:1396–1405. doi: 10.1016/S0140-6736(12)61336-1. [DOI] [PubMed] [Google Scholar]
- 77.Frobert O, Lagerqvist B, Carlsson J, Lindback J, Stenestrand U, James SK. Differences in restenosis rate with different drug-eluting stents in patients with and without diabetes mellitus: A report from the SCAAR (swedish angiography and angioplasty registry). J Am Coll Cardiol. 2009;53:1660–1667. doi: 10.1016/j.jacc.2009.01.054. [DOI] [PubMed] [Google Scholar]
- 78.Vasaiwala S, Mauri L. Clinical review of the resolute zotarolimus-eluting stent for the treatment of coronary artery disease. Interventional Cardiology. 2012;4:33–43. [Google Scholar]
- 79.Silber S, Serruys PW, Leon MB, Meredith IT, Windecker S, Neumann FJ, Belardi J, Widimsky P, Massaro J, Novack V, Yeung AC, Saito S, Mauri L. Clinical outcome of patients with and without diabetes mellitus after percutaneous coronary intervention with the resolute zotarolimus-eluting stent: 2-year results from the prospectively pooled analysis of the international global resolute program. JACC Cardiovasc Interv. 2013;6:357–368. doi: 10.1016/j.jcin.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 80.von Birgelen C, Basalus MW, Tandjung K, van Houwelingen KG, Stoel MG, Louwerenburg JH, Linssen GC, Said SA, Kleijne MA, Sen H, Lowik MM, van der Palen J, Verhorst PM, de Man FH. A randomized controlled trial in second-generation zotarolimus-eluting Resolute stents versus everolimus-eluting Xience V stents in real-world patients: The twente trial. J Am Coll Cardiol. 2012;59:1350–1361. doi: 10.1016/j.jacc.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 81.Silber S, Windecker S, Vranckx P, Serruys PW. Unrestricted randomised use of two new generation drug-eluting coronary stents: 2-year patient-related versus stent-related outcomes from the resolute all comers trial. Lancet. 2011;377:1241–1247. doi: 10.1016/S0140-6736(11)60395-4. [DOI] [PubMed] [Google Scholar]
- 82.Neumann FJ, Widimsky P, Belardi JA. One-year outcomes of patients with the zotarolimus-eluting coronary stent: Resolute international registry. EuroIntervention. 2012;7:1181–1188. doi: 10.4244/EIJV7I10A189. [DOI] [PubMed] [Google Scholar]
- 83.Farkouh ME, Boden WE, Bittner V, Muratov V, Hartigan P, Ogdie M, Bertolet M, Mathewkutty S, Teo K, Maron DJ, Sethi SS, Domanski M, Frye RL, Fuster V. Risk factor control for coronary artery disease secondary prevention in large randomized trials. J Am Coll Cardiol. 2013;61:1607–1615. doi: 10.1016/j.jacc.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 84.Standards of medical care in diabetes--2013. Diabetes Care. 2013;36:S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, Reaven P, Sherwin RS. Intensive glycemic control and the prevention of cardiovascular events: Implications of the accord, advance, and va diabetes trials: A position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119:351–357. doi: 10.1161/CIRCULATIONAHA.108.191305. [DOI] [PubMed] [Google Scholar]
- 86.Habib A, Karmali V, Polavarapu R, Akahori H, Nakano M, Yazdani S, Otsuka F, Pachura K, Davis T, Narula J, Kolodgie FD, Virmani R, Finn AV. Metformin impairs vascular endothelial recovery after stent placement in the setting of locally eluted mammalian target of rapamycin inhibitors via s6 kinase-dependent inhibition of cell proliferation. J Am Coll Cardiol. 2013;61:971–980. doi: 10.1016/j.jacc.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wiviott SD, Braunwald E, Angiolillo DJ, Meisel S, Dalby AJ, Verheugt FW, Goodman SG, Corbalan R, Purdy DA, Murphy SA, McCabe CH, Antman EM. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38. Circulation. 2008;118:1626–1636. doi: 10.1161/CIRCULATIONAHA.108.791061. [DOI] [PubMed] [Google Scholar]
- 88.James S, Angiolillo DJ, Cornel JH, Erlinge D, Husted S, Kontny F, Maya J, Nicolau JC, Spinar J, Storey RF, Stevens SR, Wallentin L. Ticagrelor vs. Clopidogrel in patients with acute coronary syndromes and diabetes: A substudy from the platelet inhibition and patient outcomes (PLATO) trial. Eur Heart J. 2010;31:3006–3016. doi: 10.1093/eurheartj/ehq325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diletti R, Serruys PW, Farooq V, Sudhir K, Dorange C, Miquel-Hebert K, Veldhof S, Rapoza R, Onuma Y, Garcia-Garcia HM, Chevalier B. Absorb II randomized controlled trial: A clinical evaluation to compare the safety, efficacy, and performance of the absorb everolimus-eluting bioresorbable vascular scaffold system against the xience everolimus eluting coronary stent system in the treatment of subjects with ischemic heart disease caused by de novo native coronary artery lesions: Rationale and study design. Am Heart J. 2012;164:654–663. doi: 10.1016/j.ahj.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 90.Park SJ, Kang SJ, Ahn JM, Shim EB, Kim YT, Yun SC, Song H, Lee JY, Kim WJ, Park DW, Lee SW, Kim YH, Lee CW, Mintz GS, Park SW. Visual-functional mismatch between coronary angiography and fractional flow reserve. JACC Cardiovasc Interv. 2012;5:1029–1036. doi: 10.1016/j.jcin.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 91.Nam CW, Mangiacapra F, Entjes R, Chung IS, Sels JW, Tonino PA, De Bruyne B, Pijls NH, Fearon WF. Functional syntax score for risk assessment in multivessel coronary artery disease. J Am Coll Cardiol. 2011;58:1211–1218. doi: 10.1016/j.jacc.2011.06.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.