Abstract
Background
Alcohol use disorders (AUDs) are a major public health problem, and the few treatment options available to those seeking treatment offer only modest success rates. There remains a need to identify novel targets for the treatment of AUDs. The neuronal nicotinic acetylcholine receptors (nAChRs) represent a potential therapeutic target in the brain, as recent human genetic studies have implicated gene variants in the α5 nAChR subunit as high risk factors for developing alcohol dependence.
Methods
Here, we evaluate the role of α5* nAChR for ethanol-mediated behaviors using male α5+/+ and α5−/− mice. We characterized the effect of hypnotic doses of ethanol and investigated drinking behavior using an adapted Drinking-in-the Dark (DID) paradigm that has been shown to induce high ethanol consumption in mice.
Results
We found the α5 subunit to be critical in mediating the sedative effects of ethanol. The α5−/− mice showed slower recovery from ethanol-induced sleep, as measured by loss of righting reflex. Additionally the α5−/− mice showed enhanced impairment to ethanol-induced ataxia. We found the initial sensitivity to ethanol and ethanol metabolism to be similar in both α5+/+ and α5−/− mice. Hence the enhanced sedation is likely due to a difference in the acute tolerance of ethanol in α5−/− mice. However the α5 subunit did not play a role in ethanol consumption for ethanol concentrations ranging from 5% to 30% using the DID paradigm. Additionally, varenicline was effective in reducing ethanol intake in α5−/− mice.
Conclusion
Together, our data suggest that the α5 nAChR subunit is important for the sedative effects of ethanol but does not play a role in ethanol consumption in male mice. Varenicline can be a treatment option even when there is loss of function of the α5 nAChR subunit.
Keywords: α5 nAChR, ethanol, mice, varenicline
Introduction
Alcohol use disorders (AUDs) are a world-wide problem with few effective treatments (Moss et al., 2007). Excessive drinking alone ranks third in leading causes of preventable deaths with a heavy economic burden on society (National Institute of Alcohol Abuse and Alcoholism, NIAAA). There remains a need to improve treatment options and strategies to help individuals with AUDs. The neuronal nicotinic acetylcholine receptors (nAChRs) represent a pharmacotherapeutic target for the treatment of AUDs (Chatterjee and Bartlett, 2010) as ethanol has been shown to interact with nAChRs in the brain (Blomqvist et al., 1992; Ericson et al., 2003; Larsson et al., 2002). The nAChRs are pentameric ligand-gated ion channels that are widely expressed in the brain (Gotti et al., 2006; Luetje et al., 1990). They are expressed as a combination of α2–6 and β2–4 subunits in the heteromeric form or as α7–10 subunit in the homomeric form.
It has been shown by many laboratories that neuronal nicotinic receptors play an important role in ethanol-mediated behaviors (Aistrup et al., 1999; Blomqvist et al., 1993; Cardoso et al., 1999; Ericson et al., 2009; Larsson et al., 2004; Narahashi et al., 1999; Steensland et al., 2007). Moreover, recent studies have shown varenicline, a smoking cessation aid, with activity at nAChRs, is effective in reducing alcohol administration in humans (McKee et al., 2009). Recent human genetic studies have implicated single nucleotide polymorphisms (SNPs) in the CHRNA5 gene, encoding the α5 nAChR subunit, to be strongly associated with an increased risk to develop alcohol dependence (Joslyn et al., 2008; Schlaepfer et al., 2008; Wang et al., 2009). The α4β2α5 subtype is present at high concentrations in the midbrain dopaminergic reward pathway (Champtiaux et al., 2002; Gaimarri et al., 2007). At this time, there is little known about the link between the susceptibility to developing alcohol dependence and variations in the α5 gene.
Transgenic mice with genetically modified nAChR subunits have become important tools for studying the importance of specific nAChR subunits in ethanol-mediated behaviors. Previous studies have shown that α4 and β2 nAChR subunits do not play a role in ethanol consumption using transgenic knockout mice (Hendrickson et al., 2010; Kamens et al., 2010). Whether the α5 subunit is important for ethanol sensitivity or ethanol consumption remains unknown. In contrast, there are a number of studies showing that the α5 nAChR subunit is strongly associated with the behavioral effects of nicotine including altered anxiety-related behavior (Gangitano et al., 2009) and increased nicotine intake (Fowler et al., 2011). Additionally, α5 deficient (−/−) mice are hyposensitive to high acute nicotine doses with reduced nicotine-induced seizures (Salas et al., 2003). The link between α5 subunit and ethanol dependence represents an important question to explore. Additionally, whether the α5 gene affects the efficacy of varenicline (Chantix®) with activity at α4β2*- containing nAChRs in reducing ethanol consumption.
In this study, we used α5 subunit deficient mice to evaluate the importance of the subunit in ethanol-mediated behaviors in vivo. We assessed both the sedative and ataxic doses of ethanol using the loss of righting reflex (LORR) and rotarod paradigms, respectively. In addition, we determined the baseline ethanol consumption patterns in the male α5 +/+ (wild type) and α5 −/− (deficient) mice. We found the α5 subunit to play an important role for the sedative effects of ethanol at the high acute doses without affecting ethanol metabolism. However, we show that the α5 subunit does not play a significant role in modulating ethanol consumption. Importantly, varenicline, shown to be efficacious in human alcoholics, reduced ethanol consumption selectively in both the α5+/+ and α5−/− mice. Our data suggest that varenicline can be administered as a potential treatment to humans with SNPs in the CHRNA5 gene.
Methods and Materials
Animals and Housing
All male mice were weaned at 21 days old and were group housed 2–5 per cage of the same sex on a 12 hour light/dark cycle (lights on 7am) in climate controlled rooms. For the drinking experiments, mice were individually housed under a reverse light cycle (lights on 10 pm). All mice had food and water available ad libitum. All procedures were pre-approved by the Gallo Center ethics committee and were in accordance with NIH guidelines for the Humane Care and Use of Laboratory Animals. The α5−/− mice were generously provided by Dr. Jerry Stitzel (University of Colorado). All mice were backcrossed to a C57BL/6J (Jackson Laboratory, Bar Harbor, ME) background over 10 generations before arrival at the Gallo Center. The male α5+/+ and matching α5−/− littermate mice used were generated from heterozygous breeding pairs. All transgenic mice used were healthy and appeared similar to their wild type littermates. Genotyping was performed using polymerase chain reaction as previously described (Salas et al., 2003).
Loss of Righting Reflex
For the loss of righting reflex (LORR) paradigm, 3.2 g/kg (20% v/v) ethanol was given as an intraperitoneal (i.p.) injection. The time for the latency to LORR was recorded as the time from ethanol injection to the time the mouse was unable to right itself three times within a 15 second period from the supine position. LORR was recorded as the time elapsed from when the mouse was unable to right itself three times within a 15 second period from the supine position until it recovered and was able to right itself 3 times within a 15 second period.
The lowest dose of ethanol required to induce LORR in approximately 50% of the sample population (LORR ED50) was assessed by the “up and down” method (Newton et al., 2004). In this paradigm, a mouse was given a 2.8 g/kg dose of ethanol (i.p.) and tested for LORR 5 minutes following the injection. If an LORR lasted at least 1 minute, the next mouse was given a dose lowered by 0.1 g/kg of ethanol. Conversely, if there was not an LORR lasting more than 1 minute, the dose was increased by 0.1 g/kg, thereby approaching the threshold dose required to achieve a LORR. The LORR ED50 value was calculated as previously described (Newton et al., 2004).
Rotarod Ataxia
Mice were trained for six sessions to remain on an accelerating rotarod (Jones and Roberts, 1968) for 6 minutes, with an initial rotating rate of 6 rpm and a final rate of 30 rpm. During the 75-min test trial, the latency to fall was tested every 15 min following a 2 g/kg (i.p.) ethanol injection. A control trial was performed with mice receiving a single saline (0.0125 ml/kg, i.p.) injection 15min prior to testing on the rotarod.
Blood Ethanol Clearance
The ethanol clearance was tested by determining blood ethanol concentration (BEC) at several time points following an i.p. injection of 3.2 g/kg ethanol (Zapata et al., 2006). Trunk blood samples were collected in tubes containing 75 μL of EDTA to prevent clotting. Whole blood was centrifuged at 4°C for 20 minutes at 4000 rpm and the serum was separated into aliquots. Samples were stored at −80°C until running the BEC assay. Analysis was done using the nicotinamide adenine dinucleotide (NAD)-ethanol dehydrogenase (ADH) spectrophotometric assay (Zapata et al., 2006). All reagents used in this assay were purchased from Sigma-Aldrich (St. Louis, MO). BECs were computed against a standard calibration curve. All samples and standards were run in triplicate.
Drinking in the Dark paradigm
To evaluate the baseline drinking consumption of α5+/+ and α5−/− mice, we adapted the drinking-in-the-dark (DID) model of ethanol consumption (Rhodes et al., 2005; Steensland et al., 2010). Briefly, male α5+/+ and α5−/− littermates (P21, week 3) were individually housed in double-grommet cages and given access to one bottle of 20% (v/v) ethanol and one bottle of filtered water for a four-hour period (1 pm–5 pm), three hours into the dark cycle, Monday to Friday, in a reverse light/dark cycle room for 6 weeks (30 exposures). The ethanol- and water-containing bottles sides were switched every presentation to prevent a preference for drinking from one side. Two bottles of filtered water were available at all other times. None of the solutions were sweetened with sucrose, nor were the solutions introduced in gradually increasing concentrations to initiate drinking. All fluids were presented in 50-mL graduated plastic centrifuge tubes (Fisher Scientific, New Jersey, USA) fitted with rubber stoppers and a 2.5 inches stainless-steel sipper tubes with a double ball bearings. Bottles were weighed at 2 and 4 hours after presentation, and measurements were taken to the nearest 0.1gram. The preference for the alcohol over water (20% ethanol (ml) divided by total fluid intake (ml) times 100) was also calculated. We also measured 5% sucrose consumption using the DID paradigm for 10 exposures in the same male α5+/+ and α5−/− littermates at 20–22 weeks. They were given access to one bottle of 5% (v/v) sucrose and one bottle of filtered water for a four-hour period (1 pm–5 pm), three hours into the dark cycle, Monday to Friday, in a reverse light/dark cycle room for 2 weeks (10 total exposures). The position of the sucrose- and water-containing bottles were switched every presentation to prevent a preference for drinking from one side. Two bottles of filtered water were available at all other times including between weeks 12–20. Bottles were weighed at 2 and 4 hours after presentation, and measurements were taken to the nearest 0.1 gram. The preference of sucrose over water was calculated. Mouse weights were measured daily to calculate the adjusted g/kg intake.
Acute effects of Varenicline on 20% ethanol intake and 5% sucrose intake
Immediately following maintained stable baseline drinking levels of 20% (v/v) ethanol solution (30 exposures, weeks 3–9) or 5% sucrose using the DID paradigm (10 exposures, weeks 20–22) we evaluated the acute effects of varenicline administration on ethanol intake in α5+/+ and α5−/− mice. Varenicline (0.3 or 2 mg/kg) or vehicle (saline) was administered (i.p.) 30 minutes before the presentation of the bottles. An average of the response to 3 saline injections was taken to be the vehicle measurement for both genotypes. Each injection was given at least two days apart using a Latin square design, thus each animal served as its own control. Varenicline was dissolved in 0.9% w/v saline and administered in a 0.01 ml/kg volume. Body weights were taken before drug administration.
Drugs
Solutions containing 95% (v/v) ethanol (Gold Shield Chemical Co., Hayward, CA), DHβE (Sigma-Aldrich, St. Louis, MO), and Varenicline (Pfizer Global Research and Development, Groton, CT) were prepared fresh daily for all experiments and dissolved in saline.
Statistics
We used Sigma Stat (Systat Software, San Jose, CA), using two-way, one-way ANOVA or unpaired t-test wherever applicable with Newman–Keuls post hoc analysis when a significant effect was found (p < 0.05).
Results
Acute Effects of Ethanol
We first studied the behavioral manifestations following high acute ethanol doses in α5+/+ and α5−/− mice, using the loss of righting reflex (LORR) and accelerating rotarod paradigms. The α5−/− mice were more sensitive to a hypnotic dose of ethanol (3.2 g/kg, i.p.) and lost their righting reflex for a significantly longer period of time (α5+/+: 26.9±4.8 mins; α5−/−: 40.7±4.3 mins; * p<0.05 two-tailed unpaired t test, n=8–13, Fig. 1A,). The latency to loss of righting reflex was not significantly different (α5+/+: 89±3.1 secs; α5−/−: 95±5.4 secs; two-tailed unpaired t test, n.s). Similarly, at the ataxic dose of ethanol (2 g/kg, i.p.), the α5−/− exhibited a difference in tolerance to ethanol compared with α5+/+ mice. They displayed a shorter latency to fall at longer testing time points (75 mins: α5+/+: 314.8±17.7 sec; α5−/−: 231.7±32.7 sec., n=11, Fig. 1B) in the rotarod paradigm. A two-way ANOVA analysis revealed a significant effect of genotype (F (1, 120) = 4.7, p < 0.05), an effect of time after injection (F (5, 120) = 18.9, p<0.001) but no genotype-time interaction (F (5, 120) = 0.9, n.s.) with post-hoc revealing significance at 75 min (*p<0.05). To examine if this effect was due to an initial different level of response at this high dose, we assessed the minimum threshold dose to achieve the LORR behavior. We found no difference in ED50 of ethanol (α5+/+: 3.0±0.1g/kg; α5−/−: 2.9±0.1 g/kg) required to produce LORR, (two-tailed unpaired t test, n.s., n=6 animals per genotype, Fig. 2A) suggesting the possibility of developing a difference in the acute tolerance of ethanol between the genotypes. To exclude a difference in ethanol metabolism between genotypes, we measured their blood ethanol clearance (BEC) following a high dose of ethanol (3.2 g/kg). We found no difference in the BEC during the 90 minutes following the 3.2 g/kg ethanol injection (B, n=3 animals per genotype for each time point, Fig. 2B). A two-way ANOVA analysis revealed no effect of genotype (F (1, 10) = 0.1, n.s.), or genotype-time interaction (F (2, 10) = 0.001, n.s.) but an effect of time after injection (F (2, 10) = 4.1, p<0.05).
Figure 1. The α5 subunit plays an important role for sedative dose of ethanol and influences ethanol-induced ataxia.
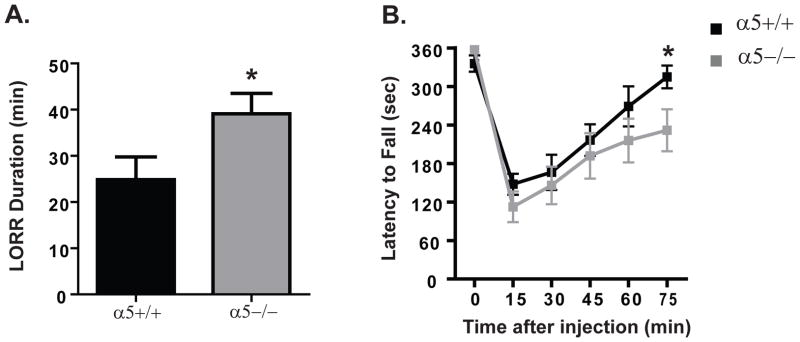
(A) The loss of righting reflex (LORR) duration for 3.2g/kg (i.p.) ethanol was shorter for α5+/+ (black) compared to α5−/− (gray) mice. (B) On the accelerating rotarod, α5−/−(gray) had an increased latency to fall compared to α5+/+ (black) mice for 2 g/kg (i.p.) ethanol dose. In A, n=8–13 animals, B, n=11 animals. The values are expressed as duration (mins) ± SEM (A) and duration (secs) ± SEM (B) (two-tailed unpaired t-test (A) and two-way ANOVA (B) followed by Newman-Keuls test, *p<0.05).
Figure 2. The initial sensitivity to ethanol and ethanol metabolism is similar for α5+/+ and α5−/− mice.
(A) The threshold dose (ED50) of ethanol required to induce LORR was similar for α5+/+ and α5−/− mice. (B) The blood ethanol concentrations were not different between α5+/+ and α5−/− mice at any of the time points measured. In A, n=6 animals per genotype, B, n=3 animals per genotype for each time point. The values are expressed as alcohol dose (g/kg) ± SEM (A) and blood ethanol concentrations (mg/dl) ± SEM (two-tailed unpaired t-test (A) and two-way ANOVA (B)).
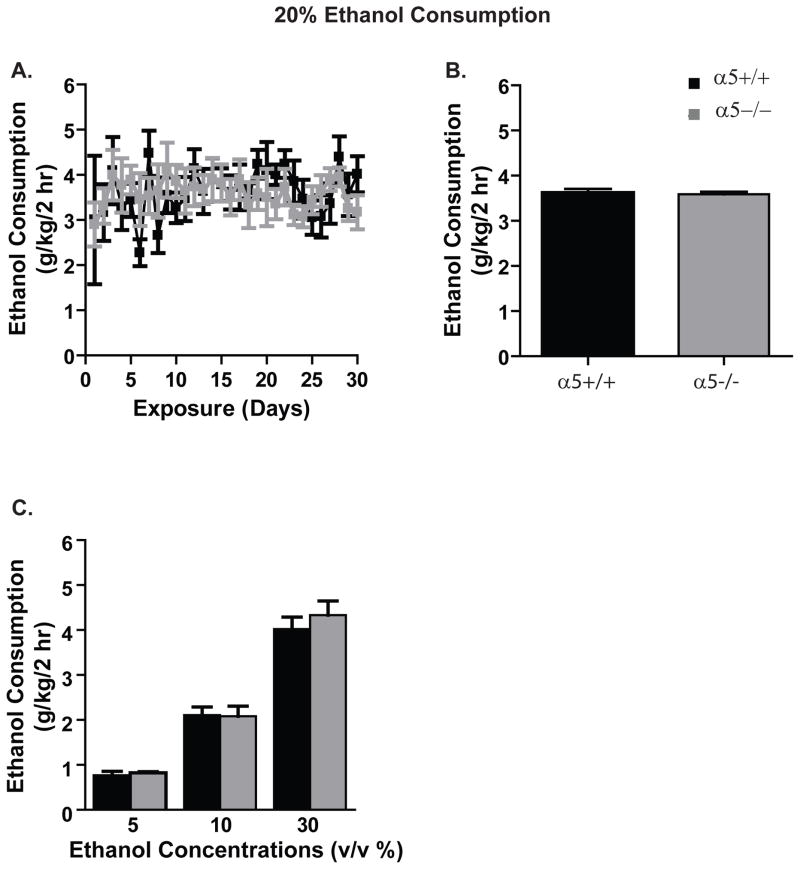
Ethanol Drinking Behavior using the Drinking-in- the-Dark paradigm
Next, we assessed baseline ethanol consumption of the α5+/+ and α5−/− mice using the Drinking-in- the-Dark (DID) paradigm for at least six weeks (30 exposures). The mice were given a four-hour access period (1pm to 5pm) for five days (Monday-Friday) with a two-bottle choice of water and 20% ethanol. We found α5+/+ and α5−/− mice consumed similar amounts of 20% ethanol (n=7–11 animals, Fig. 3A). Two-way ANOVA repeated measures revealed no significant effect at the 2 hour time point [genotype: (F (1, 464) = 0.01, n.s.)], [exposure day: (F (29, 464) = 1.1, n.s.); [genotype-exposure day interaction: (F (29, 464) = 0.8, n.s.)] and no difference between α5+/+ and α5−/− mice in average ethanol intake for the entire 6-week time period (α5+/+: 3.6±0.1g/kg/2 hrs; α5−/−: 3.6±0.05g/kg/ 2 hrs; two-tailed unpaired t test, n.s., n=7–11 animals, Fig. 3B). We found no difference in the ethanol intake at the 4-hour time point (data not shown). However, the average water intake and preference for ethanol over 6 weeks were different over time between α5+/+ and α5−/− mice at both 2 hours and 4 hours [water intake: α5+/+: 1.3±0.1g/kg/2hrs, 2.6±0.2g/kg/4hrs; α5−/−: 1.6±0.1g/kg/2hrs, 3.4±0.2g/kg/4hrs; preference: α5+/+:68.9±2.1%/2 hrs, 64.1± 2%/4hrs; α5−/−: 63.8±1.3%/2 hrs, 59.3±1.1%/4 hrs; data not shown]. Two-way repeated measures ANOVA at the 2 hr time point revealed a significant effect of water intake over time (F (29, 464) = 2.9, p<0.0001) but no effect of genotype (F (1, 464) = 1.0, n.s.) or genotype-time interaction (F (29, 464) = 1.4, n.s.). Similarly, two-way repeated measures ANOVA at 2 hr revealed a significant effect of preference over time (F (29, 464) = 3, p<0.0001) but no effect of genotype (F (1, 464) = 0.62, n.s.) or genotype-time interaction (F (29, 464) = 1.3, n.s.). Two-way repeated measures ANOVA at the 4 hr time point revealed a significant effect of water intake over time (F (29, 464) = 6.3, p<0.0001) but no effect of genotype (F (1, 464) = 1.0, n.s.) or genotype-time interaction (F (29, 464) = 0.8, n.s.). Similarly, two-way repeated measures ANOVA at 4 hr revealed a significant effect of preference over time (F (29, 464) = 5.1, p<0.0001), an effect of genotype-time interaction (F (29, 464) = 1.7, p<0.05) but no effect of genotype (F (1, 464) = 0.62, n.s.).
Figure 3. The α5 nAChR does not play a role in ethanol consumption in mice using the drinking-in-the-dark paradigm.
(A) Both the α5+/+ (black) and the α5−/− (gray) mice show similar 20% ethanol consumption during the 30 days of exposure. (B) The average ethanol intake across 30 exposures for α5+/+ and α5−/− mice is similar. (C) α5+/+ and α5−/− mice shown similar ethanol intake at 5%, 10% and 30% ethanol concentrations. In A&B, n=7–11 animals and in C, n=5–6 animals. The values are expressed as mean alcohol intake (g/kg/2 hrs) ± SEM (two-way ANOVA repeated measures (A) and two-way ANOVA (C) followed by Newman-Keuls test and two-tailed unpaired t-test (B)).
The α5−/− mice have been shown to have increased nicotine intake only at high doses compared with their wild-type littermates (Fowler et al., 2011). Hence, we assessed if concentrations apart from the 20% affected the ethanol intake in α5−/− and α5+/+ mice. For this study, we administered ethanol at 5%, 10% and 30% concentration for one week (five ethanol exposures) and 2 hr ethanol intake was measured using the DID paradigm (see Materials and Methods). We found no difference in the ethanol consumption at any of the concentrations tested here at the 2 hour time point (5%: α5+/+: 0.75±0.09g/kg/2hrs; α5−/−: 0.82±0.02g/kg/2hrs; 10%: α5+/+: 2.1±0.1g/kg/2hrs; α5−/−: 2.08±0.2g/kg/2hrs; 30%: α5+/+: 4±0.2g/kg/2hrs; α5−/−: 4.3±0.3g/kg/2hrs, n=5–6 animals, Fig. 3C). Two-way ANOVA for ethanol intake revealed an effect of ethanol concentration (F (2, 124) = 147.5, p<0.001) but no effect of genotype (F (1, 124) = 0.5, n.s.) or genotype-concentration interaction (F (2, 124) = 0.4, n.s.). We found difference in the ethanol preference only at the 5% ethanol concentration at the 2 hour time point (5%: α5+/+: 72.27±4.1%; α5−/−: 58.7±2.7%; 10%: α5+/+: 59±3%; α5−/−: 62.5±1.5%;; 30%: α5+/+: 48.7±5%; α5−/−: 46.7±5%). Two-way ANOVA for ethanol preference revealed an effect of ethanol concentration (F (2, 124) = 13.7, p<0.001) but no effect of genotype (F (1, 124) = 1.26, n.s.) or genotype-concentration interaction (F (2, 124) = 2.5, n.s.).
The Effect of Acute Treatment of Varenicline
We also evaluated the effect of varenicline with partial agonist activity at the α4β2* nAChR on voluntary ethanol consumption in the α5+/+ and α5−/− mice. Varenicline (0.3 and 2 mg/kg, i.p.) or vehicle (saline) was given to the mice that had at least 6 weeks of exposure with stable baseline drinking levels. Interestingly, one-way ANOVA repeated measures revealed that varenicline treatment had an overall main effect on 20% ethanol consumption in both genotypes at both 2 hrs and 4 hrs time point measured [2 hrs: α5+/+: (F (2,16) = 4.4, p<0.05); α5−/−: (F (2,22) = 7.7, P<0.01), 4 hrs: α5+/+: (F (2,16) = 4.5, p<0.05); α5−/−: (F (2,22) = 6.6, p<0.01)]. Post hoc analysis revealed that the highest dose of varenicline (2 mg/kg) significantly decreased the amount of ethanol consumed in both α5+/+ and α5−/−mice at 2 hrs (*p<0.05, **p<0.01, n=9–13 animals, Fig 4A&B, 4 hr data not shown). Subsequently, there was no overall main effect on water at both time points [2 hrs: α5+/+: (F (2,16) = 0.3, n.s.); α5−/−: (F (2,22) = 0.7, n.s.), Fig 4C&D, 4 hrs: α5+/+: (F (2,16) = 0.9, n.s.); α5−/−: (F (2,22) = 0.9, n.s.) (data not shown)] or preference of ethanol over water [2 hrs: α5+/+: (F (2,16) = 0.7, n.s.); α5−/−: (F (2,22) = 2.6, n.s.), 4hrs: α5+/+: (F (2,16) = 0.7, n.s.); α5−/−: (F (2,22) =0.2, n.s.) (data not shown)]. To determine the selectivity of varenicline in reducing ethanol consumption, we used the DID-model of 5% sucrose consumption (see Materials and Methods). Varenicline (0.3 and 2 mg/kg, i.p.) or saline were administered to mice that have been exposed to 5% sucrose for two weeks and had a stable baseline sucrose intake (2 hrs: α5+/+: 5.2±1 g/kg/2hrs; α5−/−: 5.9±2.6 g/kg/2hrs, 4 hrs: α5+/+: 8.8±1.5 g/kg/4hrs; α5−/−: 8.7±3.1 g/kg/4hrs). We found no effect of varenicline (2 mg/kg) treatment on 5% sucrose intake in both α5+/+ and α5−/− mice at both time points (two-tailed paired t test, n.s., n=9–13 animals, Fig. 4E&F, (4 hr data not shown)). There was also no effect on water or preference of sucrose over water in both genotypes at both time points (two-tailed paired t test, n.s., (4 hr data not shown)).
Figure 4. Varenicline is effective in selectively reducing ethanol consumption in α5+/+ and α5−/− mice without affecting water or sucrose consumption.
Varenicline (2 mg/kg, i.p.) treatment decreased voluntary ethanol consumption in both α5+/+ (A) and α5−/− (B) mice 2 hrs after the onset of drinking using the two bottle choice DID paradigm. Varenicline was selective in decreasing ethanol consumption with no effect on water consumption (C&D) and sucrose consumption (E&F) in both α5+/+ and α5−/−mice. n=9–13 animals. The values are expressed as mean ethanol or sucrose intake (g/kg) ± SEM (A,B,E,F) or water intake (ml/100g) ± SEM (C,D) (repeated measures ANOVA followed by Newman-Keuls post hoc test). *p<0.05, **p<0.01, compared with vehicle.
Discussion
In this study, we have used α5 nAChR subunit deficient mice to characterize the role of this subunit in ethanol-mediated behaviors. Also, given that the α5 nAChR subunit can modify the pharmacology of α4β2*-containing nAChRs (Kuryatov et al., 2008; Ramirez-Latorre et al., 1996), we examined whether the efficacy of varenicline, a partial agonist at α4β2 nAChR (Rollema et al., 2009) that is known to reduce ethanol consumption (Hendrickson et al., 2010; Steensland et al., 2007), would be altered by the α5 nAChR subunit.
We found that the α5 nAChR subunit plays a role in the sedative effects of high acute doses of ethanol. Both α5+/+ and α5−/− mice showed a similar minimum dose of ethanol required to achieve hypnosis, however, the α5−/− mice demonstrated a slower recovery from ethanol-induced sleep compared with α5+/+ mice, suggesting the development of reduced acute tolerance. Given that the blood ethanol clearance is similar for both genotypes, the α5−/− mice appear to be recovering at a lower blood ethanol concentration. Studies have shown that the cerebellum is the critical region for ethanol-induced mouse sedation, and a major nAChR subtype mediating this effect is the α4β2*-containing nAChR (Taslim et al., 2008). In cell-based heterologous systems, the co-expression of α5 with α4 and β2 subunit has been shown to increase the expression and alter the function of the α4β2 nAChRs (Kuryatov et al., 2008). Hence, it is plausible that the presence of α5 can influence the function of α4β2* containing nAChRs and thereby affect the sedation behavior (Al-Rejaie and Dar, 2006; Taslim et al., 2008; Taslim and Saeed Dar, 2011). It is interesting to note that the increased level of sensitivity to high doses of ethanol in α5−/− mice is in sharp contrast to their dramatically low sensitivity for acute high doses of nicotine (Salas et al., 2003). Typically, a low sensitivity or high tolerance to drugs of abuse is thought to be an indicator for development of drug dependence (Schuckit et al., 2006; 2012).
Historically, the low level of response (LR) phenotype that defines the initial sensitivity to alcohol has been an important predictive indicator for the development of alcohol use disorders (AUDs) (de Fiebre and Collins, 1992; Schuckit et al., 2012; Trim et al., 2010). Researchers have developed long-sleep (LS) and short-sleep (SS) mice that had been genetically selected for high and low ethanol sensitivity respectively. The mice were subjected to a variety of behavioral measures to determine the duration of their loss of righting reflex (LORR) and ethanol-induced ataxia after a hypnotic dose of ethanol (de Fiebre and Collins, 1992; de Fiebre et al., 1990; 1992). The SS mice had a shorter LORR duration and a longer latency to fall from the rotarod. The SS mice also drank more ethanol and had lower blood ethanol concentrations than the LS mice (de Fiebre and Collins, 1993). Based on findings such as these, it is thought that genes which influence initial sensitivity to ethanol may be crucial in the treatment of AUDs.
The high sensitivity to ethanol in α5−/− mice may initially seem contrary to the human genetic studies linking the α5 gene to high risk for alcohol dependence (Schlaepfer et al., 2008; Wang et al., 2009) and low level of response (LR) to alcohol (Joslyn et al., 2008). However, α5 SNPs have been reported to be associated with a two-fold increase in α5 mRNA (Wang et al., 2009). We hypothesize the absence of α5 leads to an increase in ethanol-induced sedation and slower recovery, the over expression of α5 will lead to a reduction in sedation and a quicker recovery from ethanol-induced sleep and hence higher tolerance.
Another key finding from this study is that the α5 subunit does not play a role in basal ethanol consumption using the two-bottle choice DID paradigm in male mice. This is not completely surprising since mice deficient of the α4 and β2 nAChR subunit show no effect on ethanol intake and α4β2 nAChR antagonist DHβE does not affect ethanol administration in rodents (Hendrickson et al., 2010; Kamens et al., 2010; Le et al., 2000). However, as this study was conducted in male mice we cannot rule out the possibility that the α5 subunit plays a role in ethanol consumption in female mice. It has been shown there is progesterone-dependent modulation of α5 nicotinic receptor expression that contributes to fluctuations in anxiety levels during the ovarian cycle (Gangitano et al., 2009). In future studies, it will be important to investigate the role of the α5 subunit in ethanol-mediated behaviors in female mice. A recent study suggests that α3β4* rather than α4β2* nAChRs may play a prominent role in ethanol consumption (Chatterjee et al., 2010). Although there is some evidence that α5 may also be present in α3β4* nAChRs (Gerzanich et al., 1998; Quick et al., 1999), most studies indicate a more frequent association of α5 subunits with the α4β2*-containing nAChRs in the brain (Gotti et al., 2009; Mao et al., 2008). Moreover, the α5 subunit appears to play no role in ethanol consumption following increasing ethanol concentrations, ruling out the possibility of a concentration-dependent factor. This is in contrast to nicotine studies where the α5−/− but not the α5+/+ mice self-administer high doses of nicotine, suggesting that the α5 subunit plays a role in limiting nicotine intake (Fowler et al., 2011). Our data suggests that the α5 subunit, most likely in the α4α5β2* nAChR subtype, plays a very minor role in ethanol consumption in mice using the drinking-in-the-dark paradigm.
Given than ~80% of smokers also drink alcohol, varenicline was tested in rats and humans and shown to dose-dependently reduce alcohol consumption (McKee et al., 2009; Steensland et al., 2007). Here, we find that varenicline is effective in reducing ethanol consumption selectively with no change in sucrose or water consumption in both the α5+/+ and α5−/− mice. Interestingly, the deletion of the α4 (Hendrickson et al., 2010) but not the β2 (Kamens et al., 2010) nAChR subunit changes the efficacy of varenicline in reducing ethanol intake. Therefore, it appears that both α5 and β2 subunits of the putative α4β2α5 nAChR subtypes are not required for the effect of varenicline. Moreover, our recent findings have shown that varenicline is a partial agonist at α3β4* nAChRs (Chatterjee et al., 2010) and this receptor subtype may contain the α4 subunit, is effective in reducing ethanol consumption in rodents,
In summary, we have demonstrated that the α5 subunit plays a significant role in the level of response to high doses of acute ethanol. However, it has no effect on voluntary ethanol consumption in the DID paradigm. We have shown that varenicline is efficacious in the presence and absence of the α5 subunit of the nAChR in rodents, this infers but remains to be examined in humans, that varenicline will be an effective treatment option in subjects carrying SNPs in the CHRNA5 gene.
Acknowledgments
We thank Stacy Taylor for excellent technical assistance in weaning and maintaining the breeding colony and Jerry Stitzel for generously providing us with the α5−/− mice used to generate the mice for this study. This work was supported by funding from the NIH 1RC2AA019429-01 (to S.E.B), NIH 1R01AA017924-01 (to S.E.B.), NIH DA015663 (to Michael Marks, University of Colorado), and the State of California for Medical Research on Alcohol and Substance Abuse through the University of California, San Francisco (to S.E.B).
References
- Aistrup GL, Marszalec W, Narahashi T. Ethanol modulation of nicotinic acetylcholine receptor currents in cultured cortical neurons. Mol Pharmacol. 1999;55:39–49. doi: 10.1124/mol.55.1.39. [DOI] [PubMed] [Google Scholar]
- Al-Rejaie S, Dar MS. Antagonism of ethanol ataxia by intracerebellar nicotine: possible modulation by mouse cerebellar nitric oxide and cGMP. Brain Res Bull. 2006;69:187–196. doi: 10.1016/j.brainresbull.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist O, Engel JA, Nissbrandt H, Soderpalm B. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol. 1993;249:207–213. doi: 10.1016/0014-2999(93)90434-j. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Soderpalm B, Engel JA. Ethanol-induced locomotor activity: involvement of central nicotinic acetylcholine receptors? Brain Res Bull. 1992;29:173–178. doi: 10.1016/0361-9230(92)90023-q. [DOI] [PubMed] [Google Scholar]
- Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999;289:774–780. [PubMed] [Google Scholar]
- Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, McIntosh JM, Changeux JP. Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci. 2002;22:1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Bartlett SE. Neuronal nicotinic acetylcholine receptors as pharmacotherapeutic targets for the treatment of alcohol use disorders. CNS Neurol Disord Drug Targets. 2010;9:60–76. doi: 10.2174/187152710790966597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Steensland P, Simms JA, Holgate J, Coe JW, Hurst RS, Shaffer CL, Lowe J, Rollema H, Bartlett SE. Partial agonists of the alpha3beta4* neuronal nicotinic acetylcholine receptor reduce ethanol consumption and seeking in rats. Neuropsychopharmacology. 2010;36:603–615. doi: 10.1038/npp.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fiebre CM, Collins AC. Classical genetic analyses of responses to nicotine and ethanol in crosses derived from long- and short-sleep mice. J Pharmacol Exp Ther. 1992;261:173–180. [PubMed] [Google Scholar]
- de Fiebre CM, Collins AC. A comparison of the development of tolerance to ethanol and cross-tolerance to nicotine after chronic ethanol treatment in long- and short-sleep mice. J Pharmacol Exp Ther. 1993;266:1398–1406. [PubMed] [Google Scholar]
- de Fiebre CM, Marks MJ, Collins AC. Ethanol-nicotine interactions in long-sleep and short-sleep mice. Alcohol. 1990;7:249–257. doi: 10.1016/0741-8329(90)90014-4. [DOI] [PubMed] [Google Scholar]
- de Fiebre CM, Marley RJ, Miner LL, de Fiebre NE, Wehner JM, Collins AC. Classical genetic analyses of responses to sedative-hypnotic drugs in crosses derived from long-sleep and short-sleep mice. Alcohol Clin Exp Res. 1992;16:511–521. doi: 10.1111/j.1530-0277.1992.tb01409.x. [DOI] [PubMed] [Google Scholar]
- Ericson M, Löf E, Stomberg R, Soderpalm B. The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther. 2009;329:225–230. doi: 10.1124/jpet.108.147058. [DOI] [PubMed] [Google Scholar]
- Ericson M, Molander A, Lof E, Engel JA, Soderpalm B. Ethanol elevates accumbal dopamine levels via indirect activation of ventral tegmental nicotinic acetylcholine receptors. Eur J Pharmacol. 2003;467:85–93. doi: 10.1016/s0014-2999(03)01564-4. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaimarri A, Moretti M, Riganti L, Zanardi A, Clementi F, Gotti C. Regulation of neuronal nicotinic receptor traffic and expression. Brain Res Rev. 2007;55:134–143. doi: 10.1016/j.brainresrev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Gangitano D, Salas R, Teng Y, Perez E, De Biasi M. Progesterone modulation of alpha5 nAChR subunits influences anxiety-related behavior during estrus cycle. Genes Brain Behav. 2009;8:398–406. doi: 10.1111/j.1601-183X.2009.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J Pharmacol Exp Ther. 1998;286:311–320. [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Pang X, Gardner PD, Tapper AR. Activation of alpha4* nAChRs is necessary and sufficient for varenicline-induced reduction of alcohol consumption. J Neurosci. 2010;30:10169–10176. doi: 10.1523/JNEUROSCI.2601-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BJ, Roberts DJ. The quantiative measurement of motor inco-ordination in naive mice using an acelerating rotarod. J Pharm Pharmacol. 1968;20:302–304. doi: 10.1111/j.2042-7158.1968.tb09743.x. [DOI] [PubMed] [Google Scholar]
- Joslyn G, Brush G, Robertson M, Smith TL, Kalmijn J, Schuckit M, White RL. Chromosome 15q25.1 genetic markers associated with level of response to alcohol in humans. Proc Natl Acad Sci U S A. 2008;105:20368–20373. doi: 10.1073/pnas.0810970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology (Berl) 2010;208:613–626. doi: 10.1007/s00213-009-1759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol Pharmacol. 2008;74:132–143. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- Larsson A, Jerlhag E, Svensson L, Soderpalm B, Engel JA. Is an alpha-conotoxin MII-sensitive mechanism involved in the neurochemical, stimulatory, and rewarding effects of ethanol? Alcohol. 2004;34:239–250. doi: 10.1016/j.alcohol.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Larsson A, Svensson L, Soderpalm B, Engel JA. Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol. 2002;28:157–167. doi: 10.1016/s0741-8329(02)00244-6. [DOI] [PubMed] [Google Scholar]
- Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Luetje CW, Patrick J, Seguela P. Nicotine receptors in the mammalian brain. Faseb J. 1990;4:2753–2760. doi: 10.1096/fasebj.4.10.2197155. [DOI] [PubMed] [Google Scholar]
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. The alpha4beta2alpha5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem. 2008;104:446–456. doi: 10.1111/j.1471-4159.2007.05011.x. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline Reduces Alcohol Self-Administration in Heavy-Drinking Smokers. Biol Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY. Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend. 2007;91:149–158. doi: 10.1016/j.drugalcdep.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T, Aistrup GL, Marszalec W, Nagata K. Neuronal nicotinic acetylcholine receptors: a new target site of ethanol. Neurochem Int. 1999;35:131–141. doi: 10.1016/s0197-0186(99)00055-8. [DOI] [PubMed] [Google Scholar]
- Newton PM, Orr CJ, Wallace MJ, Kim C, Shin HS, Messing RO. Deletion of N-type calcium channels alters ethanol reward and reduces ethanol consumption in mice. J Neurosci. 2004;24:9862–9869. doi: 10.1523/JNEUROSCI.3446-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RA. Alpha3beta4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rollema H, Hajos M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, Horner WE, Chapin DS, Hoffmann WE, Johnson DE, McLean S, Freeman J, Williams KE. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol. 2009;78:813–824. doi: 10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol. 2003;63:1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ, Lessem JM, McQueen MB, Rhee SH, Ehringer MA. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol Psychiatry. 2008;63:1039–1046. doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit M, Smith T, Pierson J, Danko G, Beltran IA. Relationships among the level of response to alcohol and the number of alcoholic relatives in predicting alcohol-related outcomes. Alcohol Clin Exp Res. 2006;30:1308–1314. doi: 10.1111/j.1530-0277.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim RS, Allen RC, Fukukura T, Knight EE, Cesario EM, Kreikebaum SA. A prospective evaluation of how a low level of response to alcohol predicts later heavy drinking and alcohol problems. Am J Drug Alcohol Abuse. 2012;37:479–486. doi: 10.3109/00952990.2011.598590. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Nielsen CK, Holgate J, Bito-Onon JJ, Bartlett SE. The neurokinin 1 receptor antagonist, ezlopitant, reduces appetitive responding for sucrose and ethanol. PLoS One. 2010;5(9):e12527. doi: 10.1371/journal.pone.0012527. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taslim N, Al-Rejaie S, Saeed Dar M. Attenuation of ethanol-induced ataxia by alpha(4)beta(2) nicotinic acetylcholine receptor subtype in mouse cerebellum: a functional interaction. Neuroscience. 2008;157:204–213. doi: 10.1016/j.neuroscience.2008.08.046. [DOI] [PubMed] [Google Scholar]
- Taslim N, Saeed Dar M. The role of nicotinic acetylcholine receptor (nAChR) alpha7 subtype in the functional interaction between nicotine and ethanol in mouse cerebellum. Alcohol Clin Exp Res. 2011;35:540–549. doi: 10.1111/j.1530-0277.2010.01371.x. [DOI] [PubMed] [Google Scholar]
- Trim RS, Simmons AN, Tolentino NJ, Hall SA, Matthews SC, Robinson SK, Smith TL, Padula CB, Paulus MP, Tapert SF, Schuckit MA. Acute ethanol effects on brain activation in low- and high-level responders to alcohol. Alcohol Clin Exp Res. 2010;34:1162–1170. doi: 10.1111/j.1530-0277.2010.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Grucza R, Cruchaga C, Hinrichs AL, Bertelsen S, Budde JP, Fox L, Goldstein E, Reyes O, Saccone N, Saccone S, Xuei X, Bucholz K, Kuperman S, Nurnberger J, Jr, Rice JP, Schuckit M, Tischfield J, Hesselbrock V, Porjesz B, Edenberg HJ, Bierut LJ, Goate AM. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol Psychiatry. 2009;14:501–510. doi: 10.1038/mp.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Gonzales RA, Shippenberg TS. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology. 2006;31:396–405. doi: 10.1038/sj.npp.1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J. Human alpha4beta2 acetylcholine receptors formed from linked subunits. J Neurosci. 2003;23:9004–9015. doi: 10.1523/JNEUROSCI.23-27-09004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]